Abstract
Adrenal incidentalomas are unsuspected, asymptomatic adrenal masses detected on imaging. Most are non-functioning benign adrenocortical adenomas but can represent other benign lesions or lesions requiring therapeutic intervention including adrenocortical carcinoma, pheochromocytoma, hormone-producing adenoma or metastasis. This review summarizes and highlights radiological recommendations within the recently issued guidelines for the management of adrenal incidentalomas from the European Society of Endocrinology Clinical Practice in collaboration with the European Network for Study of Adrenal Tumours. Four pre-defined clinical questions were addressed in the guidelines and two have specific relevance and implications for radiologists: (1) how to assess risk of malignancy on imaging and (2) what follow-up is indicated if an adrenal incidentaloma is not surgically removed? The guidelines also include recommendations for frequently encountered special circumstances, including bilateral incidentalomas, incidentalomas in patients with extra-adrenal malignancy and in the young and elderly patients. This review highlights radiological recommendations within the guidelines and evidence used for formulating the guidelines.
INTRODUCTION
An adrenal incidentaloma is defined as a mass detected on imaging not performed for the purpose of investigating adrenal disease. The imaging is for evaluation of symptoms that are not obviously related to an adrenal aetiology. An adrenal tumour in patients with a pre-disposing hereditary syndrome detected on surveillance imaging is outside the definition of an adrenal incidentaloma. Adrenal masses discovered during staging investigations for other malignancies also do not meet the strict definition of an adrenal incidentaloma. However, as this is a frequent clinical scenario, it is addressed under special circumstances.
The guidelines have included only adrenal incidentalomas larger than 1 cm. This arbitrary cut-off is in line with previous recommendations and reviews.1–12
Adrenal incidentalomas are composed of benign and malignant lesions derived from the adrenal cortex, medulla or extra-adrenal origin. The vast majority, >90%, are benign adrenocortical adenomas. The reported frequency of malignant lesions in the literature varies according to the context of the study and the size inclusion criteria of the adrenal masses within these studies. Prevalence of malignant and functional lesions is overestimated as the prevalence is based on surgical studies where indeterminate adrenal masses are resected, as they not retain typically benign features.3 Autopsy studies suggest a prevalence of incidentalomas of around 2% (range 1.0–8.7%), increasing with age.5–7 Radiological studies report a frequency close to 3% in patients below the age of 50 years, increasing up to 10% in the elderly.2,5–7,13–15 Childhood incidentalomas are extremely rare.
Target group and aim of the guidelines
The European Society of Endocrinology (ESE) Clinical Practice Guideline was developed for endocrinologists, radiologists, surgeons and general physicians. The overall purpose of the guideline is to provide practical guidance for the management of patients with adrenal incidentalomas.
Methodology
The detailed methodology is described within the guidance document.16
In summary, the guideline used the Grading of Recommendations Assessment, Development and Evaluation method, firstly defining the clinical question(s) and secondly performing a systematic literature search and rating the quality of the evidence. Recommendations by the guideline panel are worded as recommend (strong recommendation) and suggest (weak recommendation). When coming to a guideline recommendation, the quality of evidence, balance of desirable and undesirable outcomes, values and preferences (patient preferences, goals for health, costs, management inconvenience, feasibility of implementation etc.) were considered.17,18
Key information for radiologists
The guidelines addressed four important clinical questions for review: (1) how to assess risk of malignancy on imaging, (2) how to define and manage low-level autonomous cortisol secretion, the so called “sub-clinical” Cushing's syndrome, (3) which patients should have surgical treatment and how should it be performed and (4) what follow-up is indicated if the adrenal incidentaloma is not surgically removed?
Questions 1 and 4 are directly relevant to radiological practice and will be the main focus of this article.
How to assess risk of malignancy on imaging?
Question (1a): what is the most accurate diagnostic imaging procedure to determine whether an adrenal mass is benign in patients with unilateral or bilateral adrenal mass(es) on imaging with or without the history of other malignant lesions?
The recommendations were guided by a recent meta-analysis conducted by some of the guideline panel members on the performance of imaging in differentiating benign from malignant adrenal incidentalomas. The included 37 studies in this systematic review were included using strict inclusion criteria.19 No randomized studies comparing the commonly used imaging modalities met the inclusion criteria of the review. The majority of included studies had moderate to high risk of bias due to unclear study population selection, retrospective selection of the diagnostic threshold and inadequate reference standards.
The studies included and evaluated in the guidelines reviewed the performance of five commonly used radiological diagnostic criteria:
(1) tumour density >10 Hounsfield units (HU) on non-contrast CT
(2) contrast-enhanced CT including delayed intravenous contrast media washout, either absolute percentage washout or relative percentage washout
(3) MRI chemical shift analysis: loss of signal intensity between in-phase and out-of-phase images (including both qualitative and quantitative estimates of signal loss)
(4) fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET) or PET-CT, the maximum standardized uptake value (SUVmax)
(5) ratio of SUVmax in the adrenal gland compared with that of the liver (adrenal : liver ratio).
18 of the 37 studies were included but were small with a median sample size of 45 (range 12–181). Only 7 studies addressed true incidentalomas, whereas 11 studies included patients with known extra-adrenal malignancy. 2 studies with 102 true incidentalomas suggest that CT density >10 HU has 100% sensitivity for detection of adrenal malignancy (confidence interval 91–100%). In patients with a history of underlying malignancy, the performance is less reliable. Five studies evaluating the >10 HU cut-off as indicative of malignancy showed a 93% sensitivity for detection of malignancy as 7% of adrenal metastases were found to have a tumour density of ≤10 HU. The use of non-contrast CT attenuation <10 HU therefore has a better performance in excluding malignancy in patients with no underlying malignancy.
The overall performance of all other diagnostic criteria was poor and based on small numbers of studies with very few patients and accompanying wide confidence intervals. For true adrenal incidentalomas, two of three MRI studies reported sensitivities and specificities lower than CT using adrenal : liver and adrenal : spleen ratios for the loss of signal intensity. The performance of 18F-FDG PET-CT for adrenal mass : liver ratio and SUVmax in the two included studies was not clearly superior than non-contrast CT and was similar to MRI. In patients with a history of extra-adrenal malignancy, only one study included CT contrast-enhanced washout and showed very poor 16% sensitivity. Four of the five studies reported high 89–99% sensitivity and high specificity 60–93% for measures of adrenal : liver, adrenal : spleen and adrenal : muscle ratios and of loss of signal intensity on MRI. Although there were more studies evaluating CT, MRI and PET in patients with known extra-adrenal malignancy than true incidentalomas, evaluation of test performance is still based on too small numbers to be able to discriminate between tests.
Question (1b): what is the diagnostic accuracy of adrenal biopsy?
The recommendations for adrenal biopsies were guided by a systematic review conducted by members of the guideline panel with experience of adrenal biopsy and its outcomes.20 Only 8 of the 32 identified studies could be included. In the remaining studies, at least 50% of the patients lacked any or optimal reference standards. The included studies had moderate risk for bias due to limitations in biopsy techniques, patient selection, assessment of outcome and adequacy of follow-up of the study population. Pathology of adrenal lesion was reported for only 1600/2207 cases. Pooled overall complication rate derived from 1356 biopsies was 2.4% (range 5–12%), although likely underrepresented, as there were differences in assessment and reporting of complications. The most frequent complications following adrenal biopsy are haemorrhage and pneumothorax. Less common complications include pain, pancreatitis and rarely needle tract seeding. However, most are minor and self-limiting, and the rate for major complications necessitating further treatment is 0.4–2%.21
The pooled non-diagnostic rate derived from 2030 adrenal biopsy procedures was 8.6%. The diagnostic performance of adrenal biopsy using 323 adrenal biopsy procedures had a sensitivity, specificity, positive likelihood ratio and negative likelihood ratio of 87%, 100%, 229 and 0.13, respectively. Performance was lower for adrenocortical carcinoma sensitivity, specificity, positive likelihood ratio and negative likelihood ratio 70%, 98%, 100.43 and 30.9 respectively. On balance, therefore, the performance of adrenal biopsies provides no significant improvement over non-invasive imaging, but the overall complication rate is clinically significant.
Question (4): what is the optimal follow-up in patients with an apparently benign adrenal incidentaloma in order to detect malignant transformation and/or development of overt hormone excess?
Review of 14 studies assessing the natural course of 1410 pooled patients with apparently benign, non-functioning adrenal incidentalomas was performed.3,22 In the included studies, selection criteria were often not reported; information on radiological re-evaluation was not always provided or standardized; duration of follow-up was heterogeneous across studies (medians ranging from 19 to 90 months) and completeness of follow-up was difficult to assess providing overall poor quality evidence.
The pooled risk for developing malignancy in this review was 0.2%.3 In two studies, one case of adrenal non-Hodgkin lymphoma and one patient with renal cancer metastasis were reported. In the first case, imaging characteristics of the adrenal lesion at the presentation was not consistent with benign characteristics, and the lymphoma may have been misdiagnosed initially.23 In the second case, the patient had a history of renal cell carcinoma, and it is unclear whether the adrenal mass was found incidentally or during the follow-up for cancer.24 No case of malignancy was reported in the remaining patients, and no malignant transformation of a presumably benign incidentaloma was reported.
Recommendations and rationale
Risk of malignancy
(1) The guidelines recommend establishing whether the mass is benign or malignant at the time of initial detection. This is an important aim to avoid cumbersome and expensive follow-up imaging in those with benign disease. Malignant lesions may need urgent surgical intervention and other therapies, and delay may cause harm.
(2) The guidelines recommend all adrenal incidentalomas should undergo an imaging procedure to determine if the mass is homogeneous and lipid-rich and therefore benign. For this purpose, the guidelines primarily recommend the use of non-contrast CT.
(3) The guidelines suggest if non-contrast CT demonstrates a homogeneous mass with ≤10 HU, appearances are consistent with a benign adrenal mass and no further imaging is required.
Rationale
In patients presenting without known malignancy, a non-contrast CT with HU of ≤10 was only found in benign disease, whereas in patients with extra-adrenal malignancy, 7% were malignant.19
Although MRI with chemical shift imaging is based on the lipid content of masses, quantitative assessment of loss of signal intensity is not well standardized and the evidence is insufficient to make strong recommendation for its use.25,26 Qualitative interpretation of MRI is more subjective and dependent on the experience of the radiologist than quantitative CT assessment.
The guideline panel felt confident about the negative-predictive value of non-contrast CT to recommend no additional imaging when benign characteristics were found in an adrenal mass <4 cm. Additional imaging may also risk false-positive results and significant psychological and financial burden for patients and health systems.
The cut-off of 4 cm is not based on good evidence from clinical studies, but the panel felt it is necessary to provide clear guidance based on expert clinical experience.
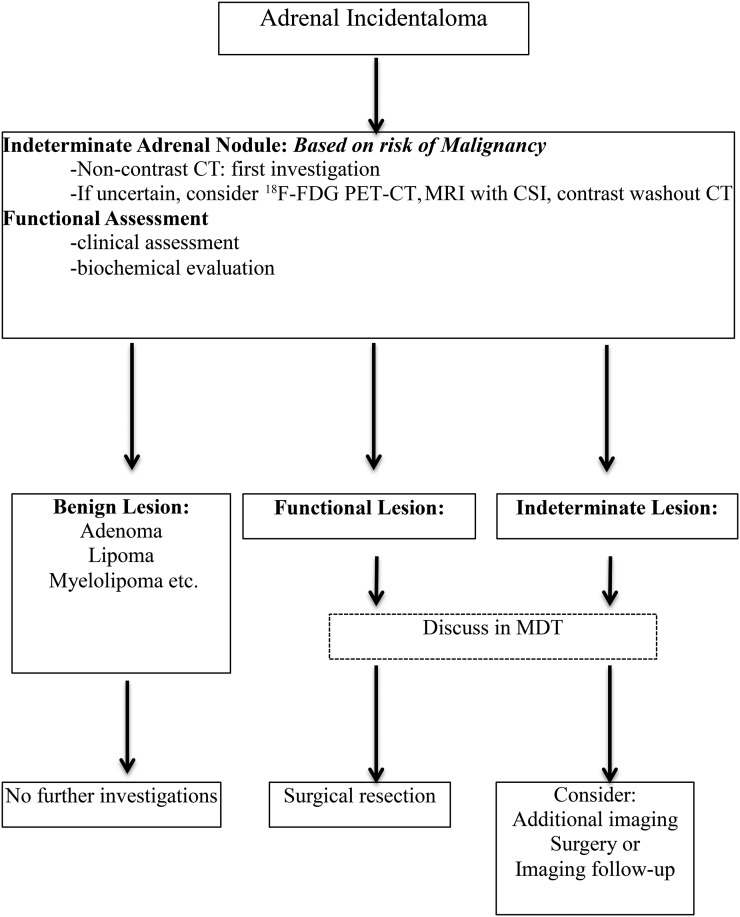
MRI with chemical shift should be the first choice only where CT is less desirable (e.g. pregnancy, children). However, if MRI with chemical shift has already been performed and the results are unambiguous, a multidisciplinary expert team (MDT) might judge this as sufficient for the individual patient. The recommendations are summarized in Figure 1.
(4) If the adrenal mass is indeterminate on non-contrast CT (>10 HU) and results of hormonal work-up do not indicate significant hormone excess, three options should be considered by a MDT acknowledging the patient's clinical context:
– immediate additional imaging with another modality
– interval imaging in 6–12 months (non-contrast CT or MRI) or
– surgery without further delay.
Figure 1.
Management of patients with adrenal incidentalomas. Based on the European Society of Endocrinology and the European Network for the Study of Adrenal Tumours adrenal incidentaloma guideline. CSI, chemical shift imaging; MDT, multidisciplinary expert team.
Rationale
Evidence for “second- or third-line” imaging modality for indeterminate adrenal mass is collectively very poor, but the guideline panel provides some guidance for clinical practice (Table 1) and emphasizes discussions which need to be individualized within a MDT setting.
Table 1.
Imaging criteria of a benign adrenal mass
| Non-contrast CT | ≤10 HU homogenous lesions only |
| A homogeneous mass is defined as a lesion with uniform density or signal intensity throughout. The ROI measurements should include at least 75% of a lesion without contamination by tissues outside the adrenal lesion | |
| Heterogeneous lesions should not be subjected to MRI or washout CT for further characterization | |
| MRI—chemical shift | Loss of signal intensity on out-of-phase imaging consistent with lipid-rich adenoma |
| CT with delayed contrast media washout | Absolute washout >60% |
| Relative washout >40% | |
| There is no clear evidence about the best time interval. We recommend 10 or 15 min for delayed images | |
| 18F-FDG PET | Absence of 18F-FDG uptake or uptake less than liver. Certain metastasis (e.g. from renal cancer or low-grade lymphoma) may be 18F-FDG negative |
18F-FDG, fluorine-18 fludeoxyglucose; HU, Hounsfield units; PET, positron emission tomography; ROI, region of interest.
These criteria can be applied only to homogeneous masses or masses with clear features consistent with benign disease, e.g. myelolipoma.
Contrast washout CT is widely available, but there is huge variability in protocols and therefore it has poor comparability.
18F-FDG PET-CT has a low risk of missing a malignant adrenal tumour, limited to lymphoma and renal cell carcinoma.27–29 It is more expensive, not always easily available and several benign adrenal tumours may have 18F-FDG uptake resulting in false-positive lesions (e.g. functional adenomas or benign pheochromocytoma).30–32
-
(5) The guidelines recommend against the use of adrenal biopsy in diagnostic work-up of patients with adrenal masses, unless there is a history of extra-adrenal malignancy and all of the following criteria are fulfilled:
(i) The lesion is hormonally inactive (in particular, a pheochromocytoma has been excluded);
(ii) The lesion has not been conclusively characterized as benign by imaging;
(iii) Management would be altered by the knowledge of the histology.
Rationale
Adrenal biopsy has a limited role mainly in confirming metastases from extra-adrenal malignancy, lymphoma or inflammatory/infectious processes. In patients with no other obvious metastatic lesions and when surgical removal of the adrenal lesion is considered, 18F-FDG PET-CT should be considered to exclude extra-adrenal metastases not visualized on CT or MRI. Only an experienced radiologist should perform adrenal biopsy and only when it is required to guide further management. The panel particularly recommends against a biopsy if the adrenal mass is likely to be an adrenocortical carcinoma, which runs a low risk of biopsy track seeding and tumour dissemination precluding a complete (R0) resection and has a significant non-diagnostic rate.20,33 The only exception is a formal confirmation of histological diagnosis in an inoperable tumour for oncological management or as part of a clinical trial.
Recommendations for imaging follow-up
(1) The guidelines recommend against further imaging for follow-up in patients with an adrenal mass <4 cm with clear benign features on imaging studies.
(2) In patients with an imaging indeterminate adrenal mass opting not to undergo adrenalectomy following initial assessment, a repeat non-contrast CT or MRI after 6–12 months can exclude significant growth. The guidelines suggest surgical resection if the lesion enlarges by >20% (in addition to at least a 5-mm increase in maximum diameter) during this period. If there is growth of the lesion below this threshold, additional imaging after 6–12 months should be performed.
Rationale
Amongst >2300 patients in follow-up studies, there is no reported occurrence of adrenal malignancy in incidentalomas displaying typical features of benign adrenocortical adenomas at initial imaging studies.3,9 Therefore, the guideline panel did not support repeating imaging investigations if the initial investigations are unequivocally consistent with a benign lesion. However, patients with adrenal incidentalomas >4 cm in diameter have undergone adrenalectomy in the past, and the literature on follow-up of non-operated large adrenal incidentalomas is scarce. As the risk of malignancy in this category is undetermined, some panel members favoured one follow-up imaging (non-contrast CT or MRI) after 6–12 months in patients not undergoing resection, as both primary adrenal malignancies or adrenal metastases are likely to increase in size whilst lack of growth may be taken as an indicator of benignity. In cases with a low likelihood of a malignant tumour, the guideline panel favoured a time interval of 12 months. As there are no published or any evidence-based size or volume cut-off to support growth suggestive of malignancy, the panel proposed adaptation of the Response Evaluation Criteria in Solid Tumors v. 1.1 criteria.34 Although Response Evaluation Criteria in Solid Tumors v. 1.1 criteria are not validated for the differentiation between benign and malignant adrenal tumours, the 20% cut-off together with an absolute increase of at least 5 mm in diameter in 6–12 months may serve as a warning for significant growth and reconsideration for surgical resection.
Exceptional cases of malignant adrenal tumour without significant growth for several years can be considered a very rare exception and does not justify follow-up of all patients with an incidentaloma with repeated imaging.35,36 In masses with no demonstrable growth, no further imaging follow-up is recommended, and only in masses with measurable growth <20%, should additional follow-up imaging be considered. The clinical necessity and interval of follow-up imaging should be discussed by a MDT.
Special circumstances
Patients with bilateral adrenal incidentalomas
(1) The guidelines recommend for patients with bilateral adrenal masses that each adrenal lesion should be assessed at the time of initial detection according to the same imaging protocol as for unilateral adrenal masses to establish if either or both masses are benign or malignant.
(2) The same recommendations regarding the indication for surgery and follow-up should be used as for patients with unilateral adrenal incidentalomas.
Rationale
Bilateral adrenal masses usually represent benign adenomas, macronodular hyperplasia or distinct bilateral nodules. In the relevant clinical setting, metastases (especially in patients with known malignancy), lymphoma or pheochromocytomas should also be considered. Each lesion should be evaluated individually as bilateral adrenal masses can represent co-occurrence of different lesions.
Adrenal incidentalomas in young or elderly patients
(1) The guidelines recommend urgent assessment of adrenal mass in children, adolescents, pregnant females and adults younger than 40 years of age because of a higher likelihood of malignancy.
(2) MRI rather than CT is suggested if dedicated adrenal imaging is required in children, adolescents, pregnant females and adults younger than 40 years of age.
(3) The guidelines recommend imaging investigations and management of patients with poor general health and a high degree of frailty should be tailored in proportion to potential clinical gain.
Rationale
Benign adrenal incidentalomas increase with age, with the majority of patients presenting in the fifth to seventh decade of life. Although 10% or more of individuals older than 70 years harbour an adrenal mass, adrenal nodules in individuals younger than 40 years are much less prevalent and are rare in children and young adults. Consequently, investigation of adrenal masses in young patients including pregnant females should be pursued with urgency, as the risk of malignancy is much higher. In the young patient, MRI is the preferred imaging technique. However, adapted low-dose unenhanced CT protocols can limit radiation exposure and offer an alternative (especially if availability of MRI is limited).
Conversely, small adrenal incidentalomas in elderly patients have a very low pre-test probability of malignancy and investigations should only be expedited where suspicion of malignancy is high and kept in proportion to the clinical performance status of the individual and the expected clinical gain.
Patients with a newly diagnosed adrenal mass and a history of extra-adrenal malignancy
(1) The guidelines suggest 18F-FDG PET-CT if performed as part of investigations for the underlying malignancy can replace other dedicated adrenal imaging techniques.
(2) Adrenal lesions characterized as benign by non-contrast CT require no further specific adrenal imaging follow-up.
(3) For indeterminate lesions, the guidelines recommend imaging at the same time interval as for the primary malignancy for assessing the growth of the lesion. Alternatively, 18F-FDG PET-CT, surgical resection or biopsy can be considered if distinction is essential to influence treatment decisions for the primary tumour.
Rationale
Both qualitative and quantitative performance of 18F-FDG PET-CT varies considerably. In 2 studies with 117 lesions in patients with history of extra-adrenal malignancy, adrenal lesion : liver ratio of 1.53–1.8 was investigated and found to have a sensitivity of 82% and specificity of 96% to detect malignant disease.37
For adrenal lesions characterized as benign by non-contrast CT, the same rationale as for patients with no history of underlying malignancy applies. However, the currently available data suggest a false-negative rate of up to 7% in this population.
In patients with advanced metastatic extra-adrenal malignancy, characterizing an adrenal incidentaloma will not alter clinical management. If, however, clinical management would be altered by the demonstration of a solitary adrenal metastasis, then further imaging, biopsy or resection should be performed.
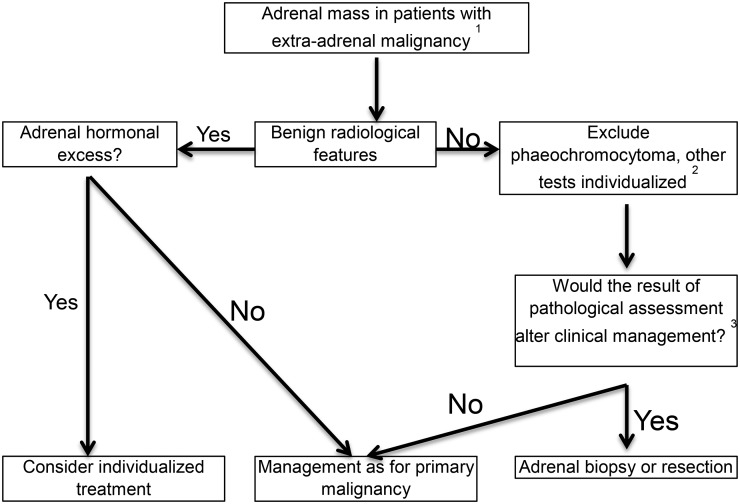
The recommendations are summarized in Figure 2.
Figure 2.
Evaluation of adrenal mass in patients with known extra-adrenal malignancy. Based on the European Society of Endocrinology and the European Network for the Study of Adrenal Tumours adrenal incidentaloma guideline. (1) Always take life expectancy into consideration. (2) If there is hormone excess, treatment individualized. (3) Fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT to exclude other metastatic deposits in patients with no other obvious metastatic lesions and for whom surgical removal of the lesion is an option.
Multidisciplinary team discussion
The guidelines recommend that patients with adrenal incidentalomas should be discussed in a MDT, if at least one of the following criteria is met:
(1) Imaging is not consistent with a benign lesion;
(2) There is evidence of hormone excess (including “autonomous cortisol secretion”);
(3) There is evidence of significant tumour growth during follow-up imaging;
(4) Adrenal surgery is considered.
Rationale
This is based mainly on expert opinion, aiming to identify subgroups of patients who would be most likely to benefit from MDT discussion. The panel recommends that the core multidisciplinary team should consist of at least a radiologist, endocrinologist and surgeon, all with significant experience in adrenal tumours. This team should have access to anaesthetists and an endocrine pathologist, who are also experienced in adrenal tumours.
Overall comment
Several guidelines exist to assist radiologists and physicians in the investigation and management of adrenal incidentalomas.38–40 In practice, it is critical to ensure that appropriate previous imaging is reviewed in the decision-making process separating benign from malignant lesions. The panel developed this guideline after critical evaluation of the studies available at the time of analysis. Several studies had to be excluded, as these did not meet the required Grading of Recommendations Assessment, Development and Evaluation selection criteria. When compared with other guidelines such as the American Appropriateness Criteria, less weight is placed on the second line of imaging investigations in view of the weak data. In line with the European Urology guidelines, this guideline emphasizes the important role of a MDT in ensuring an expert team manages indeterminate and malignant lesions. The guideline therefore emphasizes and provides the physician clear criteria for lesions that require referral for MDT discussion and management. Unlike several existing guidelines, these guidelines also aim to reduce the imaging follow-up burden. Long-term follow-up of lesions should be limited to lesions not unequivocally characterized as benign, <4 cm and with <20% increase in size over 12 months. This maintains a safe approach to the management of adrenal incidentalomas. A critical question not addressed within these guidelines is how or whether the radiologist, at first detection of an adrenal incidentaloma, should instigate biochemical investigations and further follow-up. Should all incidental adrenal lesions be referred for clinical and biochemical endocrine evaluation? These specific considerations were outside the remit of the current guidelines and the evidence to make any recommendation was not reviewed.
CONCLUSION
This targeted summary of the recent guidelines from the European Society of Endocrinology and ENSAT developed to aid the management of adrenal incidentalomas provides a management strategy for an increasingly frequent clinicoradiological problem. The guidelines aim to provide a safe, simple, cost-effective algorithm based on the evidence available, minimizing the need and psychological stress experienced by patients undergoing repeated imaging for an incidentaloma. An attenuation value of <10 HU on non-contrast-enhanced CT to confirm a benign adrenal lesion has the strongest evidence base. For these lesions, if no adrenal cortical hyperfunction is demonstrated, the lesions are homogeneous and <4 cm in size, no further imaging follow up is recommended. The evidence base for the second- and third-line imaging modalities to exclude malignancy is weak, and there is a need for clinical trials in all areas.
REFERENCES
- 1.Barzon L, Scaroni C, Sonino N, Fallo F, Paoletta A, Boscaro M. Risk factors and long-term follow-up of adrenal incidentalomas. J Clin Endocrinol Metab 1999; 84: 520–6. doi: https://doi.org/10.1210/jcem.84.2.5444 [DOI] [PubMed] [Google Scholar]
- 2.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 2003; 149: 273–85. [DOI] [PubMed] [Google Scholar]
- 3.Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol 2009; 161: 513–27. [DOI] [PubMed] [Google Scholar]
- 4.Favia G, Lumachi F, Basso S, D'Amico DF. Management of incidentally discovered adrenal masses and risk of malignancy. Surgery 2000; 128: 918–24. doi: https://doi.org/10.1067/msy.2000.109965 [DOI] [PubMed] [Google Scholar]
- 5.Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med 2003; 138: 424–9. [DOI] [PubMed] [Google Scholar]
- 6.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev 1995; 16: 460–84. [DOI] [PubMed] [Google Scholar]
- 7.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 2004; 25: 309–40. [DOI] [PubMed] [Google Scholar]
- 8.Tabarin A, Bardet S, Bertherat J, Dupas B, Chabre O, Hamoir E, et al. Exploration and management of adrenal incidentalomas. French Society of Endocrinology Consensus. Ann Endocrinol (Paris) 2008; 69: 487–500. [DOI] [PubMed] [Google Scholar]
- 9.Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol 2011; 164: 851–70. doi: https://doi.org/10.1530/EJE-10-1147 [DOI] [PubMed] [Google Scholar]
- 10.Young WF, Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007; 356: 601–10. doi: https://doi.org/10.1056/NEJMcp065470 [DOI] [PubMed] [Google Scholar]
- 11.Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical guidelines for the management of adrenal incidentalomas: executive summary of recommendations. Endocr Pract 2009; 15: 450–3. doi: https://doi.org/10.4158/ep.15.s1.1 [DOI] [PubMed] [Google Scholar]
- 12.Sahdev A, Reznek RH. Imaging evaluation of the non-functioning indeterminate adrenal mass. Trends Endocrinol Metab 2004; 15: 271–6. doi: https://doi.org/10.1016/j.tem.2004.06.012 [DOI] [PubMed] [Google Scholar]
- 13.Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, et al. A survey on adrenal incidentaloma in Italy. Study group on adrenal tumors of the Italian society of endocrinology. J Clin Endocrinol Metab 2000; 85: 637–44. [DOI] [PubMed] [Google Scholar]
- 14.Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 2006; 29: 298–302. [DOI] [PubMed] [Google Scholar]
- 15.Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K. The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer 2007; 14: 587–99. [DOI] [PubMed] [Google Scholar]
- 16.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol 2016; 175: G1–34. [DOI] [PubMed] [Google Scholar]
- 17.Hammarstedt L, Muth A, Wangberg B, Bjorneld L, Sigurjonsdottir HA, Gotherstrom G, et al. Adrenal lesion frequency: a prospective, cross-sectional CT study in a defined region, including systematic re-evaluation. Acta Radiol 2010; 51: 1149–56. [DOI] [PubMed] [Google Scholar]
- 18.Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol 2013; 66: 726–35. [DOI] [PubMed] [Google Scholar]
- 19.Dinnes J, Bancos I, Ferrante di Ruffano L, Chortis V, Davenport C, Bayliss S, et al. Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses—a systematic review and meta-analysis. Eur J Endocrinol 2016; 175: R51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamhane S, Delivanis DA, Alahdab F, Shah M, Arlt W, Fassnacht M, et al. The diagnostic performance of adrenal biopsy: a systematic review and meta-analysis. Eur J Endocrinol 2016; 175: R65–80. doi: https://doi.org/10.1530/EJE-16-0297 [DOI] [PubMed] [Google Scholar]
- 21.Welch TJ, Sheedy PF, Stephens DH. Percutaneous adrenal biopsy: review of a 10-year experience. Radiology 1994; 193: 341–4. doi: https://doi.org/10.1148/radiology.193.2.7972740 [DOI] [PubMed] [Google Scholar]
- 22.Yener S, Ertilav S, Secil M, Akinci B, Demir T, Comlekci A, et al. Natural course of benign adrenal incidentalomas in subjects with extra-adrenal malignancy. Endocrine 2009; 36: 135–40. doi: https://doi.org/10.1007/s12020-009-9191-1 [DOI] [PubMed] [Google Scholar]
- 23.Libe R, Dall’Asta C, Barbetta L, Baccarelli A, Beck-Peccoz P, Ambrosi B. Long-term follow-up study of patients with adrenal incidentalomas. Eur J Endocrinol 2002; 147: 489–94. doi: https://doi.org/10.1530/eje.0.1470489 [DOI] [PubMed] [Google Scholar]
- 24.Tsvetov G, Shimon I, Benbassat C. Adrenal incidentaloma: clinical characteristics and comparison between patients with and without extraadrenal malignancy. J Endocrinol Invest 2007; 30: 647–52. [DOI] [PubMed] [Google Scholar]
- 25.Rodacki K, Ramalho M, Dale BM, Battisti S, de Campos RO, Giardino A, et al. Combined chemical shift imaging with early dynamic serial gadolinium-enhanced MRI in the characterization of adrenal lesions. AJR Am J Roentgenol 2014; 203: 99–106. doi: https://doi.org/10.2214/ajr.13.11731 [DOI] [PubMed] [Google Scholar]
- 26.Seo JM, Park BK, Park SY, Kim CK. Characterization of lipid- poor adrenal adenoma: chemical-shift MRI and washout CT. AJR Am J Roentgenol 2014; 202: 1043–50. [DOI] [PubMed] [Google Scholar]
- 27.Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer 2006; 107: 175–83. doi: https://doi.org/10.1002/cncr.21967 [DOI] [PubMed] [Google Scholar]
- 28.Tsukamoto N, Kojima M, Hasegawa M, Oriuchi N, Matsushima T, Yokohama A, et al. The usefulness of (18)F-fluorodeoxyglucose positron emission tomography [(18)F-FDG-PET] and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer 2007; 110: 652–9. [DOI] [PubMed] [Google Scholar]
- 29.Zukotynski K, Lewis A, O'Regan K, Jacene H, Sakellis C, Krajewski K, et al. PET/CT and renal pathology: a blind spot for radiologists? Part 1, primary pathology. AJR Am J Roentgenol 2012; 199: W163–7. [DOI] [PubMed] [Google Scholar]
- 30.Ansquer C, Scigliano S, Mirallie E, Taieb D, Brunaud L, Sebag F, et al. 18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. Eur J Nucl Med Mol Imaging 2010; 37: 1669–78. [DOI] [PubMed] [Google Scholar]
- 31.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2009; 94: 4757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alencar GA, Fragoso MC, Yamaga LY, Lerario AM, Mendonca BB. (18)F-FDG-PET/CT imaging of ACTH-independent macronodular adrenocortical hyperplasia (AIMAH) demonstrating increased (18) F-FDG uptake. J Clin Endocrinol Metab 2011; 96: 3300–1. doi: https://doi.org/10.1210/jc.2011-1397 [DOI] [PubMed] [Google Scholar]
- 33.Williams AR, Hammer GD, Else T. Transcutaneous biopsy of adrenocortical carcinoma is rarely helpful in diagnosis, potentially harmful, but does not affect patient outcome. Eur J Endocrinol 2014; 170: 829–35. doi: https://doi.org/10.1530/eje-13-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 35.Nogueira TM, Lirov R, Caoili EM, Lerarion AM, Miller BS, Fragoso MC, et al. Radiographic characteristics of adrenal masses preceding the diagnosis of adrenocortical cancer. Horm Cancer 2015; 6: 176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozsari L, Kutahyalioglu M, Elsayes KM, Vicens RA, Sircar K, Jazaerly T, et al. Preexisting adrenal masses in patients with adrenocortical carcinoma: clinical and radiological factors contributing to delayed diagnosis. Endocrine 2016; 51: 351–9. [DOI] [PubMed] [Google Scholar]
- 37.Kunikowska J, Matyskiel R, Toutounchi S, Grabowska-Derlatka L, Koperski L, Krolicki L. What parameters from 18F-FDG PET/CT are useful in evaluation of adrenal lesions? Eur J Nucl Med Mol Imaging 2014; 41: 2273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garrett RW, Nepute JC, Hayek ME, Albert SG. Adrenal incidentalomas: clinical controversies and modified recommendations. AJR Am J Roentgenol 2016; 206: 1170–8. doi: https://doi.org/10.2214/AJR.15.15475 [DOI] [PubMed] [Google Scholar]
- 39.Choyke PL; ACR Committee on Appropriateness Criteria. ACR appropriateness criteria on incidentally discovered adrenal mass. J Am Coll Radiol 2006; 3: 498–504. [DOI] [PubMed] [Google Scholar]
- 40.Baltzera P, Clausera P, Klatteb T, Walzc J. Work-up of the incidental adrenal mass. Eur Urol 2016; 1: 217–22. [DOI] [PubMed] [Google Scholar]