Abstract
Objective:
To investigate the relationship between CT imaging findings and DPC4 gene expression and to determine the prognostic value of DPC4 gene expression to predict overall survival in patients with pancreatic ductal adenocarcinoma.
Methods:
Between January and December 2011, we retrospectively analyzed 163 pancreatic ductal adenocarcinomas in 163 patients who had undergone surgical resection (mean age = 61.8 years; range = 35–81 years). We divided the study patients into two groups according to DPC4 gene expression: DPC4-expression or DPC4-non-expression group. The CT findings were analyzed by two reviewers. The associations between the CT imaging findings and DPC4 gene expression were evaluated using univariate analysis and multivariate logistic regression analysis. Overall survival was compared according to the DPC4 gene expression (DPC4-expression vs DPC4-non-expression) using Kaplan–Meier analysis and log-rank testing. To avoid bias, subgroup analyses of CT findings in T3 tumour and overall survival in patients with T3 tumour and R0 resection were performed.
Results:
Between DPC4-expression group (n = 75) and DPC4-non-expression group (n = 88), three CT findings (i.e., tumour margin, peripancreatic infiltration, and the presence of background intraductal pancreatic mucinous neoplasm) were significantly different in univariate analysis. Of these, a well-defined tumour margin was significantly associated with DPC4-expression tumour (adjusted odds ratio = 2.06; p = 0.032) in multivariate analysis. Of the total 163 patients, the mean overall survival of the DPC4-expression group was significantly longer than that of the DPC4-non-expression group (30.0 vs 22.0 months; p = 0.049). Of the 150 T3 tumours, the presence of well-defined tumour margins was also a significant CT finding (adjusted odd ratio = 2.00; p = 0.044) in multivariate analysis. However, of 131 patients with T3 tumour and R0 resection, the overall survival period of the DPC4-expression group was not significantly different from that of the DPC4-non-expression group (24.0 vs 22.0 months; p = 0.240).
Conclusion:
The presence of well-defined tumour margins on CT was significantly linked with DPC4-expression tumour.
Advances in knowledge:
A well-defined tumour margin is an independent CT finding associated with DPC4-expression pancreatic ductal adenocarcinoma.
INTRODUCTION
Pancreatic ductal adenocarcinoma has a poor prognosis, typically showing aggressive local invasion and early metastasis.1,2 Although surgical resection is the known curative treatment for pancreatic cancer, the post-resection 5-year survival rate is reportedly only 9–21%.3 Adjuvant systemic chemotherapy and/or locoregional radiation therapy may provide survival benefits to some select patients; however, those therapies are generally highly toxic and may cause complications. Thus, administering tailored treatment strategies based on the patient's tumour biology may be helpful for managing patients with pancreatic ductal adenocarcinoma. Exploring the genetic and molecular biology of these tumours is an important starting point for planning such individualized treatment strategies.
Recent advances in pancreatic ductal adenocarcinoma biology have led to the discovery of recurrent genetic mutations in K-ras, p53 and DPC4 and the identification of the core signalling pathways for this disease. Several studies have attempted to correlate the genetic alterations with the clinical features of this cancer,3–9 which reportedly show that the loss of DPC4 (a tumour suppressor gene) and K-ras mutation (an oncogene) predict overall survival.3 Notably, Iacobuzio-Donahue et al9 recently reported that DPC4 gene expression can be a good predictive biomarker, in that the DPC4 non-expression is highly associated with widespread metastasis, whereas DPC4 expression is associated with locally destructive tumours. Therefore, the DPC4 gene expression may be an important factor for stratifying pancreatic ductal adenocarcinoma patients to treatment strategies such as systemic chemotherapy or local control. However, the Iacobuzio-Donahue et al9 study was conducted based on post-mortem analysis rather than in the clinic. In clinical practice, imaging studies such as CT or MRI are the best methods for evaluating tumour growth patterns. So far, there have been no studies on the association between DPC4 gene expression and the tumour growth patterns of pancreatic ductal adenocarcinoma on imaging modalities.
Based on the results of prior studies,3,8,9 we hypothesized that imaging analysis in clinical practice would show different tumour growth patterns according to the DPC4 gene expression and that DPC4 gene expression would predict overall survival in a pancreatic ductal adenocarcinoma cohort. To test our hypothesis, we conducted our present study using a retrospective cohort at a single institution.
METHODS AND MATERIALS
Study population
Asan Medical Center institutional review board approved this retrospective study and waived the requirement for informed consent. We performed a systematic computerized search of Asan Medical Center's database for the terms “pancreas ductal adenocarcinoma” (as the pathologic diagnosis) and “pancreatectomy” (as the procedure code). The patient selection process is presented in Figure 1. Using this search strategy, we identified 202 consecutive patients with pancreatic adenocarcinoma who underwent surgical resection at our hospital between January and December 2011. The inclusion criteria were as follows: (a) histopathological diagnosis of pancreatic ductal adenocarcinoma; (b) pre-operative CT; (c) surgery at our institution within 1 month of CT; and (d) the presence of visible tumours on CT. Among these 202 patients, we excluded 36 cases with the ductal adenocarcinoma variants or mixed neoplasms of the pancreas, 1 patient who did not undergo pre-operative CT within 1 month and 2 patients with an invisible pancreatic mass on CT. Therefore, 163 patients with pancreatic ductal adenocarcinoma were included in this study.
Figure 1.
Diagram for selecting the study population.
Review of the medical records
The medical records of the study patients were reviewed to identify each patient's age, sex, initial presentation, surgical history, tumour location, tumour stage, tumour grade and residual tumour. The initial presentation was categorized as “symptomatic” or “incidental”. Tumours were classified as symptomatic if the patient presented with the signs and symptoms related to the tumour, e.g. abdominal pain or jaundice etc. All other tumours were categorized as incidental. The location of the tumour was categorized as the pancreas head or body/tail based on the pathological reports. The stage of the tumour and presence of a residual tumour (R0, negative resection margins; R1, microscopically positive margins; and R2, macroscopically positive margins) were recorded according to the TNM staging criteria of the American Joint Committee on Cancer.10
Immunohistochemical staining for DPC4
Immunohistochemical staining was performed at the immunohistochemical laboratory of the Department of Pathology, Asan Medical Center. Whole, representative cancer sections from the available paraffin blocks were labelled with DPC4. Briefly, 4-µm-thick tissue sections were deparaffinized and hydrated in xylene and then in serially diluted ethanol solutions, respectively. Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide for 10 min, and then heat-induced antigen retrieval was performed. The primary antibody for DPC4/SMAD4 (SC7966, clone B-8, mouse monoclonal, 1 : 500 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX) was used to stain the section using the Ventana Autostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer's protocol. The primary antibody was incubated for 32 min at room temperature, and the sections were counterstained with haematoxylin, dehydrated in ethanol and cleared in xylene. The DPC4-labelled area was scored as 0–3 if <10%, 10–33%, 34–66% or >67% of the area was stained, respectively; a score of 0 indicated total DPC4 non-expression, whereas Scores 1–3 were considered to indicate DPC4-expression labelling.11,12
Image analysis
All CT examinations were performed using a Sensation 16 (Siemens Medical Systems, Erlangen, Germany), Somatom® Definition scanner (Siemens Medical Systems), LightSpeed® 16 (GE Healthcare, Milwaukee, WI) or LightSpeed VCT® scanner (GE Healthcare). Non-enhanced, arterial and portal venous-phase images were obtained in 150 patients. Non-enhanced and portal venous-phase images were obtained in 13 patients. For contrast-enhanced CT, 100–120 ml of iopromide (Ultravist® 370 or Ultravist 300; Bayer Schering Pharma, Berlin, Germany) was intravenously administered at a rate of 3 ml s−1 using an automatic power injector. The scan parameters, reconstruction thickness and the delay time for the arterial phase of each CT scanner are summarized in Table 1.
Table 1.
Scan parameters, reconstruction thickness and delay time for the arterial-phase images obtained using each CT scanner
| Variable | Somatom® Sensation 16 (Siemens Medical Systems, Erlangen, Germany) | Somatom Definition (Siemens Medical Systems) | LightSpeed® 16 (GE Healthcare, Milwaukee, WI) | LightSpeed VCT® (GE Healthcare) |
|---|---|---|---|---|
| Beam collimation (mm) | 16 × 0.75 | 64 × 0.6 | 16 × 1.25 | 64 × 0.625 |
| Beam pitch | 1 | 1 | 0.984 | 0.984 |
| Gantry rotation time (s) | 0.5 | 0.5 | 0.5 | 0.5 |
| kV/mAsa | 120/200 | 120/200 | 120/200 | 120/200 |
| Reconstruction thickness | ||||
| Axial pre/arterial/portal (mm) | 5/3/3 | 5/3/3 | 5/2.5/2.5 | 5/2.5/2.5 |
| Coronal arterial/portal (mm) | 5/5 | 5/5 | 5/5 | 5/5 |
| Delay time for the arterial phase (s)b | 10 | 15 | 10 | 15 |
Automated dose modulation using the maximum allowable tube current.
After attenuation of the aorta at the thoracolumbar junction had reached 100 HU.
The qualitative CT findings were reviewed by the consensus of two radiologists (14 and 7 years of experience in abdominal radiology, respectively). These reviewers knew that the patients in the study population had pancreatic ductal adenocarcinoma, but they were blinded to the initial presentations, operative findings, pathological reports, DPC4 gene expression and radiological reports. The reviewers evaluated tumour size, tumour location, homogeneity, tumour margins, intratumoural calcification, organ invasion, pancreatic duct dilatation, upstream pancreatic atrophy, bile duct dilatation, arterial invasion, venous invasion, peripancreatic infiltration, the presence of intraductal pancreatic mucinous neoplasm (IPMN) in the background parenchyma, lymph node enlargement and tumour density on the arterial and portal venous phases.
The longest diameter of the tumour on the axial images was measured by a radiologist. The tumour location was categorized as either the pancreas head or body/tail. A tumour located to the right of the left edge of the portal–superior mesenteric vein confluence was considered to be a tumour on the pancreas head, and a tumour located to the left of the left edge of the portal–superior mesenteric vein confluence was considered a tumour on the pancreas body/tail.13 The homogeneity of the tumour was categorized as homogeneous or heterogeneous. A tumour with mixed-density necrosis or haemorrhage in >70% of the lesion was considered heterogeneous; otherwise, the tumour was considered homogeneous. The tumour margins were categorized as well or ill defined. In order to evaluate the tumour margin, we used the axial image that showed the longest diameter of the tumour. On this axial image, we assessed for the presence of spiculation/infiltration at the tumour margin. If the spiculated or infiltrative involvement of tumour margins was >70% (252°/360°), the tumour was considered as ill defined; otherwise, the tumour was considered well defined. The tumour margins were evaluated in only 150 of the pancreatic ductal adenocarcinomas in our series because we could not evaluate the margins in 13 adenocarcinomas that arose from IPMNs in the background parenchyma. A tumour arising from an IPMN in the background parenchyma was determined when a soft-tissue-enhancing mass was present in the background pancreatic parenchyma and demonstrated a diffuse pattern of pancreatic ductal dilatation or a segmented cystic appearance.14,15 The presence of calcification within the tumour was evaluated on non-enhanced CT. We considered intratumoural calcification when high attenuating foci in the tumour (visually opaque as bone or >200 HU) was noted.
Pancreatic duct dilatation was defined as the main pancreatic duct that measured ≥4 mm in diameter. Upstream atrophy was defined as the presence of decreased pancreatic parenchymal volume distal to the tumour. Bile duct dilatation was defined as the dilatation of both the extrahepatic bile duct (>8 mm) and intrahepatic bile duct (>2 mm). Peripancreatic infiltration was defined as peritumoural fatty stranding, and adjacent organ invasion was defined as encasement or infiltration into the adjacent organs such as the duodenum, stomach, kidneys or spleen.16 To evaluate vascular invasion, we used the following criteria: tumour thrombus, vessel occlusion, stenosis, contour deformity and more than half of the perimeter in contact with the tumour.17 We evaluated all possible adjacent vessels, including the celiac trunk, common hepatic artery, superior mesenteric artery, gastroduodenal artery, pancreaticoduodenal artery, splenic artery, portal vein, superior mesenteric vein, splenic vein, gastroepiploic vein and gastrocolic trunk, because our aim was to evaluate the tumour characteristics and not surgical resectability. Lymph node enlargement was defined by a short axis measuring >1 cm, abnormal round morphology or central necrosis.13
The pancreatic tumours were compared in terms of density within the pancreatic parenchyma on visual assessment and classified as hypodense, isodense or hyperdense on arterial-phase and portal venous-phase images. Also, the arterial and portal enhancement ratio was determined using the Hounsfield unit (HU) values on the contrast-enhanced arterial-phase and portal venous-phase images by manually drawing a region of interest within the tumour and the downstream parenchyma. If there was insufficient pancreatic parenchyma downstream of the tumour, the region of interest was placed on the pancreatic parenchyma upstream of the tumour.18 The arterial enhancement ratio was defined as the HU value of the tumour divided by the HU value of the pancreatic parenchyma as measured on arterial-phase imaging. The portal enhancement ratio was defined as the HU value of the tumour divided by the HU value of the pancreatic parenchyma as measured on the portal-phase images. Because arterial-phase images were only available for 150 patients, the arterial-phase density and arterial enhancement ratios were evaluated in these cases.
Statistical analysis
To determine any relationship between the demographic and clinical characteristics and DPC4 gene expression, the demographic and clinical characteristics of the DPC4-expression patients and DPC4-non-expression patients were compared using the Fisher exact or χ2 tests (for categorical variables) or the Student's t-test (for continuous variables). The associations between DPC4 gene expression and the CT findings of the pancreatic ductal adenocarcinomas were analyzed using univariate analysis with the Fisher exact or χ2 tests (for categorical variables) or the Student's t-test (for continuous variables). Subsequently, among the CT findings that demonstrated potential significance on univariate analysis, multivariate logistic regression analysis was performed to determine any independently significant CT findings that predict DPC4-expression tumour.
To evaluate the relationship between overall patient survival and the DPC4 gene expression, the overall survival rates at 1, 2 and 3 years were analyzed and compared between DPC4-expression and DPC4-non-expression patients using the Fisher exact test. In addition, we evaluated the relationship between overall patient survival and the independent CT finding for predicting DPC4-expression tumour. According to the DPC4 gene expression and the presence of independent CT finding, the survival curves were drawn using the Kaplan–Meier method, and univariate comparisons were performed using the log-rank test. Overall survival was calculated from the date of pancreatic surgery to the date of death. The last date of data collection was 15 April 2015.
To minimize bias, we performed subgroup analysis of CT findings in T3 tumours using univariate analysis and multivariate logistic regression analysis. We calculated the sensitivity, specificity, positive-predictive value and negative-predictive value of this significant CT finding for predicting DPC4-expression tumour. In addition, subgroup analysis of overall survival in patients with T3 tumours and R0 resection was performed. These statistical analyses were performed using SPSS® v. 21.0 statistical software (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). In this study, p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The demographic and pathologic characteristics of the 163 included patients are summarized in Table 2. There were 92 males (mean age = 61.7 years; range = 41–81 years) and 71 females (mean = 62.0 years; range = 35–78 years), with a mean age of 61.8 years (range = 35–81 years). Most tumours were symptomatically detected (68.1%; 111 of 163 patients) and diagnosed as Stage II (93.3%; 152 of 163 patients). Among the 163 patients with pancreatic ductal adenocarcinoma, 75 patients (46.0%) demonstrated DPC4 expression and 88 patients (54.0%) demonstrated DPC4 non-expression on the immunohistochemical analysis. There were no significant differences in any basic demographic/clinical characteristics between the DPC4-expression and DPC4-non-expression groups (p ≥ 0.05).
Table 2.
Baseline demographic and pathological tumour characteristics of the 163 patients with primary pancreatic ductal adenocarcinoma
| Variable | DPC4-expression (n = 75) | DPC4-non-expression (n = 88) | p-value |
|---|---|---|---|
| Age (years) | 61.9 ± 9.5 | 61.8 ± 9.1 | 0.941 |
| Sex (M : F) | 43 : 32 | 49 : 39 | 0.832 |
| Tumour location | |||
| Head | 48 (64.0%) | 64 (72.7%) | 0.231 |
| Body/tail | 27 (36.0%) | 24 (27.3%) | |
| Initial presentation | |||
| Symptomatica | 48 (64.0%)a | 63 (71.6%)a | 0.300 |
| Incidental | 27 (36.0%) | 25 (28.4%) | |
| Type of operation | |||
| Total pancreatectomy | 11 (14.7%) | 13 (14.8%) | 0.985 |
| Pancreaticoduodenectomy | 46 (61.3%) | 51 (58.0%) | 0.661 |
| Distal pancreatectomy | 18 (24.0%) | 24 (27.3%) | 0.634 |
| Tumour grade | |||
| Grade 1 | 11 (14.7%) | 9 (10.2%) | 0.569 |
| Grade 2 | 50 (66.7%) | 65 (73.9%) | |
| Grade 3 | 14 (18.7%) | 14 (15.9%) | |
| T stage | |||
| T1 | 5 (6.7%) | 3 (3.4%) | 0.179 |
| T2 | 4 (5.3%) | 1 (1.1%) | |
| T3 | 66 (88.0%) | 84 (95.5%) | |
| Tumour stage (TNM) | |||
| I | 8 (10.7%) | 3 (3.4%) | 0.066 |
| II | 67 (89.3%) | 85 (96.6%) | |
| Residual tumour | |||
| R0 | 67 (89.3%) | 75 (85.2%) | 0.435 |
| R1 | 8 (10.7%) | 13 (14.8%) | |
| R2 | 0 (0.0%) | 0 (0.0%) | |
F, female; M, male.
Data are represented as mean ± standard deviation or number (percentage).
111 patients with initial symptoms, including 74 patients with pain, 30 patients with jaundice, 5 patients with indigestion and 2 patients with nausea and vomiting.
CT findings associated with DPC4 gene expression in overall subjects
Of the 163 tumours, there were 8 T1 tumours, 5 T2 tumours and 150 T3 tumours. There was no T4 tumour. The univariate analyses showed that three CT findings—tumour margin, background IPMN and peripancreatic infiltration—were significantly different between the DPC4-expression patients and DPC4-non-expression patients (Table 3). Notably, DPC4-expression tumours tended to be well defined (55.4%) in comparison with DPC4-non-expression tumours (37.6%) (p = 0.031 according to the Fisher exact test) (Figures 2 and 3). The presence of the background IPMN was more frequently observed in DPC4-expression tumours than DPC4-non-expression tumours (13.3% vs 3.4%; p = 0.020). Peripancreatic infiltration was observed more frequently in DPC4-non-expression tumours than DPC4-expression tumours (94.3% vs 81.3%; p = 0.010).
Table 3.
Univariate analysis of the CT features and DPC4 gene expression in the total 163 patients with pancreatic ductal adenocarcinoma
| Variable | DPC4-expression (n = 75) | DPC4-non-expression (n = 88) | p-value | |
|---|---|---|---|---|
| Tumour size (mm) | Longest axial diameter | 29.0 ± 12.2 | 28.4 ± 9.9 | 0.738 |
| Tumour location | Head | 48 (64.0%) | 64 (72.7%) | 0.231 |
| Body/tail | 27 (36.0%) | 24 (27.3%) | ||
| Tumour homogeneity | Homogeneous | 13 (17.3%) | 11 (12.5%) | 0.385 |
| Heterogeneous | 62 (82.7%) | 77 (87.5%) | ||
| Tumour marginsa | Well defined | 36 (55.4%) | 32 (37.6%) | 0.031 |
| Ill defined | 29 (44.6%) | 53 (62.4%) | ||
| IPMN background | Presence | 10 (13.3%) | 3 (3.4%) | 0.020 |
| Absence | 65 (86.7%) | 85 (96.6%) | ||
| Intratumoural calcification | Presence | 3 (4.0%) | 1 (1.1%) | 0.239 |
| Absence | 72 (96.0%) | 87 (98.9%) | ||
| Pancreatic duct dilatation | Presence | 54 (72.0%) | 68 (77.3%) | 0.439 |
| Absence | 21 (28.0%) | 20 (22.7%) | ||
| Upstream atrophy | Presence | 19 (25.3%) | 26 (29.5%) | 0.549 |
| Absence | 56 (74.7%) | 62 (70.5%) | ||
| Bile duct dilatation | Presence | 36 (48.0%) | 47 (53.4%) | 0.491 |
| Absence | 39 (52.0%) | 41 (46.6%) | ||
| Peripancreatic infiltration | Presence | 61 (81.3%) | 83 (94.3%) | 0.010 |
| Absence | 14 (18.7%) | 5 (5.7%) | ||
| Organ invasion | Presence | 15 (20.0%) | 9 (10.2%) | 0.079 |
| Absence | 60 (80.0%) | 79 (89.8%) | ||
| Artery invasion | Presence | 23 (30.7%) | 35 (39.8%) | 0.226 |
| Absence | 52 (69.3%) | 53 (60.2%) | ||
| Vein invasion | Presence | 23 (30.7%) | 32 (36.4%) | 0.443 |
| Absence | 52 (69.3%) | 56 (63.6%) | ||
| Lymph node enlargements | Presence | 39 (52.0%) | 40 (45.5%) | 0.405 |
| Absence | 36 (48.0%) | 48 (54.5%) | ||
| Arterial-phase densityb | Hypodensity | 69 (95.8%) | 74 (94.9%) | 0.780 |
| Isodensity | 3 (4.2%) | 4 (5.1%) | ||
| Hyperdensity | 0 (0.0%) | 0 (0.0%) | ||
| Portal-phase density | Hypodensity | 69 (94.5%) | 82 (93.2%) | 0.726 |
| Isodensity | 4 (5.5%) | 6 (6.8%) | ||
| Hyperdensity | 0 (0.0%) | 0 (0.0%) | ||
| Arterial enhancement ratio (%)b | 0.60 ± 0.16 | 0.56 ± 0.16 | 0.225 | |
| Portal enhancement ratio (%) | 0.64 ± 0.17 | 0.65 ± 0.17 | 0.715 | |
IPMN, intraductal papillary mucinous neoplasm.
Tumour margin was evaluated in 150 pancreatic ductal adenocarcinomas because 13 adenocarcinomas with an IPMN in the background parenchyma could not be evaluated.
Arterial-phase density was assessed in 150 pancreatic ductal adenocarcinomas due to the unavailability of arterial-phase images in 13 adenocarcinomas. The arterial enhancement ratio and arterial/portal enhancement ratio were calculated in 150 pancreatic ductal adenocarcinomas.
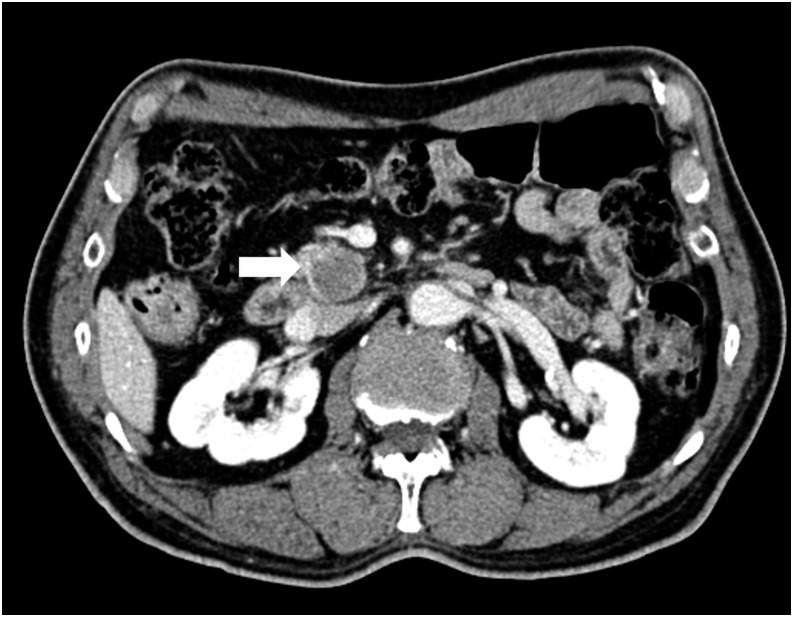
Figure 2.
A 75-year-old male with DPC4-expression pancreatic ductal adenocarcinoma. The transverse portal venous-phase CT scan showed a 4.1-cm, well-defined, hypodense tumour (arrow) in the pancreatic head. Pylorus-preserving pancreaticoduodenectomy was performed. The patient was alive throughout the 50-month follow-up period.
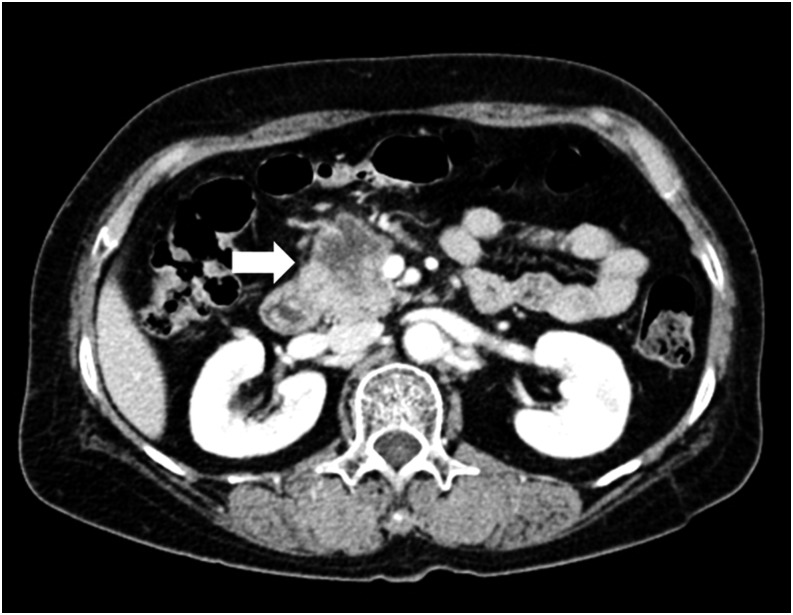
Figure 3.
A 55-year-old female with DPC4-non-expression pancreatic ductal adenocarcinoma. The transverse portal venous-phase CT scan showed a 3.6-cm, ill-defined, hypodense tumour (arrow) in the pancreatic head. The Whipple procedure was performed. This patient died 4 months after surgery.
According to the multivariate logistic regression analysis using backward elimination with tumour margins, peripancreatic infiltration and IPMN background as covariates, the presence of well-defined tumour margins was the single, independently significant CT finding for predicting a DPC4-expression tumour (adjusted odds ratio = 2.06; 95% confidence interval = 1.07–3.97; p = 0.032). The other covariates were eliminated from the multivariate model.
Relationship between the DPC4 gene expression and overall survival in overall subjects
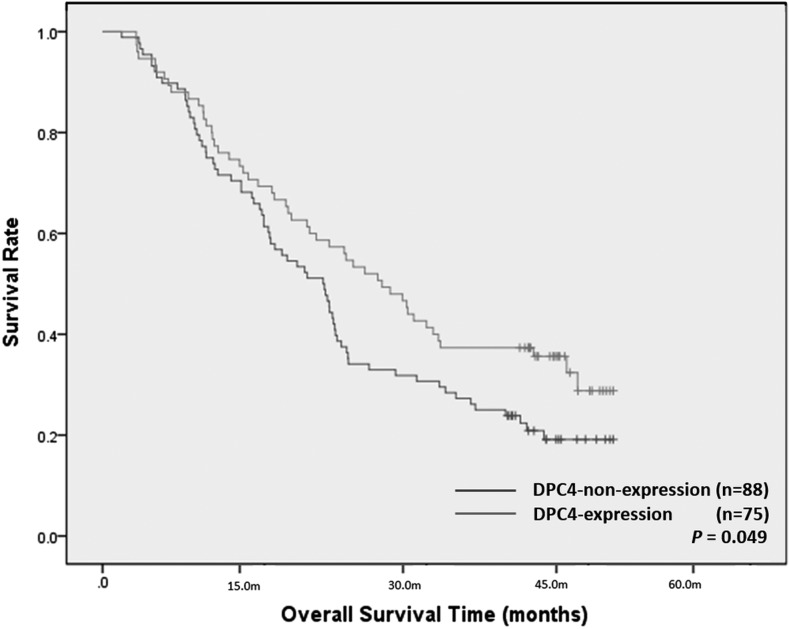
The median follow-up period for all 163 study patients was 24.0 months (95% confidential interval = 20.3–27.7 months). DPC4-non-expression significantly affected overall survival (p = 0.049; Figure 4). The median overall survival period of the DPC4-expression group was 30.0 months and that of the DPC4-non-expression group was 22.0 months. The estimated 1-, 2- and 3-year overall survival rates of the DPC4-expression group (76.0%, 57.3%, and 37.3%, respectively) were better than those of the DPC4-non-expression group (71.6%, 38.6%, 27.3%, respectively). However, the p-values for the differences (p = 0.449, 0.001, and 0.058, respectively) were only statistically significant for the 2-year survival rate. Based on the tumour margin, the median overall survival period of the well-defined tumour group was not significantly different from that of the ill-defined tumour group (27.0 vs 22.0 months; p = 0.070).
Figure 4.
Overall survival times after surgical resection in the 163 patients with pancreatic ductal adenocarcinomas. The Kaplan–Meier curves for overall survival based on DPC4 gene expression demonstrated a higher overall survival rate in the DPC4-expression group (n = 75) than in the DPC4-non-expression group (n = 88) (p = 0.049).
Subgroup analysis of CT findings in T3 tumours and overall survival in patients with T3 tumour and R0 resection
Of the 150 T3 tumours, there were 66 DPC4-expression tumours (44.0%) and 84 DPC4-non-expression tumours (56.0%). DPC4-expression tumours showed well-defined tumour margin (54.1% vs 37.0%; p = 0.043) more frequently than DPC4-non-expression tumours, and DPC4-non-expression tumour showed peripancreatic infiltration (97.6% vs 87.9%; p = 0.018) more frequently than DPC4-expression tumour. The other CT findings did not show any significant difference between the two groups (Table 4). According to the multivariate logistic regression analysis, the presence of well-defined tumour margins was the independently significant CT finding for predicting a DPC4-expression tumour (adjusted odds ratio = 2.00; 95% confidence interval = 1.02–3.94; p = 0.044). Using this CT finding, the sensitivity, specificity, positive-predictive value and negative-predictive value for predicting DPC4-expression tumour were 54.1%, 63.0%, 52.4% and 64.6%, respectively.
Table 4.
Univariate analysis of the CT features and DPC4 gene expression in 150 patients with T3 tumours
| Variable | DPC4-expression (n = 66) | DPC4-non-expression (n = 84) | p-value | |
|---|---|---|---|---|
| Tumour size (mm) | Longest axial diameter | 29.7 ± 11.8 | 28.9 ± 9.8 | 0.985 |
| Tumour location | Head | 41 (62.1%) | 61 (72.6%) | 0.217 |
| Body/tail | 25 (37.9%) | 23 (27.4%) | ||
| Tumour homogeneity | Homogeneous | 13 (19.7%) | 11 (13.1%) | 0.274 |
| Heterogeneous | 53 (80.3%) | 73 (86.9%) | ||
| Tumour marginsa | Well defined | 33 (54.1%) | 30 (37.0%) | 0.043 |
| Ill defined | 28 (45.9%) | 51 (63.0%) | ||
| IPMN background | Presence | 5 (7.6%) | 3 (3.6%) | 0.279 |
| Absence | 61 (92.4%) | 81 (96.4%) | ||
| Intratumoural calcification | Presence | 3 (4.5%) | 1 (1.2%) | 0.206 |
| Absence | 63 (95.5%) | 83 (98.8%) | ||
| Pancreatic duct dilatation | Presence | 49 (74.2%) | 66 (78.6%) | 0.534 |
| Absence | 17 (25.8%) | 18 (21.4%) | ||
| Upstream atrophy | Presence | 17 (25.8%) | 25 (29.8%) | 0.588 |
| Absence | 49 (74.2%) | 59 (70.2%) | ||
| Bile duct dilatation | Presence | 34 (51.5%) | 44 (52.4%) | 0.916 |
| Absence | 32 (48.5%) | 40 (47.6%) | ||
| Peripancreatic infiltration | Presence | 58 (87.9%) | 82 (97.6%) | 0.018 |
| Absence | 8 (12.1%) | 2 (2.4%) | ||
| Organ invasion | Presence | 14 (21.2%) | 9 (10.7%) | 0.077 |
| Absence | 52 (78.8%) | 75 (89.3%) | ||
| Artery invasion | Presence | 23 (34.8%) | 35 (41.7%) | 0.385 |
| Absence | 43 (65.2%) | 49 (58.3%) | ||
| Vein invasion | Presence | 23 (34.8%) | 32 (38.1%) | 0.682 |
| Absence | 43 (65.2%) | 52 (61.9%) | ||
| Lymph node enlargements | Presence | 36 (54.5%) | 39 (46.4%) | 0.324 |
| Absence | 30 (45.5%) | 45 (53.6%) | ||
| Arterial-phase densityb | Hypodensity | 62 (95.4%) | 70 (94.6%) | 0.832 |
| Isodensity | 3 (4.6%) | 4 (5.4%) | ||
| Hyperdensity | 0 (0.0%) | 0 (0.0%) | ||
| Portal-phase density | Hypodensity | 62 (93.9%) | 79 (94.0%) | 0.978 |
| Isodensity | 4 (6.1%) | 5 (6.0%) | ||
| Hyperdensity | 0 (0.0%) | 0 (0.0%) | ||
| Arterial enhancement ratio (%)b | 0.60 ± 0.16 | 0.56 ± 0.16 | 0.224 | |
| Portal enhancement ratio (%) | 0.65 ± 0.18 | 0.65 ± 0.17 | 0.979 | |
IPMN, intraductal papillary mucinous neoplasm.
Tumour margin was evaluated in 142 pancreatic ductal adenocarcinomas because 8 adenocarcinomas with an IPMN in the background parenchyma could not be evaluated.
Arterial-phase density was assessed in 139 pancreatic ductal adenocarcinomas due to the unavailability of arterial-phase images in 11 adenocarcinomas. The arterial enhancement ratio and arterial/portal enhancement ratio were calculated in 139 pancreatic ductal adenocarcinomas.
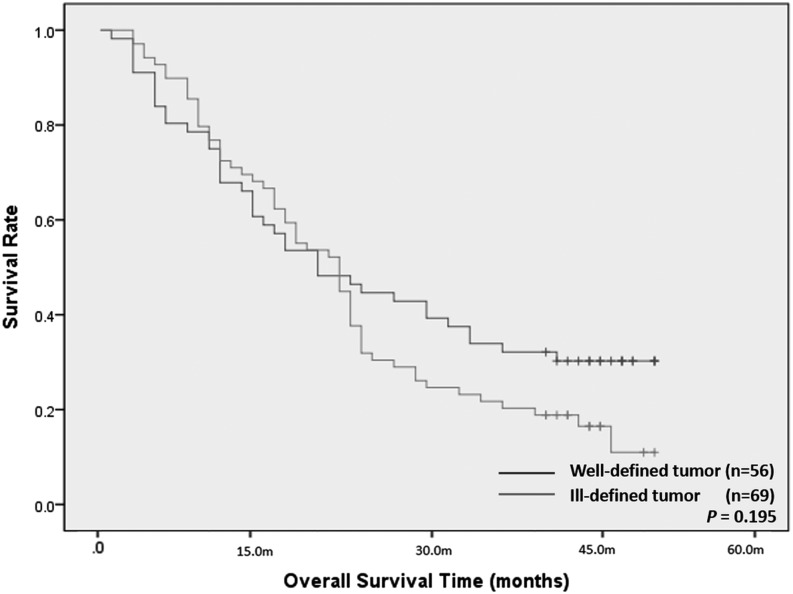
Regarding the relationship between DPC4 gene expression and overall survival, of the 131 patients with T3 tumour and R0 resection patients, the overall survival period of the DPC4-expression group was not significantly different from that of the DPC4-non-expression group (24.0 vs 22.0 months; p = 0.240). Based on the tumour margin, of the 125 patients with T3 tumour and R0 resection patients who were available for evaluation of the tumour margin, the median overall survival period of the well-defined tumour group was 20.0 months and that of the ill-defined tumour group was 22.0 months. There was no significant difference in overall survival period between the two groups (p = 0.195; Figure 5).
Figure 5.
Overall survival times after surgical resection in the 125 patients with T3 tumour and R0 resection who were available for evaluation of the tumour margin. There was no significant difference in overall patient survival between the well-defined tumour group (n = 56) and the ill-defined tumour group (n = 69) (p = 0.195).
DISCUSSION
As expected, our current study results showed that the well-defined tumour margins on CT were independently and significantly associated with the DPC4-expression tumour in both overall subjects (adjusted odds ratio = 2.06; p = 0.032) and T3 tumour subgroup (adjusted odds ratio = 2.00; p = 0.044). Although DPC4 non-expression significantly affected overall survival in the total 163 patients (p = 0.049), of the 131 patients with T3 tumour and R0 resection, the overall survival period of the DPC4-expression group was not significantly different from that of the DPC4-non-expression group (24.0 vs 22.0 months; p = 0.240).
In pancreatic ductal adenocarcinoma, the DPC4 gene is one of the most important tumour-suppressor genes,3,19 as it demonstrates a 55–66% inactivation rate.20,21 In our current study, the frequency of DPC4 gene non-expression was 54.0%, which is similar to the values reported in previous studies.3,20,21 According to the results of recent studies, DPC4-expression tumours tend to manifest as local disease rather than wide-spread disease.3,9,22 Our present study demonstrated that DPC4-expression tumours were more likely to be well defined and showed, less frequently, peripancreatic infiltration than DPC4-non-expression tumours. We observed that well-defined tumour margins are significantly associated with DPC4-expression tumour in patients with pancreatic ductal adenocarcinoma. Considering the fact that the presence of well-defined tumour margins is representative of less infiltrative disease behaviour, this imaging feature could be well correlated with the locally limited disease pattern of DPC4-expression tumour. Although our results were modest, DPC4 gene expression may enable clinicians to stratify patients to receive either chemoradiotherapy when the DPC4 gene is expressive or only systemic chemotherapy when the DPC4 gene is non-expressive.9 However, this study could not define patient management implications in patients with pancreatic ductal adenocarcinoma, as we mainly focused on the correlation between imaging findings and tumour gene expression. Further study will be needed to define the clinical implications.
Although the presence of well-defined tumour margins was significantly associated with DPC4-expression tumours, the frequency of well-defined tumours in the DPC4-expression group was not remarkably higher than that in the DPC4-non-expression group (55.4% vs 37.6%). Using this CT finding in T3 tumours, the sensitivity, specificity, positive-predictive value and negative-predictive value for predicting DPC4-expression tumour were not high (54.1%, 63.0%, 52.4% and 64.6%). In addition, of the 125 T3 tumour with R0 resection patients, there was no significant difference in overall survival between well-defined and ill-defined tumours. This could be explained due to multiple factors. In other words, the tumour margin had significant associations with multiple factors that had no significant relationship with overall survival. Although our study demonstrated that the presence of well-defined tumour margins was significantly associated with DPC4-expression tumour, our results were modest, and we should not overestimate the results because tumour margin was the best predictor out of a series of poor predictors.
Our current analysis is the simplest and most explorative form of a radiogenomic study. Radiogenomics—the identification of imaging traits that correspond to different molecular phenotypes with clinical and biological relevance—is one of the most important fields in the development of personalized medicine because it can enable individualized treatment strategies by predicting individual risk stratification, responses and toxicity before definite treatment. To date, there have been no radiogenomic studies on pancreatic cancer. Indeed, the number of clinically relevant gene mutations that have been found for this cancer remains limited (e.g. p53, Kras, DPC4, erb2 and TGF-beta). The Pancreatic Cancer Genome Project is currently ongoing,23 with the prospect of extensive radiogenomic studies on pancreatic cancer. This initial study provides baseline data and is a step towards further evaluations of the radiogenomic features of pancreatic ductal adenocarcinoma.
Our present study had several limitations of note. First, as we analyzed only patients with resectable and localized pancreatic ductal adenocarcinoma, results might be influenced by selection bias. Second, we did not obtain the interobserver variability of the qualitative image analysis. It was due to the consensus review. However, experienced abdominal radiologists performed the image analyses, and disagreements were uncommon during the consensus review. Third, we used various CT scanners and parameters due to the retrospective study design.
In summary, our present study is the first to analyze the association between DPC4 gene expression and CT imaging findings in patients with pancreatic ductal adenocarcinoma. We showed that the presence of well-defined tumour margins was significantly linked with DPC4-expression tumour. However, there was no significant difference in overall patient survival according to tumour margins on CT. Further investigations and validations are needed to confirm this finding, preferably using a larger, more comprehensive and prospective cohort.
FUNDING
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (No. 2014R1A1A1006823) and a grant from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea (No. 2015-0636).
Contributor Information
Sang Hyun Choi, Email: edwardchoi83@gmail.com.
Hyoung Jung Kim, Email: hjk@amc.seoul.kr.
Kyung Won Kim, Email: medimash@gmail.com.
Soyeon An, Email: soyan21@gmail.com.
Seung-Mo Hong, Email: shong28@amc.seoul.kr.
Song Cheol Kim, Email: drksc@amc.seoul.kr.
Myung-Hwan Kim, Email: mhkim@amc.seoul.kr.
REFERENCES
- 1.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4: 567–79. [DOI] [PubMed] [Google Scholar]
- 2.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006; 10: 1199–210; discussion 1210–11. doi: https://doi.org/10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Shin SH, Kim SC, Hong SM, Kim YH, Song KB, Park KM, et al. Genetic alterations of K-ras, p53, c-erbB-2, and DPC4 in pancreatic ductal adenocarcinoma and their correlation with patient survival. Pancreas 2013; 42: 216–22. doi: https://doi.org/10.1097/MPA.0b013e31825b6ab0 [DOI] [PubMed] [Google Scholar]
- 4.Dong M, Nio Y, Tamura K, Song MM, Guo KJ, Guo RX, et al. Ki-ras point mutation and p53 expression in human pancreatic cancer: a comparative study among Chinese, Japanese, and Western patients. Cancer Epidemiol Biomarkers Prev 2000; 9: 279–84. [PubMed] [Google Scholar]
- 5.Castells A, Puig P, Mora J, Boadas J, Boix L, Urgell E, et al. K-ras mutations in DNA extracted from the plasma of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J Clin Oncol 1999; 17: 578–84. [DOI] [PubMed] [Google Scholar]
- 6.Hu YX, Watanabe H, Ohtsubo K, Yamaguchi Y, Ha A, Motoo Y, et al. Bcl-2 expression related to altered p53 protein and its impact on the progression of human pancreatic carcinoma. Br J Cancer 1999; 80: 1075–9. doi: https://doi.org/10.1038/sj.bjc.6690466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawesha A, Ghaneh P, Andren-Sandberg A, Ograed D, Skar R, Dawiskiba S, et al. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16(INK4A), p21(WAF-1), cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer 2000; 89: 469–74. doi: https://doi.org/10.1002/1097-0215(20001120)89:6<469::aid-ijc1>3.0.co;2-l [DOI] [PubMed] [Google Scholar]
- 8.Liu F. SMAD4/DPC4 and pancreatic cancer survival. Commentary re: M. Tascilar et al., the SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res 2001; 7: 3853–6. [PubMed] [Google Scholar]
- 9.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; 27: 1806–13. doi: https://doi.org/10.1200/jco.2008.17.7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 11.Wilentz RE, Su GH, Dai JL, Sparks AB, Argani P, Sohn TA, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am J Pathol 2000; 156: 37–43. doi: https://doi.org/10.1016/s0002-9440(10)64703-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshima M, Okano K, Muraki S, Haba R, Maeba T, Suzuki Y, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg 2013; 258: 336–46. doi: https://doi.org/10.1097/SLA.0b013e3182827a65 [DOI] [PubMed] [Google Scholar]
- 13.Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014; 270: 248–60. doi: https://doi.org/10.1148/radiol.13131184 [DOI] [PubMed] [Google Scholar]
- 14.Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics 2009; 29: 1749–65. doi: https://doi.org/10.1148/rg.296095506 [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto S, Horton KM, Lawler LP, Hruban RH, Fishman EK. Intraductal papillary mucinous neoplasm of the pancreas: can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics 2005; 25: 1451–68; discussion 68–70. doi: https://doi.org/10.1148/rg.256055036. [DOI] [PubMed] [Google Scholar]
- 16.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014; 20: 7864–77. doi: https://doi.org/10.3748/wjg.v20.i24.7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 1997; 168: 1439–43. doi: https://doi.org/10.2214/ajr.168.6.9168704 [DOI] [PubMed] [Google Scholar]
- 18.Kim DW, Kim HJ, Kim KW, Byun JH, Song KB, Kim JH, et al. Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur Radiol 2015; 25: 1375–83. doi: https://doi.org/10.1007/s00330-014-3532-z [DOI] [PubMed] [Google Scholar]
- 19.Esposito I, Konukiewitz B, Schlitter AM, Kloppel G. Pathology of pancreatic ductal adenocarcinoma: facts, challenges and future developments. World J Gastroenterol 2014; 20: 13833–41. doi: https://doi.org/10.3748/wjg.v20.i38.13833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biankin AV, Kench JG, Morey AL, Lee CS, Biankin SA, Head DR, et al. Overexpression of p21(WAF1/CIP1) is an early event in the development of pancreatic intraepithelial neoplasia. Cancer Res 2001; 61: 8830–7. [PubMed] [Google Scholar]
- 21.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000; 60: 2002–6. [PubMed] [Google Scholar]
- 22.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–49. doi: https://doi.org/10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 23.Iacobuzio-Donahue CA. Genetic evolution of pancreatic cancer: lessons learnt from the pancreatic cancer genome sequencing project. Gut 2012; 61: 1085–94. doi: https://doi.org/10.1136/gut.2010.236026 [DOI] [PMC free article] [PubMed] [Google Scholar]