Abstract
Sickle cell disease (SCD) is a hereditary red cell disorder with clinical manifestations secondary to sickling or crescent-shaped distortion of the red blood cells. Major clinical manifestations of SCD include haemolytic anaemia and vaso-occlusive phenomena resulting in ischaemic tissue injury and organ damage. Chronic sequelae of the anaemia and vaso-occlusive processes involving the musculoskeletal system include complications related to extramedullary haematopoiesis, osteonecrosis, myonecrosis and osteomyelitis. Sickle cell bone disease is one of the commonest clinical presentations. Awareness and knowledge of the imaging features related to these complications are essential for early diagnosis and prompt management. In this article, the pathophysiology and key imaging findings related to these complications are reviewed.
PATHOPHYSIOLOGY OF SICKLE CELL DISEASE
Sickle cell disease (SCD) is an umbrella term for haemoglobinopathies with a clinical feature of red blood cell (RBC) “sickling”. The abnormal haemoglobin (Hb) contains haemoglobin S (HbS) molecule, and symptomatic disorders are due to homozygous HbS sickle cell anaemia (HbSS) or due to heterozygous forms resulting from combination of HbS with another abnormal globin gene (as in beta-thalassemia gene resulting in haemoglobin S-thalassemia (HbS-Thal) or HbC gene resulting in haemoglobin S-C disease (HbSC)).
The Hb molecule is a tetramer of two alpha globins and two beta globins. A point mutation in the beta globin gene results in the substitution of valine for glutamic acid as the sixth amino acid of the globin chain. This mutation leads to an increased RBC turnover and anaemia, as blood cells with two mutated beta globin chains are removed from the circulation at a higher rate by splenic macrophages. The abnormal Hb tetramer can polymerize under conditions of hypoxia and dehydration leading to vaso-occlusive phenomenon.1 The effects of haemoglobin S are responsible for hallmark features of SCD through increased red cell adherence to endothelium, activation of local vasoactivity and adhesion and inflammation leading to sickle vaso-occlusion and haemolysis.2–4
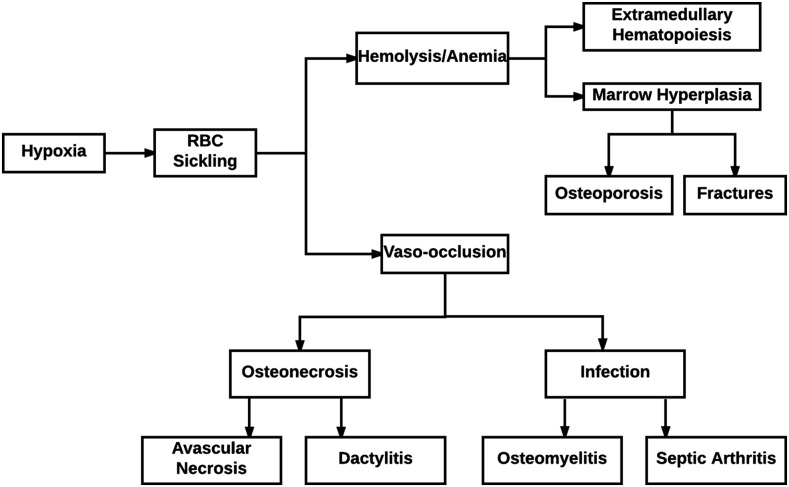
Musculoskeletal complications of SCD are common and are often the main causes for their acute and chronic morbidities. Acute manifestations include bony infarcts with vaso-occlusive crises and osteomyelitis, while osteoporosis and osteonecrosis are chronic problems. The various musculoskeletal complications and their imaging appearances are elucidated in this article, and a schematic summary showing the complications is illustrated in Figure 1.
Figure 1.
Schematic summary of the musculoskeletal complications of sickle cell disease. RBC, red blood cell.
Abnormal sickling of RBCs leads to many musculoskeletal complications relating to anaemia as well as vaso-occlusion and the resulting ischaemia, end-organ damage and infection. Anaemia related to SCD leads to conditions promoting the increased production of RBCs such as extramedullary haematopoiesis, bone expansion and pathologic fractures related to haematopoietic marrow hyperplasia and osteoporosis. Consequences of vaso-occlusion are the result of ischaemia and include avascular necrosis, bone infarcts, the premature closure of epiphyseal plates and the halting of the growth of long bones, the development of H-shaped vertebrae and dactylitis. Vaso-occlusion in SCD also leads to decreased flow of blood through small vessels supplying bones, resulting in the formation of zones of ischaemia in which pathogens can invade and thrive, causing osteomyelitis and septic arthritis. Finally, ischaemia, as a result of vaso-occlusion, can also result in muscle necrosis, soft tissue haematomas and superinfection with abscess formation.
HAEMATOPOIETIC MARROW HYPERPLASIA
Extramedullary haematopoiesis refers to the process by which blood or blood cells are formed outside of the bone marrow.5 Although extramedullary haematopoiesis is more commonly associated with sickle cell variants (e.g. HbS-thal) and other haemolytic anaemias, it can also be a feature of SCD. Extramedullary haematopoiesis occurs in response to poor RBC formation and associated chronic hypoxia. It is preceded by the conversion of the yellow marrow to the haematopoietic red marrow.
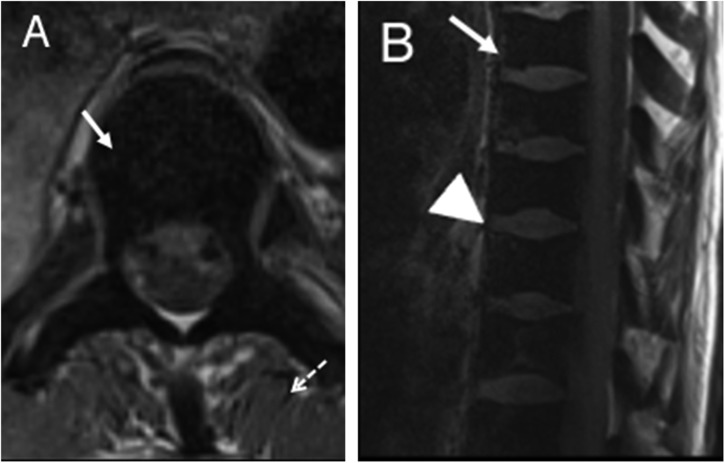
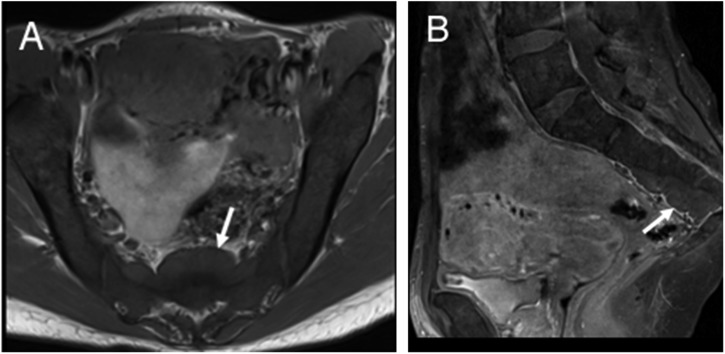
As part of the normal physiological ageing process, there is conversion of red marrow to yellow marrow with the transformation occurring in the appendicular skeleton before the axial skeleton. In patients with SCD, there is reconversion of yellow marrow and persistence of red marrow. These changes manifest as loss of normal fatty signal with abnormal low signal on T1 weighted images (Figure 2). In addition, there is marrow hyperplasia with associated widening of medullary spaces, coarsening of the normal trabecular pattern and cortical thinning. This increase in width of medullary spaces as well as the change in the trabecular pattern can lead to the appearance of osteopenia, which can be visible on imaging. These morphological features along with the associated softening of long bones can increase the risk of insufficiency fractures. One manifestation of softening that can be clearly visible on imaging in vertebral bodies is the fish vertebrae configuration in which the endplates form a smooth concave shape (Figure 2). Extramedullary haematopoiesis presents as soft tissue masses which produce RBCs in various sites of the body including the spleen, lymph nodes, liver, heart, thymus, spinal canal, breasts, pleura, prostate, adrenal glands, kidneys and peripheral and cranial nerves. The soft tissue masses of extramedullary haematopoiesis are similar to normal intramedullary haematopoietic tissues and thus have intermediate signal intensity on T1 and T2 weighted MRI (Figure 3).
Figure 2.
Axial T1 (a) and sagittal T1 (b) images of the thoracic spine showing diffuse low signal of the vertebral bodies consistent with replacement of the normal fatty marrow with haematopoietically active red marrow. It can be noted that the marrow signal (solid arrows) is abnormally low compared with that of the adjacent discs (arrowhead) and muscles (dashed arrow). The characteristic smooth biconcave deformity of vertebral body endplates is also depicted.
Figure 3.
T1 weighted axial (a) and T2 weighted sagittal (b) images of the sacral region showing an intermediate signal soft tissue mass in the presacral region representing the site of extramedullary haematopoiesis (arrows). Diffuse loss of the marrow signal in the visualized pelvic bones and vertebral bodies consistent with marrow replacement is also depicted.
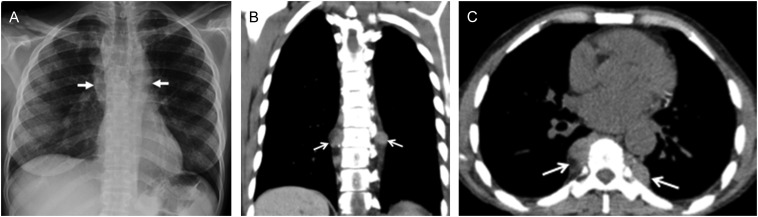
In the chest, the paravertebral region is a common site for extramedullary haematopoiesis. On imaging, it can present as paravertebral masses in the thorax which are large, have multiple lobes and are usually asymptomatic (Figure 4). On CT, these paravertebral masses are smooth, isodense to the adjacent muscle and can rarely lead to erosion of the adjacent bone.6
Figure 4.
An anteroposterior (AP) chest radiograph (a) demonstrating large smoothly marginated bilateral paravertebral soft tissue masses representing sites of extramedullary haematopoiesis (arrows). Axial (b) and coronal (c) non-contrast chest CT (soft tissue window) images of the thoracic cavity demonstrate bilateral lobulated soft tissue masses with sharp margins consistent with extramedullary haematopoiesis (arrows).
Stunted growth has been reported in children secondary to the hyperplastic marrow, with premature closure of epiphyses and impaired and asymmetrical growth of long bones.7,8 Vertebral growth may be affected by the ischaemia of the central vertebral growth plate, resulting in H-shaped vertebrae from the squared-off depression of vertebral endplates and sometimes, “tower” vertebrae formation from increasing vertebral height without a corresponding increase in girth.9
OSTEONECROSIS
Osteonecrosis or bone infarction refers to tissue hypoxia and ischaemia, which, in SCD, are consequences of RBC sickling in the bone marrow that can cause blood stasis. Blood vessels which supply the bone with nutrition may become occluded, leading to the development of osteosclerotic strands which are linear and lie parallel to the cortical margin. These osteosclerotic strands are visible on imaging and lead to a “bone within bone” appearance.10 Because of oedematous changes in the marrow, MRI may show areas of increased signal.11 Over time, these chronic infarcted regions become hypointense on T1 and T2 imaging because of the sclerosis and fibrotic changes accompanying this process (Figure 5).
Figure 5.
Axial proton density (a), axial T2 fat-saturated (b) and axial T1 fat-saturated post-contrast images (c) of the distal tibia: proton density and T2 fat-saturated images demonstrate the peripheral line of low signal intensity representing the sclerotic tissue (solid arrows) surrounding a central region of marrow oedema (dashed arrows). On T1 fat-saturated post-contrast images, deep to the low signal sclerotic tissue, there is a circumferential band-like region of enhancement representing a zone of hyperaemia (arrowhead).
The long bones are the more common sites of bone infarction. Osteonecrosis involving epiphyseal regions of long bones is generally termed as avascular necrosis. The heads of the humerus and femur are the most common sites involved (Figure 6). The infarcted portions of the medullary bone eventually evolve into regions of reactive sclerosis and new bone formation.10 Metaphyseal or diaphyseal osteonecrosis leads to a characteristic appearance of intramedullary sclerosis (Figure 7). In contrast to the avascular necrosis that can occur in other diseases, avascular necrosis in SCD can present diffusely, and lesions seen on MRI generally appear larger.12
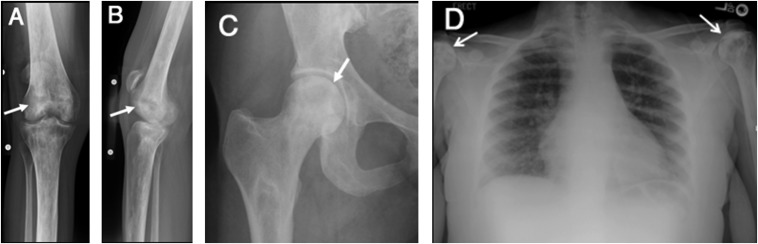
Figure 6.
Anteroposterior (AP) and lateral radiographs of the knee (a, b) demonstrating patchy areas of sclerosis and lucency in the distal femur and proximal tibia representing changes of osteonecrosis (arrows). Mild joint space narrowing related to secondary osteoarthritis is also present. AP radiographs of the right hip (c) demonstrating osteonecrosis (arrow). The patchy regions of sclerosis involving the femoral head can be noted. The AP radiograph of the chest (d) reveals patchy sclerosis of humeral heads consistent with avascular necrosis (arrows).
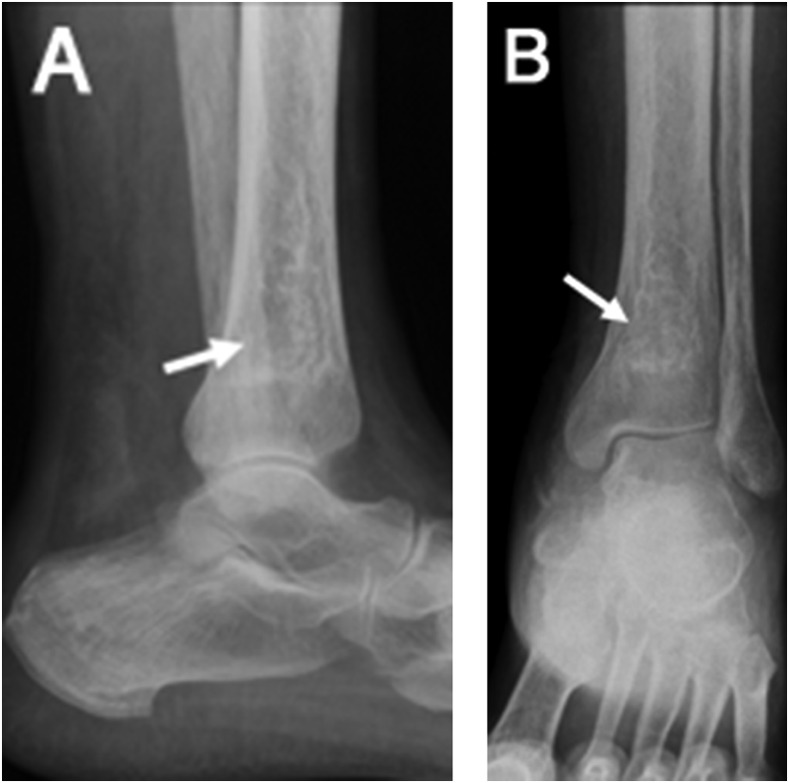
Figure 7.
Anteroposterior (AP) and lateral radiographs (a, b) of the knee demonstrating an irregular area of intramedullary sclerosis involving the distal tibia related to changes of prior infarct (arrows).
In most cases of avascular necrosis in SCD, the initial radiographic images can have a normal appearance, and the first evidence of this complication can be seen on MRI.13 MRI can be useful for illustrating infarction which manifests as oedema with increased T2 signal in the acute phase and as fibrotic and sclerotic pattern with low signal on all sequences in the chronic phase. The T2 weighted images, specifically, illustrate areas of increased signal intensity as a result of bone marrow oedema. A double line which is composed of a hypointense peripheral border and a hyperintense inner border is often visible on T2 weighted imaging (Figure 8). This consists of centrally increased T2 signal representing granulation tissues surrounded by dark lines representing the sclerotic bone.
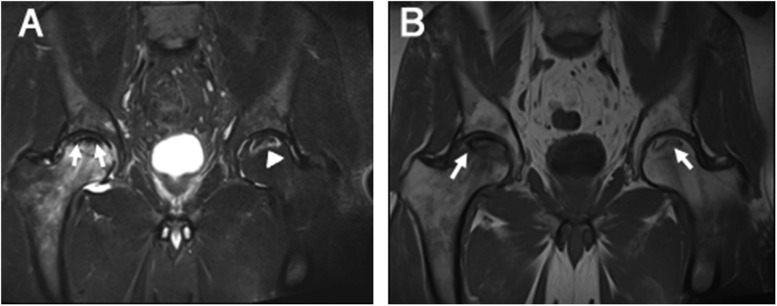
Figure 8.
Acute chronic osteonecrosis: a coronal T2 weighted MR image of the bilateral hips (a) demonstrating the classic double line sign (arrows) representing osteonecrosis of the right hip with superimposed marrow oedema of the femoral head representing acute chronic infarct. The high-signal intensity inner line represents the hypervascular granulation tissue. In the left femoral head, there is a high-signal intensity serpiginous rim delineating the area of chronic infarct (arrowhead) with no significant marrow oedema to suggest acute component. A coronal T1 weighted MR image of the bilateral hips (b) demonstrating serpiginous areas of low signal intensity in the bilateral femoral heads delineating the areas of infarct (arrows).
H-shaped vertebrae in the spine are another important manifestation of bone infarction in SCD. Vertebral bodies exhibit central endplate depression owing to chronic infarction. This deformity can be differentiated from the marrow hyperplasia by a specific step-like appearance of the endplates of the vertebrae.12 As a result of this endplate depression, vertebrae that are adjacent to the H-shaped vertebrae may exhibit lengthening to compensate for the change in shape and to support the spinal column. This deformity has been referred to as “tower” vertebrae (Figure 9).
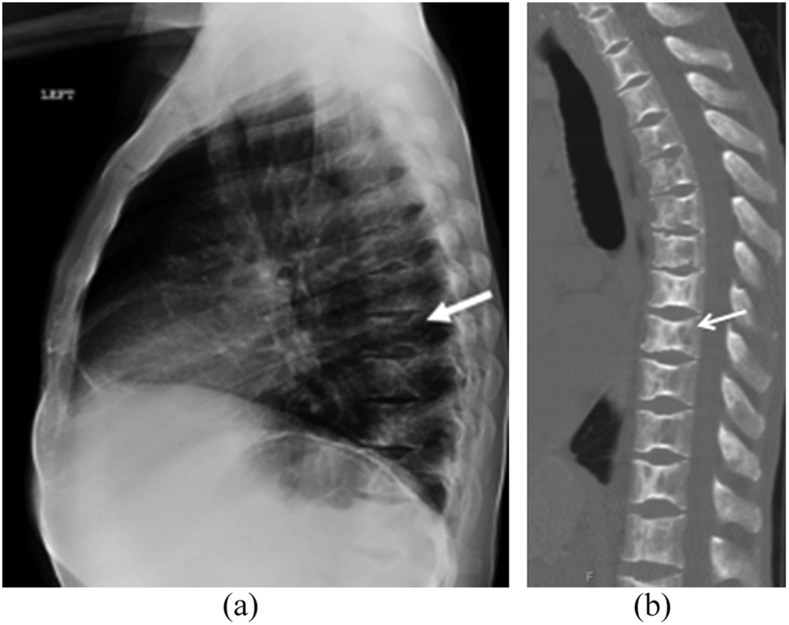
Figure 9.
A lateral radiograph of the thoracic spine (a) demonstrating sharply marginated depression of the superior and inferior endplates of multiple adjacent vertebral bodies representing changes of microvascular endplate necrosis (arrow). A sagittal image of the thoracic spine in the bone window (b) demonstrates coarse trabeculae with endplate depressions resulting in the typical “H”-shaped vertebrae of sickle cell disease.
Another important complication of ischaemia and infarction of the bone related to SCD is dactylitis, also known as “hand–foot” syndrome. This is defined as infarction of the bone that occurs in the diaphysis of the tubular bones in the hands and feet.14 This usually occurs at a young age because these regions still contain haematopoietic marrow.15 Children with SCD commonly experience dactylitis, and the clinical presentation of this complication can include fever, decrease in movement, as well as tenderness and swelling of the affected regions. Vasoconstriction induced by cold temperature can also lead to episodes of severe pain at the infarcted sites in the hands and feet of patients affected by SCD. Radiographically, dactylitis appears as lucency in a patchy distribution with periosteal reaction. On MRI, there is abnormal marrow enhancement of the involved digits with marrow and soft tissue oedema (Figure 10).
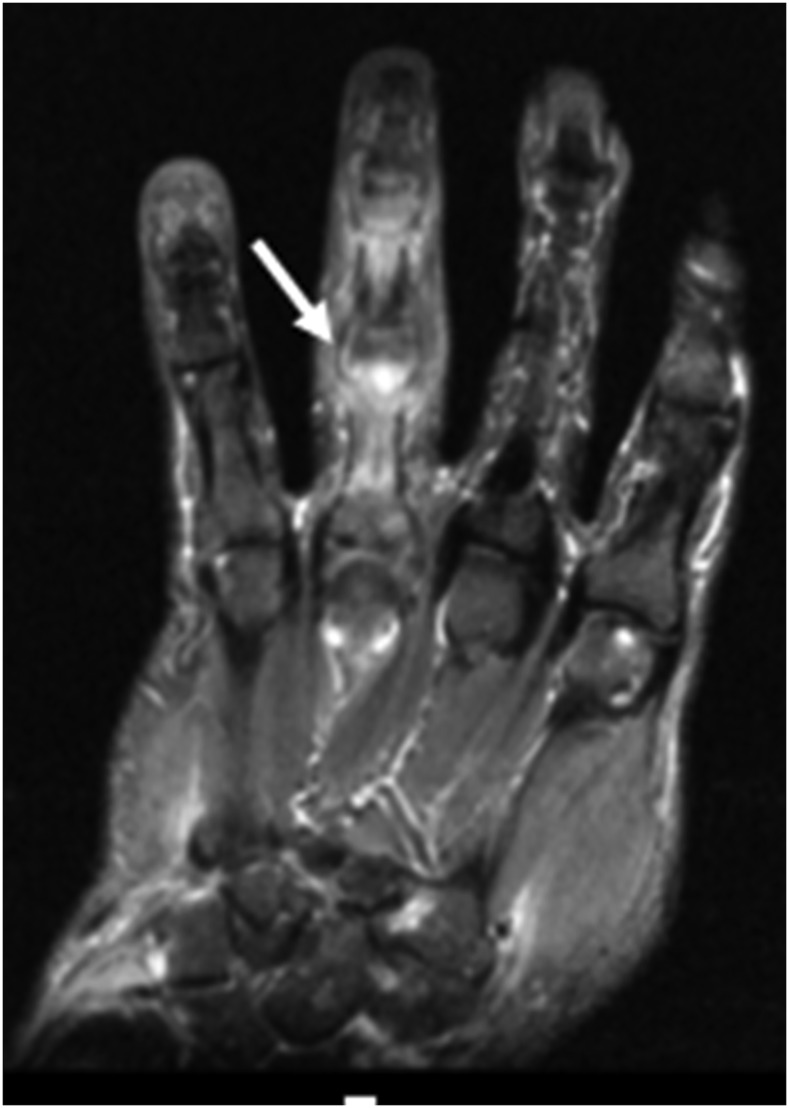
Figure 10.
A coronal T1 fat-saturated post-contrast image of the left hand demonstrating abnormal marrow enhancement and oedema involving the proximal and middle phalanges of the fourth digit. Adjacent soft tissue oedema (arrow) is also present.
The progression of osteonecrosis results in key changes that can be observed on imaging. Both sclerosis and lucency can be observed within the epiphysis, and afterwards subchondral lucencies which are crescent-shaped develop. Ultimately, during this natural progression of avascular necrosis, depression of the articular surface followed by collapse and fragmentation of the region occurs.16 This collapse can in turn result in degenerative changes of the corresponding joint. Late stages of avascular necrosis often require surgical management with joint replacement.
OSTEOMYELITIS
Infections of the bone and joint are serious and common complications caused by SCD. In one study, as many as 7% of patients with SCD were estimated to be diagnosed with septic arthritis, and 18% patients were diagnosed with osteomyelitis.17 Asplenia, impaired complement activity and phagocytosis are hypothesized to be important factors that predispose patients with SCD to infection of the bone and joints.18 In addition, infarcted areas of the medullary bone can be a good medium in which bacteria can grow, as reduced blood flow in these regions can result in locally impaired immunological response.
Infection in these cases usually occurs haematogenously, but it can also be the result of direct spread from surrounding skin ulcer. Sickling of RBCs within mesenteric vessels and the resulting infarction of parts of the gastrointestinal tract is thought to be the cause of bacteraemia involving Gram-negative organisms including salmonella.19 In cases of direct spread, usually from skin ulcers, pathogens which normally reside on the skin including anaerobic organisms and staph aureus are thought to be the cause of infection.
Osteomyelitis usually affects the diaphysis of the long bones, but other bones including vertebrae can be sites of infection. Clinical features of osteomyelitis including fever, pain, increased inflammatory markers in serum and swelling are similar to those of infarction and bone crises, thus introducing some difficulty in differentiating infection from infarction.10 Early detection of osteomyelitis is critical for prompt initiation of antibiotic therapy and to prevent surgical intervention.
Although plain films are often the initial method of evaluation for osteomyelitis, radiographic findings are often non-specific and lag histologic bone changes. These findings include soft tissue oedema, periostitis and regional osteopenia and may not be apparent until 7–14 days (Figure 11). Consequently, poor sensitivity for early detection and poor specificity of findings often in the setting of underlying chronic changes such as osteonecrosis and arthritis, and equivocal radiographic findings often prompt further evaluation with MRI.
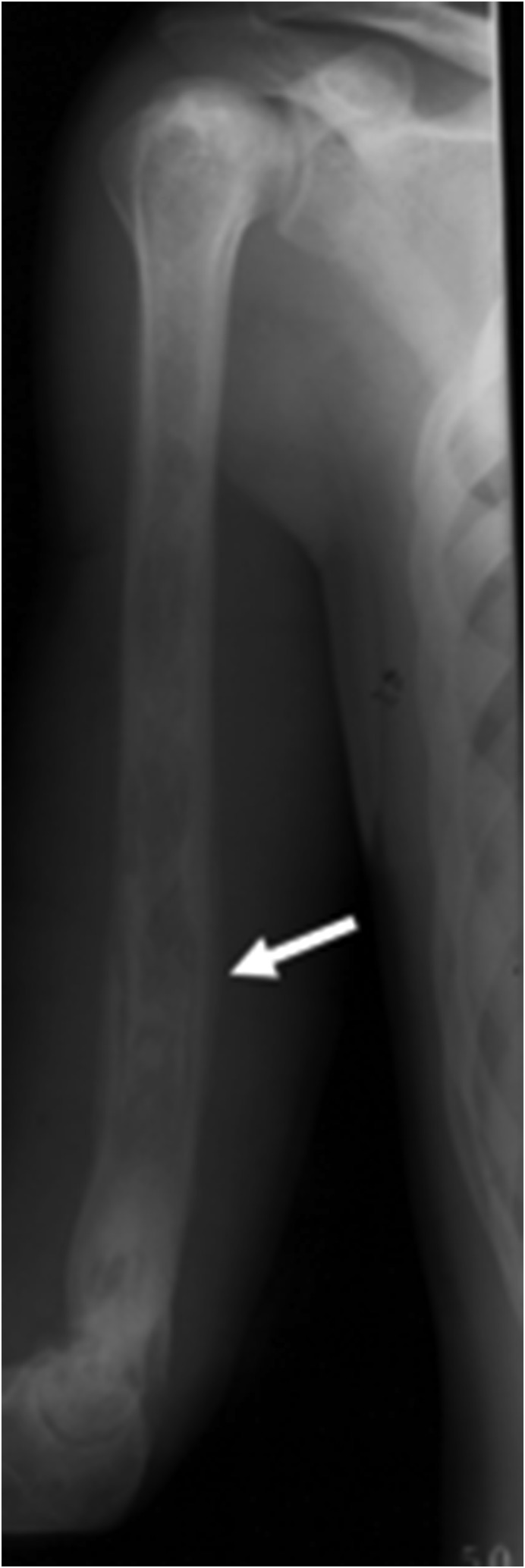
Figure 11.
An anteroposterior (AP) radiograph of the humerus demonstrates the permeative heterogeneous appearance of the humerus with endosteal scalloping and aggressive periosteal reaction (arrow).
MRI is the preferred method for evaluation, as it offers the best combination of sensitivity and specificity and allows early detection of osseous changes as well as additional soft tissue and joint findings.10 Primary MR findings of osteomyelitis include decreased marrow signal on T1 weighted images, increased signal on T2 weighted images and enhancement on post-contrast T1 weighted imaging (Figure 12). MRI is also useful for showing adjacent soft tissue fluid collections, cellulitis, cortical bone interruption sinus tracts and possible sequestra.20 In osteomyelitis, contrast-enhanced T1 weighted imaging can be used to illustrate peripheral enhancement in the bone marrow surrounding a centre that is non-enhancing.21 Both T2 weighted fat-saturated images and the contrast-enhanced T1 weighted images are important for discriminating between osteomyelitis and infarction of the bone.
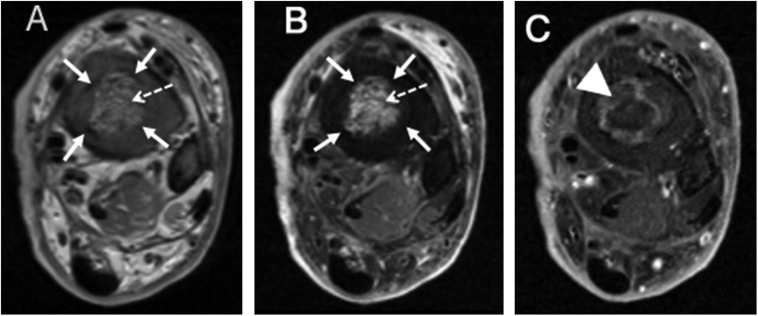
Figure 12.
Coronal T1 (a), coronal T1 post-contrast (b) and coronal short tau inversion-recovery (c) images of the femur demonstrating extensive marrow oedema with associated serpiginous and tubular marrow enhancement in the distal femoral metaphysis/diaphysis (solid arrows) with extensive associated periosteal reaction (arrowhead), adjacent soft tissue swelling and oedema (dashed arrows). These findings are characteristic of osteomyelitis.
While osteomyelitis is more common, septic arthritis is also an important complication of SCD. Septic arthritis is commonly seen in cases in which there is also vaso-occlusion and resulting infarction of the bone. Typical MR findings of septic arthritis include oedema in the perisynovial region and vivid enhancement of the synovium post-contrast.
MUSCLE AND SOFT TISSUE ABNORMALITIES
Vaso-occlusion involving the muscles, fascia and soft tissues can lead to complications such as myonecrosis, abscess formation and myositis. These complications, although less common and less reported than osseous counterparts, however, need to be recognized and treated promptly. MRI has excellent sensitivity for the identification of soft tissue and muscular changes.
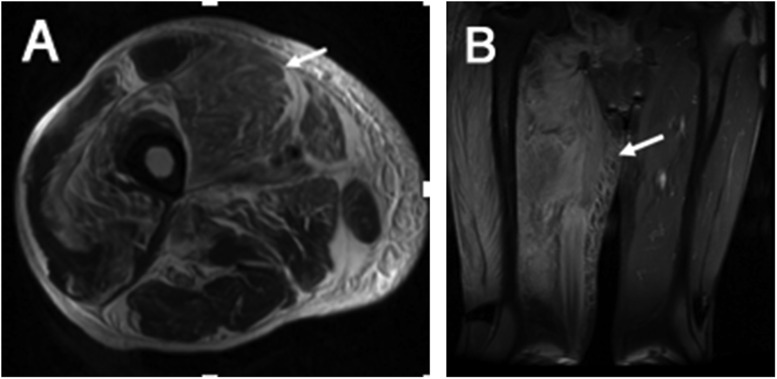
Typical MR findings include muscle oedema characterized by high signal on inversion-recovery and fat-suppressed T2 weighted images, abscesses with high T2/low T1 signal and myonecrosis characterized by asymmetric muscle enlargement with oedema and abnormal enhancement (Figure 13). Adjacent osseous involvement with necrosis and osteomyelitis may also be seen frequently.20
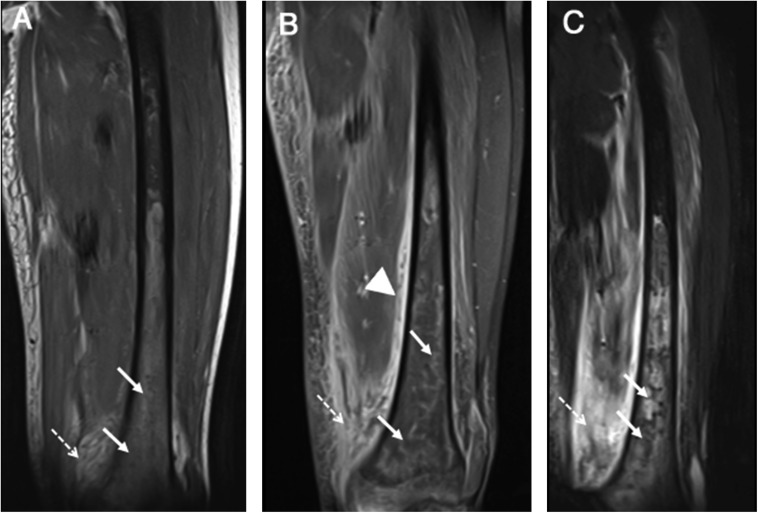
Figure 13.
Axial T2 (a) and coronal T2 (b) images of the right thigh: there is asymmetric enlargement and hyperintensity involving anterior compartment muscles (Vastus group) and to a lesser extent involving adductor and posterior compartment muscles (arrows). These changes are consistent with oedema and necrosis.
In these cases, sinus tracts which open onto the overlying skin and defects in the cortical bone that allow for communication between the marrow and the fluid collections are often noted.
CONCLUSION
Musculoskeletal complications are common causes of morbidity and mortality in SCD. Being familiar with the pathophysiology, clinical presentation and imaging manifestations of SCD is helpful for accurate diagnosis and prompt treatment.
Contributor Information
Vijaya Kosaraju, Email: vkk5@case.edu.
Alok Harwani, Email: axh332@case.edu.
Sasan Partovi, Email: sasan.partovi@uhhospitals.org.
Vasant Garg, Email: vasant.garg@uhhospitals.org.
Sabarish Ayyappan, Email: sabarish.ayyappan@uhhospitals.org.
Christos Kosmas, Email: christos.kosmas@uhhospitals.org.
Mark Robbin, Email: mark.robbin@uhhospitals.org.
REFERENCES
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997; 337: 762–9. doi: https://doi.org/10.1056/NEJM199709113371107 [DOI] [PubMed] [Google Scholar]
- 2.Frenette PS. Sickle cell vasoocclusion: heterotypic, multicellular aggregations driven by leukocyte adhesion. Microcirculation 2004; 11: 167–77. doi: https://doi.org/10.1080/mic.11.2.167.177 [PubMed] [Google Scholar]
- 3.Hebbel RP, Vercellotti G, Nath KA. A systems biology consideration of the vasculopathy of sickle cell anemia: the need for multi-modality chemo-prophylaxsis. Cardiovasc Hematol Disord Drug Targets 2009; 9: 271–92. doi: https://doi.org/10.2174/1871529x10909040271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. Semin Thromb Hemost 2011; 37: 226–36. doi: https://doi.org/10.1055/s-0031-1273087 [DOI] [PubMed] [Google Scholar]
- 5.Ginzel AW, Kransdorf MJ, Peterson JJ, Garner HW, Murphey MD. Mass-like extramedullary hematopoiesis: imaging features. Skeletal Radiol 2012; 41: 911–6. doi: https://doi.org/10.1007/s00256-011-1323-z [DOI] [PubMed] [Google Scholar]
- 6.Orphanidou-Vlachou E, Tziakouri-Shiakalli C, Georgiades CS. Extramedullary hemopoiesis. Semin Ultrasound CT MR 2014; 35: 255–62. doi: https://doi.org/10.1053/j.sult.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Claster S, Vichinsky EP. Managing sickle cell disease. BMJ 2003; 327: 1151–5. doi: https://doi.org/10.1136/bmj.327.7424.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collett-Solberg PF, Ware RE, O'Hara SM. Asymmetrical closure of epiphyses in a patient with sickle cell anemia. J Pediatr Endocrinol Metab 2002; 15: 1207–12. doi: https://doi.org/10.1515/jpem.2002.15.8.1207 [DOI] [PubMed] [Google Scholar]
- 9.Marlow TJ, Brunson CY, Jackson S, Schabel SI. “Tower vertebra”: a new observation in sickle cell disease. Skeletal Radiol 1998; 27: 195–8. doi: https://doi.org/10.1007/s002560050364 [DOI] [PubMed] [Google Scholar]
- 10.Lonergan GJ, Cline DB, Abbondanzo SL. Sickle cell anemia. Radiographics 2001; 21: 971–94. doi: https://doi.org/10.1148/radiographics.21.4.g01jl23971 [DOI] [PubMed] [Google Scholar]
- 11.Martinoli C, Bacigalupo L, Forni GL, Balocco M, Garlaschi G, Tagliafico A. Musculoskeletal manifestations of chronic anemias. Semin Musculoskelet Radiol 2011; 15: 269–80. doi: https://doi.org/10.1055/s-0031-1278426 [DOI] [PubMed] [Google Scholar]
- 12.Malizos KN, Siafakas MS, Fotiadis DI, Karachalios TS, Soucacos PN. An MRI-based semiautomated volumetric quantification of hip osteonecrosis. Skeletal Radiol 2001; 30: 686–93. doi: https://doi.org/10.1007/s002560100399 [DOI] [PubMed] [Google Scholar]
- 13.Calder JD, Hine AL, Pearse MF, Revell PA. The relationship between osteonecrosis of the proximal femur identified by MRI and lesions proven by histological examination. J Bone Joint Surg Br 2008; 90: 154–8. doi: https://doi.org/10.1302/0301-620X.90B2.19593 [DOI] [PubMed] [Google Scholar]
- 14.Babhulkar SS, Pande KC, Babhulkar SS. Clinical review of osteonecrosis of humeral head in sickle cell haemoglobinopathy (Study of 258 shoulders). J Shoulder Elbow Surg 1996; 5: S63. doi: https://doi.org/10.1016/s1058-2746(96)80300-4 [Google Scholar]
- 15.Resnick D, Kransdorf MJ. Hemoglobinopathies and Other Anemias. Bone and joint imaging: Philadelphia, PA: Elsevier BV; 2005. pp. 635–51. [Google Scholar]
- 16.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am 2006; 88: 2565–72. doi: https://doi.org/10.2106/JBJS.E.01455 [DOI] [PubMed] [Google Scholar]
- 17.Bahebeck J, Atangana R, Techa A, Monny-Lobe M, Sosso M, Hoffmeyer P. Relative rates and features of musculoskeletal complications in adult sicklers. Acta Orthop Belg 2004; 70: 107–11. [PubMed] [Google Scholar]
- 18.Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol 2005; 129: 482–90. doi: https://doi.org/10.1111/j.1365-2141.2005.05476.x [DOI] [PubMed] [Google Scholar]
- 19.Anand AJ, Glatt AE. Salmonella osteomyelitis and arthritis in sickle cell disease. Semin Arthritis Rheum 1994; 24: 211–21. doi: https://doi.org/10.1016/0049-0172(94)90076-0 [DOI] [PubMed] [Google Scholar]
- 20.Ganguly A, Boswell W, Aniq H. Musculoskeletal manifestations of sickle cell anaemia: a pictorial review. Anemia 2011; 2011: 794283. doi: https://doi.org/10.1155/2011/794283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umans H, Haramati N, Flusser G. The diagnostic role of gadolinium enhanced MRI in distinguishing between acute medullary bone infarct and osteomyelitis. Magn Reson Imaging 2000; 18: 255–62. doi: https://doi.org/10.1016/s0730-725x(99)00137-x [DOI] [PubMed] [Google Scholar]