Abstract
Objective:
Treatment of canine peripheral nerve sheath tumours (PNSTs) is challenging and prognosis after surgical resection is considered poor. The aim of this study was to evaluate the feasibility and effectiveness of stereotactic radiotherapy (RT) of these tumours.
Methods:
10 dogs with clinical symptoms and MRI findings consistent with PNSTs of the brachial plexus, branches and nerve roots were treated with linear accelerator-based volumetric-modulated arc radiotherapy (VMAT) with a dose of 35 Gy/5 fractions. Clinical and MRI follow-up examinations were planned and radiotoxicity and survival times were investigated.
Results:
Tumours involved the plexus and proximal nerves in three dogs, the plexus, proximal nerves and nerve roots in five dogs and the nerve roots and proximal nerves in two dogs. Partial response and partial or complete reductions of neurological deficits were observed in all the treated dogs. Local recurrence was observed in 9/10 of treated dogs. No symptom directly referable to radiotoxicity was observed. Mean overall survival of 371 ± 30 days [95% confidence interval (CI) of (315–427)] and mean progression-free survival of 240 ± 30 days (95% CI of 188–291) from this work are comparable with surgical literature data regarding the plexus and proximal nerve localization, but are superior in comparison with nerve root localization.
Conclusion:
VMAT can be a safe and viable alternative to surgery in cases of canine brachial plexus PNSTs involving the proximal nerves and nerve roots.
Advances in knowledge:
To our knowledge, this is the first prospective observational clinical study regarding VMAT stereotactic RT treatment for canine brachial plexus PNSTs and suggests that VMAT may achieve at least similar clinical outcome than surgery in a safer way.
INTRODUCTION
Peripheral nerve sheath tumours (PNSTs) are malignant tumours of nerve sheath origin, frequently occurring in dogs, particularly in the brachial plexus nerves (C6–T2), although other nerves may be affected.1 Histologically, they are described as heterogeneous neoplasms arising from the cells surrounding the axons of the peripheral nerves ranging from spindle cells in fascicles to sheets and cords of pleomorphic cells.1,2 Canine PNSTs resemble malignant PNSTs in humans, regarding both histology and behaviour.3,4 In detail, PNSTs of the tract C6–T2 can be classified as “peripheral” if involving nerves distal to the brachial plexus, as “of the plexus” if involving the brachial plexus itself and the emerging nerves from C5 to T2 distal to the intervertebral foramina and as “of the roots” if involving the dorsal or ventral roots within the spinal canal; tumours involving more than one location are classified according to their most proximal location.5 This kind of tumour spreads both proximally and distally along the nerve and may ultimately involve the spinal cord, causing compression and associated neurological deficits.6 Metastases are rare, although lung and uveal metastases have been reported.5,7
Concerning several published articles regarding canine PNSTs, no recent work has been conducted specifically about the treatment of PNSTs. At present, surgery is considered the treatment of choice.4,8–12 However, the overall prognosis for surgical management of canine PNSTs involving the brachial plexus or its roots is deemed unsatisfactory, with a median survival time of 12 months in dogs affected by plexus tumours and of 5 months for root tumours.5 Tumour recurrence occurs in 78% of cases, with a relapse-free interval of 7.5 months for surgically treated dogs for plexus tumours and of 1 month for plexus and root tumours.5 Recurrence is mainly caused by the impossibility of performing a wide resection owing to the closeness of critical structures such as the spinal cord, meninges, spinal vessels and vertebrae.13–15 To the authors' knowledge, there have been no studies either evaluating the efficacy of adjuvant treatments, particularly chemotherapy or radiotherapy (RT), in the management of brachial plexus and root tumours, or regarding curative-intent RT.
Thanks to the advent of new and refined irradiation techniques [intensity-modulated radiotherapy, volumetric-modulated arc radiotherapy (VMAT) etc.], lesions affecting critical neural structures can now be better addressed16 with RT.
In particular, VMAT has been successfully used in veterinary medicine for the treatment of meningiomas, gliomas, cervical paragangliomas and rabbit thymomas, allowing a highly conformal dose distribution within a short beam time compared with cone beam-based delivery when multiple isocenters are necessary owing to tumour shape complexity.17–26
The aim of this work was to evaluate the feasibility and effectiveness of curative high-dose hypofractionated frameless VMAT in canine brachial plexus PNSTs, paying particular attention to any improvement in local tumour control and damage of critical structures compared with surgical resection.
METHODS AND MATERIALS
The study and RT treatment protocol were approved by the local scientific ethics committee. Informed consent regarding all diagnostic examinations of all dogs included in the study was obtained from the owners prior to any clinical evaluation.
A prospective single-institution clinical research study was conducted from October 2010 to December 2013 on client-owned dogs suffering from brachial plexus PNSTs.
Inclusion criteria to be admitted to the study were: normal minimum diagnostic tests including complete blood cell count and biochemistry panel and a presumptive, imaging-based diagnosis of PNST assessed by a radiologist (MD).
No biopsies were performed.
Neither the severity of neurological symptoms nor the administration of any symptomatic medical therapy before diagnosis was used as an exclusion condition.
Each dog was filmed and the results of neurological examinations were recorded as a reference for subsequent follow-up. All the neurological examinations were performed by the same clinician (MD). Lameness score (0–4) was attributed according to the clinical lameness scoring system for assessment in dogs.31,32 Neurological alterations were graded according to localization and severity.
For the diagnostic investigation, animals were anaesthetized by means of propofol (Proposure; Merial Italia spa, Milan, Italy) and maintained on a constant rate infusion. All dogs underwent endotracheal intubation and were ventilated with 100% oxygen and positioned in dorsal recumbency and were pre-medicated with a combined protocol of dexmedetomidine (3 µg kg−1) (Dexdomitor; Orion Pharma, Espoo, Finland) and methadone (100 µg kg−1) (Epthadone; Molteni Farmaceutici, Granatieri Scandicci, Italy). The presumptive diagnosis of PNST was developed on the basis of clinical history, neurological and orthopaedic examination and spine and plexus MRI.27,28
The most peculiar clinical aspect of brachial plexus tumour was unilateral forelimb progressive lameness with monoparesis and muscle atrophy.5 The MRI criteria supporting PNST diagnosis were either the presence of a nodule in the axilla along the nerves of the brachial plexus or the presence of diffuse or focal thickening of the brachial plexus or its nerve roots.9,29,30
The MRI examinations were conducted using a 1.5-T superconductive whole-body MRI (Intera 1.5T; Philips Medical Systems, Eindhoven, Netherlands) scanner with gradients of 70 mT m−1. A quadrature spine coil was used. The MRI protocol provided the following scan sequences: a turbo spin-echo T2 weighted (TSE T2w) pulse sequence with repetition time (TR) 3500 ms, echo time (TE) 130 ms, two number of excitations (NEX) and 1024 × 512 matrix oriented along the sagittal plane; a short tau inversion-recovery (STIR) sequence with TR 2500 ms, TE 150 ms, inversion time 50 ms, two NEX and 1024 × 512 matrix oriented along the sagittal, dorsal and transverse planes; a T1 weighted contrast-enhanced spin-echo sequence with TR 450 ms, TE 5 ms, two NEX and 1024 × 512 matrix oriented along the sagittal and dorsal planes; and a contrast-enhanced fast-field echo T1 weighted (FFE T1w) sequence with TR 450 ms, TE 5 ms, two NEX and 512 × 512 matrix oriented along the transverse plane. Slice thickness was set at 2 mm without any intersection gap. For post-contrast images, 0.5 mmol ml−1 of gadodiamide (Omniscan; GE Healthcare, Milan, Italy) was administered at a dose of 0.2 ml kg−1 in the cephalic vein. The injection was performed using a high-pressure injection system (Medrad Spectris, Volkach, Germany) with a standardized infusion rate of 3 ml s−1 and acquisition time of 5 min post-injection.
Typically, PNST shows hyperintensity to muscle on T2w images and isointensity on T1w images, with different degrees of homogeneous or heterogeneous contrast enhancement following gadolinium intravein administration (Figures 1–3).29,30
Figure 1.
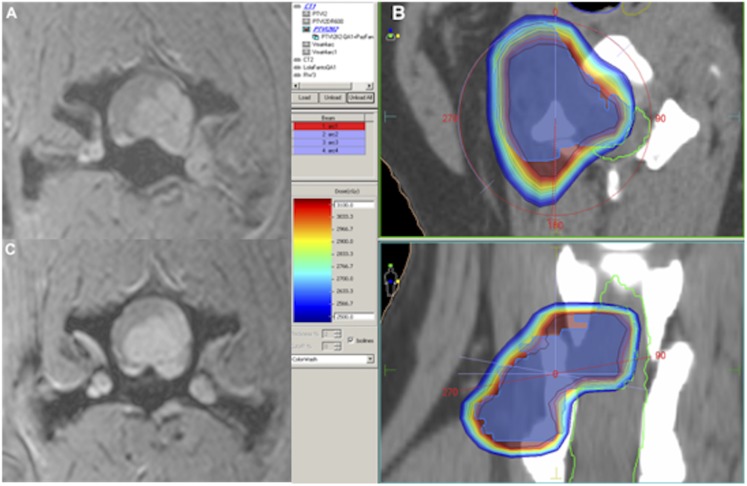
Dog no. 3 (a female mixed breed, 8 years of age): transverse MRI fast-field echo contrast-enhanced axial scan at C5–C6 at the level of peripheral nerve sheath tumour entering the corresponding neuroforamen (a); colour wash dose distribution (b); response 6 months after the end of the treatment (c).
Figure 3.
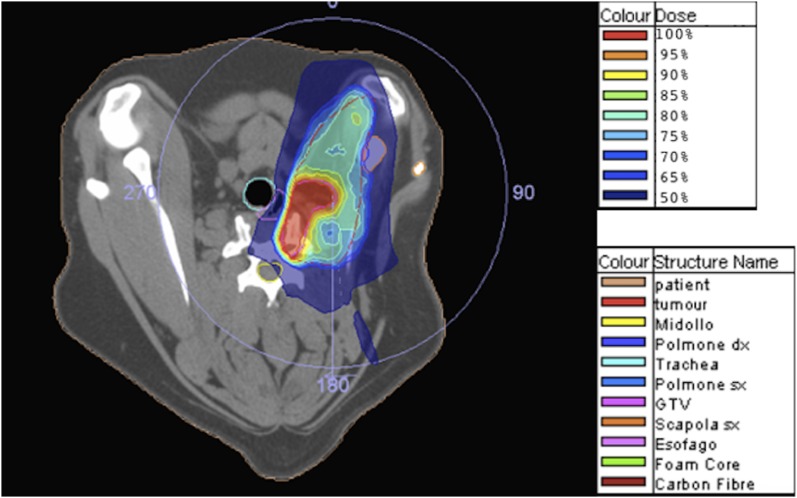
The same dog as in Figure 2: colour wash representation of dose distribution. GTV, gross tumour volume.
Brachial plexus tumours were classified as peripheral, of the plexus and proximal nerves and of the roots of the plexus, as described elsewhere.5,30
For the RT treatment, a standardized patient-positioning technique was developed: every dog was provided with a wooden cradle containing a vacuum mattress. Careful attention was paid during patient positioning in order to obtain a correct dorsal recumbence, with the head naturally extended and forelimbs taped together and placed caudally with the tip of the nails tangential to the xiphoid process. A typical patient setup is shown in Figure 4.
Figure 4.
Typical patient setup: in the photograph, both the wooden cradle and the vacuum bag with the patient who is anaesthetized are visible.
The virtual simulations for RT planning were performed within 1 week following the diagnostic MRI using a multidetector CT scanner (CT Brilliance; Philips Medical Systems, Eindhoven, Netherlands).33 The parameters used for the CT simulation were: 200 mA, 120 kV, pitch 0.6, rotation 1 s and slice thickness 1.5 mm. The provisional isocentre was marked with three radiopaque fiducials on the wooden cradle.
Fusion of the dedicated MRI and CT scans was performed with a semi-automated protocol using dedicated software (Focal®; Elekta, Stockholm, Sweden).
For well-demarcated tumours, the gross tumour volume (GTV) was defined by contouring the area of signal and structure abnormality in STIR or FFE T1w contrast-enhanced pulse sequences. For infiltrating tumours, the GTV encompassed all areas of signal abnormality in fluid-attenuated inversion-recovery or TSE T2w pulse sequences. Clinical target volume (CTV) was defined by adding 2 cm to the GTV along the involved nerves or plexus but excluding the spinal cord. Planned tumour volume (PTV) was defined by expanding the CTV 5 mm in all directions. The expansions of the CTV to PTV were limited by the spinal cord, but encompassed other nerves proximal to the brachial plexus. The contoured organs at risk (OARs) were the spinal cord, trachea, oesophagus, shoulder and lungs.
The OAR dose constraints were derived from the human ones described by the American Association of Physicists in Medicine Task Group 101 report.34
RT treatment was administered using an Elekta Synergy S linear accelerator equipped with a micromultileaf beam collimator (Elekta Beam Modulator EBM®) and an XVI cone-beam CT (CBCT) system. The prescribed dose to the PTV was 35 Gy delivered in five fractions on alternate days. VMAT treatments were planned using a Monte Carlo statistical algorithm and the CMS Monaco 3.0 treatment planning system.
For all patients, a specific plan was elaborated with a single 360° arc optimized over continuous dose rate variation, leaf position and gantry rotational speed to obtain target coverage and OAR sparing. The plan quality was evaluated by means of standard dose volume histograms In detail, the degree of PTV coverage was considered acceptable when the V95% and V107% levels (the PTV receiving <95% and >107% of the prescription dose) were, <4% and 2%, respectively.
Treatment feasibility was evaluated by checking the agreement between planned and delivered doses through an “in air” “patient-based” quality assurance procedure using the Elektai View amorphous silicon electronic portal imager device and Math Resolutions Dosimetry Check (DC) system software before giving the treatment to the patient. An additional absolute dose comparison was performed using the ScandiDos Delta4 system. In both cases, the agreement was to analyze using a gamma (γ) function,35,36 with a dose agreement of 3% and distance to the agreement of 3 mm, with acceptance criteria of γ < 1 for >95% of the comparison points. Delivery time, defined as the approximate time needed in the linear accelerator “beam-on” phase, was also investigated. Patient setup reproducibility was evaluated for each treatment session using the XVI CBCT. In detail, the discrepancy between the XVI CBCT and the simulation CT was considered acceptable, if the displacement did not exceed 2 mm in any direction. When discrepancies were found to be >2 mm and up to 5 mm, table movements were performed in accordance with the XVI CBCT software results. When discrepancies were found to be >5 mm, the patient was repositioned in the cradle and XVI CBCT was repeated to check the patient setup again; if agreement was still not achieved, the whole treatment procedure was repeated starting from the CT simulation.
Neurological and clinical examinations were performed on a daily basis during the irradiation treatment period, and then weekly for the first month. Eventually, monthly examinations were performed with regard to deambulation, involving limb dysfunction, lameness or paresis after the first month. The need for ancillary medications, in particular corticosteroids, was recorded.
With regard to the imaging response assessment protocol, serial MRI examinations were performed 2 months after irradiation and planned at 4, 6, 9, 12, 18 and 24 months. Moreover, if dictated by the clinical conditions, additional MRI examinations were scheduled. All MRI scans were performed using the same scanner and parameters used at diagnosis for comparison purposes. In particular, volumetric disease variation was analyzed. Other parameters evaluated by interobserver agreement were changes in the signal intensity of the tumour and of the spinal cord on TSE T2w pulse sequence (TR 3500 ms, TE 130 ms, two NEX and 512 × 512 matrix) and fluid-attenuated inversion-recovery pulse sequence (TR 3000 ms, TE 150 ms, inversion time 50 ms, two NEX and 512 × 512 matrix), contrast uptake of the tumour on FFE T1w pulse sequence (TR 450 ms, TE 5 ms, two NEX and 512 × 512 matrix) and the presence of a mass effect.
Specific response evaluation criteria were established to assess disease regression after irradiation.37 The volumetric MRI evaluation was performed according to response evaluation criteria in solid tumours categorization and implemented with clinical follow-up examinations.38,39
The categorical assignment was determined as follows. Patients were ascribed to the complete response group when the disappearance of all measurable enhancing tumours was observed, and stable or improved clinical status was achieved without corticosteroid administration.
Patients were ascribed to the partial response group when volumetric reduction on MR images was found to be ≥30%, and stable or improved clinical status was achieved with stable or decreased corticosteroid administration. Patients were ascribed to the stable disease group when volumetric reduction on MR images was found to be ≤30% or when the volumetric increase was found to be ≤20%, and stable or improved clinical status was achieved with stable or decreased corticosteroid administration. Patients were ascribed to the progressive disease group when either the appearance of one or more new lesions or volumetric increase was ≥20% or clinical deterioration was observed.
Radiation toxicities were evaluated clinically and graded according to Veterinary Radiation Therapy Oncology Group criteria.40
Overall, disease-specific and progression-free survival was estimated using the Kaplan–Meier curve analysis method. In particular, all deaths were considered events, while loss to follow-up or being alive at the time of data analysis warranted censoring. Deaths from PNSTs were also grouped as tumour dependent (recurrence or progression), radiation damage dependent and radiation damage independent. Median overall survival time and the 95% confidence interval (CI) were calculated considering the time to the event starting from the end of the radiation therapy.
RESULTS
10 dogs were enrolled in the study: 6 intact males and 4 females. The cohort included three Labrador Retrievers and seven middle-sized mixed breeds. The median age at diagnosis was 9 years (mean 9.3 years; range 7–13 years).
The presenting complaints at the first clinical examination were: lameness (Grade 3 or 4) and monoparesis (in 9/10 dogs), tetraparesis (in 1/10 dogs), muscular atrophy (in 7/10 dogs), axillary pain (in 6/10 dogs), neck pain (in 3/10 dogs) and Horner syndrome (in 3/10 dogs). In all affected dogs, the involved structures of the brachial plexus were enlarged, hyperintense in T2W pulse sequences and showed a contrast enhancement.
In detail, in three dogs, the tumour involved the brachial plexus and proximal nerves, with evidence of a nodular enlargement. In five dogs, the plexus, proximal nerves and roots were involved, with nodular enlargement of the plexus and diffuse thickening with hyperintensity in STIR sequence and contrast enhancement of the affected roots. In two dogs, the roots and spinal nerve proximal to the brachial plexus were evident, with a nodular enlargement of the affected roots determining a severe intradural compression on the spinal cord. The localization was C6 on the left side in one dog, C6–T1 in four dogs (three left, one right), C7–T1 in three dogs (two right, one left) and T1–T2 in two dogs (one right, one left).
No abnormalities were detected in the serum and complete blood cell count.
Regarding the tumour size, the largest diameter of the GTV in one dog was <1 cm, between 1.1 cm and 2 cm in four dogs, between 3.1 cm and 4 cm in three dogs and >4 cm in two dogs. Mean GTV measured at the first CT simulation time was 8.2 cm3 (range 2.7–33.1 cm3) and mean PTV was 114 cm3 (range 28.4–244.9 cm3).
The RT prescription was 35 Gy in five fractions given every other day. The RT treatments were planned with one 360° arc. The mean monitor units were 2300 ± 500 MU, while the mean control points, delivery time and modulation degree were 137 ± 5, 230 ± 30 s and 2.3 ± 0.4, respectively. Only 8/10 plans fulfilled the PTV and the OAR constraints; 2 plans regarding dogs with nodular enlargement of the root required a recalculation with a reduced prescription (33 and 31 Gy) owing to the unacceptable dose delivered to the spinal cord.
The 95% isodose volume coverage (V95%) was 97.1 ± 0.3% for the GTV and 96.8 ± 1.4% for the PTV. High 107% isodose volume coverage (V107%) was 1.7 ± 0.3% for the GTV and 0.3 ± 0.5% for the PTV. Agreement between planned and delivered doses measured using the DC system showed a mean value of 97 ± 2% for γ < 1 confirming the feasibility of the treatment. Similarly, the quality assurance check performed by the Delta 4 system resulted in a 96 ± 1% value, confirming a valid agreement between the two methods and between the planned and delivered doses.
The analysis related to the setup reproducibility showed no systematic errors. In particular, the mean values of the mean shift for each dog for the x, y and z directions were 0.1 ± 0.6 mm, 0 ± 0.8 mm and 0.1 ± 0.8 mm, respectively. All of the mean displacements were compatible with the zero hypothesis and in no case was there a need to repeat the whole treatment procedure starting from the CT simulation.
Table 1 shows the data obtained in plan optimization. In Table 2, the data on setup reproducibility evaluation are reported. Each data point in Table 2 represents the mean, for each patient, over all of the sessions for displacement along that axis.
Table 1.
Plan optimization and agreement between planned and measured dose results
| Dog | V95% PTV | V107% PTV | MU | CP | Mod. degree | Delivery time | DC agreement | Delta 4 agreement |
|---|---|---|---|---|---|---|---|---|
| 1 | 95.5 | 1.15 | 2125 | 135 | 2.2 | 199 | 98.7 | 98.4 |
| 2 | 96.4 | 0.18 | 1653 | 128 | 1.8 | 234 | 96.4 | 95.7 |
| 3 | 99.85 | 0.04 | 2425 | 137 | 2.2 | 208 | 99.3 | 95.3 |
| 4 | 96.3 | 0.1 | 2651 | 139 | 2.4 | 239 | 96.1 | 97.4 |
| 5 | 95.9 | 0.85 | 1581 | 141 | 1.8 | 178 | 98.3 | 97.2 |
| 6 | 96.7 | 0.01 | 2861 | 145 | 2.8 | 265 | 95.4 | 96.4 |
| 7 | 96.9 | 0.05 | 2164 | 131 | 2.1 | 229 | 95.3 | 96.4 |
| 8 | 96.4 | 0.08 | 2997 | 144 | 2.9 | 272 | 95.9 | 96.2 |
| 9 | 95.8 | 0.98 | 2067 | 134 | 2.1 | 227 | 97.1 | 96.2 |
| 10 | 98.7 | 0.04 | 2169 | 137 | 2.2 | 205 | 99.1 | 95.2 |
CP, Control Points; DC, Dosimetry Check; MU, monitor units; PTV, planned tumour volume.
Table 2.
Setup reproducibility analysis
| Dog | Mean x shift (mm) | Mean y shift (mm) | Mean z shift (mm) |
|---|---|---|---|
| 1 | 0.6 | 0.5 | −1.1 |
| 2 | −0.4 | 0.6 | 0.7 |
| 3 | 0.5 | −0.2 | 0.4 |
| 4 | 0.8 | 0.3 | 0.9 |
| 5 | −0.7 | −0.7 | −0.8 |
| 6 | −0.3 | −0.4 | 0.8 |
| 7 | 0.4 | 0.8 | 0.9 |
| 8 | 0.6 | −1.3 | −0.5 |
| 9 | 0.4 | −0.9 | 0.7 |
| 10 | −0.9 | 1.1 | −1 |
Figure 2 shows the dose distribution and the dose–volume histogram for a representative case. Similar results were obtained for all patients.
Figure 2.
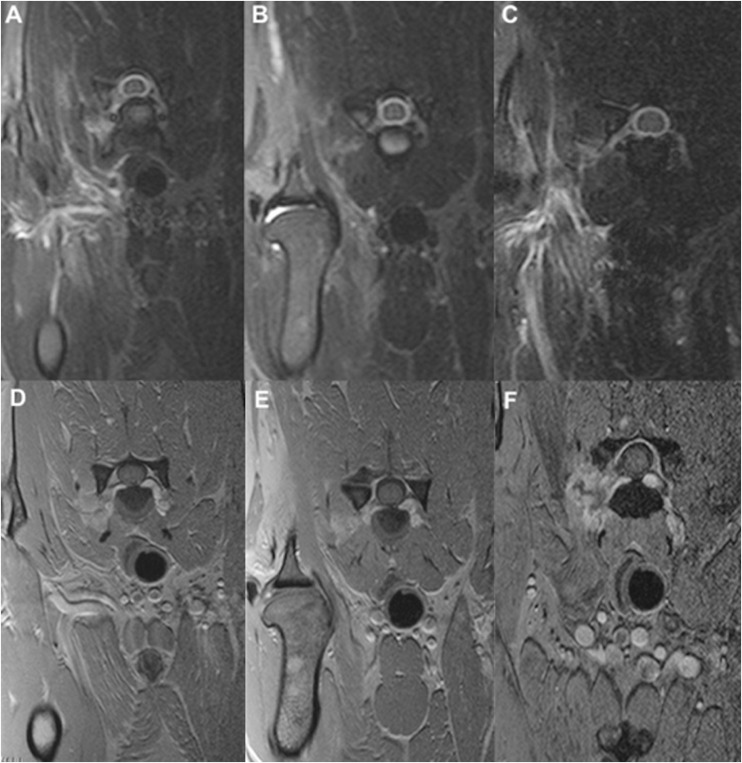
Dog no. 7 (a female Labrador, 10 years of age): above, transverse MRI short tau inversion recovery scans, below, fast-field echo contrast-enhanced scans at C6–C7, at diagnosis (a, d), after 12 months (b, e) and after 16 months (c, f).
All dogs completed the RT course. No acute effects directly referable to the treatment were recorded. All dogs underwent the planned clinical and MRI follow-up examinations.
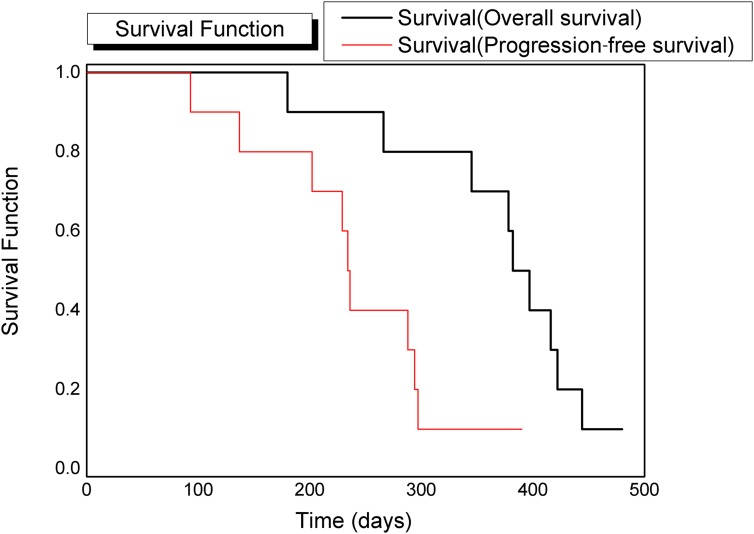
During the follow-up period, improvement or normalization of neurological status was observed in all dogs. Partial response was obtained in all treated dogs. MRI examinations performed during follow-up revealed a reduction in tumour volume and a progressive reduction of contrast enhancement (Figures 1–3). A slight muscular hyperintensity in the STIR pulse sequence within the irradiation field was observed. Local recurrences were observed in 9/10 patients, with a representation of the previous complaints. Mean progression-free survival time was 240 ± 30 days (95% CI 188–291 days). At the time of writing, only one dog was alive, 452 days after the end of the treatment. Euthanasia due to clinical symptoms related to PNST recurrence was the cause of death for all the other dogs included in this study. Mean overall survival was 371 ± 30 days (95% CI 315–427 days). The Kaplan–Meier analysis data are reported in Table 3, while the relative curves are shown in Figure 5.
Table 3.
Data for the Kaplan–Meier analysis. The only censored data are for the 10th patient
| Dog | Overall survival (days) | Progression-free survival (days) |
|---|---|---|
| 1 | 179 | 98 |
| 2 | 265 | 141 |
| 3 | 345 | 201 |
| 4 | 377 | 227 |
| 5 | 381 | 233 |
| 6 | 397 | 239 |
| 7 | 418 | 282 |
| 8 | 423 | 288 |
| 9 | 446 | 295 |
| 10 | 482 | 392 |
Figure 5.
Kaplan–Meier curve analysis: the overall survival curve is in black and the progression-free survival is in red.
DISCUSSION
To the authors' knowledge, this is the first prospective observational clinical study regarding VMAT stereotactic RT for canine brachial plexus PNSTs. The technical difficulties in treating small, highly malignant tumours close to critical structures were addressed using VMAT.40,41 The Monte Carlo statistic calculation algorithm improved dose calculation at various tissue interfaces, where wrong doses could be generated by a standard calculation algorithm.
No relevant data regarding canine PNST sensitivity to RT exist. The radiation dose was selected considering the putative tolerance of the critical structures near the field of irradiation, particularly the spinal cord. The prescribed dose was, in fact, reduced in two cases owing to the presence of the spinal cord within the field of irradiation and this could represent a bias in the survival curve analysis.
A good-quality treatment plan was achieved, as shown in Table 1, where both target coverage and OAR sparing were obtained. Owing to the small target volume, the concordance between prescribed and delivered doses is a fundamental parameter in the irradiation of canine PNSTs using typical VMAT sharp-dose gradients. Delta 4 and DC system demonstrated the treatment feasibility, as shown in Table 1. From Table 1 is also annotable the delivery time is very short and this makes fast processing with reduction of the sedation 10 times for each fraction compared with other RT techniques like non-coplanar stereotactic cone treatment.
Important data, such as the operative criteria in the definition of GTV, CTV and PTV, have never been codified. In this study, we considered the GTV as the volume of enlarged or altered signal neural structures. The expansion of GTV to CTV was 2 cm because this was considered sufficient to encompass microscopic disease along the neural structures, withholding the spinal cord and isotropic expansion. The expansion of 5 mm from CTV to PTV was considered sufficient to address setup uncertainties.
No adverse effects were detected and a consistent improvement of the affected limb functionality was observed after the treatment. Pain elicited by palpation of the involved region disappeared after RT. In particular, the lameness score passed from Grade 3 or 4 in all the patients to Grade 2 or 4 in three patients and Grade 1 or 4 for the remaining seven dogs. No corticosteroid was administered. This could also be related to the small setup margin from the CTV to the PTV that we adopted in our study. This was possible owing to the accuracy and setup reproducibility shown by the displacement analysis for our immobilization system that is composed of a vacuum lock inserted into a home-made cradle (Table 2). These results suggest that VMAT modality, for this pathology, allows a larger dose to be delivered to the target resulting in a higher tumour control probability with reduced normal tissue compliance probability. Moreover, hypofractionation has a better manageability, reducing the number of sitting sedations.
Frameless stereotactic RT demonstrated therapeutic efficacy with a consistent improvement in both life quality and expectancy compared with literature data.4,12,42–44 In a retrospective study, median survival time for dogs affected by plexus tumours was 360 days, and median survival time for root tumours was 150 days. Tumour recurrence occurred in 78% of cases affected by root and plexus tumours, with a relapse-free interval of 225 days for surgically treated plexus tumours and 30 days for root tumours.5 In our study, we observed local recurrence in 90% of treated dogs; mean overall survival was 371 days and progression-free survival was 240 days (Figure 5). Our results are comparable with those of the retrospective study concerning tumours with plexus localization; but, on the contrary, they are superior in brachial plexus PNST with root localization. In the same article,5 it is also reported that plexus and root tumours usually involve several nerves in a plexus, although all nerves may not appear affected at the time of surgery. Limb amputation must be radical with total excision of the involved tissues, but this could be hardly possible if the intracanalar portion of the tumour is located ventrally to the spinal cord or invades the cord parenchyma. The adequacy of resection based on histopathological examination can often be difficult, because en bloc or wide-margin surgical resection is almost impossible.5
Early reports on canine PNSTs describe this pathology as a benign and surgically correctable condition.45 Literature data and the present study disagree with this assumption.4,42–44
Repeated surgery in the retrospective study did not elicit a significant benefit. Among our patients, only one dog was reirradiated, gaining 2 more months of good quality of life. The absence of radiotoxic effects in our work could also be related to a too little life span to develop late damage to the spinal cord or a subjective superior tolerability of the spinal cord to radiation; if this assumption was confirmed, a dose escalation trial to assess any correlation between the response and dose could be performed in future.
Furthermore, the present work is the first prospective study in dogs where MRI was used to evaluate tumour response to irradiation. The systematic protocol for scheduled MRI examinations regarding every change in the post-treatment clinical presentation allows clinicians to gather interesting information about the history of irradiated PNSTs. The most common radiological finding for all patients who were irradiated was a reduction in tumour volume, with progressive shrinkage of the mass and a reduction of the mass effect.
The limitations of this prospective study are the lack of histopathological confirmation and immunohistochemical or molecular tests. No dogs were available for necroscopy to confirm the presence or absence of radiation effects or tumour regrowth. However, the presumptive imaging-based diagnosis of PNST, formulated for each patient by MD, correlated with the presumptive orthopaedic and neurological symptoms, is considered a valid method to detect PNSTs.6,9
CONCLUSION
In conclusion, this study shows that VMAT hypofractionated RT is a feasible and effective therapeutic option for canine brachial plexus PNSTs. Although overall survival is comparable with or superior to surgical case series, the treatment of this disease remains challenging. In cases of PNSTs involving the brachial plexus and proximal nerves or the roots, VMAT hypofractionated RT could be suggested as a standard treatment alternative to surgery that is not always a practicable solution. In future, an escalation dose protocol or a combined protocol with chemotherapy should be evaluated. Finally, the data regarding survival analysis also present a bias due to reirradiation of two dogs and a non-uniform dose between all the dogs. Further data will give more accurate results and a better volume response assessment.
Contributor Information
Mario Dolera, Email: lacittadina@alice.it.
Luca Malfassi, Email: lacittadinafondazione@gmail.com.
Cristina Bianchi, Email: cribi.bianchi@gmail.com.
Nancy Carrara, Email: nancy_carrara@hotmail.com.
Sara Finesso, Email: sarafinesso@hotmail.com.
Silvia Marcarini, Email: silvia.kant@tiscali.it.
Giovanni Mazza, Email: giovanni@arcaveterinaria.it.
Simone Pavesi, Email: pavesi.simone@gmail.com.
Massimo Sala, Email: salamas@katamail.com.
Gaetano Urso, Email: gaetano.urso@ao.lodi.it.
REFERENCES
- 1.Summers BA, Cummings JF, de Lahunta A. Neoplasia and the peripheral nervous system. In: Summers BA, Cummings JF, de Lahunta A, eds. Veterinary neuropathology. St. Louis, MO: Mosby; 1995. pp. 472–81. [Google Scholar]
- 2.Higgins RJ. Tumours of the nervous system. Part 1. Pathology. In: Thielen GH, Madewell BR, eds. Veterinary cancer medicine. 2nd edn. Philadelphia, PA: Lea & Febiger; 1987. pp. 6002–6. [Google Scholar]
- 3.Jones TC, Hunt RD. Veterinary pathology. 5th edn. Philadelphia, PA: Lea & Febiger; 1983. pp. 1685–6. [Google Scholar]
- 4.Tavasoly A, Javanbakht J, Khaki F, Hosseini E, Bahrami A, Hassan MA, et al. Ulnar malignant peripheral nerve sheath tumour diagnosis in a mixed-breed dog as a model to study human: histologic, immunohistochemical, and clinicopathologic study. Diagn Pathol 2013; 8: 86. doi: https://doi.org/10.1186/1746-1596-8-86 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Brehm DM, Vite CH, Steinberg HS, Haviland J, van Winkle T. A retrospective evaluation of 51 cases of peripheral nerve sheath tumours in the dog. J Am Anim Hosp Assoc 1995; 31: 349–59. doi: https://doi.org/10.5326/15473317-31-4-349 [DOI] [PubMed] [Google Scholar]
- 6.Platt SR. What is your diagnosis? Malignant nerve sheath tumour. J Small Anim Pract 2002; 43: 139–40. [PubMed] [Google Scholar]
- 7.Duke FD, Brudenall DK, Scott EM, Teixeira LB, Dubielzig RR. Metastatic uveal schwannoma of blue-eyed dogs. Vet Ophthalmol 2013; 16(Suppl. 1): 141–4. doi: https://doi.org/10.1111/vop.12022 [DOI] [PubMed] [Google Scholar]
- 8.Carmichael S, Griffiths IR. Tumours involving the brachial plexus in seven dogs. Vet Rec 1981; 108: 435–7. doi: https://doi.org/10.1136/vr.108.20.435 [DOI] [PubMed] [Google Scholar]
- 9.McCarthy RJ, Feeney DA, Lipowitz AJ. Preoperative diagnosis of tumours of the brachial plexus by use of computed tomography in three dogs. J Am Vet Med Assoc 1993; 202: 291–4. [PubMed] [Google Scholar]
- 10.Simpson DJ, Beck JA, Allan GS, Culvenor JA. Diagnosis and excision of a brachial plexus nerve sheath tumour in a dog. Aust Vet J 1999; 77: 222–4. doi: https://doi.org/10.1111/j.1751-0813.1999.tb11705.x [DOI] [PubMed] [Google Scholar]
- 11.Davis EG, Coates JR, Johnson GC, Cook CR, Pardo ID. What is your neurologic diagnosis? Peripheral nerve sheath tumour. J Am Vet Med Assoc 2011; 239: 189–91. doi: https://doi.org/10.2460/javma.239.2.189 [DOI] [PubMed] [Google Scholar]
- 12.Steinberg HS. Brachial plexus injuries and dysfunctions. Vet Clin North Am Small Anim Pract 1988; 18: 565–80. [DOI] [PubMed] [Google Scholar]
- 13.Lee RM, Ong CP, Jacobsen AS, Chan MY, Hwang WS. Malignant peripheral nerve sheath tumour mimicking carotid body tumour-case report and review. J Pediatr Surg 2011; 46: 554–8. [DOI] [PubMed] [Google Scholar]
- 14.Ziadi A, Saliba I. Malignant peripheral nerve sheath tumour of intracranial nerve: a case series review. Auris Nasus Larynx 2010; 37: 539–45. doi: https://doi.org/10.1016/j.anl.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 15.Gulati N, Rekhi B, Suryavanshi P, Jambhekar NA. Epithelioid malignant peripheral nerve sheath tumour of the uterine corpus. Ann Diagn Pathol 2011; 15: 441–5. doi: https://doi.org/10.1016/j.anndiagpath.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 16.Silker ML, Donahue BR, Vogelbaum MA, Tome WA, Gilbert MR, Mehta MP. Primary intracranial neoplasms. In: Halperin EC, Brady LW, Perez CA, eds. Perez and Brady's principles and practice of radiation oncology. 6th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 300–19. [Google Scholar]
- 17.Dolera M, Malfassi L, Carrara N, Marcarini S, Finesso S, Mazza G, et al. Stereotactic volume modulated arc radiotherapy in canine meningiomas: imaging-based and clinical neurological post-treatment evaluation. J Am Anim Hosp Assoc 2016; In press. [DOI] [PubMed] [Google Scholar]
- 18.Keyerleber MA, McEntee MC, Farrelly J, Thompson MS, Scrivani PV, Dewey CW. Three-dimensional conformal radiation therapy alone or in combination with surgery for treatment of canine intracranial meningiomas. Vet Comp Oncol 2015; 13: 385–97. doi: https://doi.org/10.1111/vco.12054 [DOI] [PubMed] [Google Scholar]
- 19.Axlund TW, McGlasson ML, Smith AN. Surgery alone or in combination with radiation therapy for treatment of intracranial meningiomas in dogs: 31 cases (1989–2002). J Am Vet Med Assoc 2002; 221: 1597–600. [DOI] [PubMed] [Google Scholar]
- 20.Dolera M, Malfassi L, Bianchi C, Carrara N, Corbetta L, Finesso S, et al. Frameless stereotactic radiotherapy alone and combined with temozolomide for canine gliomas. ACVIM Forum Research Report Program, 2016; Denver, CO. doi: https://doi.org/10.1111/jvim.13963
- 21.Bley CR, Sumova A, Roos M, Kaser-Hotz B. Irradiation of brain tumours in dogs with neurologic disease. J Vet Intern Med 2005; 19: 849–54. doi: https://doi.org/10.1111/j.1939-1676.2005.tb02776.x [DOI] [PubMed] [Google Scholar]
- 22.Spugnini EP, Thrall DE, Price GS, Sharp NJ, Munana K, Page RL. Primary irradiation of canine intracranial masses. Vet Radiol Ultrasound 2000; 41: 377–80. doi: https://doi.org/10.1111/j.1740-8261.2000.tb02091.x [DOI] [PubMed] [Google Scholar]
- 23.Brearley MJ, Jeffery ND, Phillips SM, Dennis R. Hypofractionated radiation therapy of brain masses in dogs: a retrospective analysis of survival of 83 cases (1991–1996). J Vet Intern Med 1999; 13: 408–12. [DOI] [PubMed] [Google Scholar]
- 24.Lester NV, Hopkins AL, Bova FJ, Friedman WA, Buatti JM, Meeks SL, et al. Radiosurgery using a stereotactic headframe system for irradiation of brain tumours in dogs. J Am Vet Med Assoc 2001; 219: 1562–7. [DOI] [PubMed] [Google Scholar]
- 25.Dolera M, Carrara N, Malfassi L. VMAT stereotactic body radiation therapy in multimodal approach of carotid paraganglioma in a dog. J Am Anim Hosp Assoc 2016. In press. [DOI] [PubMed] [Google Scholar]
- 26.Dolera M, Malfassi L, Mazza G, Urso G, Sala M, Marcarini S, et al. Feasibility for using hypofractionated stereotactic volumetric modulated arc radiotherapy (VMAT) with adaptive planning for treatment of thymoma in rabbits: 15 cases. Vet Radiol Ultrasound 2016; 57: 313–20. doi: https://doi.org/10.1111/vru.12321 [DOI] [PubMed] [Google Scholar]
- 27.Pozzo G. Compendio di risonanza magnetica. Diagnostica per immagini. 1st edn. Torino, Italy: UTET; 2001. pp. 236–370. [Google Scholar]
- 28.Todd M, Shah GV, Mukherji SK. MR imaging of brachial plexus. Top Magn Reson Imaging 2004; 15: 113–25. [DOI] [PubMed] [Google Scholar]
- 29.Kraft S, Ehrhart EJ, Gall D, Klopp L, Gavin P, Tucker R, et al. Magnetic resonance imaging characteristics of peripheral nerve sheath tumours of the canine brachial plexus in 18 dogs. Vet Radiol Ultrasound 2007; 48: 1–7. doi: https://doi.org/10.1111/j.1740-8261.2007.00195.x [DOI] [PubMed] [Google Scholar]
- 30.Van Es HW. MRI of the brachial plexus. Eur Radiol 2001; 11: 325–36. doi: https://doi.org/10.1007/s003300000644 [DOI] [PubMed] [Google Scholar]
- 31.Fossum T. Small animal surgery. St. Louis, MO: Mosby; 1997. [Google Scholar]
- 32.Slatter D. Textbook of small animal surgery. 3rd edn. Philadelphia, PA: W.B. Saunders; 2003. [Google Scholar]
- 33.Rudich SR, Feeney DA, Anderson KL, Walter PA. Computed tomography of masses of the brachial plexus and contributing nerve roots in dogs. Vet Radiol Ultrasound 2004; 45: 46–50. doi: https://doi.org/10.1111/j.1740-8261.2004.04007.x [DOI] [PubMed] [Google Scholar]
- 34.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010; 37: 4078–101. doi: https://doi.org/10.1118/1.3438081 [DOI] [PubMed] [Google Scholar]
- 35.Pollock BE. Stereotactic radiosurgery of benign intracranial tumours. J Neurooncol 2009; 92: 337–43. doi: https://doi.org/10.1007/s11060-009-9831-6 [DOI] [PubMed] [Google Scholar]
- 36.Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys 1998; 25: 656–61. doi: https://doi.org/10.1118/1.598248 [DOI] [PubMed] [Google Scholar]
- 37.Rossmeisl JH, Jr, Garcia PA, Daniel GB, Bourland JD, Debinski W, Dervisis N, et al. Invited review-neuroimaging response assessment criteria for braintumours in veterinary patients. Vet Radiol Ultrasound 2014; 55: 115–32. doi: https://doi.org/10.1111/vru.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: https://doi.org/10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 39.Ladue T, Klein MK; Veterinary Radiation Therapy Oncology Group. Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound 2001; 42: 475–6. doi: https://doi.org/10.1111/j.1740-8261.2001.tb00973.x [DOI] [PubMed] [Google Scholar]
- 40.Minniti G, Amichetti M, Enrici RM. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol 2009; 4: 42. doi: https://doi.org/10.1186/1748-717X-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasilopulos RJ, Mackin AJ, Jennings D, Read R. What is your neurologic diagnosis: nerve sheath tumour involving the brachial plexus with involvement of the spinal cord. J Am Vet Med Assoc 2002; 221: 1397–9. doi: https://doi.org/10.2460/javma.2002.221.1397 [DOI] [PubMed] [Google Scholar]
- 42.Platt SR, Graham J, Chrisman CL, Collins K, Chandra S, Sirninger J, et al. MRI and US in the diagnosis of a malignant peripheral nerve sheath tumour in a dog. Vet Radiol Ultrasound 1999; 40: 367–71. doi: https://doi.org/10.1111/j.1740-8261.1999.tb02128.x [DOI] [PubMed] [Google Scholar]
- 43.Suzuki S, Uchida K, Nakayama H. The effects of tumour location on diagnostic criteria for canine malignant peripheral nerve sheath tumours (MPNSTs) and the markers for distinction between canine MPNSTs and canine perivascular wall tumours. Vet Pathol 2014; 51: 722–36. doi: https://doi.org/10.1177/0300985813501336 [DOI] [PubMed] [Google Scholar]
- 44.Bailey CS. Long-term survival after surgical excision of a schwannoma of the sixth cervical spinal nerve in a dog. J Am Vet Med Assoc 1990; 196: 754–6. [PubMed] [Google Scholar]
- 45.Ueno H, Miyoshi K, Fukui S, Kondo Y, Matsuda K, Uchide T. Extranodal lymphoma with peripheral nervous system involvement in a dog. J Vet Med Sci 2014; 76: 723–7. doi: https://doi.org/10.1292/jvms.13-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]