Abstract
Objective:
To compare treatment outcome of patients with head and neck (HN) tumours treated with definitive radiation therapy that, mainly owing to differences in the fractionation scheme used with simultaneous integrated boost techniques, resulted in a different biological dose.
Methods:
181 patients with HN cancer, prescribed to about 70.2 Gy in the primary tumour, were included in this study. Population cohort was divided into Group <70 and Group ≥70 when the mean dose converted to a 2 Gy fractionation in the primary tumour was less or higher than 70.2 Gy, respectively. The probability of local control (LC), locoregional control (LRC), disease-free survival (DFS) and overall survival (OS) was determined for both groups. The incidence of acute and late complications was compared between the two groups.
Results:
At 24 months for Groups <70 and ≥70, LC was 83.2% and 87.5%, LRC was 79.5% and 81.6%, DFS was 56.3% and 66.8% and OS was 63.9% and 71.5% p = ns, respectively. The incidence of acute dysphagia, odynophagia and pain, and late mucositis was significantly higher in Group ≥70 than in Group <70. The rate of xerostomia, dysphonia, radiodermatitis, alopecia, dental complications, hypoacusia and weight loss was comparable between the two groups.
Conclusion:
The biological dose escalation was safe, but an increase in the incidence of the acute side effects: dysphagia, odynophagia and pain and late mucositis, was obtained.
Advances in knowledge:
Despite the significant biological dose escalation, within the range of doses delivered to this cohort, no clear dose–response effect was observed.
INTRODUCTION
With increased knowledge on tissue response to radiation therapy (RT), fractionation schedules may be optimized for each tumour type. Fractionation optimization aimed at increasing tumour cure, by minimizing tumour proliferation, or at reducing adverse effects by allowing normal tissue damage to repair between fractions. A reduction in overall treatment time (OTT) through accelerated RT, compared with conventional fractionation, resulted in a significant improvement in locoregional control (LRC).1 However, significant clinical benefits in terms of LRC and overall survival (OS) were obtained with hyperfractionated RT compared with accelerated regimens without increasing late toxicity.2,3
With the clinical implementation of intensity-modulated radiation therapy (IMRT) simultaneous integrated boost techniques or simultaneous modulated accelerated RT, using a dose per fraction larger than conventional in the primary tumour, were widely adopted in the clinical routine.4 The integration of different prescription dose levels in the same plan is straightforward with inverse treatment planning. Reducing the number of plans per patient was a necessity to decrease the workload required with pre-treatment patient-specific IMRT verifications. Theoretically, both a reduction in OTT and an increase in dose per fraction should result in a therapeutic gain in terms of the probability of tumour control. Interestingly, an increase in the average weekly dose had a significant impact on LRC for patients with squamous cell cancer of the head and neck (HN) receiving RT alone, but not for those undergoing concurrent chemotherapy.5
Inversely optimized IMRT was implemented in 2008 at IPOCFG and simultaneously integrated boost plans, with at least two prescription dose levels per plan, were used as much as possible. This fractionation methodology gradually replaced the conventional fractionation of five fractions per week of 1.8 Gy applied with forward optimized IMRT.6 As a result of the evolution in RT, a cohort of patients with the same prescribed dose but different biological doses was collected. Differences in biological dose, i.e. dose converted to a 2 Gy fraction scheme, were a consequence of the total delivered dose, the fractionation schedules used, OTT and the shape of the three-dimensional (3D) dose distribution planned with different dose algorithms. Thus, the aim of this study was to compare treatment outcome in patients with HN cancer treated with definitive RT prescribed with the same therapeutic physical dose but resulting in a different biological effective dose.
METHODS AND MATERIALS
Patients and treatments
From 2007 to 2013, 181 patients with HN squamous cell carcinoma (90.0%) or undifferentiated carcinoma, treated with definitive RT and prescribed with a total dose ranging from 68.0 to 70.6 Gy to the primary tumour, delivered with a dose per fraction varying from 1.8 to 2.15 Gy, were included in this study. The clinical data electronically stored during the routine RT medical appointments were used in this retrospective study.7 Patients were divided into two groups depending on whether the value of the mean dose converted to a 2 Gy fractionation in the primary tumour was inferior or superior to 70.2 Gy (Group <70 and Group ≥70, respectively). The reasons for selecting the dose delivered to the primary tumour were threefold: first, recurrences occurred mostly in the vicinity of the primary tumour;8 second, the biological dose escalation, through an increase in dose per fraction, was mostly applied to this volume; and third, reducing OTT resulted in clinical benefits in controlling the gross tumour but had little effect on the control of neck nodes.2,9 A dose–response effect, resulting from the biological dose escalation obtained by an increase in dose per fraction and reducing OTT, would thus be more evident in the primary tumour. Patient, disease and treatment characteristics for both groups are summarized in Table 1.
Table 1.
Patient, disease and treatment characteristics for both groups
| Characteristic | Group <70, N (%) | Group ≥70, N (%) | p-value |
|---|---|---|---|
| Age (years) | |||
| ≤55 | 37 (46.8) | 61 (59.8) | χ2 (1) = 3.0 |
| >55 | 42 (53.2) | 41 (40.2) | p = 0.082 |
| Gender | |||
| Male | 69 (87.3) | 79 (77.5) | χ2 (1) = 2.9 |
| Female | 10 (12.7) | 23 (22.5) | p = 0.087 |
| Site | |||
| Larynx | 15 (19.0) | 9 (8.8) | n.a. |
| Oral cavity | 8 (10.1) | 7 (6.9) | |
| Oropharynx | 22 (27.8) | 30 (29.4) | |
| Nasopharynx | 14 (17.7) | 31 (30.4) | |
| Pharyngeal–laryngeal | 11 (13.9) | 15 (14.7) | |
| Hypopharynx | 7 (8.9) | 8 (7.8) | |
| Others | 2 (2.5) | 2 (2.0) | |
| T stage | |||
| 1–2 | 40 (50.6) | 49 (48.0) | χ2 (1) = 0.1 |
| 3–4 | 39 (49.4) | 53 (52.0) | p = 0.715 |
| N stage | |||
| 0–1 | 30 (38.0) | 21 (20.6) | χ2 (1) = 7.5 |
| 2–3 | 49 (62.0) | 81 (79.4) | p = 0.006 |
| Type of RTCT | |||
| Concomitant CT | 53 (67.1) | 71 (69.6) | χ2 (2) = 0.1 |
| Sequential CT | 11 (13.9) | 13 (12.7) | p = 0.936 |
| Intensive RTa | 15 (19.0) | 18 (17.6) | |
| RT techniqueb | |||
| 3DCRT | 3 (3.8) | 4 (3.9) | Fisher = 53.1 |
| fIMRT | 34 (43.0) | 2 (2.0) | p < 0.001 |
| rIMRT | 29 (36.7) | 56 (54.9) | |
| IMRT | 13 (16.5) | 40 (39.2) | |
| Total | 79 | 102 | |
3DCRT, three-dimensional conformal radiotherapy; fIMRT, forwardly optimized intensity-modulated radiation therapy; IMRT, intensity-modulated radiation therapy; RTCT radiochemotherapy; rIMRT, rapid intensity-modulated radiation therapy; RT, radiation therapy.
In intensive RT, RT was used as the single oncological treatment.
fIMRT uses sequential plans manually optimized, rIMRT and IMRT are inversely optimized IMRT with simultaneous integrated boost using a different number of segments (approximately 35 and 75, respectively).
Treatment details are described elsewhere.6,8 In summary, target volume delineation followed Gregoire et al.10 Prescription dose to primary tumour volume and large adenopathies was around 70.2 Gy and to high- and low-risk lymph nodes, it ranged from 50.4 to 59.4 Gy. The main organs at risk included in plan optimization were: spinal cord, brainstem, mandible, parotid glands, thyroid and others considered relevant for each pathology.
Planning was performed in the treatment planning systems Oncentra® (Nucletron; Elekta, Stockholm, Sweden) and delivery was performed in ONCOR™ Avant-Garde from Siemens (Germany) (step-and-shoot technique for inverse IMRT). During the time frame of this study, i.e. 2007–2013, simpler target volumes were irradiated with 3D conformal treatment techniques (3DCRT, i.e., a simplified version of forward optimized IMRT), using up to 10 beams and a daily fraction of 1.8 Gy. More complex cases were irradiated with IMRT. From 2007–2012 forwardly optimized IMRT (fIMRT) was used. This technique used 5–7 gantry directions with a total of 15–25 segments manually optimized. Dose fractionation schedule was also based on the delivery of five fractions of 1.8 Gy per week. This technique was replaced by inversely optimized IMRT using 30–55 segments (rIMRT) (rapid intensity-modulated radiation therapy) or 70–80 segments (IMRT) for more complex targets and when the patient could sustain longer irradiation times. With inversely optimized IMRT, dose integration of at least two prescription dose levels per plan was made. Therefore, in most cases, the first target volume, generally low-risk lymph nodes, was being irradiated with a minimum daily fraction of 1.8 Gy and the second or third with a maximum daily fraction of 2.15 Gy.
Although in this population cohort, different delivery techniques were used for treatment, it is the delivered dose distribution that should be related to the outcome. Therefore, in this study, the analysis was based on the information gathered from the 3D dose matrix aiming to minimize the dependency on the delivery technique. Dose was calculated using collapsed cone as the dose computation algorithm for all patients. Plans made using the pencil beam dose algorithm were restored in the treatment planning system and recalculated using the most recent dose algorithm. For patients with significant anatomic deviations relatively to the planning CT, replanning was performed and the total accumulated dose was used for dosimetric assessment. Image co-registrations between different CTs were made using Velocity AI 2.7 (Varian).
Using the planned 3D dose matrices of each patient (from one to three sequential plans), total dose was converted to a 2 Gy fractionation scheme, in each structure of interest and/or voxel, using the Biological Effective Dose (BED) concept and the methodology described in detail in Ferreira et al.6
| (1) |
where Np is the number of plans and Di is the total nominal or physical dose in each voxel delivered in plan i in fractions of size di. α/β is the ratio of the linear quadratic model. Tpot is the tumour potential doubling time, T is the real OTT for the prescribed treatment and Tk is the time at which repopulation begins. D2 Gy is the total dose converted into a fractionation of 2 Gy, delivered during T2 Gy days, which results in the same biological effect. In this study, for HN tumours, an α/β of 10, a potential doubling time of 3 days and a kick-off time for repopulation of 28 days were used.11 For the organs at risk, the repopulation term was disregarded and an α/β of 3 was used.
Concomitant chemotherapy was mainly cisplatin based. Patients unable to undergo this scheme were evaluated to cetuximab. In the sequential protocol, RT was administered between cycles of chemotherapy using mostly docetaxel cisplatin and Fluorouracil (TPF) or cisplatin and fluorouracil (PF).
During treatment, all patients attended a weekly medical appointment at the RT department. After that, follow-up occurred with 3-month intervals during 2 years, after which periodicity was reduced to every 6 months. At each follow-up visit, a complete physical examination, CT and/or MRI, assessment of radiation-induced secondary effects was performed. Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer guidelines were mostly used to score observed complications.12 Routine blood tests and thyroid function assessment also made part of the clinical follow-up protocol. Mean follow-up time for Groups <70 and ≥70 was 27.8 months [95% confidence interval (CI): 23.9–31.7 months] and 18.9 months (95% CI: 16.4–21.4 months), respectively. Median follow-up time for Groups <70 and ≥70 was 24.8 months (95% CI: 21.5–28.0 months) and 17.6 months (95% CI: 13.7–21.4 months), respectively.
Statistical analysis
Local control (LC) and LRC were defined from the time complete tumour response to the initial treatment protocol was obtained. Disease-free survival (DFS) was defined from the time of complete tumour response to the therapy up to the time of recurrence, metastasis, second tumour or death. Time to distant metastasis (DM) and OS were calculated from the start of RT. LC, LRC, DFS, distant metastasis (DM) and OS were calculated using the Kaplan–Meier method. Log-rank test was used to test the equality of the survival distributions between both groups.
Summary statistics were reported as mean and standard deviation values for continuous variables and as counts and percentages for categorical variables. Comparisons between patient characteristics and groups were evaluated using χ2 test for contingency tables (when the expected cell count assumption is verified) or Fisher's exact test (otherwise). The dose statistics in the primary tumour: prescribed dose, the physical dose and the dose converted for 2 Gy fractions were compared for both patients groups using the independent t-test (normality assumption verified by Kolmogorov–Smirnov test) or using the Mann–Whitney U test (otherwise). For the difference between two independent proportions, a z-test was used.
The incidence of complications in the salivary glands (xerostomia), oesophagus (dysphagia), pharynx (odynophagia), larynx (dysphonia), skin (radiodermatitis), hair (alopecia), mucous membrane (mucositis), teeth and ear (hypoacusia), as well as pain and weight loss, for both patient groups was compared at the time maximum severity of RT side effects was observed, at 7 weeks (43.0 ± 3.2 days), at 12 ± 4 months and 24 ± 5 months after RT, selecting the closest medical appointment from the period under evaluation. χ2 test for contingency tables or Fisher's exact test was used for this comparison. For the longitudinal analysis of patient response to RT, the information gathered in all RT appointments was grouped into time intervals of increasing extent, as follow-up time became longer, i.e. as the numbers of patients significantly decreased with increasing follow-up time, even larger time intervals had to be considered for analysis.
Potential prognostic factors (hazard ratios and 95% CIs) associated with LC, LRC, DFS and OS were explored in univariate and multivariate analyses performed using the Cox regression models. These were: age, gender, tumour site (hypopharynx, pharyngeal–laryngeal, larynx vs oropharynx vs oral cavity vs nasopharynx), T stage (1–2 vs 3–4) and N stage (0–1 vs 2–3), mean dose converted to 2 Gy in the primary tumour (<70 vs ≥70 Gy), dose per fraction (≤2 vs > 2 Gy) and OTT. The variable dose per fraction and OTT were excluded from the multivariate analysis owing to high correlation with the mean dose converted to 2 Gy in the primary tumour. Cox regression was performed using a forced entry method (all the considered variables are entered into the equation in one step).
All statistical analyses were performed using SPSS® Software, v. 20.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL), and p-values under 0.05 were considered significant.
RESULTS
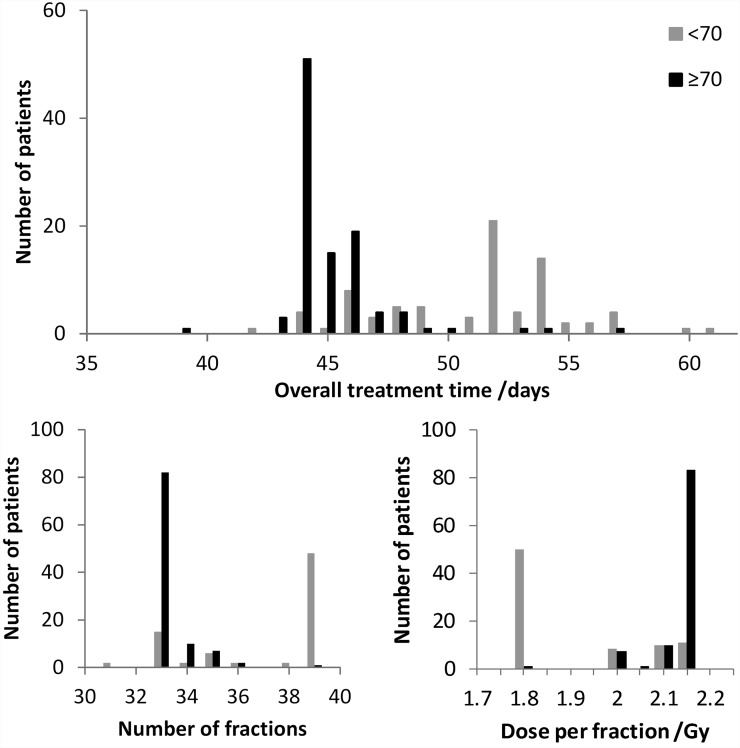
Figure 1 shows the histograms for OTT, the number of fractions and the dose per fraction for Groups <70 and ≥70. 60.8% of the patients of Group <70 were irradiated with 39 fractions of 1.8 Gy, while 79.4% of the patients of Group ≥70 were irradiated in the primary tumour with 33 fractions of around 2.13 Gy. For this population cohort, 65.7% of the patients received RT as planned. Mean OTT for Group <70 was 51.2 ± 3.9 days (range: 42–61 days) and for Group ≥70, it was 45.1 ± 2.2 days (range: 39–57 days) (p < 0.001). The percentage of treatment breaks due to different causes was similar between the two groups, except for interruptions due to holidays. Major cause for prolongation of OTT was RT toxicity and intercurrent disease (26.6% and 21.9%, respectively). Possibly, owing to the longer treatment prescribed to Group <70, breaks due to holidays were 31.3% compared with 21.9% in Group ≥70.
Figure 1.
Histograms of overall treatment time, number of fractions and dose per fraction (of the first plan) in the primary tumour for Group <70 and Group ≥70.
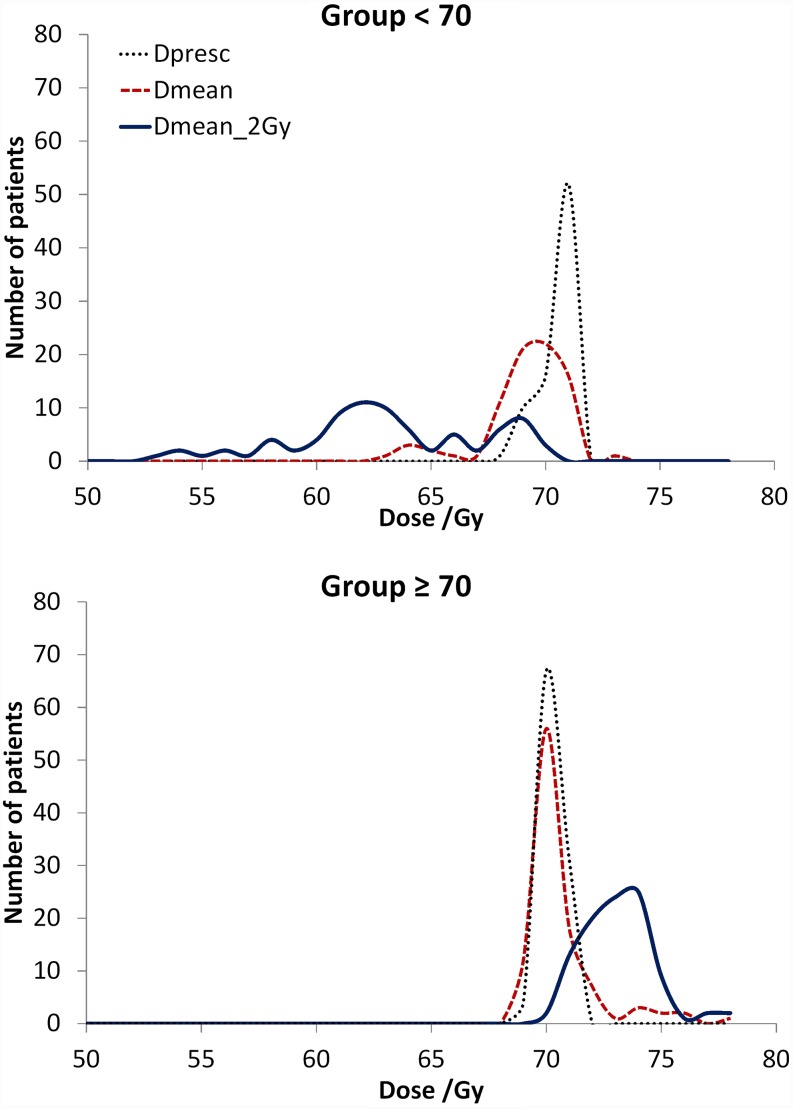
In Figure 2, the frequency distribution of the prescribed dose, the mean physical dose and the dose converted to 2 Gy fractions, or biological dose, in the primary tumour for both patient groups is shown. The average nominal mean doses in the primary tumour for Group <70 and Group ≥70 were 68.7 ± 1.9 Gy and 70.2 ± 1.6 Gy (p < 0.001), respectively, and the average mean doses converted to 2 Gy were 62.7 ± 4.2 Gy and 73.1 ± 2.7 Gy (p < 0.001), respectively. Other dose statistics in the primary tumour may be seen in Table 2. For the parotid glands, a statistically significantly larger average mean dose was delivered in Group <70 than in Group ≥70 (p ≤ 0.015).
Figure 2.
Comparison between the prescribed dose (Dpresc), the nominal mean dose in the primary target volume (Dmean) and the mean dose converted to 2 Gy (Dmean_2 Gy) for patients with a mean biological dose in the primary tumour inferior and superior to 70.2 Gy.
Table 2.
Dose statistics for Groups <70 and ≥70 for the planning target volume of the primary tumour (PTV-T) and the parotid glands
| Group | Physical dose/Gy Average ± SD (minimum–maximum) |
Dose converted to 2 Gy fractions/Gy Average ± SD (minimum–maximum) |
||||
|---|---|---|---|---|---|---|
| <70 | ≥70 | Statistics | <70 | ≥70 | Statistics | |
| PTV-T | ||||||
| D98% |
64.2 ± 3.2 (48.1–68.9) |
65.3 ± 1.9 (60.1–70.9) |
t(179) = −3.0 p = 0.005 |
54.2 ± 4.7 (36.8–63.5) |
63.7 ± 3.4 (54.6–74.2) |
t(179) = −15.8 p < 0.001 |
| Dmean |
68.7 ± 1.9 (62.4–72.4) |
70.2 ± 1.6 (68.4–77.5) |
t(179) = −5.8 p < 0.001 |
62.7 ± 4.2 (52.1–69.9) |
73.1 ± 2.7 (70.0–87.4) |
t(179) = −20.2 p < 0.001 |
| D2% |
71.7 ± 1.9 (66.9–77.3) |
73.3 ± 1.9 (70.4–81.1) |
t(179) = −5.9 p<0.001 |
68.4 ± 5.1 (55.9–79.5) |
79.3 ± 3.5 (73.9–95.7) |
t(179) = −17.0 p<0.001 |
| Contralateral parotid | ||||||
| Dmean | 36.5 ± 8.3 (0.8–58.0) |
34.2 ± 6.0 (13.6–55.1) |
t(174) = 2.1 p=0.035 |
31.5 ± 8.3 (0.5–53.7) |
28.8 ± 5.9 (9.5–50.1) |
t(174) = 2.5 p=0.015 |
| Ipsilateral parotid | ||||||
| Dmean | 41.3 ± 9.6 (0.9–67.2) |
37.1 ± 5.9 (19.1–56.8) |
t(175) = 3.6 p < 0.001 |
36.2 ± 9.6 (0.5–63.7) |
31.8 ± 6.1 (14.6–52.4) |
t(175) = 3.7 p < 0.001 |
Dmean, mean dose in the primary target volume; SD, standard devaition.
D2% and D98% are the maximum and minimum significant doses.
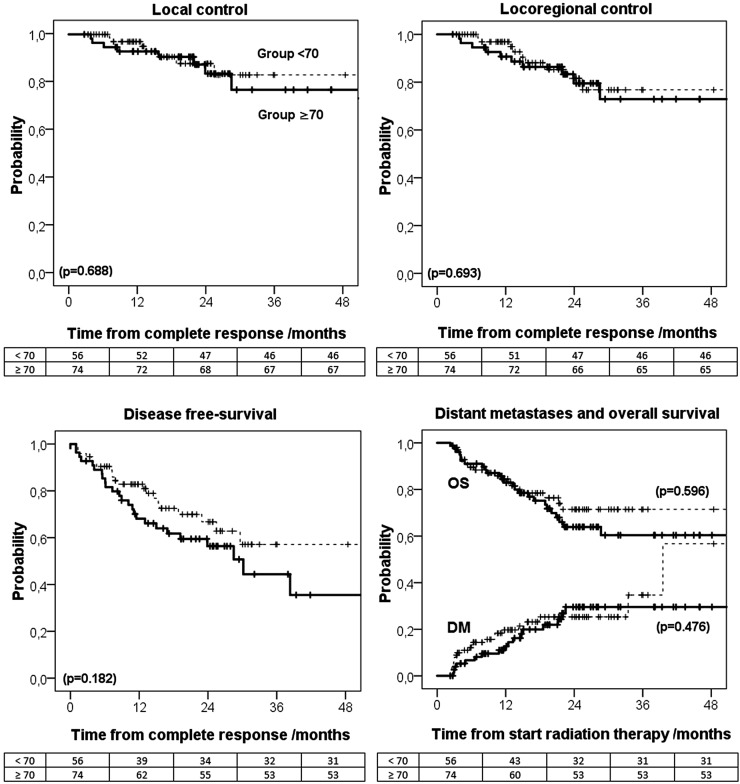
For Groups <70 and ≥70, complete tumour response was obtained in 70.9% and 72.5% of the patients (p = 0.81), respectively; partial tumour response was obtained in 19.0% vs 15.7% of the patients (p = 0.56), respectively, and tumour progression was observed in 7.6% vs 10.8% of the patients (p = 0.45), respectively. At 24 months, LC for Groups <70 and ≥70 was 83.2% and 87.5%, LRC was 79.5% and 81.6%, DFS was 56.3% and 66.8%, DM was 29.4% and 25.1% and OS was 63.9% and 71.5%, respectively (Figure 3).
Figure 3.
Kaplan–Meier for the probability of: local control, locoregional control, disease-free survival, distant metastasis (DM), and overall survival (OS) for Groups <70 and ≥70 (the numbers of patients in the last panel refer to the endpoint OS).
In univariate and multivariate analyses, non-concomitant radiochemotherapy, compared with concomitant radiochemotherapy, was significantly related to poorer LC, LRC and DFS (Table 3). More advanced tumour stages were significantly related to worst OS, while nasopharynx tumours were related to better DFS and OS compared with the reference group (hypopharynx, pharyngeal–laryngeal and larynx tumours). The total delivered dose, converted to a 2 Gy fractionation, was not significantly associated with any of the survival endpoints.
Table 3.
Multivariate results from Cox's regression for the endpoints: local control (LC), locoregional control (LRC), disease-free survival (DFS) and overall survival (OS). Gender (male) was excluded from multivariate analysis owing to poor estimation. Dose per fraction and overall treatment time were also excluded owing to high correlation with mean dose converted to 2 Gy in the primary tumour (Dmean).
| Variables | HR | 95% CI | p-value |
|---|---|---|---|
| LC | |||
| Age | 1.00 | (0.96; 1.05) | 0.88 |
| Tumour site | |||
| Hypo + Ph/la + Lar | 1 | – | – |
| Oral cavity | 3.05 | (0.51; 18.13) | 0.22 |
| Oropharynx | 0.70 | (0.20; 2.53) | 0.59 |
| Nasopharynx | 0.29 | (0.05; 1.58) | 0.15 |
| T stage (3–4) | 2.81 | (0.80; 9.87) | 0.11 |
| N stage (2–3) | 0.96 | (0.29; 3.23) | 0.95 |
| Non-concomitant RT | 3.70 | (1.09;12.50) | 0.04 |
| Dmean (≥70 Gy) | 1.14 | [0.39; 3.31] | 0.82 |
| LRC | |||
| Age | 1.02 | [0.98; 1.06] | 0.43 |
| Tumour site | |||
| Hypo + Ph/la + Lar | 1 | -- | -- |
| Oral cavity | 2.56 | [0.45; 14.39] | 0.29 |
| Oropharynx | 0.57 | [0.18; 1.78] | 0.33 |
| Nasopharynx | 0.34 | [0.08; 1.41] | 0.14 |
| T stage (3–4) | 2.47 | [0.83; 7.35] | 0.10 |
| N stage (2–3) | 0.88 | [0.30; 2.60] | 0.82 |
| Non-concomitant RT | 4.78 | [1.61;14.13] | 0.005 |
| Dmean (≥70 Gy) | 1.18 | [0.46; 3.04] | 0.73 |
| DFS | |||
| Age | 1.00 | [0.97; 1.03] | 0.80 |
| Tumour site | |||
| Hypo + Ph/la + Lar | 1 | – | – |
| Oral cavity | 1.21 | [0.34; 4.34] | 0.77 |
| Oropharynx | 0.55 | [0.26; 1.13] | 0.10 |
| Nasopharynx | 0.28 | [0.11; 0.69] | 0.006 |
| T stage (3–4) | 1.29 | [0.67; 2.48] | 0.45 |
| N stage (2–3) | 0.88 | [0.44; 1.76] | 0.72 |
| Non-concomitant RT | 2.00 | [1.03; 3.86] | 0.04 |
| Dmean (≥70 Gy) | 0.95 | [0.51; 1.79] | 0.88 |
| OS | |||
| Age | 1.02 | [0.99; 1.05] | 0.15 |
| Gender (male) | 0.64 | [0.25; 1.64] | 0.36 |
| Tumour site | |||
| Hypo + Ph/la + Lar | 1 | – | – |
| Oral cavity | 1.92 | [0.80; 4.64] | 0.15 |
| Oropharynx | 0.97 | [0.46; 2.06] | 0.94 |
| Nasopharynx | 0.24 | [0.08; 0.72] | 0.01 |
| T stage (3–4) | 2.07 | [1.01; 4.27] | 0.048 |
| N stage (2–1) | 2.12 | [0.89; 5.02] | 0.09 |
| Non-concomitant RT | 0.75 | [0.36; 1.55] | 0.43 |
| Dmean (≥70 Gy) | 1.02 | [0.55; 1.91] | 0.94 |
CI, confidence interval; Dmean, mean dose in the primary target volume; HR, hazard ratio; RT, radiation therapy.
Hypo + Ph/Lar + Lar is the group of patients with hypopharynx, pharyngeal-laryngeal and larynx tumours.
Variables in bold have p ≤ 0.05.
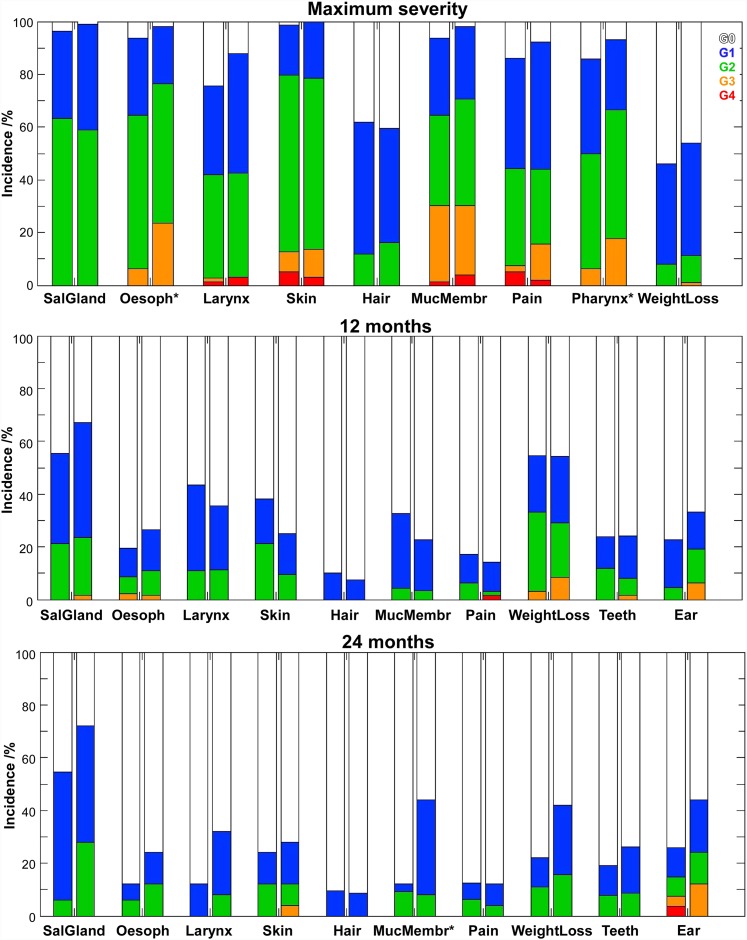
The incidence of acute and late side effects for both groups is compared in Figures 4 and 5 for several organs at risk. For maximum acute RT side effects, observed at 26.0 ± 12.4 days (range: 0–69 days), statistically significant associations between dysphagia (p = 0.006), odynophagia (p = 0.040) and pain (p = 0.025) (only for 7 weeks after RT) and the two groups were found. For Groups <70 and ≥70, the incidence of maximum acute toxicity G3–G4 for the oesophagus was 6.3% vs 23.5%; for the pharynx, it was 6.4% vs 17.6%; and for pain, it was 7.6% vs 15.7%, respectively. Acute complications G3–G4 in the mucous membrane, skin and larynx were similar between the two groups with an incidence of around 30%, 13% and 3%, respectively. G2 xerostomia, alopecia and weight loss had also comparable incidences among the two groups of about 61%, 14% and 9%, respectively.
Figure 4.
Incidence of acute and late side effects to radiation therapy (RT) for patients of Group <70 and Group ≥70 (first and second bars, respectively). The star indicates the RT side effects that resulted in statistically significant differences between the two groups. MucMembr, mucosal membrane; SalGland, salivary gland; Oesoph, oesophagus.
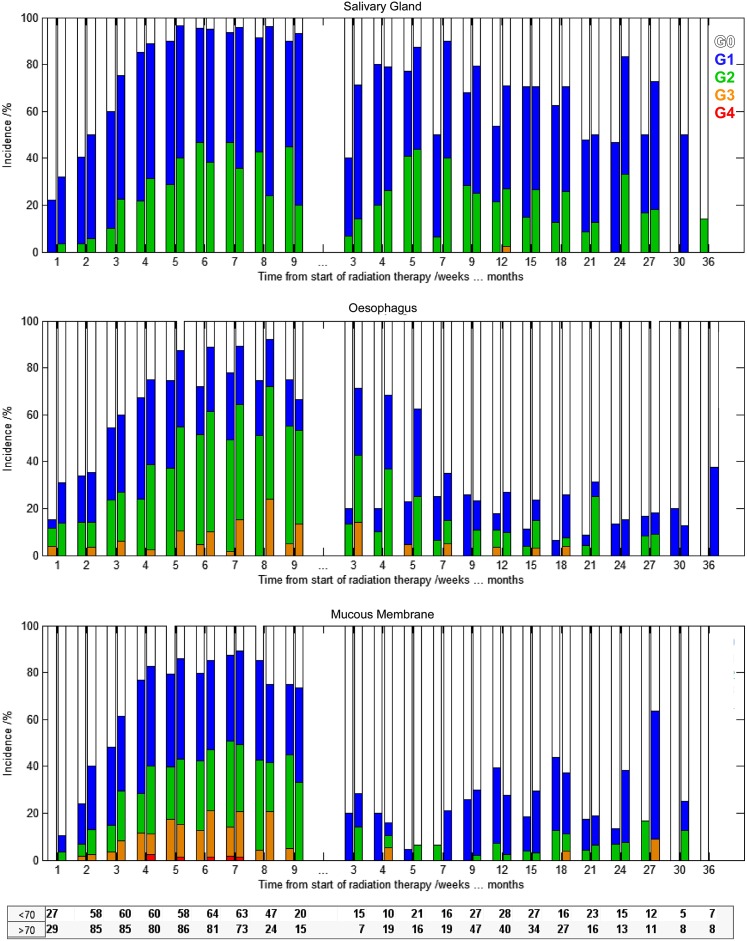
Figure 5.
Incidence of xerostomia (above), dysphagia (middle) and mucositis (below) from Week 1 up to 36 months after radiation therapy for Group <70 (first bar) and Group ≥70 (second bar).
12 months after RT [12.1 ± 1.8 months (range: 7.8–15.9 months) and 110 patients evaluated], no statistically significant differences in the incidence of complications were obtained between the two patients groups. For Groups <70 and ≥70, the incidences of G2–G3 skin complications were 21.3% vs 9.4% and the incidences of hypoacusia (ear) were 4.5% vs 19%, respectively. For the other side effects evaluated, almost no differences in the rate of RT side effects were obtained between the two patients groups. Thus, the incidence of G2–G3 complications for the salivary glands was around 22%, for oesophagus, larynx and teeth, it was about 10% and for mucous membrane and pain, it was around 5%.
For complications evaluated in 58 patients at 23.6 ± 2.3 months (range: 19.3–28.0 months) after RT, a statistical relationship in the incidence of late mucositis between Groups <70 and ≥70 was obtained (G1–G2: 12.1% vs 44.0%, respectively) (p = 0.002). Without reaching statistical significance (p ≤ 0.1), an association between late xerostomia and dysphonia with the two groups evaluated was noticed (lower panel in Figure 4).
DISCUSSION
Two or three sequential plans were commonly used in conformal RT of HN tumour cases. With IMRT and the high workload required with this treatment technique, both in treatment planning and patient-specific quality control, a reduction in the number of plans made for each patient was mandatory. Simultaneous integrated boost techniques in the IMRT context were first suggested by Butler et al in 1999,13 later discussed by Mohan et al in 200014 and widely implemented by the clinical community. This represents the delivery of a dose per fraction in the different target volumes ranging from 1.6 to 2.5 Gy.6,15,16 The conventional fractionation of 2 Gy was historically embraced because it represents the optimal balance between tumour cell kill and late side effects. Increasing the dose per fraction, relatively to this standard fractionation, should result in higher probability of tumour control. With the protective effect obtained with IMRT, by reducing both total delivered dose and the dose per fraction in the organs at risk, the levels of toxicity may be reduced or at least maintained if a dose escalation approach was adopted. Acceptable clinical results with simultaneous integrated boost techniques in HN cancer were already reported by several.15–18
In this study, the population cohort was divided into two groups depending on the biological dose delivered to the primary tumour. The heterogeneity in the values of the biological dose resulted mainly from differences in the fractionation schedule and the dose assessed by different dose algorithms at the time of planning. Group <70 was mainly composed of patients treated with 39 fractions of 1.8 Gy in 52–54 days, while Group ≥70 was mostly composed of patients treated with inverse IMRT in 44 days with a dose per fraction of 2.13 Gy in the primary tumour (Figure 1). In this cohort, 20% of the patients were planned using pencil beams (up to 2010). Thus, for Group <70, differences between the prescribed dose and the planned physical dose, recalculated with the collapsed cone algorithm in here, were obtained (Figure 2).
Physical dose was converted into biological dose so that the comparison between the outcomes of patients treated with different fractionation schedules could be made using the well-established BED concept. The real OTT, the number of fractions delivered and the planned 3D dose distribution were thus taken into account in the calculation of the biological effective dose. Owing to the influence of these factors in the biological effect, the curve of the biological dose, in Figure 2, is more spread out than the curve of the physical dose. For the patients included in Group ≥70, the curve showing the biological dose moved towards higher values than the physical dose owing to the positive effect of shortening OTT and increasing dose per fraction, compared with the reference fraction dose of 2 Gy, while the opposite happens to Group <70. As a consequence of all these factors, the dose delivered to Group ≥70 was significantly higher than the dose delivered to Group <70 (Table 2).
A trend for better survival outcome in Group ≥70, compared with Group <70, was noticed. A 10% difference in DFS and almost 8% in OS between the two groups was obtained. The difference in DFS was also related to the higher incidence of second neoplasias in Group <70 compared with Group ≥70 (6% vs 1%, respectively). By contrast, the higher DM in Group ≥70, compared with Group <70, may be related to the higher rate of N2–3 tumour cases in this group (Table 1). At short follow-up times, the difference in LC and LRC between the two groups was negligible, becoming more pronounced 30 months after RT (Figure 3). Although the level of evidence of this retrospective study is low, similar results were obtained by Miah et al18 and Leclerc et al15 for larynx and hypopharynx tumour cases. Differences in LRC, and OS, of around 10% were obtained only 2 years after RT when escalating the dose from 63 Gy to 67 Gy, delivered in 28 fractions, and from 69 Gy to 72 Gy, delivered in 30 fractions, respectively. In this study, despite the larger dose delivered with the new fractionation schedule, no statistically significant survival differences between the two groups were obtained. Thus, it is not possible to conclude whether these differences are potentially due to the biological dose escalation, as it would be expected, and significance was lost by the lack of statistical power of the study (small sample, small number of events in each category and short follow-up time), or whether the heterogeneity of this cohort, grouping tumour cases that may respond differently to fractionation, reduced the steepness of the dose–response curve, and no significant dose effect actually happens at such dose levels. With the availability of new treatment regimens based on improved knowledge of risk factors and radiobiology, therapies will be increasingly personalized.19,20 Survival and radiobiological studies are therefore needed, ideally, grouping tumour cases by factors that affect patient response to radiation such as: general health status, living habits, normal tissues and tumour biological parameters, tumour microenvironment features etc.20–23 Dose–response models on tumour response to radiation certainly need to be investigated more.
With simultaneous integrated boost IMRT, organ sparing is achieved through the highly conformal dose distributions produced by intensity-modulated beams and by avoiding the additional dose delivered by multiple plans. Thus, although the total dose in the primary tumour was larger in Group ≥70, compared with the Group <70, the total dose in the parotid glands was significantly reduced in the first group compared with the delivery of RT using sequential plans with no clear clinical differences in terms of xerostomia (Table 2, Figure 4). However, a significantly higher incidence of acute dysphagia, odynophagia and pain was obtained in Group ≥70 compared with Group <70 (Figure 4). With the present data, it remains unclear whether this increased toxicity was a consequence of the new fractionation scheme used or the larger proportion of patients with advanced N stage tumours in Group ≥70 compared with Group <70 (Table 1). Patients with more advanced tumours have a larger irradiated volume, but simultaneously these were irradiated with more sophisticated treatment techniques resulting in improved normal tissue sparing (Table 2). Further protection of oesophagus and pharynx by using IMRT are thus strongly recommended.
In Figure 5, the increase in incidence and severity of side effects with the delivery of the radiation dose can be seen (up to Week 7). Patient recovery starts immediately after the end of RT, and during Week 9, the rate of patients free from complications already increased compared with previous weeks. Interestingly, recovery from dysphagia was considerably faster in Group <70 than in Group ≥70 (Months 3–7 in Figure 5). Generally, the rates and severity of dysphagia were higher for Group ≥70, compared with Group <70, but were significantly different only for acute dysphagia. No significant dose relation was obtained for dysphagia 1 or 2 years after RT, as also seen by others.18
Late toxicity, except for mucositis, was not significantly different for both groups (Figure 4). Mucosa response to RT was usually very similar among the two groups up to Year 2. From 24 months onwards, significant differences in the incidence of late mucositis were in fact obtained (Figure 5). Similarly, in the longitudinal analysis of xerostomia, in general, the incidence of this injury was comparable between the two groups; but, after Month 15, there was a trend for worst toxicity in the high-dose group (p < 0.1). Caution is recommended here, as with increasing follow-up time the number of patients included in the analysis becomes very small and misrepresentation of the true dose–response effect may occur. Further evaluation of the long-term side effects for this new form of biological dose escalation is therefore highly recommended.
CONCLUSION
For patients with HN cancer undergoing definitive RT, a significant increase in delivered dose was obtained with the clinical implementation of simultaneous integrated boost IMRT, by increasing the dose per fraction and shortening OTT compared with conventional treatment schemes. This biological dose escalation resulted in an acceptable treatment outcome with similar rates of survival and late toxicity, except for mucositis, as the dose prescribed with the conventional fractionation. The new treatment strategy was safe, but further protective measures to minimize acute dysphagia, odynophagia and pain may be achievable by outlining the structures responsible for these side effects previously to IMRT optimization.
FUNDING
This work was financed by the Foundation for Science in Portugal within the programme UID/CTM/50025/2013. Sá-Couto's work was supported by Portuguese funds through the Center for Research and Development in Mathematics and Applications (CIDMA) within project UID/MAT/04106/2013.
Contributor Information
Brigida Costa Ferreira, Email: bcf@ess.ipp.pt.
Pedro Sá-Couto, Email: p.sa.couto@ua.pt.
Leila Khouri, Email: leilakhouri@ipocoimbra.min-saude.pt.
Maria do Carmo Lopes, Email: mclopes@ipocoimbra.min-saude.pt.
REFERENCES
- 1.Nguyen LN, Ang KK. Radiotherapy for cancer of the head and neck: altered fractionation regimens. Lancet Oncol 2002; 3: 693–701. [DOI] [PubMed] [Google Scholar]
- 2.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders MI, Bernier J, et al. Meta-Analysis of radiotherapy in carcinomas of head and neck (MARCH) collaborative group. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368: 843–54. doi: https://doi.org/10.1016/S0140-6736(06)69121-6 [DOI] [PubMed] [Google Scholar]
- 3.Beitler JJ, Zhang Q, Fu KK, Trotti A, Spencer SA, Jones CU, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014; 89: 13–20. doi: https://doi.org/10.1016/j.ijrobp.2013.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlandi E, Palazzi M, Pignoli E, Fallai C, Giostra A, Olmi P. et al. Radiobiological basis and clinical results of the simultaneous integrated boost (SIB) in intensity modulated radiotherapy (IMRT) for head and neck cancer: a review. Crit Rev Oncol Hematol 2010; 73: 111–25. doi: https://doi.org/10.1016/j.critrevonc.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Dragovic AF, Bonner JA, Spencer SA, Nabell LM, Carroll WR, Caudell JJ. Impact of average weekly dose of radiation during radiotherapy alone or chemoradiotherapy in head and neck cancer. Head Neck 2011; 33: 1551–6. doi: https://doi.org/10.1002/hed.21634 [DOI] [PubMed] [Google Scholar]
- 6.Ferreira BC, do Carmo Lopes M, Mateus J, Capela M, Mavroidis P. Radiobiological evaluation of forward and inverse IMRT using different fractionations for head and neck tumours. Radiat Oncol 2010; 5: 57. doi: https://doi.org/10.1186/1748-717X-5-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira B, Khouri L, Lopes MC, Ferreira H. RESPONSE, an Electronic Health Patient Information Software for Radiation Therapy. Lackovic, Vasic D, eds. 6th European Conference of the International Federation for medical and biological Engineering, IFMBE Proceedings 45. Switzerland: Springer International Publishing; 2014. pp. 691–4.
- 8.Ferreira BC, Marques R, Khouri L, Santos T, Sá-Couto P, do Carmo Lopes M, et al. Assessment and topographic characterization of locoregional recurrences in head and neck tumours. Radiat Oncol 2015; 10: 41. doi: https://doi.org/10.1186/s13014-015-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003; 362: 933–40. [DOI] [PubMed] [Google Scholar]
- 10.Grégoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol 2003; 69: 227–36. [DOI] [PubMed] [Google Scholar]
- 11.Fowler JF. Optimum overall times II: extended modelling for head and neck radiotherapy. Clin Oncol (R Coll Radiol) 2008; 20: 113–26. doi: https://doi.org/10.1016/j.clon.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 12.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. doi: https://doi.org/10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 13.Butler EB, Teh BS, Grant WH, 3rd, Uhl BM, Kuppersmith RB, Chiu JK, et al. Smart (simultaneous modulated accelerated radiation therapy) boost: a new accelerated fractionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 1999; 45: 21–32. [DOI] [PubMed] [Google Scholar]
- 14.Mohan R, Wu Q, Manning M, Schmidt-Ullrich R. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys 2000; 46: 619–30. [DOI] [PubMed] [Google Scholar]
- 15.Leclerc M, Maingon P, Hamoir M, Dalban C, Calais G, Nuyts S, et al. A dose escalation study with intensity modulated radiation therapy (IMRT) in T2N0, T2N1, T3N0 squamous cell carcinomas (SCC) of the oropharynx, larynx and hypopharynx using a simultaneous integrated boost (SIB) approach. Radiother Oncol 2013; 106: 333–40. doi: https://doi.org/10.1016/j.radonc.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Franzese C, Fogliata A, Clerici E, Franceschini D, Villa E, D'Agostino G, et al. Toxicity profile and early clinical outcome for advanced head and neck cancer patients treated with simultaneous integrated boost and volumetric modulated arc therapy. Radiat Oncol 2015; 10: 224. doi: https://doi.org/10.1186/s13014-015-0535-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauve A, Morris M, Schmidt-Ullrich R, Wu Q, Mohan R, Abayomi O, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: II—clinical results. Int J Radiat Oncol Biol Phys 2004; 60: 374–87. [DOI] [PubMed] [Google Scholar]
- 18.Miah AB, Bhide SA, Guerrero-Urbano MT, Clark C, Bidmead AM, St Rose S, et al. Dose-escalated intensity-modulated radiotherapy is feasible and may improve locoregional control and laryngeal preservation in laryngo-hypopharyngeal cancers. Int J Radiat Oncol Biol Phys 2012; 82: 539–47. doi: https://doi.org/10.1016/j.ijrobp.2010.09.055 [DOI] [PubMed] [Google Scholar]
- 19.Kelly JR, Husain ZA, Burtness B. Treatment de-intensification strategies for head and neck cancer. Eur J Cancer 2016; 68: 125–33. doi: https://doi.org/10.1016/j.ejca.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biau J, Chautard E, Miroir J, Lapeyre M. Radioresistance parameters in head and neck cancers and methods to radiosensitize. [In French.] Cancer Radiother 2015; 19: 337–46. [DOI] [PubMed] [Google Scholar]
- 21.Bol V, Grégoire V. Biological basis for increased sensitivity to radiation therapy in HPV-positive head and neck cancers. Biomed Res Int 2014; 1–6. doi: https://doi.org/10.1155/2014/696028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker-Schiebe M, Sperling M, Pinkert U, Hoffmann W. Impact of p16 alterations and pretreatment anemia on toxicity in head and neck cancer patients undergoing definitive radiochemotherapy. Oncol Res Treat 2015; 38: 570–6. doi: https://doi.org/10.1159/000441344 [DOI] [PubMed] [Google Scholar]
- 23.Swartz JE, Pothen AJ, Stegeman I, Willems SM, Grolman W. Clinical implications of hypoxia biomarker expression in head and neck squamous cell carcinoma: a systematic review. Cancer Med 2015; 4: 1101–16. doi: https://doi.org/10.1002/cam4.460 [DOI] [PMC free article] [PubMed] [Google Scholar]