Abstract
Objective:
Accurate pre-treatment grading and staging of bladder cancer are vital for better therapeutic decision and prognosis. The aim of the present study was to evaluate the correlation between maximum standardized uptake value (SUVmax) calculated during early dynamic and post-diuretic fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT studies with grade and pT-stage of bladder cancer.
Methods:
39 patients with suspected/proven bladder carcinoma underwent 10-min early dynamic pelvic imaging and delayed post-diuretic whole-body FDG PET/CT imaging. SUVmax of the lesions derived from both studies was compared with grade and pT-stage. Relationship of SUVmax with grade and pT-stage was analyzed using independent sample t-test and analysis of variance.
Results:
SUVmax of the early dynamic imaging showing tumour perfusion was independent from the SUVmax of delayed imaging. High-grade tumours showed higher SUVmax than low-grade tumours in the early dynamic imaging (5.4 ± 1.4 vs 4.7 ± 1.6; p-value 0.144) with statistically significant higher value in Stage pT1 tumours (6.8 ± 0.8 vs 5.5 ± 1.2; p-value 0.04). Non-invasive pTa tumours had significantly less SUVmax than higher stage tumours during early dynamic imaging [F(4,29) = 6.860, p 0.001].
Conclusion:
Early dynamic imaging may have a role in predicting the grade and aggressiveness of the bladder tumours and thus can help in treatment planning and prognostication.
Advances in knowledge:
Dynamic PET/CT is a limitedly explored imaging technique. This prospective pilot study demonstrates the utility of this modality as a potential adjunct to standard FDG PET/CT imaging in predicting the grade and aggressiveness of the bladder tumours and thus can impact the patient management.
INTRODUCTION
The management of patients with urinary bladder cancer is based on the stage and grade of the disease; hence, accurate pre-treatment clinical and radiological staging at presentation is vital for better therapeutic decision and prognosis. Traditionally, cystoscopy is considered gold standard for the detection of bladder cancer.1,2 On documentation of a tumour, the patient is scheduled for a transurethral resection of the bladder tumour (TURBT) for histological confirmation of diagnosis and to determine the grade and depth of tumour invasion.3 TURBT without the inclusion of the detrusor muscle is of inadequate quality and is associated with increased mortality. This requires restaging TURBT to reliably distinguish between high- and low-grade histologies and also the depth of invasion (pTa, pT1 and pT2), as it is imperative for accurate treatment planning.3,4 Radiological imaging such as CT and MRI play a significant role in staging and advancements in functional imaging, with MRI having further improved their utility in bladder cancer; but, these modalities may have certain limitations in evaluating the extent of local or regional disease reliably.5,6 Fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT has become a useful modality in cancer evaluation based on hypermetabolism observed in malignant cells.7 However, the high urinary excretion of FDG has limited its role in urologic malignancies. The masking effect of the urinary activity makes detection of bladder wall lesions and pelvic lymph nodes difficult. Certain interventions were adopted to detect bladder wall lesions with 18F-FDG PET/CT in the past to decrease the tracer activity in the urine such as furosemide injection before imaging, retrograde irrigation of bladder with double lumen Foley catheter, post-void imaging or by using other tracers;8–11 but, these might add inconvenience to the patients. Diuresis along with hydration had shown increased tumour to background contrast and decreased urinary tracer activity; but, holding the urine during image acquisition can be troublesome to the patients after diuresis. The best results were obtained from controlled parenteral hydration with 250 ml of normal saline solution infusion and 10 mg of i.v. injection of furosemide starting 30 min after tracer injection without increasing the risk of urinary discomfort.12 It has been shown that the number of blood vessels and blood flow (neoangiogenesis) in the cancerous sites is increased and may have prognostic implications.13 A previous study has suggested that first-pass uptake of 18F-FDG (first 2 min) provides an estimate of tumour perfusion and complements to the metabolic information obtained from standard PET/CT in a single study.14 Thus, increased blood flow in the bladder lesions can be exploited in the bladder cancer detection with early dynamic imaging of the pelvis immediately after tracer injection, circumventing the problem of high urinary tracer accumulation encountered with standard imaging at 60 min. Only one pilot study comprising seven patients with bladder cancer utilized early dynamic 18F-FDG flow imaging with encouraging results.15 Further, no previous studies have tried to find out the correlation between the maximum standardized uptake value (SUVmax) during 18F-FDG PET/CT of the bladder cancer and the aggressiveness of the disease. This prospective study was undertaken to evaluate the correlation of SUVmax values with grade and pathological T-stage of bladder tumours utilizing early dynamic pelvic imaging and standard 18F-FDG PET/CT acquisition.
METHODS AND MATERIALS
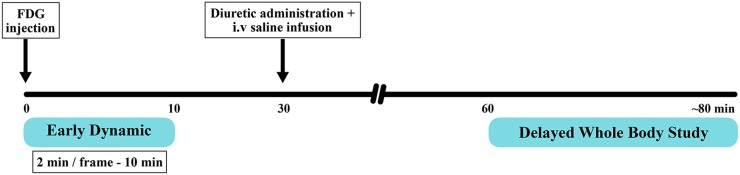
This prospective study of 39 patients with suspected or proven bladder carcinoma with their written informed consent was duly approved by the hospital institutional ethics committee. All the patients adequately fasted and had their blood glucose level <200 mg dl−1. They were encouraged not to void before early dynamic imaging. A very low current CT scout scan was taken to position the urinary bladder in the scanner field of view, followed by non-enhanced CT of the pelvis for PET attenuation correction, anatomical correlation and image fusion using a dedicated PET/CT scanner (GE Discovery 710; GE Healthcare, Milwaukee, WI). Early dynamic imaging of the pelvis was initiated immediately after i.v. administration of 370 MBq of 18F-FDG for initial 10 min at a rate of 2 min/frame with a total of five frames. The patients were advised to rest comfortably after dynamic imaging in the waiting room. 30 min post-injection of FDG, patients were given 10 mg of i.v. furosemide along with the parenteral infusion of 250 ml of normal saline. This hydration protocol was chosen, as it had shown the best results in a previous study.12 They were instructed to void frequently during waiting period. Standard whole-body 18F-FDG PET/CT imaging was performed in the caudocephalad direction from midthigh to skull 60 min post-injection (Figure 1). Both early regional dynamic and delayed whole-body standard images were acquired in three-dimensional mode, corrected for attenuation using CT-derived attenuation map and reconstructed with three-dimensional iterative algorithm (ordered subset expectation maximization). Thus, both early dynamic and standard images were acquired without increasing the overall study time or causing any additional patient discomfort.
Figure 1.
Schematic diagram depicting the timeline of early dynamic (2 min/frame for 10 min; frame mode) and whole-body fludeoxyglucose (FDG) positron emission tomography/CT acquisition protocols.
Two experienced nuclear medicine physicians analyzed the scans for positive findings along with calculation of semi-quantitative analysis (SUVmax values). Dynamic bladder blood flow images showing increased blood flow to the region of bladder walls were considered suspicious for malignancies. The SUVmax were calculated by carefully placing the volume of interest over the bladder lesions with anatomical reference from the fused PET/CT images for each 2-min frame. The standard imaging at 60 min showing increased focal FDG uptake in the bladder wall was considered suspicious for malignancy and SUVmax normalized to body weight was obtained. SUVmax of the bladder lesion with the highest tracer uptake in case of multiple lesions was derived from both the early dynamic and the standard imaging. The SUVmax obtained at 2 min, 10 min, its rate of uptake during the dynamic imaging and standard imaging at 60 min post-injection were analyzed for its correlation with the grade and pT-stage of the tumours obtained from histopathology (gold standard). The grade and invasion of the tumour was ascertained from the histopathological examination of tissues using World Health Organization/International Society of Urological Pathology revised consensus classification 2004, following TURBT or radical cystectomy. Patients with inadequate biopsies have been excluded from statistical analysis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Statistical analysis
Statistical Package for the Social Sciences (SPSS®) software v. 16 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) was used for statistical analysis. Quantitative data were expressed as mean ± standard deviation. Independent sample t-test was used to determine whether there was any significant difference between the SUVmax of the bladder lesions during the early dynamic and post-diuretic FDG PET/CT in the low-grade and high-grade lesions. Analysis of variance with Tukey Honest Significant Difference (HSD) post hoc test was used to compare the SUVmax during early dynamic and standard images in the different pT-stages of the lesion obtained from the histopathology reports. Pearson correlation was used to compare the SUVmax during early dynamic and standard images.
RESULTS
A total of 39 patients (37 males and 2 females) with a mean age and standard deviation of 58 ± 10.3 years were included in this prospective study. The patients underwent TURBT to ascertain the grade and pT-stage of bladder tumours, and six of them were further taken up for radical cystectomy after TURBT within 15–58 days (mean 34.3 days) and their histopathology from radical cystectomy was considered. The SUVmax of the tumour at the first frame (initial 2 min) of the early dynamic imaging (representing increased blood flow) and at 60 min imaging ranged from 1.4 to 8 (5.2 ± 1.5) and 2.9–51.4 (17.5 ± 11.6), respectively. SUVmax of the tumour from the initial 2 min dynamic image appeared to be independent of the SUVmax at 60 min (representing metabolic activity) (Pearson correlation coefficient r = +0.019; p-value 0.910). SUVmax at 10 min of dynamic imaging correlated positively with the post-diuretic SUVmax at 60 min (Pearson r = +0.515; p-value 0.001).
Grade
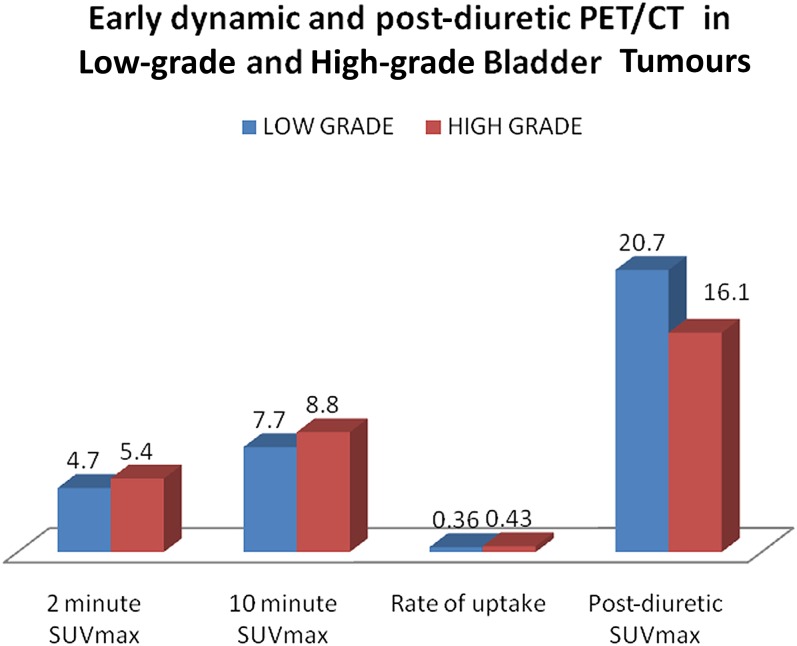
The mean SUVmax of the bladder tumours at 2 min, 10 min and the rate of uptake tended to be higher in the high-grade lesions compared with low-grade lesions in the early dynamic imaging (Figure 2), although the difference was not statistically significant (Table 1). High-grade bladder tumours revealed lower mean SUVmax compared with low-grade tumours during the standard imaging at 60 min (Table 1).
Figure 2.
Early dynamic and post-diuretic fludeoxyglucose positron emission tomography (PET)/CT in low-grade and high-grade bladder tumours. SUVmax, maximum standardized uptake value.
Table 1.
Correlation of early dynamic and post-diuretic fludeoxyglucose positron emission tomography/CT maximum standardized uptake value (SUVmax) with histological grade (any T-stage) and in T1-stage tumours
| Category |
SUVmaxvs grade (any T-stage) |
SUVmaxvs grade (T1-stage tumours) |
|||||
|---|---|---|---|---|---|---|---|
| Parameter | Grade | N | Mean ± SD | p-value | N | Mean ± SD | p-value |
| 2 min SUVmax | Low | 12 | 4.65 ± 1.60 | 0.144 | 6 | 5.50 ± 1.20 | 0.04 |
| High | 27 | 5.42 ± 1.45 | 7 | 6.82 ± 0.85 | |||
| 10 min SUVmax | Low | 12 | 7.56 ± 2.76 | 0.160 | 6 | 8.85 ± 2.54 | 0.655 |
| High | 27 | 8.77 ± 2.25 | 7 | 9.37 ± 1.48 | |||
| Rate of uptake | Low | 12 | 0.36 ± 0.20 | 0.418 | 6 | 0.41 ± 0.21 | 0.348 |
| High | 27 | 0.43 ± 0.25 | 7 | 0.32 ± 0.10 | |||
| Post-diuretic SUVmax | Low | 12 | 20.73 ± 11.73 | 0.257 | 6 | 18.05 ± 6.27 | 0.132 |
| High | 27 | 16.09 ± 11.55 | 7 | 12.30 ± 6.40 | |||
SD, standard deviation.
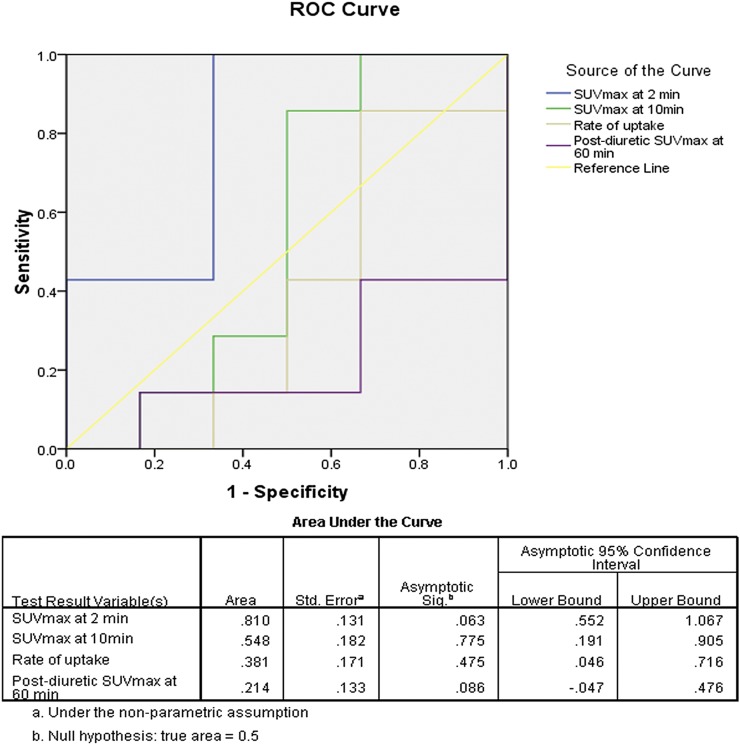
In the present study, Stage pT1 consisted of both low- and high-grade bladder tumours, whereas Stage pTa tumours were only of low grade; Stages pT2 and above had only high-grade tumours. In this subset of patients with Stage pT1, high-grade tumours had shown statistically significant higher flow of tracer than low-grade tumours at 2 min of dynamic imaging (Table 1); but, standardized uptake values (SUVs) between the low- and high-grade tumours were not statistically different for 10 min, rate of uptake during the early dynamic and post-diuretic standard imaging (Table 1). Further, receiver-operating characteristic analysis of the SUVmax values of the Stage pT1 tumours at 2 min of the dynamic study revealed a sensitivity of 100% and specificity of 67% in detecting high-grade tumours above the SUVmax of 5.45. All patients with SUVmax >7.35 at 2 min of dynamic imaging were high-grade bladder tumours (Figure 3). Thus, higher SUVmax at 2 min of early dynamic imaging (increased flow of tracer) was indicative of higher grade in pT1 bladder tumours.
Figure 3.
The figure depicts the receiver-operating characteristic (ROC) curve analysis of maximum standardized uptake value (SUVmax) at 2 min, 10 min, 60 min and rate of uptake. SUVmax at 2 min (a marker of tumour perfusion) was significantly different between low- and high-grade pT1-stage bladder tumours (p-value 0.04) (Table 1). It demonstrates that SUVmax at 2 min (area under the curve 0.810) has the potential to differentiate high-grade from low-grade pT1 lesions unlike other variables such as SUVmax at 10 min, at 60 min and rate of uptake.
T-stage
Of the 39 patients, T-stage could not be accurately determined in 5 patients on histopathology reports owing to inadequate transurethral resection biopsy.3 The detrusor muscle in four patients could not be identified in histopathology specimen to comment upon muscle invasion. There was no comment on lamina propria invasion in the fifth patient with non-muscle invasive disease, resulting in exclusion of these five patients from statistical analysis. Analysis of variance on the SUVmax at 2 min revealed statistically significant difference among the different T-stages [F(4,29) = 6.860; p 0.001]. A post hoc Tukey HSD test showed that non-invasive pTa tumours had lower SUVmax than pT1 (3.3 ± 2.6 vs 6.2 ± 1.2; p 0.018), pT2 (3.3 ± 2.6 vs 4.4 ± 0.9; p 0.665), pT3 (3.3 ± 2.6 vs 6.4 ± 1.3; p 0.041) and pT4 (3.3 ± 2.6 vs 6.7 ± 0.21; p 0.048) tumours during the early dynamic imaging at 2 min. There was no trend of increase in the SUV with pT-stage progression from pT1 to pT4 bladder tumours (Table 2). No statistically significant difference was noted in the mean SUV at 10 min, rate of uptake during the early dynamic imaging and SUVs at the post-diuretic standard imaging among the pTa, pT1, pT2, pT3 and pT4 bladder tumours.
Table 2.
Maximum standardized uptake value (SUVmax) (mean ± standard deviation) in different T-stages of bladder tumour
| Stage | Ta | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| N | 2 | 13 | 13 | 3 | 3 |
| 2 min SUVmax | 3.3 ± 2.6 | 6.2 ± 1.2 | 4.4 ± 1.0 | 6.4 ± 1.3 | 5.9 ± 1.3 |
| 10 min SUVmax | 6.3 ± 3.7 | 9.1 ± 2.0 | 8.7 ± 2.9 | 8.9 ± 1.9 | 8.4 ± 1.0 |
| Rate of uptake | 0.38 ± 0.14 | 0.37 ± 0.17 | 0.54 ± 0.31 | 0.43 ± 0.26 | 0.31 ± 0.12 |
| Post-diuretic SUVmax | 13 ± 4.6 | 14.9 ± 6.8 | 18.3 ± 15.5 | 19.9 ± 3.6 | 10.3 ± 1.5 |
DISCUSSION
The presence of urinary FDG activity and masking of some of the bladder lesions limit the role of 18F-FDG PET/CT in bladder cancer. The forced diuresis along with 18F-FDG PET/CT in bladder tumours has not provided the desired results. Belakhlef et al,15 by using the new early dynamic protocol, in a pilot study consisting of seven patients compared early blood flow imaging and the standard 18F-FDG PET/CT with histopathology. The early dynamic PET imaging in detecting bladder lesions showed five true positives, one true negative and one false positive. The novel technique of early dynamic imaging was able to detect all the bladder lesions that were seen on the post-diuretic FDG PET/CT scan at 60 min post-injection in this prospective study. It implies that non-invasive early dynamic imaging by exploiting the process of neoangiogenesis has the ability to detect bladder tumours without increasing the investigation time or patient discomfort. It may be particularly useful in patients having difficulty in holding urine during the acquisition of whole-body PET/CT after diuresis and hydration. It also revealed that the increased tumour blood flow is independent of the metabolic activity of the bladder tumours, which might be due to uncoupling of the parameters of perfusion and metabolism in tumours, also shown in a study.14
The present study demonstrated that mean SUVmax of the bladder tumours at 2 min of the early dynamic imaging was higher in the high-grade tumours (Figure 4) compared with the low-grade tumours (Figure 5), with statistically significantly higher values in Stage pT1 (p-value of 0.04). Regarding the T-stage, the non-invasive pTa bladder tumours (two patients) had significantly lower SUVmax compared with higher stage tumours at 2 min of the early dynamic imaging. The SUVmax obtained at 2 min imaging has the potential to differentiate non-invasive Stage pTa bladder tumours from the higher stage tumours. There was absence of correlation between the SUVmax of bladder tumours (representing metabolic activity at 60 min standard imaging) and their grade and aggressiveness in this study. This could be attributed to several reasons. The Glucose transporter 1 (GLUT1) expression may not be strongly correlated to the grade of the bladder tumours leading to high uptake in some of the low-grade tumours and low uptake in some of the high-grade tumours. Reis et al16 found no significant correlation between GLUT1 expression and grade/stage of the invasive urothelial carcinoma. Tumour heterogeneity may also lead to inhomogenous FDG uptake in the large lesions, and small areas of high FDG uptake in these lesions might result in underestimation of SUVmax owing to partial volume effect. Hypoxic conditions inducing increased expression of GLUT receptors and glycolytic enzymes may be the cause of high FDG uptake in some of the low-grade tumours.17 A study carried out previously has also failed to show any correlation between the SUVmax at 60 min and tumour grade and histology.18
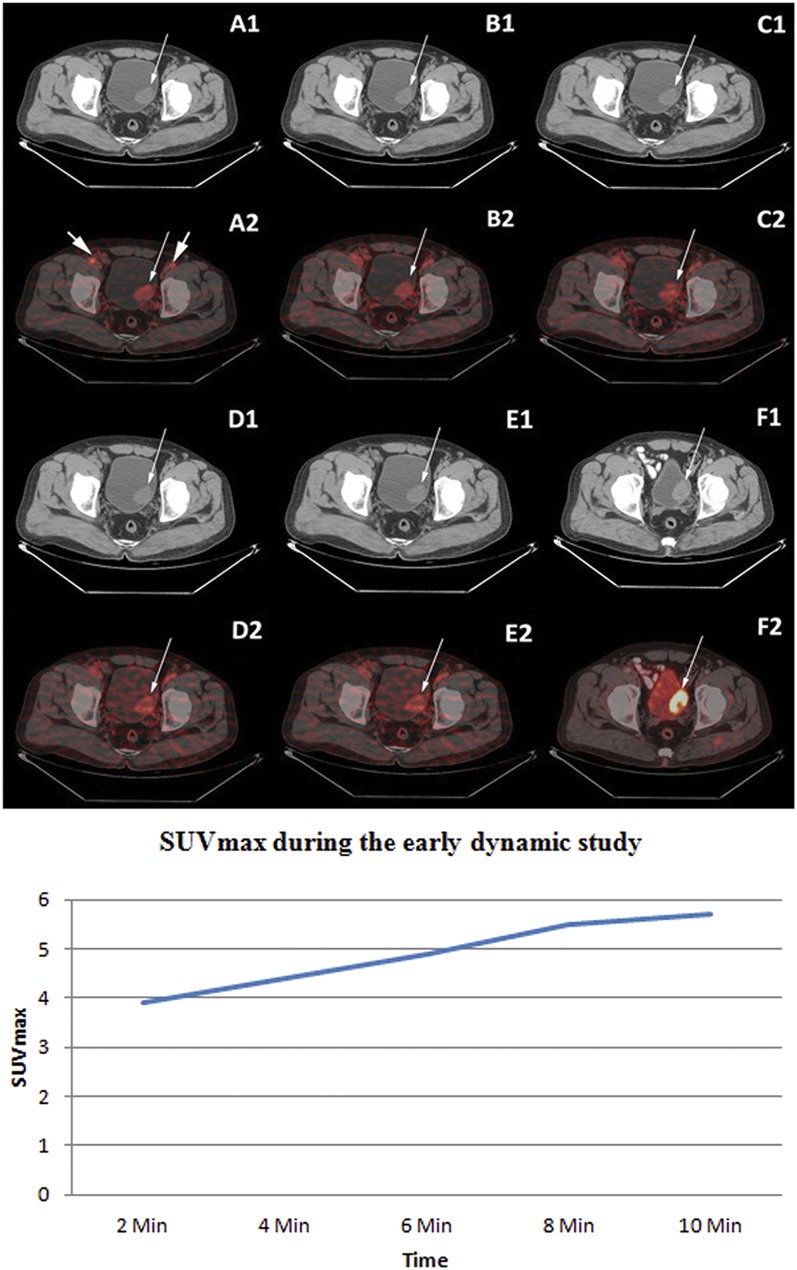
Figure 4.
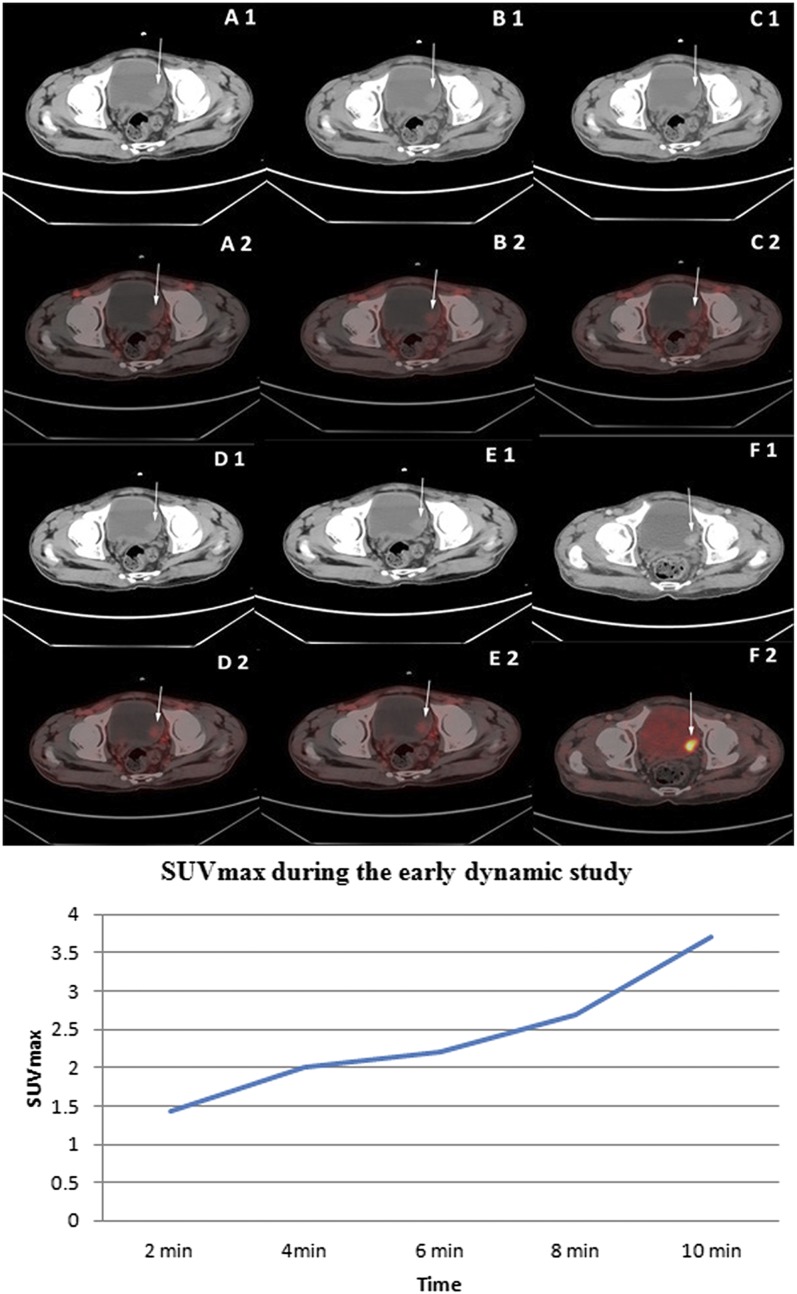
Upper panel: staging fludeoxyglucose (FDG) positron emission tomography (PET)/CT displaying the non-enhanced transaxial CT (A1–E1) and corresponding early dynamic fused PET/CT images of the bladder (A2–E2). Increased flow of tracer in the bladder tumour [maximum standardized uptake value (SUVmax) 3.9 at 2 min] in the left posterolateral wall was noted in all the dynamic images (thin arrows) as well as in the bilateral external iliac vessels (thick arrows in A2). Intense FDG uptake (SUVmax 19.9) was noted in the bladder tumour on standard FDG PET/CT images after diuresis (F1: transaxial contrast-enhanced CT and F2: fused PET/CT image). Histopathology of bladder tumour following transurethral resection of the bladder tumour was of high-grade transitional cell carcinoma. Lower panel: time activity curve of lesion SUVmax in a high-grade bladder tumour during the early dynamic imaging of patient represented in the upper panel.
Figure 5.
Upper panel: staging fludeoxyglucose (FDG) positron emission tomography (PET)/CT displaying non-enhanced transaxial CT (A1–E1) and corresponding early dynamic fused PET/CT (A2–E2) images of the bladder. Mildly increased flow of tracer in the bladder tumour (SUVmax 1.44 at 2 min) in the left lateral wall was noted in all the dynamic images (thin arrow). Intense FDG uptake [maximum standardized uptake value (SUVmax) 9.8] was noted in the bladder tumour on standard FDG PET/CT images after diuresis (F1: transaxial CECT and F2: fused PET/CT image). Histopathology following transurethral resection of the bladder tumour revealed low-grade transitional cell carcinoma with no invasion of the detrusor muscle or the lamina propria. Lower panel: time activity curve of lesion SUVmax in a low-grade bladder tumour during the early dynamic imaging of patient represented in the upper panel.
Accurate histological grading and staging of bladder cancer with an adequate TURBT is crucial for optimal management of patients. However, errors and interobserver variability are not uncommon and lead to upstaging or downstaging in some of the patients on further radical cystectomy.4 Conventional imaging modalities are essentially part of the staging but do have certain limitations.5,6 In our study, two patients showed high SUVmax (8 and 6.9, respectively) at 2 min on early dynamic PET/CT, but initial diagnosis on TURBT was low-grade, non-muscle invasive tumours. Further histopathological examination of the cystectomy specimens performed at 51 and 58 days later revealed both to be high-grade T1 and T3b tumours, respectively. Thus, initial 2 min of early dynamic FDG PET/CT imaging of the pelvis in the patients with pT1 stage tumours may have a role in predicting grade of bladder tumours as well as have the potential to change the patient management.
To the best of our knowledge, no prior study has evaluated the correlation of tumour SUVmax with grade and T-stage of the bladder tumours utilizing either early dynamic or post-diuretic PET/CT imaging. Few authors in the past have evaluated the correlation of the SUVmax with the clinicopathological characteristics of other tumours with variable results.18,19
PET/CT is valuable in the detection of bladder tumours especially after interventions such as hydration with forced diuresis.11,12 The SUVmax obtained at 60 min standard imaging are not indicative of tumour aggressiveness because metabolic activity of the bladder tumours appears to be independent of the grade and pT-stage. The novel and non-invasive technique of early dynamic FDG PET/CT imaging has shown the potential to detect bladder lesions based on the property of increased tumour blood flow without increasing patient discomfort or extending the overall time of the study. Further, SUVmax at 2 min of early dynamic study were higher with high-grade tumours (statistically significant higher values in pT1-stage) and significantly lower values in non-invasive pTa tumours. There were limited number of patients in the group showing significant differences in SUVmax at 2 min of early dynamic imaging (low-grade and high-grade tumours in pT1-stage and non-invasive Stage pTa tumours); so, further studies are required to observe this significant difference in a larger patient sample.
CONCLUSION
It is very important to accurately identify the grading and T-stage of urothelial cancer, as interobserver variations may be seen among pathologists.4 This prospective study clearly showed the difference in SUVmax for low grade vs high grade in pT1 lesions and lower SUVmax in pTa tumours compared with higher stage tumours at 2 min of early dynamic FDG PET/CT imaging. Therefore, early dynamic imaging (initial 2 min following tracer injection) as an adjunct to standard FDG PET/CT not only helps in detecting bladder tumours but may also provide complimentary information regarding the grade and aggressiveness of the bladder tumours and thus help in optimal patient management.
Contributor Information
Abhishek Sharma, Email: abhishek13pgi@gmail.com.
Uttam K Mete, Email: uttam_mete@yahoo.com.
Ashwani Sood, Email: sood99@yahoo.com.
Nandita Kakkar, Email: nandita_kakkar@yahoo.com.
Arun K R Gorla, Email: arun.gorla@outlook.com.
Bhagwant R Mittal, Email: brmittal@yahoo.com.
REFERENCES
- 1.Herr HW, Schneider M. Outpatient flexible cystoscopy in men: a randomized study of patient tolerance. J Urol 2001; 165: 1971–2. doi: https://doi.org/10.1097/00005392-200106000-00030 [PubMed] [Google Scholar]
- 2.Denzinger S, Burger M, Walter B, Knuechel R, Roessler W, Wieland WF, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 2007; 69: 675–9. doi: https://doi.org/10.1016/j.urology.2006.12.023 [DOI] [PubMed] [Google Scholar]
- 3.Chamie K, Landa EB, Bassett JC, Daskivich TJ, Leventhal M, Deapen D, et al. Quality of diagnostic staging in patients with bladder cancer: a process-outcomes link. Cancer 2015; 121: 379–85. doi: https://doi.org/10.1002/cncr.29071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostrom PJ, van Rhijn BW, Fleshner N, Finelli A, Jewett M, Thoms J, et al. Staging and staging errors in bladder cancer. Eur Urol Suppl 2010; 9: 2–9. doi: https://doi.org/10.1016/j.eursup.2010.01.005 [Google Scholar]
- 5.Maurer T, Horn T, Heck M, Gschwend JE, Eiber M, Beer AJ. Current staging procedures in urinary bladder cancer. Diagnostics (Basel) 2013; 3: 315–24. doi: https://doi.org/10.3390/diagnostics3030315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin WC, Chen JH. Pitfalls and limitations of diffusion-weighted magnetic resonance imaging in diagnosis of urinary bladder cancer. Transl Oncol 2015; 8: 217–30. doi: https://doi.org/10.1016/j.tranon.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyer T, Townsend DW, Blodgett TM. Dual-modality PET/CT tomography for clinical oncology. Q J Nucl Med 2002; 46: 24–34. doi: https://doi.org/10.1007/978-3-642-18803-9_2 [PubMed] [Google Scholar]
- 8.Kosuda S, Kison PV, Greenough R, Grossman HB, Wahl RL. Preliminary assessment of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with bladder cancer. Eur J Nucl Med 1997; 24: 615–20. doi: https://doi.org/10.1007/bf00841398 [DOI] [PubMed] [Google Scholar]
- 9.Ahlstrom H, Malmstrom PU, Letocha H, Andersson J, Langstrom B, Nilsson S. Positron emission tomography in the diagnosis and staging of urinary bladder cancer. Acta Radiol 1996; 37: 180–5. doi: https://doi.org/10.1080/02841859609173441 [DOI] [PubMed] [Google Scholar]
- 10.de Jong IJ, Pruim J, Elsinga PH, Jongen MM, Mensink HJ, Vaalburg W. Visualisation of bladder cancer using (11)C-choline PET: first clinical experience. Eur J Nucl Med Mol Imaging 2002; 29: 1283–8. doi: https://doi.org/10.1007/s00259-002-0881-7 [DOI] [PubMed] [Google Scholar]
- 11.Vicente AM, Castrejon AS, Munoz AP, Woll PP, Garcia AN. Impact of 18F-FDG PET/CT with retrograde filling of the urinary bladder in patients with suspected pelvic malignancies. J Nucl Med Technol 2010; 38: 128–37. doi: https://doi.org/10.2967/jnmt.109.074146 [DOI] [PubMed] [Google Scholar]
- 12.Ceriani L, Suriano S, Ruberto T, Giovanella L. Could different hydration protocols affect the quality of 18F-FDG PET/CT images? J Nucl Med Technol 2011; 39: 77–82. doi: https://doi.org/10.2967/jnmt.110.081265 [DOI] [PubMed] [Google Scholar]
- 13.Streeter EH, Harris AL. Angiogenesis in bladder cancer—prognostic marker and target for further therapy. Surg Oncol 2002; 11: 85–100. doi: https://doi.org/10.1016/s0960-7404(02)00013-0 [DOI] [PubMed] [Google Scholar]
- 14.Mullani NA, Herbst RS, O'Neil RG, Gould KL, Barron BJ, Abbruzzese JL. Tumor blood flow measured by PET dynamic imaging of first-pass 18F-FDG uptake: a comparison with 15O-labeled water-measured blood flow. J Nucl Med 2008; 49: 517–23. doi: https://doi.org/10.2967/jnumed.107.048504 [DOI] [PubMed] [Google Scholar]
- 15.Belakhlef S, Church C, Jani C, Lakhanpal S. Early dynamic PET/CT and 18F-FDG blood flow imaging in bladder cancer detection: a novel approach. Clin Nucl Med 2012; 37: 366–8. doi: https://doi.org/10.1097/RLU.0b013e3182443110 [DOI] [PubMed] [Google Scholar]
- 16.Reis H, Tschirdewahn S, Szarvas T, Rubben H, Schmid KW, Grabellus F. Expression of GLUT1 is associated with increasing grade of malignancy in non-invasive and invasive urothelial carcinomas of the bladder. Oncol Lett 2011; 2: 1149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dierckx RA, van de Wiele C. FDG uptake, a surrogate of tumour hypoxia? Eur J Nucl Med Mol Imaging 2008; 35: 1544–9. doi: https://doi.org/10.1007/s00259-008-0758-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karantanis D, Allen-Auerbach M, Czernin J. Relationship among glycolytic phenotype, grade, and histological subtype in ovarian carcinoma. Clin Nucl Med 2012; 37: 49–53. doi: https://doi.org/10.1097/rlu.0b013e3182291e03 [DOI] [PubMed] [Google Scholar]
- 19.Roh JL, Ryu CH, Choi SH, Kim JS, Lee JH, Cho KJ, et al. Clinical utility of 18F-FDG PET for patients with salivary gland malignancies. J Nucl Med 2007; 48: 240–6. doi: https://doi.org/10.1016/s1744-7895(07)70542-4 [PubMed] [Google Scholar]