Abstract
Objective:
To demonstrate the superiority of total psoas volume (TPV) over total psoas area (TPA) in terms of predicting post-operative complications in living-donor liver transplantation (LDLT).
Methods:
The TPA and TPV were assessed in 32 recipients who underwent CT before LDLT. The TPA was measured using an axial CT image at the level of the upper margin of the fourth lumbar vertebral body. The TPV was calculated using all the CT images from the muscle origin through the level of the pubic symphysis. Patients were divided into a sarcopenia group and no-sarcopenia group based on the medians of normalized TPA (nTPA) and normalized TPV (nTPV). We calculated the odds ratio (OR) of post-operative respiratory complications in relation to nTPA and nTPV, respectively.
Results:
Out of 32 recipients, 17 recipients experienced at least 1 post-operative respiratory complication. The OR for males according to nTPV [OR = 15.00, 95% confidence interval (CI) = 1.03–218.31; p = 0.031] was higher than that for nTPA (OR = 3.33, 95% CI = 0.36–30.70; p = 0.280). The OR for females according to nTPV (OR = 4.00, 95% CI = 0.56–28.40; p = 0.16) was the same as that for nTPA (OR = 4.00, 95% CI = 0.56–28.40; p = 0.16).
Conclusion:
Pre-operative volume of the skeletal muscle might be a better predictor for post-operative risks in LDLT recipients than pre-operative area of the skeletal muscle.
Advances in knowledge:
Post-operative risks for respiratory complications in LDLT recipients might be evaluated more accurately by using TPV instead of TPA.
INTRODUCTION
Living-donor liver transplantation (LDLT) is a life-saving intervention for many patients with end-stage liver diseases such as cirrhosis, decompensated disease, acute liver failure and hepatocellular cancer.1,2 LDLT recipients are likely to have complications such as post-operative infections and death due to the complexity of the surgical procedure.3–5 It is therefore critical to identify LDLT recipients with increased risks at the pre-operative stage.6
Recent evidence has shown that sarcopenia may identify LDLT recipients with increased risks for post-operative infections, complications and death.6,7 Sarcopenia is defined as the loss of skeletal muscle mass caused by aging, chronic medical illness and malnutrition and has been postulated to be a major factor for decline in strength.8,9 In previous studies, sarcopenia has been assessed by total psoas area (TPA), which is the sum of the area of bilateral psoas major muscles calculated by a single axial cross-sectional image.7,8 Only a few studies have assessed sarcopenia by using total psoas volume (TPV), which is the sum of the volume of bilateral psoas major muscles calculated utilizing volume data.5,10 Furthermore, to the best of our knowledge, there is no article which shows TPV to be more useful than TPA in relation to evaluating post-operative risks of LDLT patients.
The aim of this study is to demonstrate the superiority of TPV over TPA based on the idea that TPV may be able to measure skeletal muscle mass more accurately by expanding measuring range and decreasing statistical error. We measured both pre-operative TPA and pre-operative TPV of living-donor liver transplantation (LDLT) recipients and compared TPV with TPA using the odds ratio (OR) of post-operative respiratory complications of LDLT.
METHODS AND MATERIALS
Patients
32 consecutive recipients (14 males and 18 females) who underwent LDLT at a university hospital between January 2009 and December 2013 were retrospectively investigated. For all of the recipients, pre-operative abdominal CT images (i.e. within 3 months before surgery) were available. Standard demographic and clinical data were collected including data on sex, age, height, weight, body mass index (BMI) and existence of respiratory complications. In this study, respiratory complications were defined as applying to one or more of the following items: contracting pneumonia within 30 days after surgery, tracheotomy after surgery or more than 8 days with artificial respirator. 17 out of 32 recipients were classified as having respiratory complications. This study was conducted in accordance with the Declaration of Helsinki and its updates and was approved by the local ethics committee. Informed consent was obtained from all patients.
Image analysis
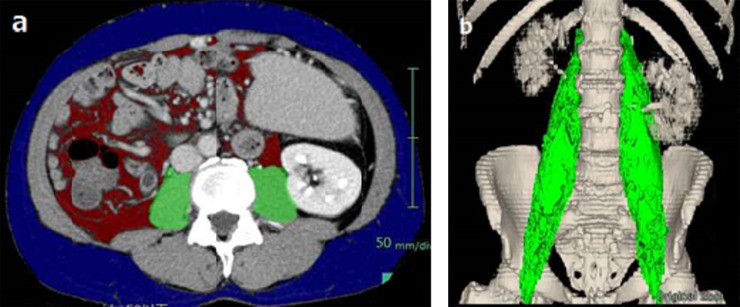
Sarcopenia was assessed by measuring the cross-sectional area of the right and left psoas muscles (total psoas muscle area = TPA) and the volume of the right and left psoas muscles (total psoas muscle volume = TPV). Equilibrium phase images of abdominal CT, covering the whole abdomen and pelvis were utilized in this study after administration of contrast at a dose of 650 mg I kg−1 of body weight over 30 s. All the images were taken within 3 months before LDLT. The tube voltage was set at 120 kVp with slice thickness of 5 mm in all cases. The tube current was not constant because automatic exposure control was applied. CT units used in this study consisted of the following 3 models: 64-detector-row Aquilion™ (Toshiba Medical Systems, Otawara, Tochigi, Japan) in 20 cases; 64-detector-row LightSpeed VCT® (GE Healthcare, Milwaukee, WI) in 7 cases; and 320-detector-row Aquilion One™ (Toshiba Medical Systems) in 5 cases. All images were analyzed using the workstation SYNAPSE VINCENT (Fujifilm Corporation, Tokyo, Japan). The measurements of TPA and TPV were performed with an automated analysis program installed in the workstation (Figure 1). The TPA was measured using axial CT images at the level of the upper edge of the fourth lumber vertebral body according to a previous study.6 The TPV was calculated for psoas major muscles using all the CT images from the muscle origin through the level of the pubic symphysis. Displacements of muscle region used to calculate TPA and TPV caused by segmentation error were manually modified by an operator and verified by a radiologist. The measured TPA and TPV were then normalized for square of height according to a previous study10 and renamed as normalized TPA (nTPA) and normalized TPV (nTPV), respectively.
Figure 1.
Sarcopenia measurement using (a) total psoas area at the level of the upper edge of the fourth lumber vertebral body and (b) total psoas volume.
Statistical analysis
The data were presented as mean ± standard deviation for all continuous variables and as number (percentage) for all categorical variables. The correlation between nTPA and nTPV was evaluated by Pearson's correlation coefficient. We separated patients into males and females and tested the difference between these groups for all continuous variables using the Mann–Whitney U test. Each group was then divided into a sarcopenia group and no-sarcopenia group based on the medians of nTPA and nTPV, and the impact of sarcopenia on post-operative respiratory complications was examined using the OR in the males group and females groups with nTPA and nTPV, respectively. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed with SPSS® v. 22 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics
The demographic and clinical characteristics of the 32 patients included in the study are outlined in Table 1. There were 14 (43.75%) males and 18 (56.25%) females in the study population. The mean age was 54.0 ± 9.6 years. The number of patients who were classified as Child–Turcotte–Pugh Class A, B and C were 4, 6 and 22, respectively. Indications for LDLT were cirrhosis (n = 12), primary biliary cirrhosis (n = 4), hepatocellular carcinoma (n = 2), fulminant hepatitis (n = 1), primary sclerosing cholangitis (n = 1), familial amyloid polyneuropathy (n = 1), autoimmune hepatitis (n = 1), polycystic kidney and liver disease (n = 1), both cirrhosis and hepatocellular carcinoma (n = 7), both fulminant hepatitis and autoimmune hepatitis (n = 1) and unknown (n = 1).
Table 1.
Clinical characteristics of patients who underwent living-donor liver transplantation
| Characteristics | All (n = 32) | Males (n = 14) | Females (n = 18) |
|---|---|---|---|
| Cirrhosis [n (%)] | 12 (37.5) | 5 (35.7) | 7 (38.9) |
| PBC [n (%)] | 4 (12.5) | 1 (7.1) | 3 (16.7) |
| HCC [n (%)] | 2 (6.3) | 2 (14.3) | 0 (0.0) |
| FH [n (%)] | 1 (3.1) | 1 (7.1) | 0 (0.0) |
| PSC [n (%)] | 1 (3.1) | 0 (0.0) | 1 (5.6) |
| FAP [n (%)] | 1 (3.1) | 1 (7.1) | 0 (0.0) |
| AH [n (%)] | 1 (3.1) | 0 (0.0) | 1 (5.6) |
| PCKLD [n (%)] | 1 (3.1) | 0 (0.0) | 1 (5.6) |
| Both cirrhosis and HCC [n (%)] | 7 (21.9) | 4 (28.6) | 3 (16.7) |
| Both FH and AH [n (%)] | 1 (3.1) | 0 (0.0) | 1 (5.6) |
| Unknown [n (%)] | 1 (3.1) | 0 (0.0) | 1 (5.6) |
| Child–Turcotte–Pugh class | |||
| A (5–6 points) [n (%)] | 4 (12.5) | 3 (21.4) | 1 (5.6) |
| B (7–9 points) [n (%)] | 6 (18.8) | 2 (14.3) | 4 (22.2) |
| C (10–15 points) [n (%)] | 22 (68.8) | 9 (64.3) | 13 (72.2) |
| Post-operative respiratory CC [n (%)] | 17 (53.1) | 8 (57.1) | 9 (50.0) |
| Only pneumonia [n (%)] | 3 (9.4) | 2 (14.3) | 1 (5.6) |
| Only tracheotomy [n (%)] | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Only extubation delay [n (%)] | 10 (31.3) | 5 (35.7) | 5 (27.8) |
| Pneumonia and tracheotomy [n (%)] | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pneumonia and extubation delay [n (%)] | 2 (6.3) | 0 (0.0) | 2 (11.1) |
| Tracheotomy and extubation delay [n (%)] | 2 (6.3) | 1 (7.1) | 1 (5.6) |
| Pneumonia, tracheotomy and extubation delay [n (%)] | 0 (0.0) | 0 (0.0) | 0 (0.0) |
AH, autoimmune hepatitis; CC, complications; FAP, familial amyloid polyneuropathy; FH, fulminant hepatitis; HCC, hepatocellular carcinoma; PBC, primary biliary cirrhosis; PCKLD, polycystic kidney and liver disease; PSC, primary sclerosing cholangitis.
Differences in psoas major muscle mass between the sexes
The average nTPA was 633.3 ± 202.0 mm2 m−2 and the average nTPV was 111.6 ± 39.0 cm3 m−2 after normalizing for patient height (Table 2). The mean nTPA was lower for females than for males (506.8 ± 146.4 vs 796.0 ± 136.6 mm2 m−2, p < 0.001) (Figure 2a). Similar to nTPA, the mean nTPV was lower among females than among males (84.2 ± 23.4 vs 146.9 ± 23.8 cm3 m−2, p < 0.001) (Figure 2b). There were significant differences between the sexes of both nTPA and nTPV. Pearson's correlation coefficients evaluating the correlation between nTPA and nTPV were 0.77 in males and 0.89 in females.
Table 2.
Demographic, total psoas area (TPA) and total psoas volume (TPV) data of patients who underwent living-donor liver transplantation
| Characteristics | All (n = 32) | Males (n = 14) | Females (n = 18) | p-value |
|---|---|---|---|---|
| Age (years) | 54.0 ± 9.6 | 49.5 ± 11.0 | 57.5 ± 6.6 | 0.037 |
| nTPA (mm2 m−2) | 633.3 ± 202.0 | 796.0 ± 136.6 | 506.8 ± 146.4 | <0.001 |
| nTPV (cm3 m−2) | 111.6 ± 39.0 | 146.9 ± 23.8 | 84.2 ± 23.4 | <0.001 |
| Height (cm) | 160.1 ± 10.0 | 169.8 ± 5.4 | 153.6 ± 6.4 | <0.001 |
| Weight (kg) | 62.7 ± 12.9 | 71.6 ± 11.7 | 55.7 ± 8.9 | 0.001 |
| BMI (kg m−2) | 24.2 ± 3.9 | 24.8 ± 3.6 | 23.7 ± 4.0 | 0.403 |
BMI, body mass index; nTPA, normalized TPA; nTPV, normalized TPV.
Figure 2.
The difference of (a) normalized total psoas area (nTPA) and (b) normalized total psoas volume (nTPV) between males and females.
Impact of sarcopenia on post-operative respiratory complications
Out of the 32 patients who underwent LDLT, 17 experienced at least 1 post-operative respiratory complication with a morbidity of 53.1%. Morbidity for post-operative respiratory complications after LDLT included pneumonia (n = 5), tracheotomy (n = 2) and extubation delay (n = 14). Males were divided into a sarcopenia group (n = 7) and no-sarcopenia group (n = 7) based on a median nTPA of 791.6 mm2 m−2. Females were divided into a sarcopenia group (n = 9) and no-sarcopenia group (n = 9) based on a median nTPA of 488.8 mm2 m−2. Similarly, males were divided into a sarcopenia group (n = 7) and no-sarcopenia group (n = 7) based on a median nTPV of 149.0 cm3 m−2. Females were divided into a sarcopenia group (n = 9) and no-sarcopenia group (n = 9) based on a median nTPV of 83.3 cm3 m−2. Patients who were classified as sarcopenia according to TPA had a higher risk of post-operative respiratory complications than those who were not classified as sarcopenia [males: OR = 3.33, 95% confidence interval (CI) = 0.36–30.70; p = 0.28; and females: OR = 4.00, 95% CI = 0.56–28.40; p = 0.16] (Table 3). In the same way as TPA, patients who were classified as sarcopenia according to TPV also had higher risk of post-operative respiratory complications than patients who were not classified as sarcopenia (males: OR = 15.00, 95% CI = 1.03–218.31; p = 0.031; and females: OR = 4.00, 95% CI = 0.56–28.40; p = 0.16) (Table 3).
Table 3.
Odds ratio of post-operative respiratory complications
| Sex | Measurement | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|
| Males | nTPA | 3.33 | 0.36–30.70 | 0.28 |
| Males | nTPV | 15.00 | 1.03–218.31 | 0.031 |
| Females | nTPA | 4.00 | 0.56–28.40 | 0.16 |
| Females | nTPV | 4.00 | 0.56–28.40 | 0.16 |
CI, confidence interval; nTPA, normalized total psoas area; nTPV, normalized total psoas volume.
DISCUSSION
Prior works have documented the correlation between increasing risks for recipients in liver transplantation and sarcopenia. In the first place, the reason that sarcopenia correlates with increasing risks for liver transplantation is that sarcopenia reflects poor nutritional status, which deteriorates prognosis.11–13 In a previous study, sarcopenia was evaluated by TPA and was shown to negatively impact morbidity and mortality after liver transplantation.7,8 A few studies have been conducted using TPV. For example, Valero et al5 have shown that sarcopenia assessed by TPV is an independent factor predictive of post-operative complications following surgical intervention for primary hepatic malignancies. Amini et al10 have also shown that sarcopenia assessed by TPV is strongly associated with short- and long-term outcomes after pancreatic resection. However, there is no article which shows TPV to be more useful than TPA in relation to evaluating the post-operative risks of LDLT patients. We tested the predictive power for post-operative respiratory complications of LDLT patients with TPA and TPV using the OR. Although the OR of TPV was higher than that of TPA in males, the OR of TPV was the same as that of TPA in females. This discrepancy may be explained by the fact that the correlation between TPA and TPV in females was stronger than in males. This means that TPV may have a stronger predictive power for post-operative respiratory complications than TPA.
This study has some unique characteristics, different from other studies. First, subjects of this research were all Japanese. To reduce the effect caused by the difference of skeletal muscle mass based on race, we set no absolute threshold of sarcopenia such as that of previous studies.5,10,14 Instead, we used the median as a relative threshold of sarcopenia. Second, subjects of this research were not deceased-donor liver transplantation but LDLT.
Several limitations must be kept in mind when considering the present study. First, the number of cases is small. This means that selection bias for patients may have been large. Actually, CIs of the OR were considerably wide. However, because the aim of this study was to demonstrate the feasibility of TPV comparing TPA, the small number of cases may not be a fatal problem. To confirm the conclusions of this study, more cases must be accumulated. Second, this study has no gold standard for TPA/TPV, although every study dealing with sarcopenia suffers from the lack of true muscle volume data. If psoas major muscles were not correctly contoured by software, we modified manually under the leadership of an experienced radiologist. Third, because there were variations in CT models involved in this study, the Hounsfield unit values of these models may be different. However, it is thought that the impact of this is negligibly small.
CONCLUSION
Pre-operative volume of skeletal muscles might be a better predictor for post-operative risks in LDLT recipients rather than area. Further studies with larger numbers of cases are required to confirm this.
Contributor Information
Yuki Wada, Email: w-0522@eis.hokudai.ac.jp.
Tamotsu Kamishima, Email: ktamotamo2@yahoo.co.jp.
Tsuyoshi Shimamura, Email: tshima@cocoa.ocn.ne.jp.
Norio Kawamura, Email: norirv@live.jp.
Kenichiro Yamashita, Email: kenchan@med.hokudai.ac.jp.
Kenneth Sutherland, Email: kensuth@med.hokudai.ac.jp.
Hiroshi Takeda, Email: h_takeda@pharm.hokudai.ac.jp.
REFERENCES
- 1.Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol 2008; 48: 567–77. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira R, Júnior M, Salvalaggio P, Rezende MB, Evangelista AS, Guardia BD, et al. Liver transplantation: history, outcomes and perspectives. Einstein (Sao Paulo) 2015; 13: 149–52. doi: https://doi.org/10.1590/S1679-45082015RW3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol 2014; 20: 6211–20. doi: https://doi.org/10.3748/wjg.v20.i20.6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller AR, Platz K. Early postoperative complications following liver transplantation. Best Pract Res Clin Gastroenterol 2004; 18: 881–900. [DOI] [PubMed] [Google Scholar]
- 5.Valero V, 3rd, Amini N, Spolverato G, Weiss MJ. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg 2015; 19: 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krell RW, Kaul DR, Martin AR. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl 2013; 19: 1396–402. doi: https://doi.org/10.1002/lt.23752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014; 20: 401–7. doi: https://doi.org/10.1002/lt.23811 [DOI] [PubMed] [Google Scholar]
- 8.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010; 211: 271–8. doi: https://doi.org/10.1016/j.jamcollsurg.2010.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M. The loss of skeletal muscle strength mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006; 61: 1059–64. [DOI] [PubMed] [Google Scholar]
- 10.Amini N, Spolverato G, Gupta R. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 2015; 20: 1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010; 39: 412–23. doi: https://doi.org/10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition 2002; 18: 978–86. doi: https://doi.org/10.1016/s0899-9007(02)00984-x [DOI] [PubMed] [Google Scholar]
- 13.Millwala F, Nguyen GC, Thuluvath PJ, Millwala F, Nguyen GC, Thuluvath PJ. Outcomes of patients with cirrhosis undergoing non-hepatic surgery: risk assessment and management. World J Gastroenterol 2007; 13: 4056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011; 13: 439–46. doi: https://doi.org/10.1111/j.1477-2574.2011.00301.x [DOI] [PMC free article] [PubMed] [Google Scholar]