Abstract
This article discusses the significance of microcalcifications on mammography and the changes in technology that have influenced management; it also describes a pragmatic approach to investigation of microcalcification in a UK screening programme.
BACKGROUND AND PREVALENCE OF MICROCALCIFICATIONS
Microcalcifications result from the deposition of calcium oxalate and calcium phosphate within the breast tissue. The mechanism by which calcium deposition occurs is not clearly understood; it may be an active cellular process, or an effect of cellular degeneration. Calcification deposits are found within the ductal system, the breast acini, stroma and vessels, mainly as calcium oxalate and calcium phosphate.
Calcium oxalate is produced by apocrine cells in the breast. The crystals are usually colourless and may be difficult to see on routine histopathology without polarization. They are mainly related to benign cystic change, but can also be seen in association with breast cancer. Calcium oxalate cannot be metabolized by mammalian cells and there is emerging evidence that exposure to high levels of oxalate may affect epithelial cells by triggering cellular and genetic changes.1
Calcium phosphate, usually in the form of calcium hydroxyapatite (similar to the form of calcium laid down in bone during skeletal growth2), is more easily recognized in histopathology as it stains purple with haematoxylin and eosin. It is more commonly associated with malignant lesions than calcium oxalate.3 Magnesium-substituted hydroxyapatite has also been reported.4 There is evidence that a change in levels and calcium carbonate content of hydroxyapatite may influence breast cancer cell growth.5
Radiographic microcalcification was first described in 1913 by Albert Salomon, a surgeon in Berlin. He imaged over 3000 surgical specimens describing the association of microcalcifications with breast cancer, demonstrated tumour spread to the lymph nodes and postulated that there were different types of breast cancer.6
Mammography developed as a speciality through the late 1950s and 1960s, with the first screening equipment introduced in the late 1960s.7 Improvements in technology with low kilovoltage, high-definition screen/film combinations and magnification views allowed the diagnosis of preclinical breast cancer. In 1986, Sickles8 proposed an interpretation scheme for microcalcifications utilizing a structured approach of classification into “benign” (requiring no further intervention), “probably benign” (managed by periodic mammography) and “suggestive of malignancy” (requiring biopsy).
The advent of organized screening during the late 1980s led to an increase in the detection of microcalcifications and, as a result, an increase in the detection of ductal carcinoma in situ (DCIS). The age-standardized incidence of DCIS in the UK has increased from around 3 per 100,000 before the advent of the National Health Service Breast Screening Programme (NHSBSP) to 23 per 100,000 in 2013. It continues to increase with the introduction of digital mammography and the UK national trial assessing the effect of increasing the age range of females invited for screening.9
There is no routinely published data from the UK national screening programmes describing the radiological features prompting further assessment, but data from other national screening programmes are available. This indicates that the recall rate for calcifications ranges from 0.4 to 2% of females screened. Investigation of mammographic microcalcification results in a diagnosis of malignancy in up to 0.3% of females screened (Table 1).
Table 1.
Published data on investigation of microcalcifications in population screening programmes
| Comparative screening data | Germany (Wiegel 201010) | United States (Glynn 201111) | Netherlends (Bluekens 201212) | Australia (Farshid 201413) | ||
|---|---|---|---|---|---|---|
| Screening interval | Biennial | Not stated? Annual |
Biennial |
Biennial | ||
| Modality | Digital | Analogue | Digital (Years 1 and 2) | Analogue | Digital | Not stated |
| Number screeneda | 24,067 | 32,600 | 19,282 | 1,045,978 | 152,515 | 1494,809 |
| Recall rate (%) | 7.5 | 6.0 | 8.5 | 1.5 | 2.4 | 4.6 |
| Cancer detection rate (%) | 1.0 | 0.33 | 0.55 | 0.52 | 0.6 | 0.52 |
| Assessment data for calcificationsb | ||||||
| Recall for calcifications | 1.7% | 0.79% | 1.82% | 0.20% | 0.67% | 0.42% had biopsy for calc |
| DCIS from calcifications | 0.20% | 0.04% | 0.09% | |||
| % of women diagnosed with malignancy from calcifications | 0.32% | 0.12% | 0.20% | 0.15% | ||
| PPV of biopsy of calcification | 36% | 41.1% | 22.6% | 35.8% | ||
DCIS, ductal carcinoma in situ; PPV, positive-predictive value.
Proportion of initial and subsequent attenders may differ between cohorts.
Rates are estimated from numbers of lesions/cancers and number of women screened.
Changes due to evolving technology—imaging
Triple assessment by clinical examination, imaging and biopsy remains the fundamental approach to breast diagnosis. The conversion to digital mammography has increased the conspicuity of microcalcification on mammography and the introduction of increasingly sophisticated biopsy techniques has facilitated tissue diagnosis.
Analogue mammography used high-resolution film/screen combinations, which were designed for optimal spatial and contrast resolution at low dose. Computed radiography has been used as an interim step for cost reasons, but digital mammography is now widely used. Digital mammography employs post-processing of the image to enhance the appearance of microcalcifications: the comparative data from the Netherlands in Table 1 demonstrate an increase in calcium detection with the change to digital imaging. Computer-aided diagnosis algorithms can further increase the detection of microcalcifications, but do not improve cancer detection in a screening setting when mammograms are double-read.14 Magnification views can be used to enhance the morphology of calcifications. Digital breast tomosynthesis does not substantially improve the interpretation of microcalcifications.15
Changes due to evolving technology—localization techniques
Microcalcification on mammography is relatively non-specific, and non-operative diagnosis by image-guided needle sample is essential. In the early days, localization was performed using craniocaudal and lateral mammograms with a localization compression grid. Early stereotactic approaches using two-angled views to give a three-dimensional coordinate for needle placement were hampered by the delays of analogue film processing and patient movement.16 The breakthrough into small-field digital technology was a spin-off of the Hubble Space Telescope in the mid-1990s, when a joint project between National Aeronautics and Space Administration and Scientific Imaging Technologies developed a new charged-couple device.17 The technology allowed a high resolution, wide dynamic range and low light sensitivity, shortening exposure time while preserving image quality, resulting in the LORAD Stereo Guided Breast Biopsy System. This has been incorporated into two approaches to stereotactic guided biopsy; it can be performed on dedicated equipment in the prone position that may be more comfortable for the patient and reduces the risk of fainting, but does not provide a conventional mammography facility. Alternatively, biopsy may be performed with the patient seated or recumbent using an add-on device to an upright mammography machine.
More recently, tomosynthesis-guided biopsy technology has become available; this is reported to be quicker and more effective for sampling low-contrast soft-tissue lesions because it requires less repositioning. However, a recent technology evaluation on behalf of the NHSBSP indicated that a stereotactic approach was preferred over tomosynthesis-guided biopsy for soft microcalcifications.18
Changes due to evolving technology—biopsy devices
At the advent of the NHSBSP in 1988, needle sampling was performed by fine needle aspiration cytology to achieve pre-operative diagnosis. Cytological analysis of fine needle specimens is a specialized technique requiring particular expertise on the part of the operator and the cytologist. It is difficult to assess sample adequacy at the time of procedure, and cytology cannot distinguish between non-invasive and invasive malignancy. NHSBSP guidance (2001) indicates a median absolute sensitivity of cytology of 57%—just over half the carcinomas identified had pre-operative malignant cytology.19
In 1994, Parker et al20 published data on the outcomes of 6152 core biopsies from 20 institutions, concluding that 14-G core breast biopsy is a reproducible and reliable alternative to surgical biopsy. This became the percutaneous biopsy method of choice, used as a reference for subsequent developments.
The shortcomings of 14-G biopsies led to the introduction of larger cores assisted by vacuum to ensure retrieval, which also allowed multiple samples to be collected with a single percutaneous introduction.21,22 Such devices range from 7–12 G and can be used to remove tissue volumes equivalent to the weight of a surgical specimen. This allows the pathologist considerably more tissue for analysis improving diagnostic accuracy, but requires additional processing and reporting time to ensure the sample has been sufficiently scrutinized.
RADIOLOGICAL IDENTIFICATION AND INTERPRETATION OF MICROCALCIFICATION
Microcalcifications are seen on many mammograms and there are well-described patterns that help to distinguish benign from potentially malignant changes. The Breast Imaging-Reporting and Data System lexicon supports consistency in nomenclature and provides descriptions to discriminate between benign and malignant changes.23 Approaches to interpretation include appreciation of the extent, morphology and distribution of the calcifications. The Royal College of Radiologists Breast Group has described a five-point scale to communicate the level of suspicion (Table 2).24 Review of prior mammograms to assess interval change is critical, although malignant calcifications may occasionally show minimal change in appearance over several years.25
Table 2.
The Royal College of Radiologists Breast Group Classification for Breast Imaging24
| Mammographic grade | Description |
|---|---|
| M1 | Normal |
| M2 | Benign |
| M3 | Indeterminate/probably benign |
| M4 | Suspicious of malignancy |
| M5 | Highly suspicious of malignancy |
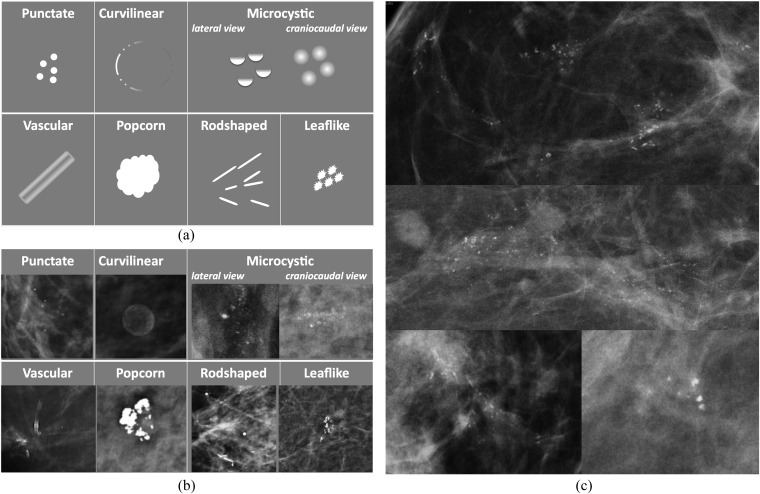
The primary feature of calcifications that prompts further analysis is clustering (>5 calcifications in a square centimetre) Figure 1 illustrates characteristic appearances of benign and malignant calcification. (Figure 1).
Figure 1.
(a) Patterns of calcification associated with a benign change; (b) examples of calcification associated with a benign change; and (c) examples of malignant calcification.
Features that suggest benign change include:
– multiple similar clusters in more than one quadrant in one or both breasts
– uniformity of the individual flecks
– lack of interval change.
Features that indicate further evaluation is required include:
– pleomorphism (variability in shape, size and density)
– linear and branching forms
– segmental distribution within a lobe of the breast
– interval change.
The characteristic morphological features of calcification are less reliable in small clusters and just under 50% of DCIS calcification clusters contain punctate calcifications.25
Microcalcifications associated with a mass lesion should be reviewed carefully. Some patterns are clearly benign (such as popcorn calcification in a fibroadenoma), but malignant change may arise in any area of breast tissue and it is possible, for example, to find DCIS colonizing a fibroadenoma.
If microcalcifications cannot reasonably be assumed to be benign, the appearance is classified as indeterminate to malignant (M3, M4 and M5) and further evaluation is required. Magnification views may be used to demonstrate the morphology more clearly and display very fine calcifications not visible on routine mammography. Lateral mammograms are useful to display the layering of calcifications in the dependent aspect of microcysts, eliminating the need for biopsy. MRI may be used for further evaluation of calcifications26 and has potential to improve specificity by reliably identifying benign change, reducing the number of cases requiring biopsy.
Any calcification that is not clearly benign should be considered for biopsy.
LOCALIZATION TECHNIQUE AND SAMPLING DEVICE
Biopsy is recommended when further imaging of calcification has not shown that changes are clearly benign. In general, calcification is biopsied using a stereotactic approach, or increasingly tomosynthesis, for localization. This requires a team approach to enable accurate positioning and recognition of the mammographic lesion. It is important to understand the geometry of the localization device to ensure precise needle placement.
When appropriate, biopsy can be performed using ultrasound. Successful identification of calcification on ultrasound relies on accurate localization of the cluster in the correct quadrant, distance from the nipple and depth below the skin surface. Calcification tends to be more conspicuous if there is any change in the adjacent soft tissue, and ultrasound-guided biopsy of calcification therefore may have a higher yield of malignancy than mammographic imaging.27,28 The size of the biopsy needle varies with local protocols; some practitioners favour a large vacuum-assisted sample and others prefer a 14-G sample, with vacuum-assisted biopsy (VAB) for selected cases.
The accuracy of 14-G biopsy depends on the size of the microcalcification cluster, the volume of the representative tissue obtained and the nature of the pathology. Today, there is a range of automated needles available commercially. Most studies describe the use of 14-G needles with a long throw (around 2 cm); a smaller gauge or shorter throw provides less tissue for analysis, which reduces the accuracy of the biopsy. The needles are single use, either for use with a reusable biopsy device or a fully disposable needle. The reusable device usually has a more rigorous spring, but can become contaminated with blood tracking back up the needle and therefore should be sterilized between procedures. The fully disposable devices are marginally more costly and are available in a range of gauge and throw. Some needles have a single action of advancing the inner stylet with sample trough, followed by the outer cutting cannula. Others, which allow initial advancement of the stylet followed by advancement of the cutting cannula, aid more precise needle placement in the case of small lesions or minimal tissue depth. All require multiple insertions to retrieve multiple samples and may only obtain scanty samples in dense or fibrous breast tissues.
Immediate specimen radiography is invaluable to assess the adequacy of the specimen—multiple calcifications are essential, preferably in more than one core, depending on the extent of calcification on the mammogram. At least five flecks of calcification should be seen or flecks in three separate cores to ensure that the sample is representative.29 It is important to ensure that calcification seen on specimen radiography correlates to the size and morphology of the calcification on mammography. A 14-G needle sample may be confidently used to establish a benign diagnosis such as microcystic or fibroadenomatoid change, or a malignant diagnosis of invasive cancer, but underestimates the nature of disease in approximately 27% of cases when DCIS and indeterminate lesions such as atypia are present.30
Much of the current literature on percutaneous biopsy of the breast describes outcomes of large sample volumes obtained through a vacuum-assisted needle. This has the advantage of reaching a definitive diagnosis with a single procedure, the duration of the procedure is reduced as the needle is introduced only once and the samples are large in volume. Haemostasis may take longer, but there is no significant difference in complication rates and the procedure is well tolerated by patients.31
Although vacuum-assisted large core biopsy has many benefits, the cost of consumables is substantially greater than 14-G biopsy. In most instances, a 14-G biopsy will be sufficient to make the diagnosis, and even small clusters may be successfully sampled if sufficient care is taken over localization. It is not essential to leave a marker clip in situ if calcium remains visible after biopsy, although an ultrasound visible marker makes it easier to identify the biopsy area when surgery is anticipated. Exceptions include very small or scattered clusters, when VAB is preferred in the first instance. In 2016, the cost of a 14-G needle and needle guides is approximately £20. A vacuum needle requires a dedicated vacuum system and marker clip placement is considered essential. The cost of a vacuum needle, guides and marker clip is around £300. In addition, the increase in work for the pathologist is considerable, as the greater volume of tissue may mean that it is difficult to identify small clusters of calcification and that more levels of multiple blocks need to be examined.
THE ROLE OF THE PATHOLOGIST IN INTERPRETATION OF BIOPSIES
Accurate diagnosis of microcalcifications depends on effective collaboration between the radiologist and pathologist. It is important that the radiologist understands the process of sample preparation. The specimen and request should be fully labelled, including adequate information regarding the nature of the lesion sampled. The radiologists should comment on the presence of calcification and give their opinion of the likely pathology: the pathologist should have access to the specimen radiograph. Segregating the samples containing calcification is useful after VAB so that subsequent levelling can be concentrated on the relevant material. Adequate fixation is necessary and larger volume samples take longer to fix. At embedding, despite the use of heated forceps, it is possible for tiny fragments of biopsy to be conveyed into subsequent samples. In this event, the pathologist may see fragments of irrelevant tissue separate from the main sample, which rarely present a diagnostic dilemma. This effect can be minimized by ensuring that breast biopsies are interspersed with non-breast samples during embedding. The samples are embedded in wax and the block is then rough-cut until the sample is apparent. Occasionally, tissues discarded during this process may include the relevant calcification.
Successive levels are then cut for staining. Current guidance indicates a minimum of 3 levels; a 0.004-mm level is cut, then 10 levels are cut and discarded, the next level is preserved and 10 further levels discarded. This means that approximately 10% of the first 0.13 mm of the block is available for review. A 14-G core biopsy is approximately 1.6-mm thick; so, the first three levels represent <10% of the specimen. Further levels are necessary if the calcification is not visible. In practice, it is more efficient to cut six levels in the first instance and review, before cutting further levels if required. A 9-G vacuum sample is approximately 3.6-mm thick and three levels constitute <4% of the tissue volume. For reference, microcalcifications are demonstrated on mammography at 0.1 mm or larger and cancer cells are approximately 0.03 mm.
The sections are routinely stained with haematoxylin and eosin. Additional staining, including immunohistochemistry, may be used to assist in diagnosis.32
The pathological entities that are associated with microcalcification have been well described in the NHSBSP guidance.33 Some commonly encountered entities are included in Table 3.
Table 3.
Pathology identified on percutaneous breast biopsy
| Benign proliferative change (B2) |
| Fibroadenoma |
| Fibrocystic change |
| Sclerosing adenosis |
| Columnar cell change |
| Indeterminate lesions (B3) |
| Atypical ductal proliferation (AEDIP) |
| In situ lobular neoplasia, including lobular carcinoma in situ and atypical lobular hyperplasia (ILN) |
| Papilloma |
| Radial scar |
| Mucinous lesions |
| Non-invasive cancer (B5a) |
| Ductal carcinoma in situ (DCIS) and intracystic carcinoma |
| Pleomorphic lobular carcinoma in situ |
| Invasive cancer (B5b) |
| Invasive ductal carcinoma |
| Invasive lobular carcinoma |
| Special type including papillary, tubular and mucinous carcinomas |
AEDIP, typical epithelial ductal proliferation; DCIS, ductal carcinoma in situ.
Cancer which extends <1 mm outside the duct wall is classified as microinvasive (B5c).
There is a spectrum of benign changes described, which may be associated with epithelial proliferation with or without atypia. A variety of lesions are classified as “indeterminate”, some because they show atypical morphology and others such as radial scar, papilloma and mucinous lesions because they may be associated with malignancy and are deemed inadequately sampled until completely removed. The diagnosis and management of indeterminate lesions will be discussed in a subsequent review. The distinction between atypia and low-grade in situ carcinoma depends on the extent of changes. If the abnormality measures >2 mm, or more than one duct system is involved, the lesion is best described as low-grade DCIS rather than atypia. In these circumstances, a larger volume of tissue at pre-operative diagnosis supports more accurate assessment by the pathologist.
THE IMPACT OF INVESTIGATING MICROCALCIFICATION
Calcification represents a challenge in both perception and interpretation. Small clusters of calcification are easy to miss and difficult to interpret. An aggressive approach to recall and investigation may result in high rates of benign biopsies, but reducing the number of females recalled is likely to mean some significant changes are not investigated. The benefit of biopsy is early diagnosis, meaning treatment can be easier and more effective, with a mortality benefit. The balance of overdiagnosis and overtreatment are difficult to model but were described for the NHSBSP in 2012.34 Some of the challenges of choosing an approach that balances risk and benefit are discussed below.
The rate of microcalcification and DCIS identified at screening depends on the age of the population and the frequency of screening. It is therefore difficult to establish baseline expected levels for assessment and rate of cancer diagnosis from calcifications.
Farshid et al35 published a series of 2545 cases investigated between 1992 and 2007, where microcalcification without soft-tissue change was biopsied. Almost half (47.7%) of the cases were graded as indeterminate, 28.3% cases as suspicious and 24.0% cases as highly suspicious. After assessment, 47.9% of cases were malignant, 4.8% cases were indeterminate (including atypia) and 47.3% cases were benign. Less than one-third (30.9%) of DCIS was low grade, and the features predicting higher grade included radiological suspicion, extent and the presence of a palpable mass.
National audit data for UK screening units in 2014–15 indicate that the rate of diagnosis of DCIS ranges from 0.5 to 3.1 cases per 1000 females screened (average 1.8).36 It is likely that this variation is due to different thresholds for biopsy. Maintaining a high threshold for sampling microcalcifications will reduce the number of females recalled and subjected to needle sampling. This minimizes unnecessary stress and discomfort in many cases and reduces the potential for overdiagnosis of low-grade DCIS and indeterminate lesions that are treated but may never affect a female in her lifetime. However, this is at the cost of missing some cases of both DCIS and invasive cancer, which may present at the next screen or as an interval cancer.
A recent analysis of data for over 5 million females screened between 2003 and 2007 investigated the relationship between the detection of DCIS and subsequent diagnosis of interval cancer in the UK. This showed that the average frequency of DCIS detected at screening was 1.6 per 1000 females screened (unit range: 0.54–3.56 per 1000 females screened). There was a significant negative association of screen-detected DCIS cases with the rate of invasive interval cancer; for every three cases of DCIS diagnosed, there was one less interval cancer.37
Microcalcification was seen more frequently in cancers that were identified by only one of two readers than in cancers detected by both readers in a screening environment.38 Reviews of imaging of females presenting with screen-detected and interval cancers show that approximately 30% of cancers were missed on the prior mammogram. Further analysis of the cases with findings on previous imaging showed that 18% of cases showed microcalcifications with digital mammography and 32% of cases showed microcalcifications with screen/film mammography.39 Warren et al40 reviewed 193 cases where cancer was diagnosed after assessment and found that microcalcifications were more likely to have been inadequately assessed than other lesions. A review of the prior mammograms of females with DCIS showed abnormality in 22% of cases.41 The calcification morphology on the prior mammogram was more indeterminate, indicating that a lower threshold for sampling indeterminate calcifications would increase the diagnosis of early DCIS.
In light of the discussion regarding overdiagnosis and overtreatment, alternatives to surgical excision for low-grade lesions are being considered. The LORIS trial (a trial comparing surgery with active monitoring for low risk DCIS) has been designed to test the efficacy of vacuum-assisted excision and regular surveillance for low-grade DCIS.42
The appearance and effect of treating screen-detected DCIS is being recorded by the Sloane Project, a UK-wide prospective audit of screen-detected DCIS and atypical hyperplasias of the breast.43 The Sloane Project began collecting data in 2003–4, including information about pre-operative findings as well as the management of DCIS. It has identified variation in the use of post-operative radiotherapy, oestrogen receptor measurement and surveillance protocols in the UK. Of interest to radiologists, the Sloane Project has demonstrated that typical calcifications in DCIS change with size of lesion. Casting calcifications are typical of larger areas of DCIS, including low grade, but small clusters of punctate or granular calcifications may represent high-grade DCIS, where an aggressive clinical approach is recommended.44
A PRAGMATIC APPROACH TO INVESTIGATION OF MICROCALCIFICATIONS
Assessment of microcalcification is described in UK national guidance.45 Calcifications that are not clearly benign at screening mammography are recalled for assessment, including further views, ultrasound and clinical examination. Biopsy is recommended in all cases where further imaging is not entirely normal or benign. A summary of assessment and microcalcification biopsy outcomes for Southwest London Breast Screening Service is shown in Table 4.
Table 4.
Data for females assessed in Southwest London Breast Screening Service between April 2013 and March 2016 (from National Breast Screening System* assessment report)
| Final non-operative diagnosis | Number of lesions sampled | % of total biopsies |
|---|---|---|
| B1 (no calcification) | 45 | 2.5% |
| B2 (benign and concordant) | 1212 | 66.5% |
| B3 (indeterminate pathology) | 152 | 8.3% |
| B4 (suspicious for malignancy) | 5 | 0.3% |
| B5a/c (in situ/microinvasive cancer) | 360 | 19.7% |
| B5b (invasive cancer) | 50 | 2.7% |
| No biopsy | 332 |
7443 females were recalled for assessment.
4338 biopsies were performed.
1824 (42%) of biopsies were performed for microcalcification.
69 (3.8%) of biopsies were repeated for non-concordance (B1).
12 (0.65%) biopsies were repeated after B4 diagnosis.
0 cancers were identified arising from an area previously assessed for calcification.
If the microcalcification is confidently seen on ultrasound, biopsy may be performed under ultrasound guidance. Ultrasound guidance allows real-time visualization of the needle and is more comfortable for both the patient and the operator. Occasionally, more calcification is seen on ultrasound than on mammography and it is advisable to place a marker clip at the site of ultrasound-guided biopsy for calcification to ensure that the site of biopsy may be subsequently demonstrated on mammography.
If the microcalcification is not seen with confidence on ultrasound, then stereotactic biopsy with in-room specimen radiography is necessary. In our practice, 14-G biopsy is chosen as first-line approach for most microcalcifications. First-line vacuum biopsy is used if the cluster is small (<5 mm) or the calcification is scanty.
Occasionally, stereotactic biopsy is not possible because the individual is unable to tolerate the procedure or the calcification cannot be targeted on the small-field biopsy device. When stereotactic biopsy is not possible, it can help to draw a skin mark over the calcifications during attempted stereotactic localization to aid localization on ultrasound.
When a firm diagnosis of DCIS or invasive cancer is made, the radiologist aims to define the extent of disease such that the surgeon is able to remove all disease in a single operation. If the lesion is focal and amenable for local excision, only then the area of most concern is biopsied. If the microcalcification is extensive and heterogeneous, or multifocal, such that mastectomy might be considered, two (or more) areas may need to be biopsied and marker clips deployed to determine disease extent.
Examination of the ipsilateral breast and axilla with ultrasound may demonstrate soft-tissue change associated with invasion and can give further information on axillary node changes. Nodes in the lower axilla with a thickened cortex are sampled by fine needle aspiration cytology or core biopsy.
DOCUMENTATION AND COMMUNICATION WITH THE PATIENT
There should be thorough documentation of the procedure, including identification of the clinician and radiographer, radiation dose, drugs administered and confirmation of the correct site check, in keeping with the National Safety Standards for Invasive Procedures.46 Details of implanted marker clips should be recorded. As with all procedures, it is important to have formal training and update procedures in place and to evaluate the service continuously through audit and comparison with local and national standards and targets.
The cooperation of the patient is critical and is best gained by providing a calm environment and avoiding delay during the intervention. The patient should be fully informed regarding the nature of the procedure and the need to stay still. It helps if she can be supported by a healthcare assistant throughout. Written information should be given well before the procedure so that the patient has sufficient time to digest the information and ask questions if necessary. There is variation in approach to confirmation of consent depending on local protocols. As the patient is fully conscious, written consent is not essential.47
The two main risks associated with biopsy are firstly, the harm of recall and intervention in a normal female who is not diagnosed with cancer and secondly, the treatment of females who have an abnormal diagnosis which would not cause harm during their lifetime. This is explained in the screening invitation leaflet.48 In addition, specific risks include haematoma, which can occasionally be extreme, and ongoing haemorrhage, which may need surgical intervention. Infection is rare. Post-biopsy pain is described but appears sporadic and unpredictable; it may be related to the extent of anxiety prior to the procedure.49
Communication of biopsy results to females is important. When the biopsy is benign, females often ask whether they need more frequent follow-up, but they should be reassured that the area of the breast sampled is no more likely to develop malignant change than surrounding tissues. Identification and management of indeterminate lesions will be discussed separately. DCIS may be a difficult diagnosis to communicate and it is often helpful to use diagrams to demonstrate the difference between DCIS and invasive cancer. Clinicians vary in the phrases they use to describe non-invasive disease; some refer to it as “early cancer”, others as “pre-cancer”, and some feel strongly that it should not be referred to as cancer at all, because DCIS is not an obligate precursor of invasive disease. The BBC has an iWonder Interactive Guide that can be helpful.50 Females may wish to know whether the biopsy can cause seeding along the biopsy tract. This may occur, but research has shown that the transplanted cells are not viable.51
SUMMARY
The identification and investigation of microcalcifications found on mammography have become more common with improving technology and there has been a parallel increase in the variety of associated lesions in pathology. This has resulted in an increase in the diagnosis of DCIS. In some cases, females may not benefit (overdiagnosis), but in others early treatment may pre-empt the development of invasive cancer. This is likely to have contributed to the reduction in mortality from breast cancer seen since the advent of screening. Clinicians responsible for the investigation of mammographic calcification should remain mindful of the need to balance harm and benefit.
Contributor Information
Louise Wilkinson, Email: louise.wilkinson@stgeorges.nhs.uk.
Val Thomas, Email: val.thomas@stgeorges.nhs.uk.
Nisha Sharma, Email: nishe.sharma2@nhs.net.
REFERENCES
- 1.Castellaro AM, Tonda A, Cejas HH, Ferreyra H, Caputto BL, Pucci OA, et al. Oxalate induces breast cancer. BMC Cancer 2015; 15: 761. doi: https://doi.org/10.1186/s12885-015-1747-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox RF, Morgan MP. Microcalcifications in breast cancer: lessons from physiological mineralization. Bone 2013; 53: 437–50. doi: https://doi.org/10.1016/j.bone.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? J Mammary Gland Biol Neoplasia 2005; 10: 181–7. doi: https://doi.org/10.1007/s10911-005-5400-6 [DOI] [PubMed] [Google Scholar]
- 4.Scimeca M, Giannini E, Antonacci C, Pistolese CA, Spagnoli LG, Bonanno E. Microcalcifications in breast cancer: an active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer 2014; 14: 286. doi: https://doi.org/10.1186/1471-2407-14-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi S, Coonrod S, Estroff L, Fischbach C. Chemical and physical properties of carbonated hydroxyapatite affect breast cancer cell behavior. Acta Biomater 2015; 24: 333–42. doi: https://doi.org/10.1016/j.actbio.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weerakkody Y, Kruger G; 2016. Available from: http://radiopaedia.org/articles/dr-albert-salomon-1
- 7.Gold RH, Bassett LW, Widoff BE. Highlights from the history of mammography. RadioGraphics 1990; 10: 1111–31. doi: https://doi.org/10.1148/radiographics.10.6.2259767 [DOI] [PubMed] [Google Scholar]
- 8.Sickles EA. Breast calcifications: mammographic evaluation. Radiology 1986; 160: 289–93. doi: https://doi.org/10.1148/radiology.160.2.3726103 [DOI] [PubMed] [Google Scholar]
- 9. Cancer Research UK. Updated June 2016. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-in-situ#heading-Twoge.
- 10.Weigel S, Decker T, Korsching E, Hungermann D, Böcker W, Heindel W. Calcifications in digital mammographic screening: improvement of early detection of invasive breast cancers? Radiology 2010; 255: 738–45. doi: https://doi.org/10.1148/radiol.10091173 [DOI] [PubMed] [Google Scholar]
- 11.Glynn CG, Farria DM, Monsees BS, Salcman JT, Wiele KN, Hildebolt CF. Effect of transition to digital mammography on clinical outcomes. Radiology 2011; 260: 664–70. doi: https://doi.org/10.1148/radiol.11110159 [DOI] [PubMed] [Google Scholar]
- 12.Bluekens AM, Holland R, Karssemeijer N, Broeders MJ, den Heeten GJ. Comparison of digital screening mammography and screen-film mammography in the early detection of clinically relevant cancers: a multicenter study. Radiology 2012; 265: 707–14. doi: https://doi.org/10.1148/radiol.12111461 [DOI] [PubMed] [Google Scholar]
- 13.Farshid G, Sullivan T, Jones S, Roder D. Performance indices of needle biopsy procedures for the assessment of screen detected abnormalities in services accredited by BreastScreen Australia. Asian Pac J Cancer Prev 2014; 15: 10665–73. doi: https://doi.org/10.7314/APJCP.2014.15.24.10665 [DOI] [PubMed] [Google Scholar]
- 14.Gilbert FJ, Astley SM, McGee MA, Gillan MG, Boggis CR, Griffiths PM, et al. Single reading with computer-aided detection and double reading of screening mammograms in the United Kingdom National Breast Screening Program. Radiology 2006; 241: 47–53. doi: https://doi.org/10.1148/radiol.2411051092 [DOI] [PubMed] [Google Scholar]
- 15.Spangler ML, Zuley ML, Sumkin JH, Abrams G, Ganott MA, Hakim C, et al. Detection and classification of calcifications on digital breast tomosynthesis and 2D digital mammography: a comparison. AJR Am J Roentgenol 2011; 196: 320–4. doi: https://doi.org/10.2214/AJR.10.4656 [DOI] [PubMed] [Google Scholar]
- 16.Bolmgren J, Jacobson B, Nordenström B. Stereotaxic instrument for needle biopsy of the mamma. AJR Am J Roentgenol 1977; 129: 121–5. doi: https://doi.org/10.2214/ajr.129.1.121 [DOI] [PubMed] [Google Scholar]
- 17. Updated June 2016. Available from: https://www.spacefoundation.org/programs/space-technology-hall-fame/inducted-technologies/stereotactic-breast-biopsy-technology.
- 18. Practical evaluation of Hologic Affirm digital breast tomosynthesis biopsy system NHS Breast Screening Programme Equipment Report 1501. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/488948/Practical_evaluation_of_Hologic_tomosynthesis_biopsy_system_FINAL_291215.pdf.
- 19. Non-operative Diagnosis Subgroup of the National Coordinating Group for Breast Screening Pathology. Guidelines for non-operative diagnostic procedures and reporting in breast cancer screening. NHSBSP publication no 50; 2001. Available from: https://www.gov.uk/government/publications/nhs-breast-screening-non-operative-diagnostic-procedures.
- 20.Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, Cink TM, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 1994; 193: 359–64. doi: https://doi.org/10.1148/radiology.193.2.7972743 [DOI] [PubMed] [Google Scholar]
- 21.Park HL, Hong J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg 2014; 3: 120–7. doi: https://doi.org/10.3978/j.issn.2227-684X.2014.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrading S, Distelmaier M, Dirrichs T, Detering S, Brolund L, Strobel K, et al. Digital breast tomosynthesis-guided vacuum-assisted breast biopsy: initial experiences and comparison with prone stereotactic vacuum-assisted biopsy. Radiology 2015; 274: 654–62. doi: https://doi.org/10.1148/radiol.14141397 [DOI] [PubMed] [Google Scholar]
- 23.Sickles EA, D'Orsi CJ, Bassett LW. Acr BI-RADS® mammography. In: ACR BI-RADS®Atlas, Breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 24.Maxwell AJ, Ridley NT, Rubin G, Wallis MG, Gilbert FJ, Michell MJ; Royal College of Radiologists Breast Group. The Royal College of Radiologists Breast Group breast imaging classification. Clin Radiol 2009; 64: 624–7. doi: https://doi.org/10.1016/j.crad.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 25.Evans A. The diagnosis and management of pre-invasive breast disease: radiological diagnosis. Breast Cancer Res 2003; 5: 250–3. doi: https://doi.org/10.1186/bcr621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stehouwer BL, Merckel LG, Verkooijen HM, Peters NH, Mann RM, Duvivier KM, et al. 3-T breast magnetic resonance imaging in patients with suspicious microcalcifications on mammography. Eur Radiol 2014; 24: 603–9. doi: https://doi.org/10.1007/s00330-013-3029-1 [DOI] [PubMed] [Google Scholar]
- 27.Bae S, Yoon JH, Moon HJ, Kim MJ, Kim EK. Breast microcalcifications: diagnostic outcomes according to image-guided biopsy method. Korean J Radiol 2015; 16: 996–1005. doi: https://doi.org/10.3348/kjr.2015.16.5.996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TE, Kim DB, Jung JH, Lee EK. Sonographic visibility and feasibility of biopsy under ultrasound guidance of suspicious microcalcification-only breast lesions: a single-centre study. Hong Kong J Radiol 2015; 18: 125–33. doi: https://doi.org/10.12809/hkjr1514264 [Google Scholar]
- 29.Bagnall MJ, Evans AJ, Wilson AR, Burrell H, Pinder SE, Ellis IO. When have mammographic calcifications been adequately sampled at needle core biopsy? Clin Radiol 2000; 55: 548–53. doi: https://doi.org/10.1053/crad.1999.0483 [DOI] [PubMed] [Google Scholar]
- 30.Houssami N, Ciatto S, Ellis I, Ambrogetti D. Underestimation of malignancy of breast core-needle biopsy: concepts and precise overall and category-specific estimates. Cancer 2007; 109: 487–95. [DOI] [PubMed] [Google Scholar]
- 31.Soo AE, Shelby RA, Miller LS, Balmadrid MH, Johnson KS, Wren AA, et al. Predictors of pain experienced by women during percutaneous imaging-guided breast biopsies. J Am Coll Radiol 2014; 11: 709–16. doi: https://doi.org/10.1016/j.jacr.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 32.Members of the National Coordinating Committee for Breast Pathology. Professor Ian Ellis, Nottingham City Hospital (Writing Group Lead). Tissue pathways for breast pathology: The Royal College of Pathologists; 2010.
- 33. Pathology reporting of breast disease: a joint document incorporating the third edition of the NHS Breast Screening Programme’s guidelines for pathology reporting in breast cancer screening and the second edition of The Royal College of Pathologists’ minimum dataset for breast cancer histopathology. NHSBSP publication no 58; 2005. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/465530/nhsbsp58-high-resolution.pdf.
- 34.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M, et al. ; The Independent UK. Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review: a report jointly commissioned by Cancer Research UK and the Department of Health (England) October. Br J Cancer 2013; 108: 2205–40. doi: https://doi.org/10.1038/bjc.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farshid G, Sullivan T, Downey P, Gill PG, Pieterse S. Independent predictors of breast malignancy in screen-detected microcalcifications: biopsy results in 2545 cases. Br J Cancer 2011; 105: 1669–75. doi: https://doi.org/10.1038/bjc.2011.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NHS breast screening programme and Association of Breast Surgery. An audit of screen detected breast cancers for the year of screening April 2014 To March 2015. Public Health England. Published May 2016. Available from: http://www.associationofbreastsurgery.org.uk/media/63035/nhsbsp_abs_breast_screening_audit_201415_full_audit_v3.pdf
- 37.Duffy SW, Dibden A, Michalopoulos D, Offman J, Parmar D, Jenkins J, et al. Screen detection of ductal carcinoma in situ and subsequent incidence of invasive interval breast cancers: a retrospective population-based study. Lancet Oncol 2016; 17: 109–14. doi: https://doi.org/10.1016/S1470-2045(15)00446-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofvind S, Geller BM, Rosenberg RD, Skaane P. Screening-detected breast cancers: discordant independent double reading in a population-based screening program. Radiology 2009; 253: 652–60. doi: https://doi.org/10.1148/radiol.2533090210 [DOI] [PubMed] [Google Scholar]
- 39.Hoff SR, Abrahamsen AL, Samset JH, Vigeland E, Klepp O, Hofvind S. Breast cancer: missed interval and screening-detected cancer at full-field digital mammography and screen-film mammography—results from a retrospective review. Radiology 2012; 264: 378–86. doi: https://doi.org/10.1148/radiol.12112074 [DOI] [PubMed] [Google Scholar]
- 40.Warren R, Allgood P, Hunnam G, Godward S, Duffy S; East Anglian Breast Screening Programme. An audit of assessment procedures in women who develop breast cancer after a negative result. J Med Screen 2004; 11: 180–6. doi: https://doi.org/10.1258/0969141042467395 [DOI] [PubMed] [Google Scholar]
- 41.Evans AJ, Wilson AR, Burrell HC, Ellis IO, Pinder SE. Mammographic features of ductal carcinoma in situ (DCIS) present on previous mammography. Clin Radiol 1999; 54: 644–9. doi: https://doi.org/10.1016/S0009-9260(99)91083-8 [DOI] [PubMed] [Google Scholar]
- 42. LORIS: a Phase III trial of surgery versus active monitoring for low risk ductal carcinoma in Situ (DCIS). Updated June 2016. Available from: http://www.birmingham.ac.uk/research/activity/mds/trials/crctu/trials/loris/index.aspx.
- 43. The Sloane Project. Updated June 2016. Available from: http://www.sloaneproject.co.uk/
- 44.Evans A, Clements K, Maxwell A, Bishop H, Hanby A, Lawrence G, et al. Lesion size is a major determinant of the mammographic features of ductal carcinoma in situ: findings from the Sloane Project. Clin Radiol 2010; 65: 181–4. doi: https://doi.org/10.1016/j.crad.2009.05.017 [DOI] [PubMed] [Google Scholar]
- 45.Liston J, Wilson R. Clinical Guidelines for Breast Cancer Screening Assessment. 3rd edn. NHSBSP publication no 49; June 2010. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/465528/nhsbsp49_June2010.pdf
- 46. National Safety Standards for Invasive Procedures (NatSSIPs). v. 1. First published: 7 September 2015. NHS England Patient Safety Domain and the National Safety Standards for Invasive Procedures Group. Available from: https://www.england.nhs.uk/patientsafety/wp-content/uploads/sites/32/2015/09/natssips-safety-standards.pdf.
- 47. The Royal College of Radiologists. Standards for patient consent particular to radiology. 2nd edn: The Royal College of Radiologists; 2012. Available from: https://www.rcr.ac.uk/standards-patient-consent-particular-radiology-second-edition.
- 48. NHS Breast Screening: Helping you decide: NHS Cancer Screening Programmes; 2013. Updated June 2016. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/440798/nhsbsp.pdf.
- 49.Miller SJ, Sohl SJ, Schnur JB, Margolies L, Bolno J, Szabo J, et al. Pre-biopsy psychological factors predict patient biopsy experience. Int J Behav Med 2014; 21: 144–8. doi: https://doi.org/10.1007/s12529-012-9274-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. BBC iWonder. Why isn't breast cancer screening totally reliable? Updated June 2016. Available from: http://www.bbc.co.uk/guides/zcq7xnb.
- 51.Loughran CF, Keeling CR. Seeding of tumour cells following breast biopsy: a literature review. Br J Radiol 2011; 84: 869–74. doi: https://doi.org/10.1259/bjr/77245199 [DOI] [PMC free article] [PubMed] [Google Scholar]