Abstract
Objective:
To assess the impact on the final outcome at surgery of flat epithelial atypia (FEA) when found concomitantly with lobular neoplasia (LN) in biopsy specimens compared with pure biopsy-proven FEA.
Methods:
The approval from the institutional review board of the CHUM (Centre Hospitalier Universitaire de Montréal) was obtained. A retrospective review of our database between 2009 and 2013 identified 81 females (mean age 54 years, range 38–90 years) with 81 FEA biopsy-proven lesions. These were pure or associated with LN only in 59/81 (73%) and 22/81 (27%) cases, respectively. Overall, 57/81 (70%) patients underwent surgery and 24/81 (30%) patients underwent mammographic surveillance with a mean follow-up of 36 months.
Results:
FEA presented more often as microcalcifications in 68/81 (84%) patients and were mostly amorphous in 49/68 (72%). After excluding radio pathologically discordant cases, pure FEA proved to be malignant at surgery in 1/41 (2%; 95% confidence interval 0.06–12.9). There was no statistically significant difference in the upgrade to malignancy whether FEA lesions were pure or associated to LN at biopsy (p = 0.4245); however, when paired in biopsy specimens, these lesions were more frequently associated with atypical ductal hyperplasia (ADH) at surgery than with pure FEA (p = 0.012).
Conclusion:
Our results show a 2% upgrade rate to malignancy of pure FEA lesions. When FEA is found in association with LN at biopsy, surgical excision yields more frequently ADH than pure FEA thus warranting close surveillance or even surgical excision.
Advances in knowledge:
The association of LN with FEA at biopsy was more frequently associated with ADH at surgery than with pure FEA. If a biopsy-proven FEA lesion is deemed concordant with the imaging finding, when paired with LN at biopsy, careful surveillance or even surgical excision is suggested.
INTRODUCTION
The development of breast cancer is believed to be a multistep process originating in terminal duct lobular units and progressing towards invasive cancer. Many precursor lesions separating normal and malignant epithelium have been known including atypical duct hyperplasia (ADH), lobular neoplasia (LN) encompassing atypical lobular hyperplasia and lobular carcinoma in situ (LCIS), ductal carcinoma in situ (DCIS) and the more recently described columnar cell lesions.1 The term flat epithelial atypia (FEA) has been given to columnar cell lesions in which the native epithelium is replaced by one to multiple layers of cells that show low-grade cytologic atypia, whereby the adjective flat denotes the absence of more complex architectural patterns.1 These lesions are often found in association with microcalcifications.1–4 They, however, lack specific characteristics but are mostly described as amorphous5 as well as coarse heterogeneous and rarely fine pleomorphic.6
With the widespread use of imaging-guided biopsies as an alternative to surgical biopsy in the diagnosis of subclinical breast anomalies, increased number of high-risk lesions, including FEA, is encountered. Of these high-risk lesions, some harbour a significant risk of demonstrating cancer at excision as ADH and for which the current recommendation remains surgical excision, whereas others, such as LN, may only indicate an increased risk of breast cancer over a female's lifetime, hence close follow-up might be sufficient. In a recent review of the literature,7 the rate of upgrade of ADH to DCIS or invasive cancer ranges between 10% and 20% in studies submitted to radiological–pathological correlation. The true clinical significance of LN and FEA remains not clear, and straight guidelines for surgical excision or only surveillance are still lacking for both, especially in cases when the criteria of radiological–pathological concordance are met.
FEA is differentiated from ADH and low-grade ductal carcinoma by the presence of only low-grade cytologic atypia in the absence of architectural atypia. Since these lesions frequently merge into ADH and low-grade DCIS, deeper levels are sometimes necessary to exclude a more serious histological lesion.3 FEA lesions are also frequently observed in close association with the foci of LN and tubular carcinomas, with which they share cytological features such as prominent apical snouts and low-grade nuclear atypia.8,9 This suggests that FEA may be a non-obligate precursor in the low-grade breast neoplasia pathway.1,7,8,10,11
The reported upgrade rate to malignancy after surgical excision of FEA lesions ranges between 13% and 67% in the studies reported without radiological–pathological correlation hence favouring surgical excision.6,12 This upgrade rate, however, drops to a range from 0% to 7% in recent studies when all microcalcifications were removed at biopsy and the discordant cases excluded after careful radiological–pathological correlation.6,7,12 In a review of the literature published in 2012, Verschuur-Maes et al13 reported an upgrade rate to malignancy at surgery of 9% for surgically excised pure FEA and of 20% when FEA is found in association with ADH at biopsy. Based on this review, we were interested to evaluate the effect of LN on the final outcome at surgery of biopsy-proven FEA lesions found concomitantly with LN on specimens of biopsy.
This study aimed:
(1) To assess the impact of LN on the final outcome at surgery of FEA lesions when these are found together in biopsy specimens
(2) To determine the frequency of malignancy at surgical excision of biopsy-proven pure FEA in the breast center of the CHUM.
METHODS AND MATERIALS
Institutional review board approval was obtained, and informed consent from patients was waived. Using our breast centre pathology database, the retrospective review of 8907 imaging-guided core needle biopsies performed between 1 January 2009 and 1 January 2013 identified 110 cases of FEA. Patients were included in the study when the biopsy result yielded pure FEA or FEA with only LN as the most advanced atypical lesion at pathological analysis. Patients with associated atypical ductal hyperplasia (ADH) and ipsilateral breast cancer were excluded, so that 81 patients with 81 FEA lesions constituted our study.
A retrospective review of the imaging features (mammogram, ultrasound and/or MRI) was performed in consensus by two readers with 10 and 25 years' experience in breast imaging using the American College of Radiology, Breast Imaging Reporting and Data System (BI-RADS®) lexicon14 on dedicated workstations including two high-resolution monitors on the picture archive and communication system (Impax 6; Agfa Healthcare, Belgium).
The lesions sampled were divided into: microcalcifications, masses, distortions and abnormal enhancement on MRI. The histopathological diagnosis was obtained under stereotactic or MR guidance using a vacuum-assisted 10- or 11-gauge device when the radiological anomaly was microcalcifications or distortion on mammogram or abnormal enhancement on MR or sonographic guidance with a 14-gauge spring-loaded needle when presenting as a mass.
Based on its radiological characteristics, the radiological anomaly was categorized according to the BI-RADS lexicon and the degree of suspicion of malignancy into BI-RADS category 4A (low suspicion of malignancy), 4B (moderate suspicion of malignancy), 4C (high suspicion of malignancy) or 5 (highly suggestive of malignancy). The histopathological results at biopsy and at surgery of those lesions that underwent excision were collected from patients' files. The data were analyzed using Pearson's χ2 tests.
A p-value <0.05 was considered significant throughout this study.
RESULTS
Study population
81 FEA lesions were diagnosed in 81 females (mean age 54 years, range 38–80 years) in our institution between 2009 and 2013. The 81 FEA lesions were pure or associated with LN in, respectively, 59/81(73%) and 22/81 (27%) patients.
Imaging-guided needle biopsies were performed under stereotactic guidance with an 11-gauge vacuum-assisted needle (Mammotome Ethicon Endo-Surgery, Johnson and Johnson, Cincinatti, OH) (70/81; 86%), sonographic guidance with a 14-gauge spring-loaded needle (Bard biopsy system Tempe, AZ) (9/81; 12%) or MR guidance with a 10-gauge vacuum-assisted needle (Suros, Hologic Marlborough, MA) (2/81; 2%).
All patients in our breast centre who underwent a percutaneous imaging-guided biopsy with a pathological result yielding atypia are referred to surgical consult for counselling. Subsequently, surgical excision or follow-up is considered depending on the radiological–pathological correlation, patients' risk factors as assessed by the surgeon and patients' preference.
Overall, 57 patients (57/81; 70%) underwent surgery and 24 (24/81; 30%) mammographic surveillance with a mean follow-up of 36 months (range, 12–84 months). One patient had a repeated ultrasound-guided biopsy 48 months after the initial biopsy despite the fact that the mass was stable, and the histopathological analysis showed only pseudoangiomatous stromal hyperplasia without atypia; six patients were lost to follow-up 1 year after the biopsy and one died from pneumonia the following year. None of the patients followed-up developed a malignancy within the period of surveillance.
Imaging findings
In our study, FEA presented as microcalcifications in 68 patients (68/81; 84%), masses in 9 patients (9/81; 12%), distortions in 2 patients (2/81; 2%) and MR enhancement in 2 patients (2/81; 2%). The radiological anomaly was classified as BI-RADS 4A in 23 patients (23/81; 28%), BI-RADS 4B in 40 patients (40/81; 50%), BI-RADS 4C in 17 patients (17/81; 21%) and BI-RADS 5 in 1 patient (1/81; 1%). When presenting as microcalcifications, they were more frequently grouped in 58/68 (85%) patients or showing a segmental or regional distribution in, respectively, 4/68 (6%) and 6/68 (9%) patients. The microcalcifications were described as fine pleomorphic in 12/68 (18%) patients, as amorphous in 49/68 (72%) patients and as coarse/heterogeneous in 7/68 (10%) patients (Figure 1).
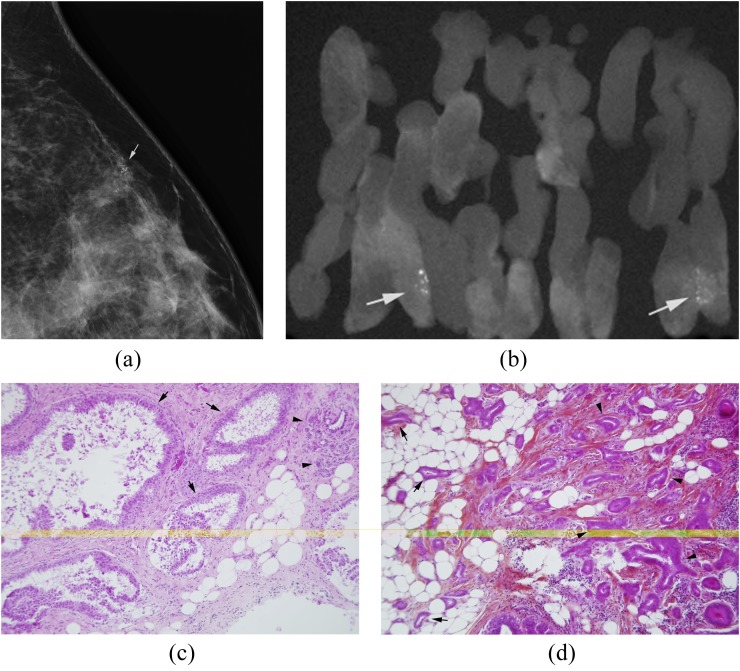
Figure 1.
(a–d) 47-year-old female; first screening mammogram. (a) Magnified lateral view demonstrating a cluster of suspicious pleomorphic microcalcifications (arrow) for which stereotactic vacuum-assisted 11-gauge core needle biopsy was recommended and performed. (b) Radiograph of core specimen obtained reveals numerous microcalcifications (arrows). (c) Photomicrograph of core biopsy specimen [haematoxylin phloxine saffron (HPS) stain, magnification ×10] reveals dilated acini lined by stratified columnar cells with low-grade atypia diagnostic of FEA (arrows). Please note the normal-size acini in this terminal ductal lobular unit (arrowheads) contrasting with FEA. (d) Photomicrograph of surgical specimen (HPS stain; magnification ×10) shows atypical tubules invading the fat (arrows) and fibrous stroma (arrowheads) consisting with low-grade tubular invasive carcinoma.
The anomaly was measured with calipers on the picture archive and communication system either on sonographic or, mammographic or MR images. It was 0.5 cm or less in 14//81 (17%) patients, 0.5–1 cm in 43/81 (53%) patients, 1–2 cm in 14/81 (18%) patients and >2 cm in 10/81 (12%) patients. Post-biopsy mammogram showed total removal of the microcalcifications in 41/68 (60%) patients, less or more than half of the cluster of microcalcifications remaining in the breast in, respectively, 15/68 (22%) and 12/68 (18%) of the cases. Sonographic correlates were found in 11 patients (11/81; 14%) presenting as masses that were irregular (4/11; 36%), oval (6/11; 55%) or round (1/11; 9%) with either microlobulated, indistinct or spiculated margins in, respectively, 7/11 (64%), 3/11 (27%) and 1/11 (9%) patients. They were classified as BI-RADS 4A in 4/11 (36%) patients, BI-RADS 4B in 5/11 (46%) patients, BI-RADS 4C in 1/11 (9%) patient and BI-RADS 5 in 1/11 (9%) patient.
Results at biopsy
Pathological analysis of the samples obtained at imaging-guided biopsy showed pure FEA in 59/81 (73%) biopsies and FEA with LN in 22/81(27%) of the biopsies.
Of these 81 FEA lesions, 57 (57/81; 70%), stratified into pure FEA (41/57; 72%) and FEA associated with LN (16/57; 28%), underwent surgical excision.
Results at surgical excision
At surgery, benign pathology or FEA with or without LN were found in 32 patients, 32/57 [56%; 95% confidence interval (CI) 42.4–69.3]; ADH in 17 patients, 17/57 (30%; 95% CI 18.4–43.4); DCIS in 4 patients, 4/57 (7%; 95% CI 1.9–17.0); and invasive cancer in 4 patients, 4/57 (7%; 95% CI 1.9–17.0) (Table 1). All cancers found at surgery were of low grade except one case of high-grade DCIS.
Table 1.
Histopathological results at surgery
| Surgical pathology | Number (%) |
|---|---|
| Benign or FEA ± LN | 32 (56) |
| ADH | 17 (30) |
| DCIS | 4 (7) |
| IDC | 4 (7) |
| Total | 57a (100) |
ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ; FEA, flat epithelial atypia; IDC, invasive ductal carcinoma; LN, lobular neoplasia.
57/81 patients underwent surgical excision.
No statistically significant difference could be found in the upgrade to malignancy of FEA lesions whether or not these were paired to LN in biopsy specimens; pure FEA and FEA with LN were, respectively, upgraded to cancer in 4/41 (10%) and 4/16 (25%) cases (p = 0.4245; χ2 = 0.6378, degree of freedom = 1) (Table 2).
Table 2.
Detailed surgical results of biopsy-proven pure flat epithelial atypia (FEA) and FEA associated with lobular neoplasia (LN)
| Surgical pathology results | ||||
|---|---|---|---|---|
| Biopsy results | Benign or FEA ± LN | ADH | Cancer (in situ or invasive) | Total (%) |
| Pure FEA | 28 (68%) | 9 (22%) | 4 (10%) | 41 (72%) |
| FEA and LN | 4 (25%) | 8 (50%) | 4 (25%) | 16 (28%) |
| Total | 32 | 17 | 4 | 57 (100%) |
ADH, atypical ductal hyperplasia.
The total upgrade to cancer after surgical excision of FEA is in our study, 8/57 (14%; 95% CI 6.3–25.8). Of these eight patients in whom surgery showed malignancy, the biopsy result yielding only atypia was considered discordant and surgery was highly recommended in four patients (Table 3). One of them presented with linear microcalcifications, the biopsy of which, revealed FEA associated with LCIS and the three others as a mass and two distortions where the biopsy yielded pure FEA. If we exclude these four cases of which the radiological presentation was deemed discordant with the post-biopsy pathological result of atypia, the total upgrade rate to cancer at surgery in our study would drop from 8/57 (14%; 95% CI 6.3–25.8) to 4/57 (7%; 95% CI 1.9–17.0) and that of pure FEA would be 1/41 (2%; 95% CI 0.06–12.9) instead of 4/41 (10%).
Table 3.
Summary of clinicoradiological findings of patients with malignant outcome after surgical excision of a biopsy-proven flat epithelial atypia (FEA) lesion
| Patient | Age, years | Radiological presentation | BI-RADS | Biopsy guidance | Needle, gauge | Biopsy result | Concordance | Surgical result |
|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Amorphous microcalcifications | 4B | Stereotactic | 11 | Pure FEA | Yes | DCIS |
| 2 | 47 | Mass | 5 | Sonographic | 14 | Pure FEA | No | IDC grade 1 |
| 3 | 63 | Distortion | 4B | Stereotactic | 11 | Pure FEA | No | IDC grade 1 |
| 4 | 55 | Distortion | 4B | Stereotactic | 11 | Pure FEA | No | IDC grade 1 |
| 5 | 47 | Linear microcalcifications | 4C | Stereotactic | 11 | FEA + LCIS | No | IDC grade 1 |
| 6 | 50 | Amorphous microcalcifications | 4A | Stereotactic | 11 | FEA + LCIS | Yes | DCIS |
| 7 | 67 | Non-mass enhancement | 4A | MRI | 9 | FEA + ALH | Yes | DCIS |
| 8 | 59 | Amorphous microcalcifications | 4B | Stereotactic | 11 | FEA + ALH | Yes | DCIS |
ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; BI-RADS, Breast Imaging Reporting and Data System; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; LCIS, lobular carcinoma in situ.
There was a significant association between concomitant presence of LN with FEA at imaging-guided biopsy and final outcome at surgery (p = 0.012; χ2 = 8.7836, degree of freedom = 2) whereby FEA found concomitantly with LN at biopsy proved to be more frequently ADH at surgery than pure FEA.
When the mammographic anomaly was a cluster of microcalcifications, which underwent a stereotactic-guided vacuum-assisted needle core biopsy, there was no association between the presence of post-biopsy residual microcalcifications (p = 0.4602) and final malignant outcome. Furthermore, no significant association could be found between the size of the cluster of microcalcifications (p = 1), and the BI-RADS classification (p = 0.4454) on one hand and upgrade to malignancy at surgery on the other hand.
The final malignant outcome was significantly associated with the radiological presentation whereby FEA lesions presenting initially as masses or distortions were more frequently upgraded to malignancy at surgery than those presenting as microcalcifications (p = 0.0091).
DISCUSSION
In our series, pure FEA was found in 59 of 8907 (0.7%) biopsies over 4 years. This is slightly lower to what has been reported, 1.2% and 1.5%.2,15 Previous studies have observed coexistence of FEA with other forms of atypia and low-grade carcinoma.1,16 In our study, FEA was associated with LN (atypical lobular hyperplasia and/or LCIS) in 22/81 (27%) cases, which is higher than was previously reported by Peres et al17 in 8/271 (3%) cases and by Bianchi et al18 in 90/589 (15.3%) cases.
In agreement with the literature, our results show that most of the FEA lesions (68/81, 84%) present at imaging as microcalcifications which is within the range reported by Peres et al17 in 95.6% (259/271) cases; Khoumais et al2 in 75% (78/104) cases; Biggar et al19 in 69% (35/51) cases; and Solorzano et al5 in 61% (20/33). These microcalcifications were considered indeterminate, lacking specific features and were often described as amorphous5 as in our study (49/68, 72%) and fine pleomorphic.2,20 FEA presenting as masses or asymmetrical densities have been reported by Peres et al17 in 4.1% (12/271) cases; Khoumais et al2 in 25% (25/104) cases; Biggar et al19 in 25% (13/51) cases; and Solorzano et al5 in 33% (11/33) cases and were found in our study in 11 patients (11/81, 14%) which is within this range. On ultrasound, Solorzano et al5 described FEA as an irregular mass with microlobulated margins similar to the reported features of DCIS and ADH.5,21,22 Most of the FEA cases presenting in our study as masses shared these sonographic characteristics and displayed an irregular shape and microlobulated margins in 4/11 (36%) and 7/11 (64%) cases, respectively. Three of them, however, were found associated with a fibroadenoma and one with fibrocystic changes. This could account for some of the benign appearing features encountered at sonography in 6 of our patients [6/11 (55%)], raising the hypothesis that FEA was, in these cases, an incidentaloma at biopsy.
Unlike few studies,2,17,19 we have found that FEA lesions presenting as masses or distortions were more often upgraded to cancer at surgery than microcalcifications. This could be explained by an undersampling factor considering that these masses were mostly sampled with a 14-gauge needle under ultrasound guidance. Nonetheless, since FEA is frequently associated with low-grade invasive tubular or lobular carcinoma9 and since these malignancies are known to often present mammographically as distortions, we assume that FEA result obtained on core needle biopsy of a distortion should be considered discordant and prompt surgical excision.
After careful radiological–pathological correlation and exclusion of the discordant cases, the total upgrade rate to malignancy after surgical excision of pure FEA lesions in our study is 2% (1/41) which is within the range reported in the literature;6,12 this would be equivalent to a BI-RADS 3 lesion for which follow-up is acceptable; larger studies are, however, needed to confirm this finding.
Our results show that the upgrade rate to cancer at surgery of the lesions, combining FEA and LN, of 25% (4/16) is similar to the results reported by Peres et al17 of 25% (2/8) but higher than that reported by Bianchi et al18 of 14.4% (13/90).
In our study, ADH was more often encountered in the excisional biopsies of FEA when these are paired to LN in biopsy specimens than alone. This finding is interesting since many studies have shown that FEA lesions are often linked with atypical hyperplasia, including atypical ductal and lobular hyperplasia, and low-grade carcinoma with which they share many similar molecular alterations suggesting that FEA may be a non-obligate precursor of low-grade carcinoma.1,4,7,10,11 This may be pertinent to identify, as many authors have suggested that patients with FEA may be at risk of natural sequential progression from FEA to ADH to cancer.3,7 As suggested by many studies, when columnar cell lesions are found in isolation, the risk of progression to a more advanced lesion is minor.4,7 Nonetheless, their presence is an excellent indicator for the coexistence of other atypical breast lesions that are associated with elevated cancer risk.4,16 Since currently there is no clear consensus as to the appropriate management decisions of isolated FEA, especially when deemed concordant with the imaging findings, it might be more prudent for patients with concomitant FEA and LN at biopsy to undergo either close surveillance or even surgical excision in order to exclude more worrisome lesions.
It is known that the amount of tissue obtained on core needle biopsy is determined by the gauge and type of needle used, automated vs vacuum-assisted, and may reflect the upgrade rate.23,24 Although we have found a statistically significant association between the needle type (i.e. 14-gauge automated needle vs 11-gauge vacuum-assisted needle) and final upgrade rate to malignancy (p = 0.0015), this finding could be more likely biased by the fact that different needles sampled different types of lesions. In fact, all FEA lesions presenting as masses were sampled by a 14-gauge needle, whereas vacuum-assisted 11-gauge needles sampled microcalcifications.
Unlike few studies17,25 that have found a statistically significant association between incomplete removal of the anomaly during sampling and malignant underestimation rate, our results as others'18,26 show that the final underestimation malignancy rate was not associated with the presence of residual microcalcifications post biopsy.
Our study has some limitations, mainly (1) it is retrospective in design and small in size; (2) it includes few cases of LN associated with FEA; (3) the pathological results were retrieved from the patients' files and were not retrospectively reviewed by a pathologist. This could account for a major limitation of our study since variability in the diagnosis of challenging cases including FEA was reported in many studies27,28 and consultation with colleagues regarding these cases can improve diagnostic accuracy;29 nonetheless, our results highlight the importance of the radiological–pathological correlation that each breast radiologist should undertake after each imaging-guided biopsy. In fact, despite the lack of consensus that persists in the management of FEA and LN, any discordance found between the radiological presentation and pathological result after core needle biopsy should lead to the appropriate management including rebiopsy or surgical excision aimed at detecting any malignancy that could have been otherwise missed.
CONCLUSION
FEA presents most often as microcalcifications, mostly of amorphous type, and less often as a mass displaying features similar to ADH on ultrasound. Our study shows that LN found in association with FEA at core needle biopsy may be an indicator for the presence of other atypical breast lesions that are associated with higher risk of breast cancer, for instance, ADH or even DCIS that can be overlooked by relying only on radiological–pathological concordance as in three of our cases. Hence, in cases where the biopsy-proven FEA result is deemed concordant with the imaging findings, it might be more prudent to recommend close surveillance or even surgical excision of those FEA lesions found concomitantly with LN on biopsy specimens.
Contributor Information
Mona El Khoury, Email: monelkhoury@gmail.com.
Lilia Maria Sanchez, Email: limasanchezb@yahoo.com.
Lucie Lalonde, Email: lalucie@sympatico.ca.
Isabelle Trop, Email: itrop@yahoo.com.
Julie David, Email: jdavis@vl.videotron.ca.
Benoît Mesurolle, Email: bmesurolle@yahoo.fr.
REFERENCES
- 1.Abdel-Fatah TM, Powe DG, Hodi Z, Lee AH, Reis-Filho JS, Ellis IO. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol 2007; 31: 417–26. [DOI] [PubMed] [Google Scholar]
- 2.Khoumais NA, Scaranelo AM, Moshonov H, Kulkarni SR, Miller N, McCready DR, et al. Incidence of breast cancer in patients with pure flat epithelial atypia diagnosed at core-needle biopsy of the breast. Ann Surg Oncol 2013; 20: 133–8. doi: https://doi.org/10.1245/s10434-012-2591-0 [DOI] [PubMed] [Google Scholar]
- 3.Schnitt SJ, Vincent-Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol 2003; 10: 113–24. doi: https://doi.org/10.1097/00125480-200305000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Lerwill MF. Flat epithelial atypia of the breast. Arch Pathol Lab Med 2008; 132: 615–21. doi: https://doi.org/10.1043/1543-2165(2008)132[615:FEAOTB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 5.Solorzano S, Mesurolle B, Omeroglu A, El Khoury M, Kao E, Aldis A, et al. Flat epithelial atypia of the breast: pathological-radiological correlation. AJR Am J Roentgenol 2011; 197: 740–6. doi: https://doi.org/10.2214/AJR.10.5265 [DOI] [PubMed] [Google Scholar]
- 6.Senetta R, Campanino PP, Mariscotti G, Garberoglio S, Daniele L, Pennecchi F, et al. Columnar cell lesions associated with breast calcifications on vacuum-assisted core biopsies: clinical, radiographic, and histological correlations. Mod Pathol 2009; 22: 762–9. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun BC, Collins LC. Recommendations for excision following core needle biopsy of the breast: a contemporary evaluation of the literature. Histopathology 2016; 68: 138–51. doi: https://doi.org/10.1111/his.12852 [DOI] [PubMed] [Google Scholar]
- 8.Aulmann S, Elsawaf Z, Penzel R, Schirmacher P, Sinn HP. Invasive tubular carcinoma of the breast frequently is clonally related to flat epithelial atypia and low-grade ductal carcinoma in situ. Am J Surg Pathol 2009; 33: 1646–53. doi: https://doi.org/10.1097/PAS.0b013e3181adfdcf [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy S, Bevers T, Kuerer H, Yang WT. Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol 2012; 198: W132–40. doi: https://doi.org/10.2214/AJR.11.7799 [DOI] [PubMed] [Google Scholar]
- 10.Simpson PT, Gale T, Reis-Filho JS, Jones C, Parry S, Sloane JP, et al. Columnar cell lesions of the breast: the missing link in breast cancer progression? A morphological and molecular analysis. Am J Surg Pathol 2005; 29: 734–46. doi: https://doi.org/10.1097/01.pas.0000157295.93914.3b [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchió C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 2010; 57: 171–92. doi: https://doi.org/10.1111/j.1365-2559.2010.03568.x [DOI] [PubMed] [Google Scholar]
- 12.Bibeau F, Chateau MC, Masson B. Management of non-palpable breast lesions with vacuum-assisted large core needle biopsies (Mammotome). Experience with 560 procedures at the Val d'Aurelle Center. [In French.] Ann Pathol 2003; 23: 582–92. [PubMed] [Google Scholar]
- 13.Verschuur-Maes AH, van Deurzen CH, Monninkhof EM, van Diest PJ. Columnar cell lesions on breast needle biopsies: is surgical excision necessary? A systematic review. Ann Surg 2012; 255: 259–65. doi: https://doi.org/10.1097/SLA.0b013e318233523f [DOI] [PubMed] [Google Scholar]
- 14.ACR BI-RADS Atlas, Breast imaging reporting and data system; 5th edn. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 15.Lavoué V, Roger CM, Poilblanc M, Proust N, Monghal-Verge C, Sagan C, et al. Pure flat epithelial atypia (DIN 1a) on core needle biopsy: study of 60 biopsies with follow-up surgical excision. Breast Cancer Res Treat 2011; 125: 121–6. doi: https://doi.org/10.1007/s10549-010-1208-1 [DOI] [PubMed] [Google Scholar]
- 16.Boulos FI, Dupont WD, Simpson JF, Schuyler PA, Sanders ME, Freudenthal ME, et al. Histologic associations and long-term cancer risk in columnar cell lesions of the breast: a retrospective cohort and a nested case-control study. Cancer 2008; 113: 2415–21. doi: https://doi.org/10.1002/cncr.23873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peres A, Barranger E, Becette V, Boudinet A, Guinebretiere JM, Cherel P. Rates of upgrade to malignancy for 271 cases of flat epithelial atypia (FEA) diagnosed by breast core biopsy. Breast Cancer Res Treat 2012; 133: 659–66. doi: https://doi.org/10.1007/s10549-011-1839-x [DOI] [PubMed] [Google Scholar]
- 18.Bianchi S, Bendinelli B, Castellano I, Piubello Q, Renne G, Cattani MG, et al. ; VANCB Study Group. Morphological parameters of flat epithelial atypia (FEA) in stereotactic vacuum-assisted needle core biopsies do not predict the presence of malignancy on subsequent surgical excision. Virchows Arch 2012; 461: 405–17. doi: https://doi.org/10.1007/s00428-012-1279-y [DOI] [PubMed] [Google Scholar]
- 19.Biggar MA, Kerr KM, Erzetich LM, Bennett IC. Columnar cell change with atypia (flat epithelial atypia) on breast core biopsy-outcomes following open excision. Breast J 2012; 18: 578–81. doi: https://doi.org/10.1111/tbj.12039 [DOI] [PubMed] [Google Scholar]
- 20.Pandey S, Kornstein MJ, Shank W, de Paredes ES. Columnar cell lesions of the breast: mammographic findings with histopathologic correlation. Radiographics 2007; 27(Suppl. 1): S79–89. doi: https://doi.org/10.1148/rg.27si075515 [DOI] [PubMed] [Google Scholar]
- 21.Mesurolle B, Perez JC, Azzumea F, Lemercier E, Xie X, Aldis A, et al. Atypical ductal hyperplasia diagnosed at sonographically guided core needle biopsy: frequency, final surgical outcome, and factors associated with underestimation. AJR Am J Roentgenol 2014; 202: 1389–94. doi: https://doi.org/10.2214/AJR.13.10864 [DOI] [PubMed] [Google Scholar]
- 22.Mesurolle B, El-Khoury M, Khetani K, Abdullah N, Joseph L, Kao E. Mammographically non-calcified ductal carcinoma in situ: sonographic features with pathological correlation in 35 patients. Clin Radiol 2009; 64: 628–36. doi: https://doi.org/10.1016/j.crad.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 23.Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: should it be excised? Hum Pathol 2007; 38: 35–41. doi: https://doi.org/10.1016/j.humpath.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 24.Villa A, Chiesa F, Massa T, Friedman D, Canavese G, Baccini P, et al. Flat epithelial atypia: comparison between 9-gauge and 11-gauge devices. Clin Breast Cancer 2013; 13: 450–4. doi: https://doi.org/10.1016/j.clbc.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 25.Dialani V, Venkataraman S, Frieling G, Schnitt SJ, Mehta TS. Does isolated flat epithelial atypia on vacuum-assisted breast core biopsy require surgical excision? Breast J 2014; 20: 606–14. doi: https://doi.org/10.1111/tbj.12332 [DOI] [PubMed] [Google Scholar]
- 26.Noël JC, Buxant F, Engohan-Aloghe C. Immediate surgical resection of residual microcalcifications after a diagnosis of pure flat epithelial atypia on core biopsy: a word of caution. Surg Oncol 2010; 19: 243–6. doi: https://doi.org/10.1016/j.suronc.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Elmore JG, Longton GM, Carney PA, Geller BM, Onega T, Tosteson AN, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 2015; 313: 1122–32. doi: https://doi.org/10.1001/jama.2015.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elmore JG, Pepe MS, Weaver DL. Discordant interpretations of breast biopsy specimens by pathologists—reply. JAMA 2015; 314: 83–4. doi: https://doi.org/10.1001/jama.2015.6239 [DOI] [PubMed] [Google Scholar]
- 29.Rakha EA, Ahmed MA, Aleskandarany MA, Hodi Z, Lee AH, Pinder SE, et al. Diagnostic concordance of breast pathologists: lessons from the National Health Service Breast Screening Programme Pathology External Quality Assurance Scheme. Histopathology 2016. doi: https://doi.org/10.1111/his.13117. Epub ahead of print. [DOI] [PubMed] [Google Scholar]