Abstract
MRI is an essential tool in breast imaging, with multiple established indications. Dynamic contrast-enhanced MRI (DCE-MRI) is the backbone of any breast MRI protocol and has an excellent sensitivity and good specificity for breast cancer diagnosis. DCE-MRI provides high-resolution morphological information, as well as some functional information about neoangiogenesis as a tumour-specific feature. To overcome limitations in specificity, several other functional MRI parameters have been investigated and the application of these combined parameters is defined as multiparametric MRI (mpMRI) of the breast. MpMRI of the breast can be performed at different field strengths (1.5–7 T) and includes both established (diffusion-weighted imaging, MR spectroscopic imaging) and novel MRI parameters (sodium imaging, chemical exchange saturation transfer imaging, blood oxygen level-dependent MRI), as well as hybrid imaging with positron emission tomography (PET)/MRI and different radiotracers. Available data suggest that multiparametric imaging using different functional MRI and PET parameters can provide detailed information about the underlying oncogenic processes of cancer development and progression and can provide additional specificity. This article will review the current and emerging functional parameters for mpMRI of the breast for improved diagnostic accuracy in breast cancer.
INTRODUCTION
MRI of the breast is an essential tool in breast imaging, with multiple indications such as pre-operative staging, therapy monitoring, detection of recurrence, assessment of breast implants, screening of females at high risk, in patients with cancers of unknown primary syndrome and as a problem-solving tool for equivocal findings on mammography and sonography.1,2 Dynamic contrast-enhanced MRI (DCE-MRI) is the backbone of any given MRI protocol and the most sensitive method for the detection of breast cancer, with a negative-predictive value ranging between 89 and 99%, but variable specificities ranging from 47 to 97%.1,3–7 DCE-MRI provides high-resolution morphological information, as well as some functional information about neoangiogenesis as a tumour-specific feature. To overcome limitations in specificity, several functional MRI parameters have been investigated and the application of these combined parameters is defined as multiparametric MRI (mpMRI) of the breast. In its development, cancer acquires several functional capabilities that are defined as the hallmarks of cancer: resistance to growth inhibitory factors; proliferation in the absence of exogenous growth factors; evasion of apoptosis; limitless replication potential via the reactivation of telomerase; abnormal angiogenesis; evasion of destruction by the immune system; invasion; and metastasis.8,9 There is evidence that mpMRI, using different functional parameters, can provide detailed information about the hallmarks of cancer8,9 and can also provide additional specificity.5,10–16
MpMRI of the breast aims to quantify and visualize biological, physiological and pathological processes at the cellular and molecular levels to further elucidate the development and progression of breast cancer and the response to treatment. MpMRI of the breast can be performed at different field strengths (1.5–7 T) and includes several functional MRI parameters, as well as hybrid imaging techniques such as positron emission tomography (PET)/MRI. This article will review the current and emerging functional parameters for mpMRI to improve diagnostic accuracy. We will discuss established MRI parameters [DCE-MRI with kinetic analysis, diffusion-weighted imaging (DWI) and proton MR spectroscopy (1H-MRSI)], high-field and ultrahigh-field MRI at 3.0 T and abbreviated MRI. In addition, we will explain novel MRI parameters, such as sodium imaging (23Na-MRI), phosphorus MRSI (31P-MRSI), chemical exchange saturation transfer (CEST) imaging, blood oxygen level-dependent (BOLD) and hyperpolarized MRI (HP MRI), and briefly review the emerging application of hybrid imaging with PET/MRI.
DYNAMIC CONTRAST-ENHANCED MRI
A hallmark of cancer development and metastatic potential is tumour angiogenesis, i.e. the development of a dedicated vasculature with abnormal vessel permeability that supports the high metabolic demand for oxygen and nutrients, especially in aggressive tumours.8 Specific peptide hormones released by cancer cells promote tumour angiogenesis as soon as they exceed 2 mm in size.17 DCE-MRI is able to depict and characterize this abnormal vasculature and permeability as a tumour-specific feature through the assessment of breast kinetic enhancement features, after the i.v. application of gadolinium chelates.18,19
High-resolution, high-field and ultrahigh-field dynamic contrast-enhanced MRI
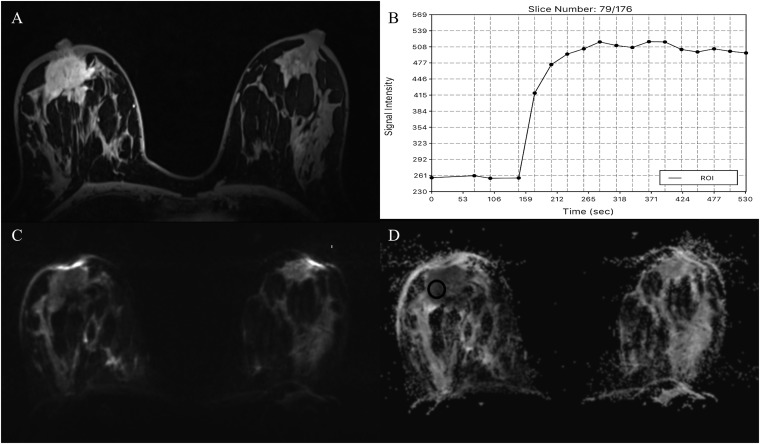
Both assessment of tumour morphology and enhancement kinetics are necessary for the optimal diagnosis of breast lesions.7 Parallel imaging techniques and the utilization of higher field strengths (≥3 T) allow both high-resolution spatial and temporal MRI with increases in sensitivity and specificity and thus, breast MRI is steadily moving to 3.0 T.3,4,7,20,21 Currently, ultrahigh-field MR scanners operating at a field strength of 7.0 T have become available. Ultrahigh-field MRI at 7.0 T offers a further significant increase in intrinsic signal-to-noise ratio, which can be translated into even higher temporal and spatial resolution imaging or functional and metabolic imaging.12,22,23 Initial studies have demonstrated the feasibility of this technique and highlighted the limitations of breast MRI at 7.0 T such as longer T1 relaxation times, shorter T2* decay time, greater radiofrequency specific absorption rate and reduced transmit field (B1+) homogeneity, all of which can limit the 7.0-T image quality in practice.24 However, since then, several studies investigating unilateral DCE-MRI of the breast at 7.0 T, in healthy volunteers and a few patients, have demonstrated that these challenges can be overcome.25–28 In the first clinical study, Pinker et al evaluated the application of bilateral contrast-enhanced MRI (CE-MRI) at 7.0 T in patients with breast tumours.12 The authors concluded that bilateral, high-resolution CE-MRI of the breast at 7.0 T is clinically applicable and enables a breast cancer diagnosis with a high diagnostic accuracy (96.6%) and excellent interrater agreement and image quality (Figure 1). Gruber et al22 performed an intraindividual comparison of the image quality, the contrast enhancement behaviour and the diagnostic value of bilateral high-spatial resolution and high-temporal resolution CE-MRI at 7.0 T and at 3.0 T in patients with breast tumours. When using optimized T1 weighted (T1W) three-dimensional (3D) sequences at 3.0 and 7.0 T, with a high temporal (both 14 s) and spatial resolution [1.1 × 1.1 × 1.1 mm3 (3.0 T), 0.7 × 0.7 × 0.7 mm3 (7.0 T)], 7.0-T CE-MRI provided simultaneous high temporal and spatial resolution, which was a significant improvement over lower field strengths, with an excellent sensitivity (100%) and specificity (91.67%) for breast cancer diagnosis. To circumvent the limitations in T2 weighted imaging due to the greater specific absorption rate at 7.0 T, Bogner et al developed a DWI protocol which simultaneously yields high-quality apparent diffusion coefficient (ADC) maps and high-spatial resolution T2 weighted MR images that can be used to assess tumour and breast morphology29.
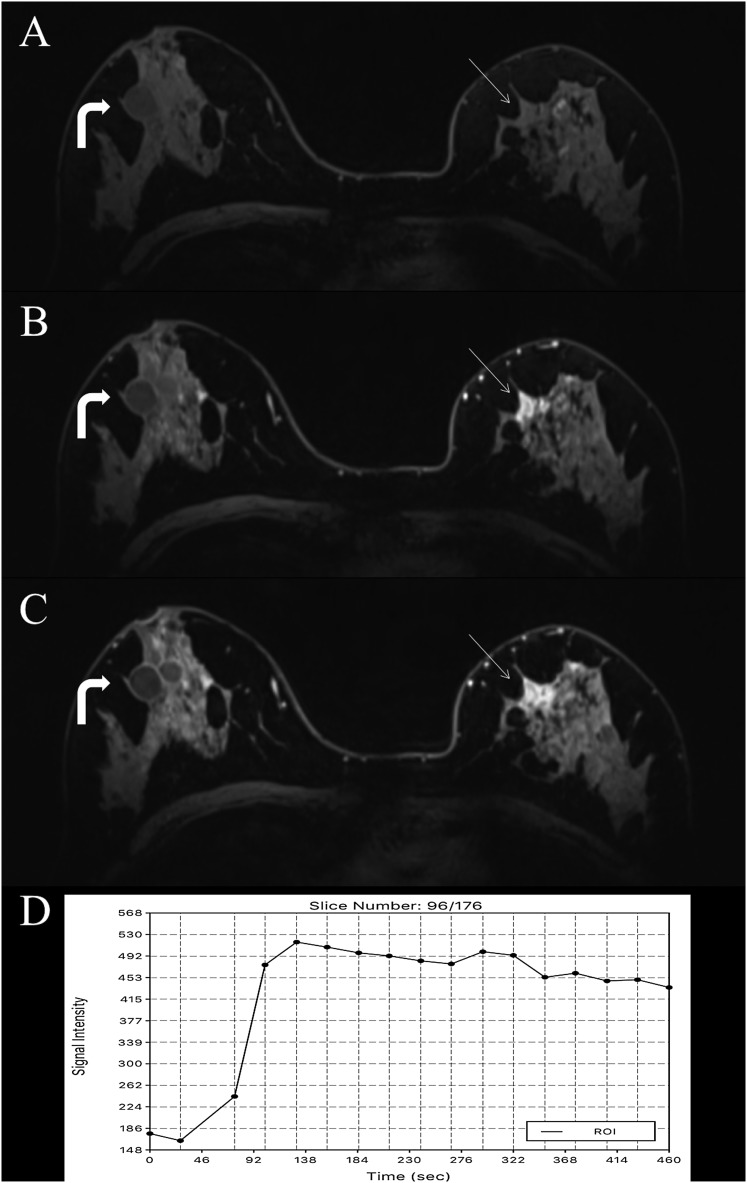
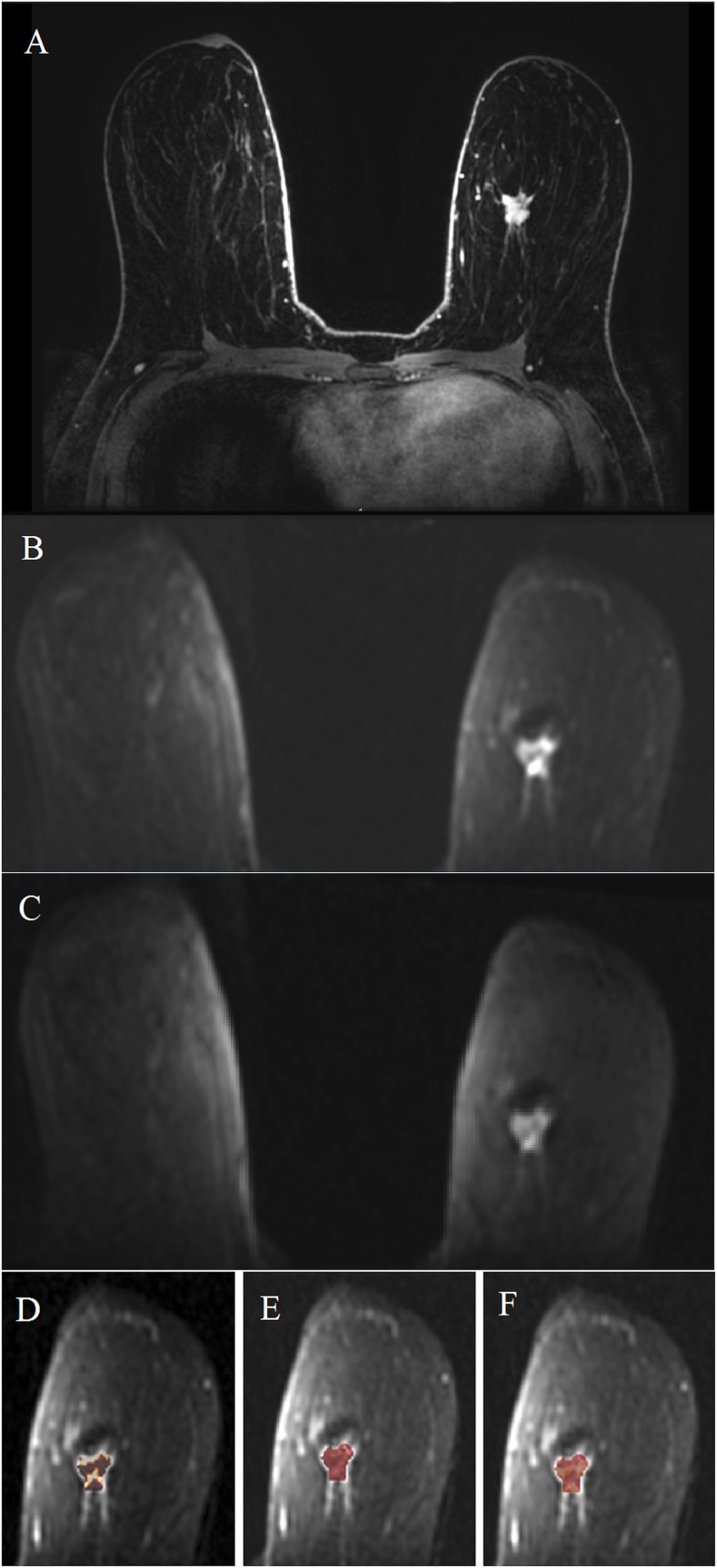
Figure 1.
MRI of the breast at 7.0 T: invasive ductal carcinoma grade 3 in the left breast in a 62-year-old female—high-resolution dynamic contrast-enhanced MRI (DCE-MRI) at 7.0 T is showing an irregular shaped and marginated mass lesion retromammillary right [(a) pre-contrast media, (b) initial and (c) delayed enhancement] with fast/washout enhancement kinetics (d). The non-enhancing cysts on the right breast (curved arrows) can be noted. High-resolution DCE-MRI at 7.0 T has accurately classified the lesion as malignant, breast imaging-reporting and data system 5 (highly suggestive of malignancy). ROI, region of interest.
Abbreviated MRI
Recently, abbreviated as well as ultrafast dynamic imaging protocols have been evaluated for both breast cancer diagnosis and screening. Several studies investigated whether an abbreviated protocol, consisting of either a pre-contrast T1W image and a single early post-contrast T1W image30–34 or high-resolution ultrafast dynamic imaging,35 was suitable to detect breast carcinoma (Figure 2). All authors concluded that the abbreviated MRI protocol for breast MRI screening allows detection of breast lesions and classification with high accuracy. These results indicate that a substantial shortening of scan protocols is possible and support the possibility of refining breast MRI screening protocols.
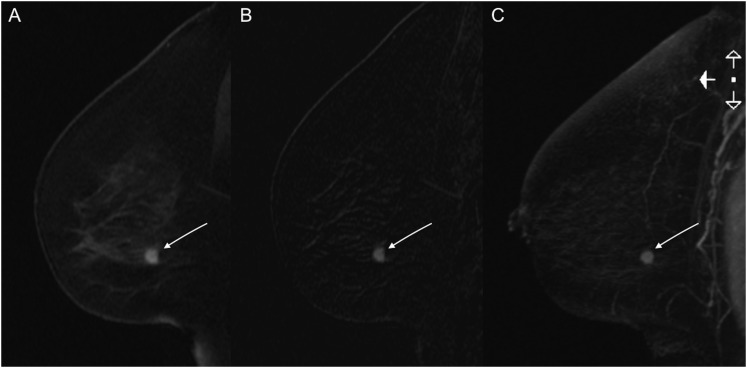
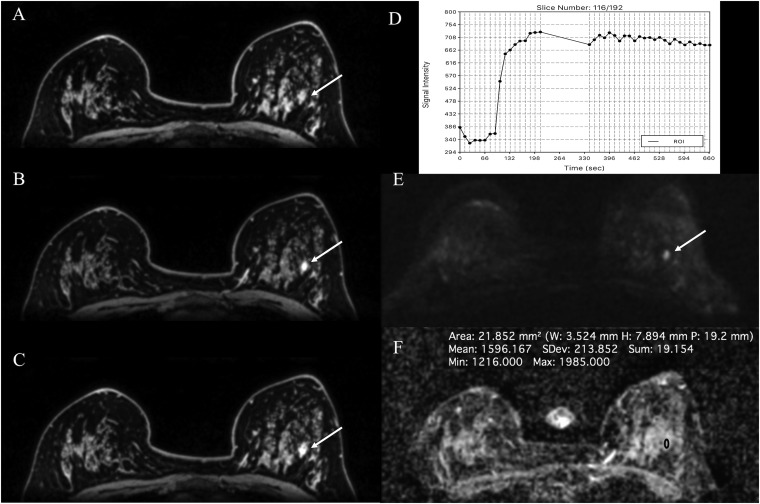
Figure 2.
MSKCC abbreviated MRI: invasive ductal carcinoma in the left breast in a 59-year-old female—abbreviated dynamic contrast-enhanced-MRI at 3.0 T is showing an irregular shaped and marginated enhancing mass in the lower quadrant of the left breast [(a) first post-contrast sequence, (b) first post-contrast subtraction sequence and (c) subtraction maximum intensity projection sequence].
Quantitative contrast-enhanced MRI
Kinetic enhancement analysis of breast tumours is routinely performed semi-quantitatively by time–intensity curves, using a modest temporal resolution with at least 2–3 post-contrast T1W acquisitions and with k-space centred at approximately 90–120 s after contrast injection for the first post-contrast images.1,6,7,15 However, high-temporal resolution MRI techniques enable a quantitative kinetic enhancement analysis through pharmacokinetic modelling. Pharmacokinetic models quantify the contrast agent exchange between the intravascular and the interstitial space, providing measures of tumour blood flow, microvasculature and capillary permeability. The Tofts two-compartment model is the most commonly used approach and measures the exchange between the breast tissue plasma and the plasma space.36,37 Contrast agent concentrations for each compartment vary with time after bolus injection, and quantitative metrics can be derived using the following relationship: Kep = Ktrans/Ve. Ktrans (min−1) is the volume transfer constant, which describes the rate of transfer of contrast agent from the plasma to the tissue. Kep (min−1) is the transfer rate constant, which describes the reflux of contrast agent from the extravascular extracellular space to the plasma compartment. Ve (%) is the leakage of fractional volume from the extravascular extracellular space into the plasma compartment (Figure 3). Pharmacokinetic parameters such as Ktrans and Kep can potentially improve the differentiation of benign and malignant breast tumours and distinguish different breast cancer subtypes.15 Huang et al investigated pharmacokinetic parameters in suspicious lesions on standard clinical breast MRI and the results indicate that the application of a cut-off for Ktrans values could be used to obviate unnecessary biopsies in lesions.38 Li et al assessed morphological and quantitative DCE-MRI for breast cancer diagnosis and as an imaging biomarker for the differentiation of subtypes. Ktrans and Kep values were significantly higher in invasive ductal carcinoma and ductal carcinoma in situ than in the borderline and benign lesions or healthy breast tissue.39
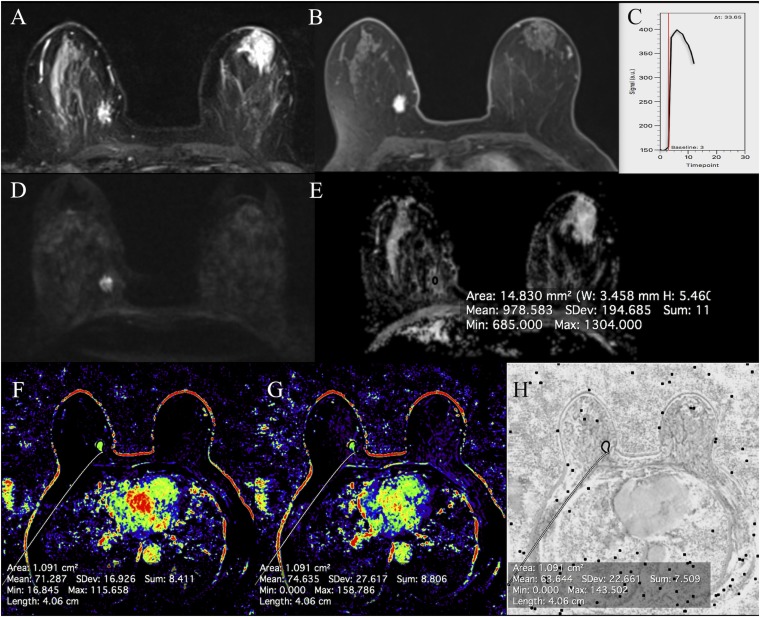
Figure 3.
MRI of the breast at 3.0 T: invasive ductal carcinoma grade 1 medially in the right breast in a 59-year-old female: short tau inversion-recovery images are showing an isointense to hyperintense irregular mass (a) with a restricted diffusivity (d) and decreased apparent diffusion coefficient values (0.98 × 10−3 mm2 s) (e). The irregular shaped and marginated mass (b) is showing fast/washout enhancement (c) and is having a mean plasma flow of 71.3 ml/100 ml min−1 (f), a mean volume distribution of 74.6 ml/100 ml (g) and a mean transit time of 63.6 s (h).
Another application for quantitative DCE MRI is the response assessment to neoadjuvant chemotherapy (NAC) in patients with breast cancer. In a recent meta-analysis, Marinovich et al41 showed that the measurement of alterations in tumour perfusion in response to pre-operative therapy, using Ktrans, is a powerful predictor of response to NAC and outperforms standard measures, such as tumour size.40
Nevertheless, quantitative DCE-MRI with pharmacokinetic modelling remains challenging, as several parameters, such as the pre-contrast T1 relaxation times of the tumour/tissue and the arterial input function, i.e. the concentration of contrast agent as it changes over time within the arterial blood, must be known. The measurement of both parameters comes with unique challenges and introduces the potential for error. To overcome these limitations, different strategies have been developed, and it seems that quantitative DCE-MRI benefits from the high-resolution MRI techniques now available.38,39,41–43 However, owing to different modelling algorithms, several challenges and various potential solutions, there are significant differences in quantitative measurements and thus, the results of individual studies cannot be readily generalized. Thus, further data—derived using standardized techniques—are warranted to fully explore the true potential of quantitative DCE-MRI.
DIFFUSION-WEIGHTED IMAGING
DWI measures the random movement of water molecules, i.e. Brownian movement, and depicts the diffusivity of the examined tissues. DWI is a strong surrogate marker for tissue microstructure, membrane integrity and cell density and can be quantified by calculating the ADC. Changes in tissue water diffusion properties can be used to detect and characterize pathological processes in any given body part.44 Developments in imaging techniques (e.g. parallel imaging) and hardware (stronger gradient systems and multichannel coils) have overcome previous limitations (susceptibility and respiratory motion artefacts) and DWI is now an integral part of oncologic imaging, including breast imaging.45,46 In short, malignant tumours tend to have a more restricted diffusion and lower ADC values than normal tissue or benign tumours owing to the high cellular density and abundance of intracellular and intercellular membranes.
DWI for breast cancer diagnosis has been evaluated with encouraging results by numerous studies using different ADC thresholds and b-values.47 Optimal ADC determination and DWI quality was found with a combined b-value protocol of 50 and 850 s mm−2, yielding a diagnostic accuracy of 96% (Figure 4).48 In a recent meta-analysis that included 26 studies, Dorrius et al confirmed that ADC values of breast lesions are influenced by the choice of b-values46. For the most accurate differentiation of benign and malignant lesions, the combination of b = 0 and 1000 s mm−2 was recommended. Nevertheless, there is a consensus that DWI yields a higher specificity (75–84%) than DCE-MRI (67–72%) and is a promising imaging biomarker that provides additional functional information to DCE-MRI.13,49 In addition to breast cancer detection, DWI can potentially be used as a non-invasive biomarker for the identification of different tumour subtypes, invasive vs non-invasive disease, tumour receptor status and tumour grading.50,51 Moreover, DWI shows promise for the monitoring of treatment response in breast cancer. Changes in ADC values occur earlier than lesion size changes or vascularity, as measured with DCE-MRI, and therefore, DWI can provide a valuable early indication of treatment efficacy.52,53
Figure 4.
MRI of the breast at 3.0 T: invasive ductal carcinoma grade 3 in the left breast in a 48-year-old female—high-resolution dynamic contrast-enhanced MRI [(a) pre-contrast media, (b) initial and (c) delayed enhancement] and T2 weighted imaging (d) at 3.0 T are showing an 8-mm irregular shaped and marginated mass lesion medially in the left breast with fast/washout enhancement kinetics (g). Diffusion-weighted imaging is showing a restricted diffusivity (e) and low apparent diffusion coefficient values (0.89 × 10−3 mm2 s) (f), indicating high cellularity which is indicative of tumour malignancy. ROI, region of interest.
Several advanced modelling approaches for DWI to provide further insights into tumour biology are currently under investigation.
Intravoxel incoherent motion
DWI is also sensitive to perfusion because the flow of blood in randomly oriented capillaries mimics a diffusion process through the intravoxel incoherent motion effect.54 Several studies have investigated intravoxel incoherent motion in breast tumours and preliminary data suggest that it can provide valuable information about both tissue microstructure and the microvasculature for improved breast cancer diagnosis,55–57 as well as differentiation of different breast cancer subtypes and molecular prognostic factors (Figure 5).56,58
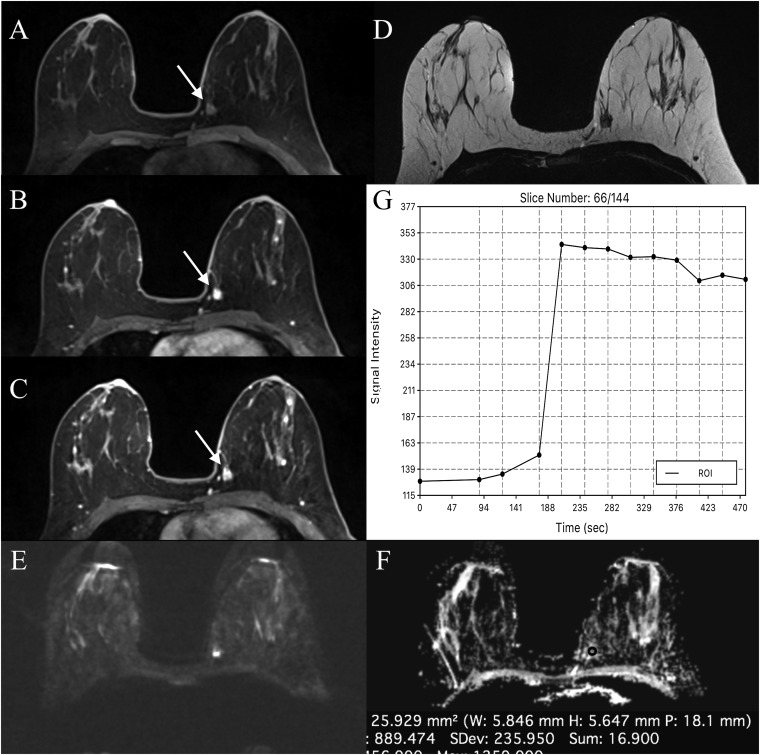
Figure 5.
Invasive ductal carcinoma in the left breast in a 42-year-old female: the mass is visible on dynamic contrast-enhanced MRI (a) and intravoxel incoherent motion (IVIM) images (b, c) at b = 0 s mm−2 and b = 500 s mm−2. IVIM protocol b-values = 0, 10, 192 and 500 s mm−2; d, e and f are showing the IVIM parameter maps for f, D and D*, respectively. Histogram analysis has gave median values of f = 22%, D = 147 × 10−6 mm2 s and D* = 7773 × 10−6 mm2 s.
Diffusion-weighted kurtosis
In living tissues, DWI is affected by Brownian incoherent motion and microperfusion or blood flow that demonstrates non-Gaussian phenomena. Diffusion-weighted kurtosis quantifies the deviation of tissue diffusion from a Gaussian pattern.59 Diffusion-weighted kurtosis has demonstrated a substantially higher sensitivity and specificity in cancer detection than ADCs and thus could provide valuable information about the diffusion properties related to the tumour microenvironment and increase diagnostic confidence for breast tumours.60–62
Diffusion tensor imaging
Diffusion tensor imaging (DTI) is an extension of DWI, which provides information about water motion in six or more directions and thereby characterizes the motion of water in more detail.63 DTI measures the two parameters, mean diffusivity and fractional anisotropy (FA). Mean diffusivity reflects the average anisotropy, whereas FA describes the degree of anisotropy.64,65 Partidge et al investigated whether DTI measures of anisotropy in breast tumours are different from those in normal breast tissue and could improve the discrimination between benign and malignant lesions65. Diffusion anisotropy is significantly lower in breast cancers than that in normal tissues, which may reflect alterations in tissue organization, but cannot reliably differentiate between benign and malignant lesions. Baltzer et al proved that DTI can visualize microanatomical differences between benign and malignant breast tumours and breast parenchyma66, but FA did not have an incremental value compared with ADC.64,65 In addition, a recent study showed that DTI parameters are influenced by background parenchymal enhancement within the normal breast tissue and should be considered in DTI evaluation.67
PROTON MR SPECTROSCOPIC IMAGING
MRSI reflects the chemical composition of a given tissue by demonstrating spatially localized signal spectra, which provide information about the varying levels of detectable metabolites. In breast imaging, the additional value of 1H-MRSI is largely based on the detection of choline (Cho), a biomarker of increased cellular turnover, which is typically increased in malignant tumours and thus aids in the characterization of breast tumours.68–70 1H-MRSI can be performed as single-voxel or multivoxel MRSI. For a detailed review of acquisition techniques and analysis of breast MRSI, a recent review article by Bolan et al71 can be referred to. Gruber et al72 developed a high-spatial resolution 3D 1H-MRSI protocol at 3.0 T, designed to cover a large fraction of the breast in a clinically acceptable measurement time of 12–15 min. Results indicated that 3D 1H-MRSI at 3.0 T yields excellent data quality and allows a differentiation of benign and malignant breast lesions with an excellent sensitivity (97%) and good specificity (84%). In a recent meta-analysis, which included 19 studies, Baltzer et al evaluated the diagnostic performance and feasibility of 1H-MRSI for differentiating malignant and benign breast lesions.73 The pooled sensitivity and specificity of 1H-MRSI was 73% and 88%, respectively. There was a substantial heterogeneity of sensitivity in the studies (42–100%), with little variation in specificity. This meta-analysis did not show any significant performance advantages of 3.0 T over 1.5 T field strength, for multivoxel over single-voxel techniques or for qualitative over quantitative tCho assessments. 1H-MRSI seems to be limited in the diagnosis of early breast cancer and small breast tumours, as well as in non-mass-enhancing lesions.
Available data show that 1H-MRSI might also be a valuable tool in the assessment of response to NAC.74,75 Breast tumour tCho levels and the changes in these levels during treatment are reflective of treatment-induced alterations in cell proliferation prior to any changes in tumour size. Therefore, 1H-MRSI can provide an early predictive imaging biomarker of response to treatment. In addition, tCho seems to be indicative of not only an increased proliferation, but also an imminent malignant transformation.76,77 Ramadan et al demonstrated that in BRCA-1 and BRCA-2 carriers, the healthy breast tissue is likely to differ from patient to patient, as well as from non-mutation carriers with regard to levels of triglycerides, unsaturated fatty acids and cholesterol, in the absence of any other imaging findings.78 Further studies are warranted, but if these findings are confirmed, there might be relevant clinical implications for the screening of high-risk females.
MULTIPARAMETRIC MRI
To overcome limitations in the specificity of DCE, several functional MRI parameters have been investigated, and the application of these combined parameters is defined as mpMRI of the breast. There is evidence that mpMRI using different functional parameters can provide detailed information about the hallmarks of cancer8,9 and provide additional specificity.5,10–16 MpMRI with DCE-MRI and DWI has been investigated for breast cancer diagnosis in multiple studies and results indicated that MpMRI with DCE-MRI and DWI increases diagnostic accuracy in breast cancer diagnosis.10,11,79 To solve the dilemma of how to combine the unique information from DCE-MRI and DWI, and how to implement mpMRI in the clinical routine, several different approaches have been introduced. Pinker et al developed a reading scheme that adapted ADC thresholds to the assigned breast imaging-reporting and data system (BI-RADS) classification.80 In that study, the sensitivity of the BI-RADS-adapted reading was not significantly different from the high sensitivity of DCE-MRI (p = 0.4), whereas the BI-RADS-adapted reading maximized specificity to 89.4%, which was significantly higher compared with DCE-MRI (p < 0.001). The authors concluded that BI-RADS-adapted reading, which combines DCE-MRI and DWI, improves diagnostic accuracy and is fast and easy to use in routine clinical practice (Figures 6 and 7).11 Baltzer et al investigated improvements in the specificity of breast MRI by integrating ADC values with DCE-MRI using a simple sum score.16 The additional integration of ADC scores achieved an improved specificity (92.4%) compared with DCE-MRI-only reading (specificity of 81.8%), with no false-negative results. In a very recent study, Dijkstra et al investigated whether the specificity in the work-up of BI-RADS 3 and 4 breast tumours can be increased when a semi-automated breast lesion analysis of quantitative DWI is implemented after DCE-MRI. When quantitative DWI was added to DCE-MRI, the combined specificity improved significantly.81 Recently, the concept of mpMRI using DCE-MRI and DWI has been translated to ultrahigh field strengths (7.0 T).10 MpMRI, which combines high-resolution DCE-MRI and DWI at 7.0 T, yielded a sensitivity and specificity of 100% and 88.2%, respectively, with an area under the curve (AUC) of 0.941, which was significantly greater than that of DCE-MRI (p = 0.003). In that study, mpMRI of the breast at 7.0 T accurately detected all cancers, reduced false positives from eight with DCE-MRI to two and thus could have obviated unnecessary breast biopsies (p = 0.031) (Figures 8 and 9).
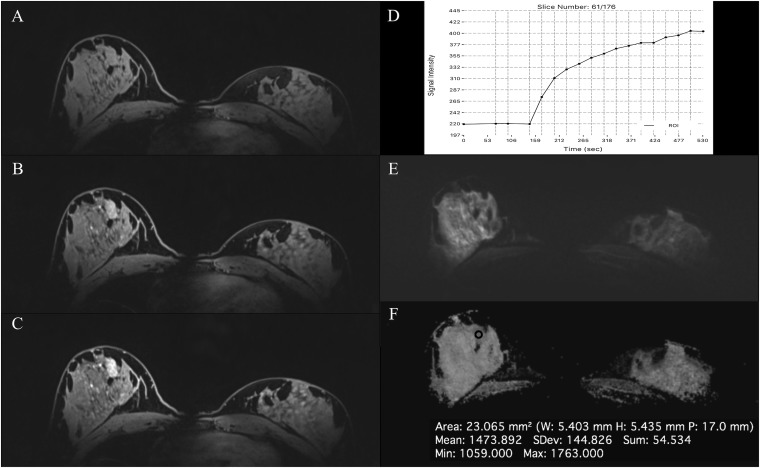
Figure 6.
MRI of the breast at 3.0 T: invasive ductal carcinoma in the right breast in a 60-year-old female—the extensive lesion occupying almost the whole right breast is demonstrating an irregular shape and margins [(a) pre-contrast media, (b) initial and (c) delayed enhancement], fast/washout enhancement (e), a restricted diffusivity (f) and decreased apparent diffusion coefficient (ADC) values (881 × 10−3 mm2/s) (g). In baseline MRI before neoadjuvant cytotoxic chemotherapy, an additional enhancing lesion has been detected in the left breast lateral. The 7-mm non-mass enhancement (arrows) is demonstrating an irregular shape and margins [(a) pre-contrast media, (b) initial and (c) delayed enhancement], fast/plateau enhancement (d), restricted diffusivity (f) and decreased ADC values (1004 × 10−3 mm2/s) (g) and has been confirmed to be high-grade ductal carcinoma in situ. ROI, region of interest.
Figure 7.
MRI of the breast at 3.0 T: fibroadenoma in a 42-year-old female at 2 o'clock in the right breast—the round and partly irregularly marginated mass [(a) pre-contrast media, (b) initial and (c) delayed enhancement] is demonstrating a homogeneous fast/washout contrast enhancement (d) and has been classified as breast imaging-reporting and data system (BI-RADS) 4 on dynamic contrast-enhanced MRI. On diffusion-weighted imaging (e), the apparent diffusion coefficient values (1.596 × 10−3 mm2/s) (f) were well above the threshold for malignancy, thus allowing an accurate classification as a benign finding (BI-RADS 3—probably benign) with the BI-RADS-adapted reading. ROI, region of interest.
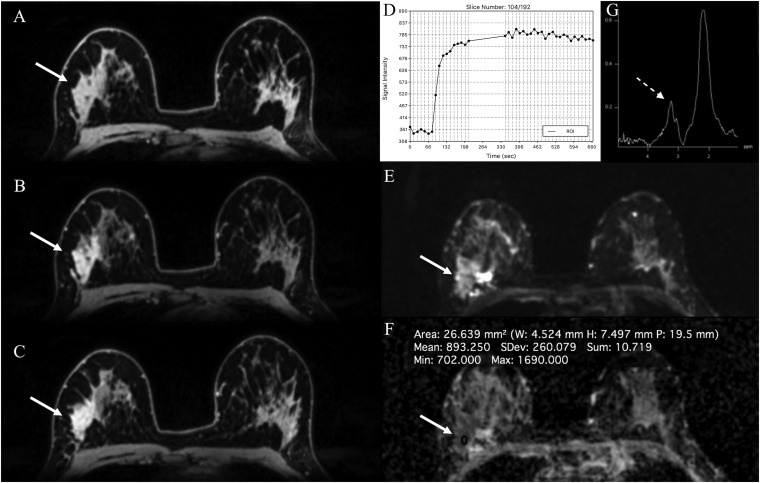
Figure 8.
MRI of the breast at 7.0 T: invasive ductal carcinoma grade 3 and associated high-grade ductal carcinoma in situ in the right breast in a 36-year-old female—high-resolution dynamic contrast-enhanced MRI at 7.0 T is showing an irregular shaped, partly spiculated mass lesion retromammillary right (a) with fast/washout enhancement kinetics (b). Diffusion-weighted imaging is showing a restricted diffusivity (c) and low apparent diffusion coefficient values (0.68 × 10−3 mm2 s) (d), indicating high cellularity, which is indicative of tumour malignancy (breast imaging-reporting and data system 5—highly suggestive of malignancy). ROI, region of interest.
Figure 9.
MRI of the breast at 7.0 T: fibroadenoma in a 32-year-old female in the right breast retromammillary—the round and partly irregularly marginated mass [(a) pre-contrast media, (b) initial and (c) delayed enhancement] is demonstrating a homogeneous medium/persistent contrast enhancement (d) and has been classified as breast imaging-reporting and data system (BI-RADS) 4 in dynamic contrast-enhanced MRI. On diffusion-weighted imaging (e), the apparent diffusion coefficient values (1.473 × 10−3 mm2/s) (f) are well above the threshold for malignancy, thus allowing an accurate classification as a benign finding (BI-RADS 3—probably benign) with BI-RADS-adapted reading.
To further increase specificity, Pinker et al compared the diagnostic accuracy of DCE-MRI as a single parameter with mpMRI with two (DCE-MRI and DWI) and three (DCE-MRI, DWI and 1H-MRSI) parameters in breast cancer diagnosis (Figure 10).82 MpMRI with three parameters yielded significantly higher AUCs (0.936) than DCE-MRI alone (0.814) (p < 0.001). MpMRI at 3.0 T with only two parameters did not yield higher AUCs (0.808) than DCE-MRI alone (0.814). MpMRI with three parameters eliminated all false-negative lesions and significantly reduced false positives (p = 0.002). Because MpMRI with three parameters increased the diagnostic accuracy of breast cancer, compared with DCE-MRI alone and mpMRI with two parameters, mpMRI should be considered for future implementation in breast cancer care. The concept of mpMRI with three parameters has been recently extended to 7.0 T. Schmitz et al investigated mpMRI with three parameters, i.e. DCE-MRI, DWI and phosphorus MRSI (31P-MRSI), at 7.0 T for the characterization of breast cancer.83 Results indicate that mpMRI of the breast at 7.0 T with three parameters is feasible in the clinical setting and shows an association between ADC and tumour grade and between 31P-MRSI and mitotic count.
Figure 10.
MRI of the breast at 3.0 T: invasive lobular carcinoma grade 3 laterally in the right breast in a 44-year-old female at 3 T—the lesion (arrow) is presenting as a segmental heterogeneous non-mass enhancement [(a) pre-contrast media, (b) initial and (c) delayed enhancement] with a fast/plateau contrast enhancement (d). On diffusion-weighted imaging, there is a restricted diffusivity (e) with decreased ADC values (0.89 × 10−3 mm2/s) (f). On proton MR spectroscopic imaging, there is a choline peak at 3.2 ppm (dashed arrow) (g). Multiparametric MRI has accurately classified the lesion as malignant, BI-RADS 5 (highly suggestive of malignancy).
EMERGING MRI PARAMETERS
In addition to already established functional MRI parameters, novel MRI parameters are rapidly being developed and translated into clinical imaging.
Sodium imaging
Sodium MRI (23Na-MRI) is an emerging MRI technique for the detection and therapy monitoring of breast cancer. 23Na-MRI provides information about the physiological and biochemical state of tissues, and sodium concentration is a sensitive indicator of cellular metabolic integrity and ion homeostasis.84 23Na-MRI depicts increased sodium levels secondary to failure of the Na+/K+-ATPase pump due to the breakdown of cell membranes as a marker for malignancy. Ouwerkerk et al investigated the potential of 23Na-MRI for the differentiation of benign and malignant breast tumours at 1.5 T84,85 and found that an increased total sodium concentration in breast tumours is a sensitive cellular level indicator of malignancy. In addition, 23Na-MRI is a sensitive imaging biomarker for response assessment in patients receiving NAC. Initial results at 1.5 T indicated that responders to NAC demonstrated significant changes in multiparametric imaging biomarkers with CE-MR, 1H-MRSI and 23Na-MRI parameters, even after the first cycle of NAC, and thus, mpMRI of the breast can provide new surrogate imaging biomarkers to predict response.90,91 However, at field strengths of 1.5 and 3.0 T, 23Na-MRI is limited. Zaric et al investigated quantitative 23Na-MRI at 7.0 T compared with DWI and proved that quantitative 23Na-MRI of the breast at 7.0 T is feasible, with good resolution and image quality, in clinically acceptable measurement times. Similar to DWI (p = 0.002), 23Na-MRI enabled differentiation of benign and malignant breast tumours (p = 0.002). 23Na-MRI adds complementary information about pathophysiologic changes in tumours and thus has the potential to improve the detection, characterization and treatment monitoring of breast lesions.86
Phosphorus spectroscopic imaging
Phosphorus spectroscopic imaging (31P-MRSI) measures the bioenergetics of tissue and membrane phospholipid metabolism, and the signals of phospholipid precursors and catabolites can be used as imaging biomarkers for tumour progression and response to therapy.87,88 However, at field strengths of 1.5 and 3.0 T, the clinical application of 31P-MRSI is limited. Recently, the feasibility of 31P-MRSI at 7.0 T has been demonstrated in healthy volunteers and in patients with breast cancer. 31P-MRS provides endogenous biomarkers for phospholipid/phosphate energy metabolism and intracellular pH and allows in vivo monitoring of tumour metabolism during NAC. 31P-MRSI is expected to be used as a targeted imaging tool for breast cancer diagnosis, tumour staging and monitoring response to therapy.87,88
Chemical exchange saturation transfer imaging
CEST is an MRI parameter that enables visualization of chemical exchange processes between protons bound to solutes and surrounding bulk water molecules.89–91 Endogenous CEST can discriminate tumour from healthy breast tissue, based on the information about protons associated with mobile proteins, through the amide proton transfer (APT) effect and has been implicated as a prognosticator of response to therapy. Initial feasibility studies hint at a significant potential for APT CEST-MRI in breast imaging.90,92 Recently, animal studies have investigated CEST contrasts other than APT, exploiting the entire CEST spectrum. Desmond et al found that imaging of the amide, amine and aliphatic signal allows non-invasive differentiation of areas of apoptosis and/or necrosis from actively progressing tumour.93 Analogous to fluorine-18 fludeoxyglucose ([18F]FDG) PET, dynamic CEST imaging after the administration of glucose enables the non-invasive evaluation of the kinetics of glycolysis and thus, CEST imaging after the administration of glucose might serve as a potential substitute for PET/CT or PET/MRI without the need for radiolabelled isotopes.94 Nevertheless, further studies will be necessary to explore the true potential of CEST imaging in breast cancer.
Blood oxygen level-dependent MRI
BOLD MRI, or intrinsic susceptibility-weighted imaging, is a non-invasive method for the indirect measurement of tumour perfusion and hypoxia. Hypoxia is a feature of most solid tumours, including breast cancer, and is associated with tumour progression, treatment resistance, local recurrence and metastasis. Initial results indicate that BOLD MRI is a simple and non-invasive technique with which to obtain information about hypoxia in breast cancer.95,96 Hypoxia imaging with BOLD MRI might, therefore, have the potential to serve as an imaging biomarker for breast cancer diagnosis and prognosis, as well as treatment response.97
Hyperpolarized MRI
HP MRI is one of the most recent advances in molecular imaging. HP MRI allows a rapid, radiation-free, non-invasive investigation of tumour metabolism by exploiting exogenous contrast agents that have been “hyperpolarized”, resulting in an extensive increase in signal intensity.98,99 Recently, 13C-labelled substrates have been polarized to obtain enhancements of the 13C nuclear MR signals, e.g. >50,000-fold at 3.0 T in the substrate, and the subsequent metabolic products. The HP 13C probes can be injected into living organisms and their metabolism can be observed in real time by chemical shift imaging. Currently, (13C) pyruvate is the most widely used probe for HP MR studies, since it polarizes well, has a long T1 relaxation time and is rapidly taken up by the cell and metabolized at the juncture of glycolysis, tricarboxylic acid, amino acid biosynthesis and other critical pathways. Several animal studies have confirmed that the real-time measurement of the relative transformation of pyruvate into lactate and alanine with HP MRI enables the differentiation of benign and malignant tumours, as well as cancer progression.99–101 Other novel probes for redox (13C dehydroascorbate), necrosis (13C fumarate) and glutamine metabolism (13C glutamine) have been developed to interrogate other metabolic pathways, and initial results are promising.102 To date, there is no specific clinical application for HP MRI in breast cancer, but pre-clinical results indicate that this technique may be applicable, in the future, for the detection of breast cancer and assessment of treatment response.103
HYBRID IMAGING WITH POSITRON EMISSION TOMOGRAPHY/MRI
In recent years, mpMRI and PET of the breast have emerged as promising imaging tools5,104,105 that provide morphologic and functional data and are of complementary value.106,107 To overcome the individual limitations of morphologic and functional imaging techniques, hybrid imaging systems have been developed and introduced into the clinical routine. Initial studies investigating fused [18F]FDG PET and DCE-MRI for breast cancer diagnosis demonstrated that fused [18F]FDG PET/MRI provides accurate morphological and functional data.108 Pinker et al investigated mpPET/MRI using DCE-MRI, DWI, 1H-MRSI and [18F]FDG for the assessment of breast tumours at 3.0 T.109 Multiparametric [18F]FDG PET/MRI provided an improved differentiation of benign and malignant breast tumours when several MRI and PET parameters were combined (Figure 10). In addition, the authors concluded that multiparametric [18F]FDG PET/MRI may lead to an up to 50% reduction of unnecessary breast biopsies. In a recent feasibility study, Pinker et al investigated combined PET/MRI of breast tumours with DCE-MRI, DWI, the radiotracer [18F]FDG and the hypoxia tracer [18F]fluoromisonidazole at 3.0 T, in eight patients, and correlated MRI and PET parameters with pathological features, grading, proliferation rate (ki67), immunohistochemistry and the clinical end-point metastasis and death.110 Preliminary results showed several moderate-to-excellent correlations between quantitative imaging markers, grading, receptor status and proliferation rate. Multiparametric criteria provided independent information. DCE-MRI, [18F]FDG and [18F]FMISO avidity strongly correlated with the presence of metastasis [r = 0.75 (p < 0.01), 0.63 (p = 0.212) and 0.58 (p = 0.093)] and death [r = 0.60 (p = 0.09), 0.62 (p = 0.08) and 0.56 (p = 0.11)]. These results demonstrate that multiparametric [18F]FDG/[18F]FMISO PET/MRI can provide quantitative prognostic information in patients with breast cancer and thus might have the potential to enable tailored therapy through improved risk stratification.
CONCLUSION
Within the past few years, mpMRI has been established in the field of breast imaging. MpMRI of the breast comprises different established MRI parameters (DCE-MRI, DWI and 1H-MRSI), as well as hybrid imaging with PET/MRI. Novel MRI parameters, such as 23Na-MRI, 31P-MRSI, CEST, BOLD and HP MRI, are being rapidly developed and translated into clinical imaging. A paradigm shift, from morphologic to functional imaging in cancer imaging, is imminent. MpMRI of the breast has the potential to significantly enhance our understanding of tumour biology, and it can be expected that mpMRI will play a pivotal role in the genomic era of cancer care, enabling personalized medicine in patients with breast cancer.
FUNDING
Funding was provided by the Austrian Nationalbank ‘Jubiläumsfond’ Project No. 13652, 16219 and 15082, the 2020 Research and Innovation Framework Programme PHC-11-2015, No. 667211–2 and seed grants from Siemens Austria, Novomed, Medicor, Austria, and Guerbet, France. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Acknowledgments
ACKNOWLEDGMENTS
We gratefully acknowledge the productive cooperation with the MR Centre of Excellence (Director: Prof. S. Trattnig).
Contributor Information
Katja Pinker, Email: katja.pinker@meduniwien.ac.at.
Thomas H Helbich, Email: thomas.helbich@meduniwien.ac.at.
Elizabeth A Morris, Email: morrise@mskcc.org.
REFERENCES
- 1.Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 2010; 46: 1296–316. doi: https://doi.org/10.1016/j.ejca.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 2.Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E, et al. Breast MRI: EUSOBI recommendations for women's information. Eur Radiol 2015. doi: https://doi.org/10.1007/s00330-015-3807-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinker K, Grabner G, Bogner W, Gruber S, Szomolanyi P, Trattnig S, et al. A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: initial results. Invest Radiol 2009; 44: 553–8. doi: https://doi.org/10.1097/rli.0b013e3181b4c127 [DOI] [PubMed] [Google Scholar]
- 4.Pinker-Domenig K, Bogner W, Gruber S, Bickel H, Duffy S, Schernthaner M, et al. High resolution MRI of the breast at 3 T: which BI-RADS(R) descriptors are most strongly associated with the diagnosis of breast cancer? Eur Radiol 2012; 22: 322–30. [DOI] [PubMed] [Google Scholar]
- 5.Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin North Am 2007; 45: 863–80. doi: https://doi.org/10.1016/j.rcl.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Morrow M, Waters J, Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 2011; 378: 1804–11. doi: https://doi.org/10.1016/S0140-6736(11)61350-0 [DOI] [PubMed] [Google Scholar]
- 7.D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS®Atlas, Breast Imaging Reporting and Data System. 5th edn. Reston, VA: American College of Radiology; 2013.
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. doi: https://doi.org/10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. doi: https://doi.org/10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 10.Pinker K, Baltzer P, Bogner W, Leithner D, Trattnig S, Zaric O, et al. Multiparametric MR imaging with high-resolution dynamic contrast-enhanced and diffusion-weighted imaging at 7 T improves the assessment breast tumors: a feasibility study. Radiology 2015: 141905.
- 11.Pinker K, Bickel H, Helbich T, Gruber S, Dubsky P, Pluschnig U, et al. Combined contrast enhanced magnetic resonance and diffusion weighted imaging reading adapted to the “Breast Imaging Reporting and Data System” for multiparametric 3 T imaging of breast lesions. Eur Radiol 2013. [DOI] [PubMed] [Google Scholar]
- 12.Pinker K, Bogner W, Baltzer P, Trattnig S, Gruber S, Abeyakoon O, et al. Clinical application of bilateral high temporal and spatial resolution dynamic contrast-enhanced magnetic resonance imaging of the breast at 7 T. Eur Radiol 2014; 24: 913–20. doi: https://doi.org/10.1007/s00330-013-3075-8 [DOI] [PubMed] [Google Scholar]
- 13.Spick C, Pinker-Domenig K, Rudas M, Helbich TH, Baltzer PA. MRI-only lesions: application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. Eur Radiol 2014; 24: 1204–10. doi: https://doi.org/10.1007/s00330-014-3153-6 [DOI] [PubMed] [Google Scholar]
- 14.Partridge SC, Singer L, Sun R, Wilmes LJ, Klifa CS, Lehman CD, et al. Diffusion-weighted MRI: influence of intravoxel fat signal and breast density on breast tumor conspicuity and apparent diffusion coefficient measurements. Magn Reson Imaging 2011; 29: 1215–21. doi: https://doi.org/10.1016/j.mri.2011.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahbar H, Partridge SC. Multiparametric MR imaging of breast cancer. Magn Reson Imaging Clin N Am 2016; 24: 223–38. doi: https://doi.org/10.1016/j.mric.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltzer A, Dietzel M, Kaiser CG, Baltzer PA. Combined reading of contrast enhanced and diffusion weighted magnetic resonance imaging by using a simple sum score. Eur Radiol 2016; 26: 884–91. doi: https://doi.org/10.1007/s00330-015-3886-x [DOI] [PubMed] [Google Scholar]
- 17.Folkman J. Seminars in medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995; 333: 1757–63. [DOI] [PubMed] [Google Scholar]
- 18.Preda A, Novikov V, Moglich M, Floyd E, Turetschek K, Shames DM, et al. Magnetic resonance characterization of tumor microvessels in experimental breast tumors using a slow clearance blood pool contrast agent (carboxymethyldextran-A2-Gd-DOTA) with histopathological correlation. Eur Radiol 2005; 15: 2268–75. doi: https://doi.org/10.1007/s00330-005-2823-9 [DOI] [PubMed] [Google Scholar]
- 19.El Khouli RH, Macura KJ, Kamel IR, Jacobs MA, Bluemke DA. 3-T dynamic contrast-enhanced MRI of the breast: pharmacokinetic parameters versus conventional kinetic curve analysis. AJR Am J Roentgenol 2011; 197: 1498–505. doi: https://doi.org/10.2214/AJR.10.4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhl CK, Jost P, Morakkabati N, Zivanovic O, Schild HH, Gieseke J. Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: Initial experience. Radiology 2006; 239: 666–76. doi: https://doi.org/10.1148/radiol.2392050509 [DOI] [PubMed] [Google Scholar]
- 21.Merckel LG, Verkooijen HM, Peters NH, Mann RM, Veldhuis WB, Storm RK, et al. The added diagnostic value of dynamic contrast-enhanced MRI at 3.0 T in nonpalpable breast lesions. PloS One 2014; 9: e94233. doi: https://doi.org/10.1371/journal.pone.0094233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber S, Pinker K, Zaric O, Minarikova L, Chmelik M, Baltzer P, et al. Dynamic contrast-enhanced magnetic resonance imaging of breast tumors at 3 and 7 T: a comparison. Invest Radiol 2014; 49: 354–62. doi: https://doi.org/10.1097/RLI.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 23.Gruber S, Minarikova L, Pinker K, Zaric O, Chmelik M, Strasser B, et al. Diffusion-weighted imaging of breast tumours at 3 Tesla and 7 Tesla: a comparison. Eur Radiol 2016; 26: 1466–73. doi: https://doi.org/10.1007/s00330-015-3947-1 [DOI] [PubMed] [Google Scholar]
- 24.Umutlu L, Maderwald S, Kraff O, Theysohn JM, Kuemmel S, Hauth EA, et al. Dynamic contrast-enhanced breast MRI at 7 Tesla utilizing a single-loop coil: a feasibility trial. Acad Radiol 2010; 17: 1050–6. doi: https://doi.org/10.1016/j.acra.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 25.Brown R, Storey P, Geppert C, McGorty K, Klautau Leite AP, Babb J, et al. Breast MRI at 7 Tesla with a bilateral coil and robust fat suppression. J Magn Reson Imaging 2014; 39: 540–9. doi: https://doi.org/10.1002/jmri.24205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown R, Storey P, Geppert C, McGorty K, Leite AP, Babb J, et al. Breast MRI at 7 Tesla with a bilateral coil and T1-weighted acquisition with robust fat suppression: image evaluation and comparison with 3 Tesla. Eur Radiol 2013; 23: 2969–78. doi: https://doi.org/10.1007/s00330-013-2972-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stehouwer BL, Klomp DW, Korteweg MA, Verkooijen HM, Luijten PR, Mali WP, et al. 7T versus 3T contrast-enhanced breast magnetic resonance imaging of invasive ductulolobular carcinoma: first clinical experience. Magn Reson Imaging 2013; 31: 613–7. doi: https://doi.org/10.1016/j.mri.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Stehouwer BL, Klomp DW, van den Bosch MA, Korteweg MA, Gilhuijs KG, Witkamp AJ, et al. Dynamic contrast-enhanced and ultra-high-resolution breast MRI at 7.0 Tesla. Eur Radiol 2013; 23: 2961–8. doi: https://doi.org/10.1007/s00330-013-2985-9 [DOI] [PubMed] [Google Scholar]
- 29.Bogner W, Pinker K, Zaric O, Baltzer P, Minarikova L, Porter D, et al. Bilateral diffusion-weighted MR imaging of breast tumors with submillimeter resolution using readout-segmented echo-planar imaging at 7 T. Radiology 2015; 274: 74–84. doi: https://doi.org/10.1148/radiol.14132340 [DOI] [PubMed] [Google Scholar]
- 30.Mango VL, Morris EA, David Dershaw D, Abramson A, Fry C, Moskowitz CS, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol 2015; 84: 65–70. doi: https://doi.org/10.1016/j.ejrad.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 31.Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 2015; 22: 1157–62. doi: https://doi.org/10.1016/j.acra.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 32.Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol 2016; 13: 374–80. doi: https://doi.org/10.1016/j.jacr.2016.09.031 [DOI] [PubMed] [Google Scholar]
- 33.Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer 2016; 16: 207–11. doi: https://doi.org/10.1016/j.clbc.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 34.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 2014; 32: 2304–10. doi: https://doi.org/10.1200/JCO.2013.52.5386 [DOI] [PubMed] [Google Scholar]
- 35.Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol 2014; 49: 579–85. doi: https://doi.org/10.1097/rli.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 36.Tofts PS, Berkowitz B, Schnall MD. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumors using a permeability model. Magn Reson Med 1995; 33: 564–8. doi: https://doi.org/10.1002/mrm.1910330416 [DOI] [PubMed] [Google Scholar]
- 37.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999; 10: 223–32. doi: https://doi.org/10.1002/(SICI)1522-2586(199909)10:3<223::AID-JMRI2>3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Tudorica LA, Li X, Thakur SB, Chen Y, Morris EA, et al. Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology 2011; 261: 394–403. doi: https://doi.org/10.1148/radiol.11102413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Wang K, Sun X, Wang K, Sun Y, Zhang G, et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit 2015; 21: 376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst 2013; 105: 321–33. doi: https://doi.org/10.1093/jnci/djs528 [DOI] [PubMed] [Google Scholar]
- 41.Yankeelov TE, Cron GO, Addison CL, Wallace JC, Wilkins RC, Pappas BA, et al. Comparison of a reference region model with direct measurement of an AIF in the analysis of DCE-MRI data. Magn Reson Med 2007; 57: 353–61. doi: https://doi.org/10.1002/mrm.21131 [DOI] [PubMed] [Google Scholar]
- 42.Yankeelov TE, DeBusk LM, Billheimer DD, Luci JJ, Lin PC, Price RR, et al. Repeatability of a reference region model for analysis of murine DCE-MRI data at 7T. J Magn Reson Imaging 2006; 24: 1140–7. doi: https://doi.org/10.1002/jmri.20729 [DOI] [PubMed] [Google Scholar]
- 43.Yankeelov TE, Luci JJ, Lepage M, Li R, Debusk L, Lin PC, et al. Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magn Reson Imaging 2005; 23: 519–29. doi: https://doi.org/10.1016/j.mri.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 44.Taouli B, Beer AJ, Chenevert T, Collins D, Lehman C, Matos C, et al. Diffusion-weighted imaging outside the brain: consensus statement from an ISMRM-sponsored workshop. J Magn Reson Imaging 2016. doi: https://doi.org/10.1002/jmri.25196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Partridge SC, McDonald ES. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn Reson Imaging Clin N Am 2013; 21: 601–24. doi: https://doi.org/10.1016/j.mric.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol 2014; 24: 2835–47. doi: https://doi.org/10.1007/s00330-014-3338-z [DOI] [PubMed] [Google Scholar]
- 47.Thomassin-Naggara I, De Bazelaire C, Chopier J, Bazot M, Marsault C, Trop I. Diffusion-weighted MR imaging of the breast: advantages and pitfalls. Eur J Radiol 2013; 82: 435–43. doi: https://doi.org/10.1016/j.ejrad.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 48.Bogner WP, Gruber S, Grabner G, Stadlbauer A, Weber M, Moser E, et al. Diffusion-weighted MRI for differentiation of breast lesions at 3.0 Tesla: how does selection of diffusion schemes affect diagnosis? Radiology 2009; In press. [DOI] [PubMed] [Google Scholar]
- 49.Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, et al. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging 2007; 25: 1–13. doi: https://doi.org/10.1016/j.mri.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martincich L, Deantoni V, Bertotto I, Redana S, Kubatzki F, Sarotto I, et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol 2012; 22: 1519–28. doi: https://doi.org/10.1007/s00330-012-2403-8 [DOI] [PubMed] [Google Scholar]
- 51.Bickel H, Pinker-Domenig K, Bogner W, Spick C, Bago-Horvath Z, Weber M, et al. Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 2015; 50: 95–100. doi: https://doi.org/10.1097/rli.0000000000000104 [DOI] [PubMed] [Google Scholar]
- 52.Park SH, Moon WK, Cho N, Song IC, Chang JM, Park IA, et al. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology 2010; 257: 56–63. doi: https://doi.org/10.1148/radiol.10092021 [DOI] [PubMed] [Google Scholar]
- 53.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging 2006; 24: 843–7. doi: https://doi.org/10.1016/j.mri.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 54.LeBihan D. IVIM method measures diffusion and perfusion. Diagn Imaging 1990; 12: 6. [PubMed] [Google Scholar]
- 55.Bokacheva L, Kaplan JB, Giri DD, Patil S, Gnanasigamani M, Nyman CG, et al. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J Magn Reson Imaging 2014; 40: 813–23. doi: https://doi.org/10.1002/jmri.24462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho GY, Moy L, Kim SG, Baete SH, Moccaldi M, Babb JS, et al. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C, Wang K, Chan Q, Liu Z, Zhang J, He H, et al. Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol 2016. [DOI] [PubMed] [Google Scholar]
- 58.Kim Y, Ko K, Kim D, Min C, Kim SG, Joo J, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of breast cancer: association with histopathological features and subtypes. Br J Radiol 2016; 89: 20160140. doi: https://doi.org/10.1259/bjr.20160140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nogueira L, Brandao S, Matos E, Nunes RG, Loureiro J, Ramos I, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol 2014; 24: 1197–203. doi: https://doi.org/10.1007/s00330-014-3146-5 [DOI] [PubMed] [Google Scholar]
- 60.Sun K, Chen X, Chai W, Fei X, Fu C, Yan X, et al. Breast cancer: diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 2015; 277: 46–55. doi: https://doi.org/10.1148/radiol.15141625 [DOI] [PubMed] [Google Scholar]
- 61.Wu D, Li G, Zhang J, Chang S, Hu J, Dai Y. Characterization of breast tumors using diffusion kurtosis imaging (DKI). PLoS One 2014; 9: e113240. doi: https://doi.org/10.1371/journal.pone.0113240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iima M, Yano K, Kataoka M, Umehana M, Murata K, Kanao S, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol 2015; 50: 205–11. doi: https://doi.org/10.1097/rli.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 63.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001; 13: 534–46. doi: https://doi.org/10.1002/jmri.1076 [DOI] [PubMed] [Google Scholar]
- 64.Partridge SC, Murthy RS, Ziadloo A, White SW, Allison KH, Lehman CD. Diffusion tensor magnetic resonance imaging of the normal breast. Magn Resonance Imaging 2010; 28: 320–8. doi: https://doi.org/10.1016/j.mri.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 65.Partridge SC, Ziadloo A, Murthy R, White SW, Peacock S, Eby PR, et al. Diffusion tensor MRI: preliminary anisotropy measures and mapping of breast tumors. J Magn Reson Imaging 2010; 31: 339–47. doi: https://doi.org/10.1002/jmri.22045 [DOI] [PubMed] [Google Scholar]
- 66.Baltzer PA, Schafer A, Dietzel M, Grassel D, Gajda M, Camara O, et al. Diffusion tensor magnetic resonance imaging of the breast: a pilot study. Eur Radiol 2011; 21: 1–10. doi: https://doi.org/10.1007/s00330-010-1901-9 [DOI] [PubMed] [Google Scholar]
- 67.Plaza MJ, Morris EA, Thakur SB. Diffusion tensor imaging in the normal breast: influences of fibroglandular tissue composition and background parenchymal enhancement. Clin Imaging 2016; 40: 506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartella L, Huang W. Proton (1H) MR spectroscopy of the breast. Radiographics 2007; 1: S241–52. doi: https://doi.org/10.1148/rg.27si075504 [DOI] [PubMed] [Google Scholar]
- 69.Pinker K, Stadlbauer A, Bogner W, Gruber S, Helbich TH. Molecular imaging of cancer: MR spectroscopy and beyond. Eur J Radiol 2012; 81: 566–77. doi: https://doi.org/10.1016/j.ejrad.2010.04.028 [DOI] [PubMed] [Google Scholar]
- 70.Sardanelli F, Fausto A, Podo F. MR spectroscopy of the breast. Radiol Med 2008; 113: 56–64. doi: https://doi.org/10.1007/s11547-008-0228-y [DOI] [PubMed] [Google Scholar]
- 71.Bolan PJ. Magnetic resonance spectroscopy of the breast: current status. Magn Reson Imaging Clin N Am 2013; 21: 625–39. doi: https://doi.org/10.1016/j.mric.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 72.Gruber S, Debski BK, Pinker K, Chmelik M, Grabner G, Helbich T, et al. Three-dimensional proton MR spectroscopic imaging at 3 T for the differentiation of benign and malignant breast lesions. Radiology 2011; 261: 752–61. doi: https://doi.org/10.1148/radiol.11102096 [DOI] [PubMed] [Google Scholar]
- 73.Baltzer PA, Dietzel M. Breast lesions: diagnosis by using proton MR spectroscopy at 1.5 and 3.0 T—systematic review and meta-analysis. Radiology 2013; 267: 735–46. doi: https://doi.org/10.1148/radiol.13121856 [DOI] [PubMed] [Google Scholar]
- 74.Jagannathan NR, Kumar M, Seenu V, Coshic O, Dwivedi SN, Julka PK, et al. Evaluation of total choline from in-vivo volume localized proton MR spectroscopy and its response to neoadjuvant chemotherapy in locally advanced breast cancer. Br J Cancer 2001; 84: 1016–22. doi: https://doi.org/10.1054/bjoc.2000.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meisamy S, Bolan PJ, Baker EH, Bliss RL, Gulbahce E, Everson LI, et al. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy–a pilot study at 4 T. Radiology 2004; 233: 424–31. doi: https://doi.org/10.1148/radiol.2332031285 [DOI] [PubMed] [Google Scholar]
- 76.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer 2011; 11: 835–48. doi: https://doi.org/10.1038/nrc3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res 1999; 59: 80–4. [PubMed] [Google Scholar]
- 78.Ramadan S, Arm J, Silcock J, Santamaria G, Buck J, Roy M, et al. Lipid and metabolite deregulation in the breast tissue of women carrying BRCA1 and BRCA2 genetic mutations. Radiology 2015; 275: 675–82. doi: https://doi.org/10.1148/radiol.15140967 [DOI] [PubMed] [Google Scholar]
- 79.Marino MA, Clauser P, Woitek R, Wengert GJ, Kapetas P, Bernathova M, et al. A simple scoring system for breast MRI interpretation: does it compensate for reader experience? Eur Radiol 2015. doi: https://doi.org/10.1007/s00330-015-4075-7 [DOI] [PubMed] [Google Scholar]
- 80.Pinker K, Bickel H, Helbich TH, Gruber S, Dubsky P, Pluschnig U, et al. Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the “Breast Imaging Reporting and Data System” for multiparametric 3-T imaging of breast lesions. Eur Radiol 2013; 23: 1791–802. doi: https://doi.org/10.1007/s00330-013-2771-8 [DOI] [PubMed] [Google Scholar]
- 81.Dijkstra H, Dorrius MD, Wielema M, Pijnappel RM, Oudkerk M, Sijens PE. Quantitative DWI implemented after DCE-MRI yields increased specificity for BI-RADS 3 and 4 breast lesions. J Magn Reson Imaging 2016. doi: https://doi.org/10.1002/jmri.25331 [DOI] [PubMed] [Google Scholar]
- 82.Pinker K, Bogner W, Baltzer P, Gruber S, Bickel H, Brueck B, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol 2014; 49: 421–30. doi: https://doi.org/10.1097/rli.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 83.Schmitz AM, Veldhuis WB, Menke-Pluijmers MB, van der Kemp WJ, van der Velden TA, Kock MC, et al. Multiparametric MRI with dynamic contrast enhancement, diffusion-weighted imaging, and 31-phosphorus spectroscopy at 7 T for characterization of breast cancer. Invest Radiol 2015; 50: 766–71. doi: https://doi.org/10.1097/rli.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 84.Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology 2003; 227: 529–37. doi: https://doi.org/10.1148/radiol.2272020483 [DOI] [PubMed] [Google Scholar]
- 85.Ouwerkerk R, Jacobs MA, Macura KJ, Wolff AC, Stearns V, Mezban SD, et al. Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res Treat 2007; 106: 151–60. doi: https://doi.org/10.1007/s10549-006-9485-4 [DOI] [PubMed] [Google Scholar]
- 86.Zaric O, Pinker K, Zbyn S, Strasser B, Robinson S, Minarikova L, et al. Quantitative sodium MR imaging at 7 T: initial results and comparison with diffusion-weighted imaging in patients with breast tumors. Radiology 2016; 280: 39–48. doi: https://doi.org/10.1148/radiol.2016151304 [DOI] [PubMed] [Google Scholar]
- 87.Klomp DW, van de Bank BL, Raaijmakers A, Korteweg MA, Possanzini C, Boer VO, et al. 31P MRSI and 1H MRS at 7 T: initial results in human breast cancer. NMR Biomed 2011; 24: 1337–42. doi: https://doi.org/10.1002/nbm.1696 [DOI] [PubMed] [Google Scholar]
- 88.Wijnen JP, van der Kemp WJ, Luttje MP, Korteweg MA, Luijten PR, Klomp DW. Quantitative (31)P magnetic resonance spectroscopy of the human breast at 7 T. Magn Reson Med 2011. [DOI] [PubMed] [Google Scholar]
- 89.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000; 143: 79–87. doi: https://doi.org/10.1006/jmre.1999.1956 [DOI] [PubMed] [Google Scholar]
- 90.Schmitt B, Trattnig S, Schlemmer HP. CEST-imaging: a new contrast in mr-mammography by means of chemical exchange saturation transfer. Eur J Radiol 2012; 1: S144–6. doi: https://doi.org/10.1016/S0720-048X(12)70060-8 [DOI] [PubMed] [Google Scholar]
- 91.Klomp DW, Dula AN, Arlinghaus LR, Italiaander M, Dortch RD, Zu Z, et al. Amide proton transfer imaging of the human breast at 7T: development and reproducibility. NMR Biomed 2013; 26: 1271–7. doi: https://doi.org/10.1002/nbm.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmitt B, Zamecnik P, Zaiss M, Rerich E, Schuster L, Bachert P, et al. A new contrast in MR mammography by means of chemical exchange saturation transfer (CEST) imaging at 3 Tesla: preliminary results. Rofo 2011; 183: 1030–6. doi: https://doi.org/10.1055/s-0031-1281764 [DOI] [PubMed] [Google Scholar]
- 93.Desmond KL, Moosvi F, Stanisz GJ. Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn Reson Med 2014; 71: 1841–53. doi: https://doi.org/10.1002/mrm.24822 [DOI] [PubMed] [Google Scholar]
- 94.Nasrallah FA, Pages G, Kuchel PW, Golay X, Chuang KH. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J Cereb Blood Flow Metab 2013; 33: 1270–8. doi: https://doi.org/10.1038/jcbfm.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rakow-Penner R, Daniel B, Glover GH. Detecting blood oxygen level-dependent (BOLD) contrast in the breast. J Magn Reson Imaging 2010; 32: 120–9. doi: https://doi.org/10.1002/jmri.22227 [DOI] [PubMed] [Google Scholar]
- 96.O'Flynn EA, DeSouza NM. Functional magnetic resonance: biomarkers of response in breast cancer. Breast Cancer Res 2011; 13: 204. doi: https://doi.org/10.1186/bcr2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li SP, Taylor NJ, Makris A, Ah-See ML, Beresford MJ, Stirling JJ, et al. Primary human breast adenocarcinoma: imaging and histologic correlates of intrinsic susceptibility-weighted MR imaging before and during chemotherapy. Radiology 2010; 257: 643–52. doi: https://doi.org/10.1148/radiol.10100421 [DOI] [PubMed] [Google Scholar]
- 98.Kurhanewicz J, Bok R, Nelson SJ, Vigneron DB. Current and potential applications of clinical 13C MR spectroscopy. J Nucl Med 2008; 49: 341–4. doi: https://doi.org/10.2967/jnumed.107.045112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brindle KM, Bohndiek SE, Gallagher FA, Kettunen MI. Tumor imaging using hyperpolarized 13C magnetic resonance spectroscopy. Magn Reson Med 2011; 66: 505–19. doi: https://doi.org/10.1002/mrm.22999 [DOI] [PubMed] [Google Scholar]
- 100.Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res 2008; 68: 8607–15. doi: https://doi.org/10.1158/0008-5472.CAN-08-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tessem MB, Swanson MG, Keshari KR, Albers MJ, Joun D, Tabatabai ZL, et al. Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1H HR-MAS spectroscopy of biopsy tissues. Magn Reson Med 2008; 60: 510–6. doi: https://doi.org/10.1002/mrm.21694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keshari KR, Sai V, Wang ZJ, Vanbrocklin HF, Kurhanewicz J, Wilson DM. Hyperpolarized [1-13C]dehydroascorbate MR spectroscopy in a murine model of prostate cancer: comparison with 18F-FDG PET. J Nucl Med 2013; 54: 922–8. doi: https://doi.org/10.2967/jnumed.112.115402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Asghar Butt S, Sogaard LV, Ardenkjaer-Larsen JH, Lauritzen MH, Engelholm LH, Paulson OB, et al. Monitoring mammary tumor progression and effect of tamoxifen treatment in MMTV-PymT using MRI and magnetic resonance spectroscopy with hyperpolarized [1-13C]pyruvate. Magn Reson Med 2015; 73: 51–8. doi: https://doi.org/10.1002/mrm.25095 [DOI] [PubMed] [Google Scholar]
- 104.Avril N, Adler LP. F-18 fluorodeoxyglucose-positron emission tomography imaging for primary breast cancer and loco-regional staging. Radiol Clin North Am 2007; 45: 645–57. doi: https://doi.org/10.1016/j.cpet.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 105.Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E. Dual time point FDG-PET/CT imaging. Potential tool for diagnosis of breast cancer. Clin Radiol 2008; 63: 1213–27. doi: https://doi.org/10.1016/j.crad.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 106.Moy L, Ponzo F, Noz ME, Maguire GQ, Murphy-Walcott AD, Deans AE, et al. Improving specificity of breast MRI using prone PET and fused MRI and PET 3D volume datasets. J Nucl Med 2007; 48: 528–37. doi: https://doi.org/10.2967/jnumed.106.036780 [DOI] [PubMed] [Google Scholar]
- 107.Kumar R, Lal N, Alavi A. 18F-FDG PET in detecting primary breast cancer. J Nucl Med 2007; 48: 1751; author reply 2. doi: https://doi.org/10.2967/jnumed.107.043265 [DOI] [PubMed] [Google Scholar]
- 108.Moy L, Noz ME, Maguire GQ, Jr, Melsaether A, Deans AE, Murphy-Walcott AD, et al. Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J 2010; 16: 369–76. doi: https://doi.org/10.1111/j.1524-4741.2010.00927.x [DOI] [PubMed] [Google Scholar]
- 109.Pinker K, Bogner W, Baltzer P, Karanikas G, Magometschnigg H, Brader P, et al. Improved differentiation of benign and malignant breast tumors with multiparametric 18fluorodeoxyglucose positron emission tomography magnetic resonance imaging: a feasibility study. Clin Cancer Res 2014; 20: 3540–9. doi: https://doi.org/10.1158/1078-0432.CCR-13-2810 [DOI] [PubMed] [Google Scholar]
- 110.Pinker K, Baltzer P, Andrzejewski P, Magometschnigg H, Georg D, Karanikas G, et al. , eds. Dual tracer PET/MRI of breast tumors: insights into tumor biology. World Molecular Imaging Conference; 2015. [Google Scholar]