Abstract
Objective:
To investigate risk factors for radiation-induced pneumonitis (RP) after hypofractionated stereotactic body radiotherapy (SBRT) in patients with lung tumours.
Methods:
From May 2004 to January 2016, 66 patients with 71 primary or metastatic lung tumours were treated with SBRT; these 71 cases were retrospectively analyzed for RP. To explore the risk factors for RP, the following factors were investigated: age, sex, performance status, operability, number of treatments, respiratory gating, pulmonary emphysema, tumour location and subclinical interstitial lung disease (ILD). Irradiated underlying lung volumes of more than 5 Gy, 10 Gy, 20 Gy and 30 Gy (Lung V5, V10, V20 and V30), mean lung dose and volumes of gross tumour volume (in cubic centimetre) and planning target volume were calculated for possible risk factors of RP.
Results:
The median follow-up period was 32 months. RP of Grade 2 or more, according to the Common Terminology Criteria for Adverse Events v. 4.0, was detected in 6 (8.4%) of the 71 cases. Grade 5 RP was identified in two cases. Of the risk factors of RP, subclinical ILD was the only factor significantly associated with the occurrence of RP of Grade 2 or more (p < 0.001). Both cases with Grade 5 RP had ILD with a honeycombing image.
Conclusion:
Subclinical ILD was the only significant factor for Grade 2–5 RP. In addition, the cases with honeycombing had a high potential for fatality related to severe RP. Patients with subclinical ILD should be carefully monitored for the occurrence of severe RP after SBRT.
Advances in knowledge:
Hypofractionated SBRT for primary or metastatic lung tumours provides a high local control rate and safe treatment.
INTRODUCTION
Hypofractionated stereotactic body radiotherapy (SBRT) for primary or metastatic lung tumours provides a high local control rate and safe treatment.1 Several reports have suggested that SBRT provides high local control rates of around 90% for patients with lung tumours.1–5 SBRT provides not only a high local control rate, but also a completely painless treatment with a low incidence of severe complications. The incidence of late toxicity of more than Grade 2 was <10% in most studies.6–8 However, rare fatalities related to severe toxicities after SBRT have been reported.9,10
Although a few patients treated with SBRT experience radiation-induced pneumonitis (RP), this is one of the most frequent toxicities in patients with lung tumours treated with SBRT. Severe RP is the most common cause of death shortly after radiotherapy. The risk factors for RP after conventional thoracic radiation therapy were reported in several studies.11–15 Compared with conventional radiation therapy, the reports of risk factors for RP after SBRT were few. Therefore, investigation of factors for severe RP is important to improve the safety of SBRT. In this study, we retrospectively analyzed the risk factors of RP after SBRT in patients with primary or metastatic lung tumours.
METHODS AND MATERIALS
Patients
From May 2004 to January 2016, SBRT was performed for 83 consecutive patients with a total of 89 primary or metastatic lung tumours at the Hachioji Center of Tokyo Medical University. All patients provided written informed consent. For this study, we retrospectively collected data for patients who were followed up for a minimum of 6 months. Of the 83 patients, 17 patients who were monitored for less than 6 months or lost to follow-up were excluded. As 5 patients were treated twice with SBRT for metastatic lung tumours at different times, 66 patients with 71 primary or metastatic lung tumours were included in the analysis. No cases received radiation therapy for lung tumours before the SBRT study.
The patient characteristics are summarized in Table 1. 44 patients were males and 22 patients were females. The median age of the patients was 80 years (range, 58–88 years). Of the total, 97% of patients had Eastern Cooperative Oncology Group performance status of 0 or 1. There were 51 primary lung tumours, 3 metastatic lung tumours developed after SBRT for primary lung tumours and 17 metastatic lung tumours from 15 patients with various cancers. Regarding primary lung tumours, 42 tumours were histologically identified as follows: adenocarcinoma, 22 tumours; squamous cell carcinoma, 17 tumours; non-small cell carcinoma, 2 tumours; and small cell carcinoma, 1 tumour. The remaining nine tumours were considered to be lung cancer without pathologically proven evidence. These tumours were diagnosed based on successive increases in tumour sizes observed on CT and/or increased uptake on positron emission tomography. Among the 20 metastatic lung tumours, the primary sites were the lung in 8 patients, the colorectum in 9 patients and other sites in 3 patients. All metastatic lung tumours were controlled at primary tumour sites or no other metastatic sites. 25 tumours were considered medically operable and 46 inoperable. This study was approved by the Ethical Review Board of the authors' institution.
Table 1.
Patient and tumour characteristics
| Number of patients (tumours) | 66 (71) |
| Sex (percentage) | |
| Male | 44 (67) |
| Female | 22 (33) |
| Age (years), median (range) | 80 (58–88) |
| Performance status | |
| 0 | 53 |
| 1 | 11 |
| 2 | 2 |
| Tumours (%) | |
| Primary lung tumour | 51 (72) |
| Metastatic lung tumour | 20 (28) |
| Pathology of primary lung cancer | |
| Adenocarcinoma | 22 |
| Squamous cell carcinoma | 17 |
| Non-small-cell carcinoma | 2 |
| Small-cell carcinoma | 1 |
| Clinically diagnosed | 9 |
| Primary sites with metastatic lung tumours | |
| Lung | 8 |
| Colorectum | 9 |
| Others | 3 |
| Number of tumours by operability (%) | |
| Operable | 25 (35) |
| Inoperable | 46 (65) |
Simulation and immobilization techniques
A body fixation device (EBS-2000, ESFORM; Engineering System, Matsumoto, Nagano, Japan), which used a vacuum cushion, was used for patient immobilization during the initial simulation and subsequent treatments. A CT scanner for radiation treatment planning was used, namely the LightSpeed RT 4 slice (GE Healthcare, Mickleton, NJ). 34 patients received four-dimensional (4D)-CT scans, in which CT data of 2.5 mm slices were acquired synchronously with a respiratory signal. During the CT examination, a series of light-emitting diodes were placed on the abdominal wall and monitored by a ceiling-mounted infrared camera in the simulation room. The planning CT scans were reconstructed from a series of 4D-CT data at the end-expiratory phase. The remaining 37 patients had CT scans at the end-expiratory and end-inspiratory phases for confirmation of internal motion because 4D-CT scanners had not been installed. Planning CT scans were obtained using a slow CT technique involving acquisition of a single 2.5-mm slice every 4 s. Audio was played during the initial simulation and subsequent treatments to induce a comfortable breathing rhythm.
Radiotherapy
Treatment planning was performed using the Eclipse™ (Varian Medical Systems, Palo Alto, CA) treatment planning system. SBRT plans were calculated with pencil beam convolution with heterogeneity correction using the Batho power law. The gross tumour volume (GTV) was contoured on the planning CT images. The lungs were contoured by automatic segmentation, as an area from −1000 to −300 Hounsfield unit was defined for the lung. For 4D-CT planning, the internal target volume (ITV) was determined using the Advantage Workstation (GE Healthcare, Chalfont St Giles, UK). For non-4D-CT planning, ITV was determined using CT scans obtained at the end-expiratory and end-inspiratory phases. The planning target volume (PTV) was determined by adding a margin of 6–8 mm to the ITV.
Patients who underwent 4D-CT were treated using a Real-time Position Management™ System (Varian Medical Systems, Palo Alto, CA) for real-time tumour targeting. The light-emitting diodes were placed on the abdomen wall, and their movement was followed by wall-mounted cameras in the treatment room. Throughout the procedure, the Real-time Position Management™ motion-tracking software corrected external body surface movement with internal tumour fiducial movement to follow and adjust for tumour motion. SBRT was planned and administered by non-coplanar static beams using six fields generated by a linear accelerator with energy of 10 MV (CLINAC® 2100C; Varian Medical Systems, Palo Alto, CA). Image guidance was performed to set up the patients before daily treatment delivery by megavoltage X-ray using an electric portal imaging device based on the spine.
Our dose prescription policies were based on the percentage of the prescribed dose covering 80% of the volume of the PTV. We principally used 50 Gy per five fractions in 5 days as the prescribed dose. When the tumour was adjacent to a high clinical risk organ (e.g. the oesophagus, spinal cord or the main trachea) or was relatively large, the dose and number of fractions were altered. The dose limitation for pulmonary parenchyma was a mean lung dose (MLD) < 18 Gy, percentage of total lung volume receiving ≥20 Gy (V20) < 20% and V15 < 25% according to the Japan Clinical Oncology Group 0403 study protocol.16 There was no constraint for maximum or minimum dose to PTV. As a result, the median prescribed dose was 50 Gy (range, 40–60 Gy) in five fractions (range, 5–10 fractions) over 5 days (range, 5–12 days).
Follow-up procedures
Regular follow-up visits were performed at 1 and/or 3 months after completing SBRT, at 3–4 month intervals for the first 2 years, and at every 4–6 months thereafter, in case of the absence of clinical symptoms. At each follow-up visit, evaluation consisted of a medical history and physical examination, CT scans and tumour marker assessment. The toxicity data were collected retrospectively from patient files. The RP was graded according to Common Terminology Criteria for Adverse Events v. 4.0. The RP grading system was as follows: Grade 1, asymptomatic (radiographic finding only); Grade 2, symptomatic and medical intervention indicated; Grade 3, severe symptomatic and oxygen indicated; Grade 4, life threatening (ventilator support indicated); and Grade 5, death.
The risk factors for radiation-induced pneumonitis
For exploring the clinical risk factors for RP, the following were investigated: age, sex, performance status, operability, number of treatments with SBRT, respiratory gating, pulmonary emphysema, tumour location and subclinical interstitial lung disease (ILD). The presence of ILD was determined based on pre-SBRT CT. The images were reviewed using CT findings usually present in ILD, such as ground-glass attenuation, reticulation, patchy ground-glass abnormalities and honeycombing. Of a total of 71 cases, 11 cases had subclinical ILD before SBRT, and 4 cases were identified as having honeycombing. CT findings were evaluated by a single radiologist.
For dosimetric factors, the total underlying lung volume was defined as the total lung volume minus the GTV. The dosimetric parameters were calculated from the dose–volume histogram for the total underlying lung volume. The irradiated total underlying lung volumes of more than 5 Gy, 10 Gy, 20 Gy and 30 Gy (Lung V5, V10, V20 and V30), MLD and volumes of GTV and PTV (in cubic centimetre) were evaluated as risk factors for RP.
Statistical analysis
The relationships among Grade 2–5 RP and the clinical factors were calculated using Fisher's exact probability test. The relationships between Grade 2–5 RP and dosimetric factors were analyzed using the Mann–Whitney U test. Univariate logistic regression analyses were performed to evaluate the data using IBM SPSS® Statistics v. 20.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Differences with p-values <0.05 were considered statistically significant. The onset time of RP after SBRT was calculated from the first day of SBRT.
RESULTS
The median follow-up period was 32 months (range, 2–135 months). Grade 2–5 RP was recognized in 6 (8.4%) of the 71 cases; Grade 2 in 3 cases, Grade 3 in 1 case and Grade 5 in 2 cases. The median time to developing symptoms was 4 months (range, 2–8 months) after the start of SBRT.
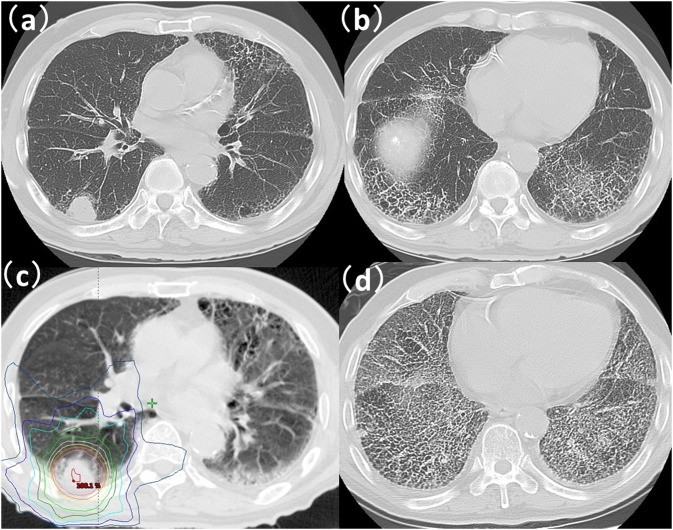
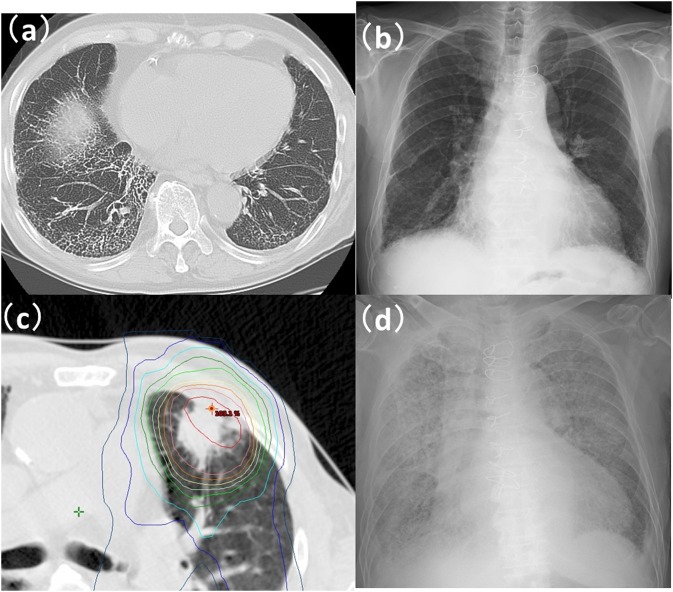
The relationships between the clinical factors and Grade 2–5 RP are summarized in Table 2. Grade 2–5 RP was observed in 5 (45%) of the 11 cases of ILD; Grade 2 in 2 cases, Grade 3 in 1 case and Grade 5 in 2 cases. By univariate analysis, ILD was the only factor significantly associated with the occurrence of Grade 2–5 RP (p < 0.001). Both cases with Grade 5 RP had ILD with honeycombing (Figures 1 and 2) prior to SBRT. The relationship between Grade 2–5 RP in an in-field region of ILD and in an out-of-field region of ILD is shown in Table 3. The region of ILD was not a significant factor for Grade 2–5 RP. A multivariate analysis was not performed because of limited data.
Table 2.
Clinical factors associated with radiation-induced pneumonitis (RP)
| RP |
Univariate | ||
|---|---|---|---|
| Grade 2–5, n = 6 | p-value | Hazard ratio (95% confidence interval) | |
| Age (<80 years vs ≥80 years) | 4/35 vs 2/36 | 0.429 | 0.456 (0.078–2.665) |
| Sex (male vs female) | 5/46 vs 1/25 | 0.414 | 0.342 (0.038–3.1) |
| PS (0 vs ≥1) | 5/58 vs 1/13 | >0.999 | 0.883 (0.094–8.269) |
| Operability (yes vs no) | 1/25 vs 5/46 | 0.414 | 2.927 (0.323–26.556) |
| Number of SBRT (once vs twice) | 6/66 vs 0/5 | >0.999 | Acalculia |
| Respiratory gating (yes vs no) | 4/34 vs 2/37 | 0.417 | 2.333 (0.399–13.645) |
| Pulmonary emphysema (yes vs no) | 3/28 vs 3/43 | 0.674 | 1.600 (0.299–8.555) |
| Tumour location (upper/middle vs lower) | 4/49 vs 2/22 | >0.999 | 1.125 (0.190-6.653) |
| Subclinical ILD (yes vs no) | 5/11 vs 1/60 | <0.001 | 49.167 (4.903–463.078) |
ILD, interstitial lung disease; PS, performance status; SBRT, stereotactic body radiotherapy.
Figure 1.
A case with Grade 5 radiation-induced pneumonitis after stereotactic body radiotherapy (SBRT): (a, b) CT images prior to SBRT for the lung. CT finding of honeycombing was recognized in both inferior lobes of the lung. (c) CT with dose distribution. Prescription dose was 56 Gy per 7 fractions. Lung V5, V10, V20, V30 and mean lung dose were 21.4%, 13.4%, 3.3%, 1.7% and 3.7%, respectively. (d) A CT image taken 7 months after SBRT showed expanding honeycombing.
Figure 2.
Another case with Grade 5 radiation-induced pneumonitis after stereotactic body radiotherapy (SBRT): (a, b) CT image and X-ray photograph (X-P) prior to SBRT for lung. CT finding of honeycombing was recognized in the right inferior lobe of lung. X-P finding of reticulonodular shadow was recognized in the right inferior lung. (c) CT with dose distribution. Prescription dose was 56 Gy per 7 fractions. Lung V5, V10, V20, V30 and mean lung dose were 14.0%, 9.3%, 3.7% 2.5% and 3.1%, respectively. (d) An X-P image taken 3 months after SBRT showed expanded shadow in both lungs.
Table 3.
The relationship between the region of subclinical interstitial lung abnormality and Grade 2–5 radiation-induced pneumonitis (RP)
| RP |
||||
|---|---|---|---|---|
| Grade 0–1, n = 6 | Grade 2–5, n = 5 | p-value | ||
| Subclinical intestinal lung abnormality n = 11 | In-field, n = 8 | 5 | 3 | |
| Out-of-field, n = 3 | 1 | 2 | 0.545 | |
Table 4 shows the relationships between the dosimetric factors and Grade 2–5 RP in all cases. No significant factor was found. Although Lung V5, V10 and MLD did not reach statistical significance in this small data set as significant confounding factors, their p-values were reasonably low, confirming their importance.
Table 4.
Relationship between dosimetric factors and Grade 2–5 radiation-induced pneumonitis (RP)
| Median (range) | RP |
p-value | |
|---|---|---|---|
| Grade 0–1 | Grade 2–5 | ||
| GTV | 6.0 cm3 (1.0 cm3–53.1 cm3) | 10.9 cm3 (2.9 cm3–27.8 cm3) | 0.222 |
| PTV | 24.0 cm3 (9.0 cm3–100.8 cm3) | 31.7 cm3 (12.9 cm3–66.6 cm3) | 0.342 |
| V5 | 13.8 Gy (3.2 Gy–28.0 Gy) | 18.4 Gy (14.0 Gy–30.0 Gy) | 0.061 |
| V10 | 8.5 Gy (1.7 Gy–16.0 Gy) | 11.4 Gy (7.9 Gy–21.4 Gy) | 0.072 |
| V20 | 3.4 Gy (0.5 Gy–7.9 Gy) | 3.5 Gy (2.2 Gy–7.7 Gy) | 0.402 |
| V30 | 1.9 Gy (0.3 Gy–4.5 Gy) | 2.1 Gy (1.4 Gy–4.7 Gy) | 0.357 |
| MLD | 2.7 Gy (0.7 Gy–4.9 Gy) | 3.4 Gy (2.5 Gy–5.8 Gy) | 0.080 |
GTV, gross tumour volume; MLD, mean lung dose; PTV, planning target volume.
The clinical data and dosimetric factors for all cases and tumours are shown in Tables 5 and 6.
Table 5.
| Number | Age (years) | Sex | PS | Operability | Number of SBRT | Respiratory gating (yes vs no) | Pulmonary emphysema (yes vs no) | Tumour location (upper/middle vs lower) | Subclinical ILD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | 0 | Inoperable | 1 | No | No | Lower | Normal |
| 2 | 75 | M | 1 | Inoperable | 1 | No | No | Upper/middle | Normal |
| 3 | 81 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Normal |
| 4 | 83 | M | 0 | Inoperable | 2 | No | Yes | Upper/middle | Normal |
| 5 | 64 | F | 1 | Inoperable | 1 | No | Yes | Upper/middle | Normal |
| 6 | 67 | F | 0 | Inoperable | 1 | Yes | Yes | Lower | Normal |
| 7 | 72 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Normal |
| 8 | 58 | F | 0 | Operable | 1 | No | No | Upper/middle | Normal |
| 9 | 65 | F | 0 | Operable | 2 | Yes | No | Lower | Normal |
| 10 | 86 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Ground glass attenuation |
| 11 | 82 | M | 0 | Operable | 1 | Yes | Yes | Lower | Normal |
| 12 | 82 | M | 0 | Operable | 2 | Yes | Yes | Lower | Normal |
| 13 | 81 | F | 0 | Operable | 1 | No | No | Upper/middle | Normal |
| 14 | 85 | F | 1 | Operable | 1 | No | No | Upper/middle | Normal |
| 15 | 77 | M | 0 | Inoperable | 1 | No | No | Lower | Normal |
| 16 | 85 | M | 0 | Inoperable | 1 | Yes | Yes | Lower | Normal |
| 17 | 78 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Honeycombing |
| 18 | 83 | F | 1 | Operable | 1 | No | Yes | Upper/middle | Normal |
| 19 | 80 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Reticulation |
| 20 | 80 | F | 0 | Inoperable | 1 | No | No | Upper/middle | Normal |
| 21 | 84 | M | 0 | Operable | 1 | Yes | No | Lower | Normal |
| 22 | 81 | M | 0 | Operable | 1 | Yes | No | Upper/middle | Normal |
| 23 | 76 | F | 0 | Operable | 1 | Yes | No | Upper/middle | Normal |
| 24 | 70 | M | 0 | Inoperable | 1 | No | Yes | Lower | Honeycombing |
| 25 | 84 | M | 2 | Inoperable | 1 | No | Yes | Lower | Normal |
| 26 | 82 | M | 0 | Operable | 1 | Yes | No | Upper/middle | Normal |
| 27 | 81 | M | 1 | Operable | 1 | Yes | No | Upper/middle | Normal |
| 28 | 60 | F | 0 | Inoperable | 1 | Yes | No | Lower | Normal |
| 29 | 83 | M | 0 | Inoperable | 1 | No | No | Upper/middle | Reticulation |
| 30 | 83 | F | 0 | Inoperable | 1 | No | No | Lower | Normal |
| 31 | 87 | F | 0 | Inoperable | 1 | No | No | Upper/middle | Normal |
| 32 | 68 | F | 0 | Operable | 1 | Yes | No | Upper/middle | Normal |
| 33 | 58 | M | 0 | Operable | 1 | Yes | No | Upper/middle | Normal |
| 34 | 73 | F | 0 | Inoperable | 1 | No | No | Upper/middle | Normal |
| 35 | 64 | M | 0 | Operable | 1 | Yes | No | Upper/middle | Normal |
| 36 | 79 | M | 0 | Operable | 1 | Yes | No | Lower | Normal |
| 37 | 71 | F | 0 | Inoperable | 1 | No | No | Upper/middle | Normal |
| 38 | 73 | F | 0 | Inoperable | 2 | Yes | No | Upper/middle | Normal |
| 39 | 79 | M | 0 | Inoperable | 1 | Yes | Yes | Upper/middle | Reticulation |
| 40 | 75 | M | 0 | Inoperable | 1 | No | No | Lower | Normal |
| 41 | 88 | M | 0 | Inoperable | 1 | Yes | Yes | Upper/middle | Normal |
| 42 | 71 | M | 0 | Operable | 1 | No | No | Lower | Normal |
| 43 | 74 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Normal |
| 44 | 84 | F | 0 | Operable | 1 | No | No | Upper/middle | Normal |
| 45 | 85 | F | 0 | Operable | 2 | No | No | Lower | Normal |
| 46 | 78 | M | 0 | Inoperable | 1 | Yes | Yes | Upper/middle | Normal |
| 47 | 78 | F | 0 | Inoperable | 1 | No | No | Lower | Reticulation |
| 48 | 85 | M | 0 | Inoperable | 1 | No | No | Upper/middle | Normal |
| 49 | 70 | M | 0 | Operable | 1 | No | No | Upper/middle | Normal |
| 50 | 83 | M | 1 | Inoperable | 1 | Yes | No | Upper/middle | Normal |
| 51 | 84 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Honeycombing |
| 52 | 82 | M | 0 | Inoperable | 1 | Yes | No | Lower | Normal |
| 53 | 87 | M | 1 | Inoperable | 1 | Yes | Yes | Upper/middle | Patchy ground glass abnormalities |
| 54 | 82 | M | 0 | Inoperable | 1 | Yes | Yes | Upper/middle | Normal |
| 55 | 83 | F | 0 | Inoperable | 1 | Yes | No | Upper/middle | Normal |
| 56 | 75 | M | 0 | Inoperable | 1 | Yes | Yes | Upper/middle | Normal |
| 57 | 77 | M | 0 | Inoperable | 1 | Yes | No | Upper/middle | Honeycombing |
| 58 | 76 | M | 1 | Operable | 1 | Yes | Yes | Upper/middle | Normal |
| 59 | 77 | F | 0 | Inoperable | 1 | Yes | No | Upper/middle | Normal |
| 60 | 83 | M | 0 | Operable | 1 | Yes | No | Upper/middle | Ground glass attenuation |
| 61 | 82 | M | 2 | Inoperable | 1 | No | Yes | Upper/middle | Normal |
| 62 | 70 | F | 0 | Inoperable | 1 | Yes | Yes | Upper/middle | Normal |
| 63 | 67 | M | 0 | Inoperable | 1 | Yes | Yes | Upper/middle | Normal |
| 64 | 65 | M | 0 | Operable | 1 | No | No | Upper/middle | Normal |
| 65 | 86 | M | 0 | Inoperable | 1 | No | No | Upper/middle | Normal |
| 66 | 83 | F | 0 | Operable | 1 | Yes | No | Lower | Normal |
| 67 | 86 | F | 1 | Operable | 1 | No | No | Lower | Normal |
| 68 | 85 | M | 0 | Inoperable | 1 | No | Yes | Upper/middle | Normal |
| 69 | 72 | M | 1 | Inoperable | 1 | Yes | Yes | Lower | Normal |
| 70 | 86 | M | 1 | Inoperable | 1 | Yes | No | Lower | Normal |
| 71 | 58 | F | 0 | Inoperable | 1 | No | No | Upper/middle | Normal |
F, female; ILD, interstitial lung disease; M, male; PS, performance status; SBRT, stereotactic body radiotherapy.
Table 6.
| No | Isosentre dose (Gy/fraction/days) | GTV (cm3) | PTV (cm3) | GTV D95 (Gy) | Lung V5 (%) | Lung V10 (%) | Lung V20 (%) | Lung V30 (%) | Lung MLD (%) | Local response | Local control | Local control duration (months) | RP grading |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54/9/11 | 1.0 | 26.5 | 53.2 | 7.0 | 5.0 | 1.7 | 0.9 | 1.4 | PR | Control | 84 | G1 |
| 2 | 60/10/12 | 2.9 | 24 | 58.4 | 19.6 | 11.6 | 4.3 | 2.6 | 3.7 | CR | Control | 135 | G1 |
| 3 | 50/10/12 | 10.3 | 34.4 | 44.8 | 8.1 | 4.1 | 1.8 | 0.9 | 1.4 | PR | Control | 33 | G0 |
| 4 | 40/5/5 | 7.1 | 30.7 | 38.1 | 9.7 | 4.4 | 1.7 | 0.9 | 1.6 | CR | Control | 17 | G0 |
| 5 | 60/10/12 | 1.8 | 13.2 | 58.6 | 11.2 | 5.0 | 1.8 | 1.0 | 1.9 | CR | Control | 118 | G0 |
| 6 | 50/5/5 | 3.0 | 12.6 | 48.9 | 10.5 | 6.0 | 2.1 | 1.2 | 2.1 | CR | Control | 32 | G1 |
| 7 | 60/10/15 | 23.2 | 58.2 | 56.5 | 13.8 | 9.5 | 3.7 | 1.9 | 2.7 | PR | Control | 10 | G0 |
| 8 | 60/10/12 | 1.1 | 16.3 | 59.3 | 11.6 | 8.5 | 3.6 | 2.1 | 2.4 | PR | Control | 118 | G1 |
| 9 | 50/5/5 | 1.4 | 14.2 | 48.9 | 14.9 | 10.2 | 3.1 | 1.7 | 2.6 | PR | Control | 32 | G1 |
| 10 | 60/10/12 | 14.7 | 61.1 | 58.1 | 21.0 | 15.0 | 7.9 | 4.5 | 4.9 | PR | Control | 8 | G0 |
| 11 | 40/5/5 | 1.1 | 11.4 | 39.1 | 6.6 | 2.0 | 0.6 | 0.3 | 1.1 | CR | Control | 35 | G0 |
| 12 | 40/5/5 | 1.5 | 12.86 | 39.2 | 7.1 | 1.7 | 0.6 | 0.3 | 1.1 | CR | Control | 35 | G0 |
| 13 | 60/10/12 | 9.6 | 47.7 | 57.2 | 17.0 | 12.5 | 6.5 | 3.7 | 3.8 | PR | Control | 52 | G1 |
| 14 | 40/5/5 | 4.1 | 21.8 | 39 | 21.6 | 8.5 | 3.0 | 1.4 | 3.2 | PR | PD | 17 | G1 |
| 15 | 35/5/5 | 2.0 | 20 | 34.1 | 6.5 | 2.0 | 0.6 | 0.3 | 1.0 | CR | PD | 12 | G0 |
| 16 | 56/7/8 | 9.2 | 42.5 | 54.4 | 14.3 | 9.3 | 3.5 | 1.8 | 2.7 | CR | Control | 43 | G1 |
| 17 | 56/7/9 | 6.2 | 25 | 54.6 | 11.3 | 7.0 | 2.6 | 1.5 | 2.4 | PR | Control | 51 | G1 |
| 18 | 49/7/9 | 18.7 | 59.2 | 47.5 | 24.0 | 15.0 | 5.7 | 3.7 | 4.4 | CR | Control | 18 | G1 |
| 19 | 40/5/5 | 1.6 | 10.1 | 39 | 6.4 | 2.4 | 1.0 | 0.5 | 1.1 | PR | PD | 39 | G0 |
| 20 | 40/5/5 | 1.4 | 9 | 38.7 | 5.9 | 2.2 | 0.5 | 0.9 | 1.1 | CR | PD | 33 | G0 |
| 21 | 49/7/9 | 5.5 | 24.5 | 46.7 | 9.8 | 6.6 | 2.7 | 1.6 | 2.1 | PR | PD | 23 | G1 |
| 22 | 56/7/9 | 13.8 | 39.5 | 53.3 | 17.2 | 10.6 | 4.0 | 2.3 | 3.4 | PR | Control | 100 | G1 |
| 23 | 56/7/9 | 7.1 | 24.5 | 53.3 | 24.3 | 14.9 | 5.7 | 3.1 | 4.4 | PR | Control | 68 | G1 |
| 24 | 56/7/9 | 14.7 | 40.3 | 54 | 21.4 | 13.4 | 3.3 | 1.7 | 3.7 | CR | Control | 8 | G5 (8M) |
| 25 | 45/5/5 | 7.2 | 39.5 | 43.4 | 10.3 | 6.0 | 2.1 | 1.2 | 1.8 | PR | Control | 6 | G0 |
| 26 | 56/7/9 | 10.7 | 36.7 | 53.5 | 20.7 | 11.6 | 3.5 | 1.9 | 3.5 | PR | Control | 7 | G1 |
| 27 | 40/5/5 | 1.6 | 13.1 | 38.9 | 7.0 | 2.4 | 1.1 | 0.5 | 1.3 | CR | Control | 13 | G1 |
| 28 | 45/5/5 | 1.0 | 10 | 44.1 | 3.2 | 2.2 | 1.1 | 0.7 | 0.7 | CR | Control | 9 | G0 |
| 29 | 56/7/9 | 6.5 | 31.9 | 54.5 | 11.8 | 9.4 | 4.8 | 3.1 | 2.9 | PR | Control | 77 | G1 |
| 30 | 49/7/9 | 5.3 | 21.6 | 44.5 | 8.7 | 5.7 | 1.4 | 0.8 | 1.5 | PR | PD | 11 | G1 |
| 31 | 56/7/9 | 6.2 | 34.9 | 54.7 | 15.7 | 11.1 | 5.3 | 3.1 | 3.5 | PR | Control | 54 | G1 |
| 32 | 50/5/5 | 1.2 | 13.8 | 49.2 | 12.8 | 5.0 | 1.5 | 0.9 | 1.9 | PR | Control | 65 | G0 |
| 33 | 49/7/9 | 2.4 | 15.6 | 47 | 8.2 | 4.2 | 1.3 | 0.8 | 1.4 | PR | Control | 86 | G1 |
| 34 | 56/8/10 | 28.0 | 63.7 | 53.7 | 28.0 | 16.0 | 6.3 | 3.7 | 4.9 | PR | Control | 7 | G0 |
| 35 | 50/5/5 | 2.9 | 12.4 | 48.1 | 15.0 | 9.8 | 4.5 | 2.0 | 3.1 | SD | Control | 9 | G0 |
| 36 | 60/10/12 | 9.0 | 38.2 | 57.5 | 13.0 | 7.2 | 2.3 | 1.4 | 2.5 | PR | Control | 10 | G0 |
| 37 | 50/5/5 | 8.3 | 23.3 | 48 | 8.6 | 6.8 | 3.4 | 2.3 | 2.0 | CR | Control | 70 | G0 |
| 38 | 50/5/5 | 3.3 | 15.1 | 49 | 13.0 | 8.5 | 3.7 | 2.2 | 2.7 | CR | Control | 43 | G1 |
| 39 | 50/5/5 | 10.0 | 38 | 48.4 | 17.2 | 9.2 | 4.1 | 2.6 | 3.3 | PR | Control | 30 | G1 |
| 40 | 50/5/5 | 10.0 | 27.9 | 48.5 | 18.5 | 8.3 | 2.8 | 1.8 | 2.9 | PR | Control | 11 | G0 |
| 41 | 45/5/5 | 1.8 | 11.7 | 44.3 | 14.5 | 7.7 | 2.1 | 1.1 | 2.3 | PR | Control | 39 | G1 |
| 42 | 60/10/12 | 5.6 | 17.2 | 58 | 15.7 | 8.8 | 3.2 | 1.9 | 2.9 | PR | Control | 61 | G1 |
| 43 | 60/10/12 | 2.5 | 10.8 | 58.6 | 12.3 | 5.3 | 1.7 | 0.9 | 2.0 | PR | PD | 14 | G0 |
| 44 | 49/7/9 | 22.4 | 49.5 | 46.5 | 26.5 | 16.0 | 6.0 | 3.7 | 4.9 | PR | Control | 65 | G1 |
| 45 | 49/7/9 | 6.0 | 22.2 | 48 | 12.1 | 6.0 | 2.3 | 1.5 | 2.1 | PR | Control | 56 | G1 |
| 46 | 50/5/5 | 4.2 | 24.2 | 49 | 14.1 | 7.9 | 2.5 | 1.4 | 2.5 | PR | Control | 6 | G2 (5M) |
| 47 | 60/10/12 | 2.9 | 12.9 | 56.6 | 15.4 | 7.9 | 2.2 | 1.4 | 2.6 | CR | Control | 6 | G2 (3M) |
| 48 | 60/10/12 | 17.9 | 47.5 | 57.8 | 9.7 | 7.5 | 4.0 | 2.1 | 2.3 | CR | Control | 18 | G1 |
| 49 | 50/5/5 | 5.3 | 17.1 | 48.8 | 12.1 | 6.7 | 1.9 | 1.1 | 2.1 | PR | Control | 45 | G1 |
| 50 | 50/5/5 | 2.9 | 13.9 | 48.7 | 22.7 | 12.6 | 4.5 | 2.7 | 3.9 | PR | Control | 57 | G1 |
| 51 | 50/5/5 | 13.3 | 35.8 | 48.5 | 16.0 | 9.8 | 3.9 | 2.3 | 3.0 | PR | PD | 14 | G1 |
| 52 | 50/5/5 | 2.1 | 18.1 | 49.6 | 17.7 | 9.5 | 3.3 | 1.9 | 3.1 | PR | Control | 56 | G1 |
| 53 | 49/7/9 | 27.8 | 66.6 | 47.4 | 30.0 | 21.4 | 7.7 | 4.7 | 5.8 | PR | PD | 33 | G3 (3M) |
| 54 | 50/5/5 | 11.7 | 32.6 | 47.8 | 11.4 | 7.2 | 3.4 | 2.1 | 2.4 | PR | Control | 48 | G1 |
| 55 | 40/5/5 | 6.4 | 17.1 | 37.6 | 13.1 | 6.4 | 2.4 | 1.3 | 2.3 | CR | Control | 29 | G1 |
| 56 | 50/5/5 | 3.4 | 17.9 | 48.3 | 16.4 | 9.4 | 3.4 | 1.9 | 2.9 | CR | Control | 12 | G0 |
| 57 | 56/7/9 | 14.8 | 36.5 | 53.3 | 14.0 | 9.3 | 3.7 | 2.5 | 3.1 | PR | Control | 2 | G5 (2M) |
| 58 | 50/5/5 | 8.7 | 38.6 | 49.1 | 18.8 | 10.9 | 4.0 | 2.6 | 3.6 | PR | Control | 38 | G1 |
| 59 | 50/5/5 | 3.2 | 14.6 | 49 | 20.0 | 10.7 | 3.7 | 2.2 | 3.2 | PR | Control | 35 | G1 |
| 60 | 50/5/5 | 7.1 | 26.9 | 49 | 24.0 | 16.5 | 5.7 | 3.6 | 4.8 | PR | Control | 28 | G2 (4M) |
| 61 | 50/5/5 | 17.3 | 42.3 | 48.3 | 24.5 | 15.9 | 6.6 | 4.0 | 4.7 | PR | Control | 8 | G1 |
| 62 | 50/5/5 | 7.5 | 23.4 | 50.6 | 15.0 | 9.5 | 5.4 | 3.1 | 3.2 | CR | PD | 11 | G1 |
| 63 | 50/5/5 | 16.1 | 42 | 49.9 | 20.0 | 13.0 | 5.2 | 3.2 | 3.9 | CR | Control | 10 | G0 |
| 64 | 50/5/5 | 3.9 | 18.8 | 49 | 5.5 | 3.5 | 2.0 | 1.3 | 1.2 | PR | Control | 31 | G1 |
| 65 | 50/10/12 | 25.4 | 42.7 | 48.2 | 24.1 | 15.7 | 6.7 | 4.1 | 4.9 | PR | Control | 30 | G1 |
| 66 | 50/5/5 | 3.6 | 14.6 | 50.3 | 12.0 | 9.3 | 2.5 | 1.5 | 2.3 | PD | PD | 5 | G0 |
| 67 | 50/5/5 | 4.7 | 15.5 | 50.4 | 19.0 | 10.0 | 3.9 | 2.3 | 3.4 | CR | PD | 16 | G0 |
| 68 | 50/10/15 | 53.1 | 100.8 | 49.5 | 25.0 | 15.0 | 5.4 | 3.9 | 4.9 | PR | Control | 17 | G1 |
| 69 | 50/5/5 | 18.9 | 50.7 | 44.2 | 15.0 | 7.8 | 2.7 | 1.6 | 2.9 | CR | Control | 10 | G0 |
| 70 | 50/5/5 | 5.4 | 28.1 | 47.5 | 15.0 | 9.8 | 5.3 | 3.1 | 3.4 | PR | Control | 5 | G0 |
| 71 | 60/8/11 | 14.6 | 32.7 | 57.3 | 11.4 | 8.6 | 5.8 | 4.1 | 3.6 | PR | Control | 6 | G1 |
CR, complete response; D95, the dose that covers 95% of the gross tumour volume; GTV, gross tumour volume; Lung Vx, irradiated lung volume more than x Gy; MLD, mean lung dose; PD, progressive disease; PR, partial response; PTV, planning target volume; RP, radiation-induced pneumonitis; SD, stable disease.
DISCUSSION
SBRT has been widely used as a safe and effective treatment for primary or metastatic lung tumours.1 Several trials have confirmed the safety of SBRT for patients with lung tumours.16–19 In the Radiation Therapy Oncology Group Trial 0236,17 Grade 3 and Grade 4 toxicities related to SBRT occurred in 12.7% (7/59) and 3.6% (2/59) of cases, respectively. No Grade 5 toxicities were reported. In the Nordic Phase II study of SBRT,19 Grade 3 toxicities were observed in 12 (21%) of the 57 patients, but no Grade 4 or 5 toxicities were reported. According to the protocol of the Japan Clinical Oncology Group 0403 study,16 the only patients restricted from participation are pregnant females. Rates of serious toxicity in most studies are low; however, rare fatalities related to severe toxicities after SBRT have been reported.9,10
RP is one of the most frequent causes of toxicity after SBRT, as well as after conventional radiotherapy, for patients with lung tumours. Although most of RP was Grade 1 or 2, a few cases had the potential to be severe or mortal.10,20, and 21 Yamashita et al10 reported that the incidence of RP Grade 2 or higher was 29% at 18 months after the completion of SBRT, and 3 (12%) of the 25 patients died of RP. Investigation of the method to predict the risk of RP after SBRT for patients with lung tumours is very important to increase safety. With regard to conventional radiotherapy, many clinical and dosimetric factors have frequently been analyzed and reported to be significantly associated with RP.14,15,22,23 Recently, the risk factors of RP after SBRT in patients with lung tumours have been investigated,19,22–29 and some studies reported about the clinical and dosimetric risk factors of RP.21,29–42 Table 7 summarizes published reports of the clinical and dosimetric risk factors associated with Grade 2 or worse RP after SBRT.
Table 7.
| First author | Prescription dose (Gy/fraction) | Number of patients | Median follow-up (months) | CTCAE | Number of patients with RP | RP factor | Detail of RP factor | p-value |
|---|---|---|---|---|---|---|---|---|
| Kanemoto A37 | 52/4 | 231 | 31.3 | v. 4 | ≥G2; 30 (13.0%) | Median V10 of all lung | G0–1: 9.4% G2–5:11.6% |
0.001 |
| Matsuo Y25 | 48/4 | 74 | 31.4 | v. 3 | ≥G2; 15 (20.3%) | V25 of all lung | G2–5; <4.2%, 14.8% ≥4.2%, 46.2% |
0.019 |
| Volume of PTV | G2–5; <37.7 ml, 11.1% ≥37.7 ml, 34.5% |
0.02 | ||||||
| Barriger RB27 | 60/3 | 251 | 17 | v. 2 | ≥G2; 23 (9.4%) | MLD of all lung | G2–5; ≤4 Gy, 4.3% >4 Gy, 17.6% |
0.02 |
| V20 of all lung | G2–5; ≤4%, 4.3% >4%, 16.4% |
0.03 | ||||||
| Yamashita H21 | 48/4 | 117 | 14.7 | v. 3 | G4-5; 9 (7.7%) | KL-6 level | G4–5; ≤500 U/mL, 3% >500 U/mL, 32% |
0.0002 |
| SP-D level | G4–5; ≤110 ng/mL, 3% >110 ng, 29% |
0.0002 | ||||||
| IP shadow in CT | G4–5; −,2% +, 57% |
<0.0001 | ||||||
| Alite F38 | 48–60/4–5 | 189 | 24.8 | v. 4 | ≥G2; 25 (13.2%) | ACEi | G2–5; non-user, 16.3% user, 4.2% |
0.03 |
| Ueki N33 | 48/4 | 157 | 39.5 | v. 3 | ≥G2; 29 (18.7%) | ILD | G2–5; −, 13.3% +, 55.0% |
<0.001 |
| Chang JY39 | 50/4 | 130 | 26 | v. 3 | G2–3; 15 (11.5%) | MLD of ipsilateral lung | G2-3; <9.14 Gy, 1.5% ≥9.14 Gy, 22% |
<0.001 |
| Inoue T40 | 48/4 | 109 | 25 | v. 3 | G2–3; 18 (16.5%) | Median MLD of all Lung | G0–1: 3.8 Gy G2–3: 4.8 Gy |
0.002 |
| Median V20 of all Lung | G0–1: 5.4% G2–3: 7.6% |
<0.001 |
ACEi, angiotensin-converting enzyme inhibitors; CTCAE, Common Terminology Criteria for Adverse Events; ILD, interstitial lung disease; IP, interstitial pneumonitis; KL-6, Krebs von den Lungen-6; MLD, mean lung dose; PTV, planning target volume; RP, radiation-induced pneumonitis; SP-D, serum surfactant protein-D.
In this study, the clinical and dosimetric risk factors for RP after SBRT for patients with primary and metastatic lung tumours were retrospectively investigated. Grade 2–5 RP was noted in 6 (8.4%) of the 71 cases. For clinical risk factors of RP, subclinical ILD was the only factor significantly associated with the occurrence of Grade 2–5 RP (p < 0.001). Among the 11 cases with ILD prior to SBRT, Grade 2–5 RP was observed in 5 (45%) cases and 2 of the 4 patients with honeycombing died of RP. The region, in-field or out-of-field, of ILD was not a significant factor for Grade 2–5 RP. According to Yamashita et al,21 severe Grade 4–5 RP was reduced from 18.8% to 3.5% on excluding patients with an obvious interstitial pneumonitis shadow on the CT and high levels of serum Krebs von den Lungen-6 (KL-6) and serum surfactant protein-D (SP-D) before performing SBRT. Even in this study, we should have investigated the serum KL-6 and SP-D levels before performing SBRT as risk factors of severe RP. However, we had little data about serum KL-6 and SP-D because these data were obtained for only patients who were symptomatic at our institution. Ueki et al33 reported that the presence of pre-existing ILD was a significant risk factor of RP worse than Grade 2, and the incidence of RP worse than Grade 2 for those with ILD was 55.0% (11/20) cases. Their data were similar to our results. In addition, in this study, we confirmed that the cases with honeycombing had a high potential for fatality related to severe RP after SBRT, and the location of ILD was not related to the incidence of RP. Indeed, it was possible that some inflammatory response was triggered for fatality, but only two of four cases with honeycombing had Grade 5 RP and other cases without honeycombing had no shadows extending far beyond the radiation field. Lungs with ILD may have properties of interstitial pneumonia (IP). Because IP is a diffuse disease, the whole sphere of the lung with ILD may have IP properties, even if it is only partial on diagnostic imaging. Thus, we considered that the existence of ILD is a risk factor of RP after SBRT, regardless of the region of ILD. Morgan et al43 indicated that sporadic pneumonitis, including extensive RP, appears to be an entirely different disease process involving immune modulation and genetic factors, as opposed to classical RP, which is characterized by the inflammatory consequences of direct irradiation injury to pulmonary tissues. Roberts et al44 demonstrated that lymphocytic alveolitis developed in both lung fields after strictly unilateral thoracic irradiation and was more pronounced in patients who developed clinical pneumonitis. They concluded that radiotherapy might cause generalized lymphocyte-mediated hypersensitivity reactions. We do not know how to reduce the risk of severe RP. However, there is some possibility that we can decrease fatal RP by conducting the radiation planning as soon as possible and taking into consideration the factors for RP of Grade 2 or more (Table 7) in cases with ILD, especially honeycombing. After SBRT, strict and careful follow-up is necessary. With regard to the dosimetric risk factors for RP, there were no significant factors; however, although Lung V5, V10 and MLD did not reach statistical significance in this small data set as significant confounding factors, their p-values were reasonably low, confirming their importance. This result might suggest that the dose level of low-dose areas of the lung such as Lung V5, V10 and MLD compared with high-dose areas such as Lung V20 and V30 has more potential correlation with Grade 2–5 RP. Guckenberger et al34 evaluated the relationship between MLD and the incidence of RP after SBRT. They reported that a significant dose–response relationship was observed; the MLD was 12.5 ± 4.3 Gy and 9.9 ± 5.8 Gy for patients with and without development of RP, respectively. Recently, Zhao et al42 analyzed 88 published studies (7752 patients) to investigate the lung toxicity after SBRT. In this report, older age, larger tumour size, Lung V20 and MLD were significantly related to RP of Grade 2 or more. These were not significant factors of RP of Grade 2 or more in our study; however, we think that the reason might be that our data set was small.
The limitation of this study is that possible selection bias with regard to the predictive factors cannot be ruled out because the present study was a retrospective series. Formal prospective studies are needed to confirm our findings.
In conclusion, subclinical ILD before SBRT was the only factor significantly associated with the occurrence of Grade 2–5 RP (p < 0.001). Moreover, the cases with honeycombing had high potential for fatality owing to severe RP. Therefore, patients with subclinical ILD, especially those with honeycombing, should be carefully monitored, with caution, for the occurrence of severe RP after SBRT.
Acknowledgments
ACKNOWLEDGMENTS
The authors wish to thank radiological technologist H Tiba, and Dr Y Tajima and Dr K Tokumasu for their professional assistance.
Contributor Information
Mitsuru Okubo, Email: okubo@tokyo-med.ac.jp.
Tomohiro Itonaga, Email: ti19850704@yahoo.co.jp.
Tatsuhiko Saito, Email: saito19880704@gmail.com.
Sachika Shiraishi, Email: s-nogi@tokyo-med.ac.jp.
Ryuji Mikami, Email: mikami-r@tokyo-med.ac.jp.
Hidetugu Nakayama, Email: h7nakayam@gmail.com.
Akira Sakurada, Email: kouayu0306@yahoo.co.jp.
Shinji Sugahara, Email: ssuga@tokyo-med.ac.jp.
Kiyoshi Koizumi, Email: kkoi@tokyo-med.ac.jp.
Koichi Tokuuye, Email: ktokuue@tokyo-med.ac.jp.
REFERENCES
- 1.Nagata Y, Negoro Y, Aoki T, Mizowaki T, Takayama K, Kokubo M, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2002; 52: 1041–6. doi: https://doi.org/10.1016/s0360-3016(01)02473-7 [DOI] [PubMed] [Google Scholar]
- 2.Hara R, Itami J, Kondo T, Aruga T, Uno T, Sasano N, et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer 2006; 106: 1347–52. doi: https://doi.org/10.1002/cncr.21747 [DOI] [PubMed] [Google Scholar]
- 3.Onimaru R, Fujino M, Yamazaki K, Onodera Y, Taguchi H, Katoh N, et al. Steep dose-response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70: 374–81. doi: https://doi.org/10.1016/j.ijrobp.2007.06.043 [DOI] [PubMed] [Google Scholar]
- 4.Hof H, Muenter M, Oetzel D, Hoess A, Debus J, Herfarth K. Stereotactic single-dose radiotherapy (radiosurgery) of early stage non small-cell lung cancer (NSCLC). Cancer 2007; 110: 148–55. doi: https://doi.org/10.1002/cncr.22763 [DOI] [PubMed] [Google Scholar]
- 5.Le QT, Loo BW, Ho A, Cotrutz C, Koong AC, Wakelee H, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol 2006; 1: 802–9. doi: https://doi.org/10.1097/01243894-200610000-00008 [PubMed] [Google Scholar]
- 6.Onishi H, Araki T. Stereotactic body radiation therapy for stage I non-small-cell lung cancer: a historical overview of clinical studies. Jpn J Clin Oncol 2013; 43: 345–50. doi: https://doi.org/10.1093/jjco/hyt014 [DOI] [PubMed] [Google Scholar]
- 7.Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol 2010; 94: 1–11. doi: https://doi.org/10.1016/j.radonc.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 8.Simone CB, 2nd, Wildt B, Haas AR, Pope G, Rengan R, Hahn SM. Stereotactic body radiation therapy for lung cancer. Chest 2013; 143: 1784–90. doi: https://doi.org/10.1378/chest.12-2580 [DOI] [PubMed] [Google Scholar]
- 9.Onimaru R, Shirato H, Shimizu S, Kitamura K, Xu B, Fukumoto S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys 2003; 56: 126–35. doi: https://doi.org/10.1016/s0360-3016(03)00095-6 [DOI] [PubMed] [Google Scholar]
- 10.Yamashita H, Nakagawa K, Nakamura N, Koyanagi H, Tago M, Igaki H, et al. Exceptionally high incidence of symptomatic Grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007; 2: 21. doi: https://doi.org/10.1186/1748-717x-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley JD, Hope A, El Naqa I, Apte A, Lindsay PE, Bosch W, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys 2007; 69: 985–92. doi: https://doi.org/10.1016/j.ijrobp.2007.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hope AJ, Lindsay PE, El Naqa I, Alaly JR, Vicic M, Bradley JD, et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys 2006; 65: 112–24. doi: https://doi.org/10.1016/j.ijrobp.2005.11.046 [DOI] [PubMed] [Google Scholar]
- 13.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999; 45: 323–9. doi: https://doi.org/10.1016/s0360-3016(99)00183-2 [DOI] [PubMed] [Google Scholar]
- 14.Goto K, Kodama T, Sekine I, Kakinuma R, Kubota K, Hojo F, et al. Serum levels of KL-6 are useful biomarkers for severe radiation pneumonitis. Lung Cancer 2001; 34: 141–8. doi: https://doi.org/10.1016/s0169-5002(01)00215-x [DOI] [PubMed] [Google Scholar]
- 15.Kong FM, Ao X, Wang L, Lawrence TS. The use of blood biomarkers to predict radiation lung toxicity: a potential strategy to individualize thoracic radiation therapy. Cancer Control 2008; 15: 140–50. [DOI] [PubMed] [Google Scholar]
- 16.Nishio T, Kunieda E, Shirato H, Ishikura S, Onishi H, Tateoka K, et al. Dosimetric verification in participating institutions in a stereotactic body radiotherapy trial for stage I non-small cell lung cancer: Japan clinical oncology group trial (JCOG0403). Phys Med Biol 2006; 51: 5409–17. doi: https://doi.org/10.1088/0031-9155/51/21/002 [DOI] [PubMed] [Google Scholar]
- 17.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. doi: https://doi.org/10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann P, Nyman J, Hoyer M, Gagliardi G, Lax I, Wennberg B, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer—a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol 2008; 88: 359–67. doi: https://doi.org/10.1016/j.radonc.2008.07.019 [DOI] [PubMed] [Google Scholar]
- 19.Lindberg K, Nyman J, Riesenfeld Källskog V, Hoyer M, Lund JÅ, Lax I, et al. Long-term results of a prospective phase II trial of medically inoperable stage I NSCLC treated with SBRT—the Nordic experience. Acta Oncol 2015; 54: 1096–104. doi: https://doi.org/10.3109/0284186x.2015.1020966 [DOI] [PubMed] [Google Scholar]
- 20.Takahashi W, Yamashita H, Kida S, Masutani Y, Sakumi A, Ohtomo K, et al. Verification of planning target volume settings in volumetric modulated arc therapy for stereotactic body radiation therapy by using in-treatment 4-dimensional cone beam computed tomography. Int J Radiat Oncol Biol Phys 2013; 86: 426–31. doi: https://doi.org/10.1016/j.ijrobp.2013.02.019 [DOI] [PubMed] [Google Scholar]
- 21.Yamashita H, Kobayashi-Shibata S, Terahara A, Okuma K, Haga A, Wakui R, et al. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat Oncol 2010; 5: 32. doi: https://doi.org/10.1186/1748-717X-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005; 63: 5–24. doi: https://doi.org/10.1016/j.ijrobp.2005.03.047 [DOI] [PubMed] [Google Scholar]
- 23.Oh D, Ahn YC, Park HC, Lim DH, Han Y. Prediction of radiation pneumonitis following high-dose thoracic radiation therapy by 3 Gy/fraction for non-small cell lung cancer: analysis of clinical and dosimetric factors. Jpn J Clin Oncol 2009; 39: 151–7. doi: https://doi.org/10.1093/jjco/hyn158 [DOI] [PubMed] [Google Scholar]
- 24.Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys 2013; 85: 190–5. doi: https://doi.org/10.1016/j.ijrobp.2012.03.041 [DOI] [PubMed] [Google Scholar]
- 25.Matsuo Y, Shibuya K, Nakamura M, Narabayashi M, Sakanaka K, Ueki N, et al. Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: e545–9. doi: https://doi.org/10.1016/j.ijrobp.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 26.Borst GR, Ishikawa M, Nijkamp J, Hauptmann M, Shirato H, Onimaru R, et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol 2009; 91: 307–13. doi: https://doi.org/10.1016/j.radonc.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 27.Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012; 82: 457–62. doi: https://doi.org/10.1016/j.ijrobp.2010.08.056 [DOI] [PubMed] [Google Scholar]
- 28.Iwata H, Shibamoto Y, Baba F, Sugie C, Ogino H, Murata R, et al. Correlation between the serum KL-6 level and the grade of radiation pneumonitis after stereotactic body radiotherapy for stage I lung cancer or small lung metastasis. Radiother Oncol 2011; 101: 267–70. doi: https://doi.org/10.1016/j.radonc.2011.05.031 [DOI] [PubMed] [Google Scholar]
- 29.Onishi H, Marino K, Terahara A, Kokubo M, Onimaru R, Shioyama Y, et al. Case series study of 26 patients who developed fatal radiation pneumonitis (RP) after stereotactic body radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2009; 75: S62. doi: https://doi.org/10.1016/j.ijrobp.2009.07.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda A, Enomoto T, Sanuki N, Nakajima T, Takeda T, Sayama K, et al. Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Radiat Med 2008; 26: 504–7. doi: https://doi.org/10.1007/s11604-008-0261-8 [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi S, Ohguri T, Ide S, Aoki T, Imada H, Yahara K, et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis. Lung Cancer 2013; 82: 260–5. doi: https://doi.org/10.1016/j.lungcan.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 32.Ishijima M, Nakayama H, Itonaga T, Tajima Y, Shiraishi S, Okubo M, et al. Patients with severe emphysema have a low risk of radiation pneumonitis following stereotactic body radiotherapy. Br J Radiol 2015; 88: 20140596. doi: https://doi.org/10.1259/bjr.20140596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueki N, Matsuo Y, Togashi Y, Kubo T, Shibuya K, Iizuka Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol 2015; 10: 116–25. doi: https://doi.org/10.1097/JTO.0000000000000359 [DOI] [PubMed] [Google Scholar]
- 34.Guckenberger M, Baier K, Polat B, Richter A, Krieger T, Wilbert J, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 2010; 97: 65–70. doi: https://doi.org/10.1016/j.radonc.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 35.Takeda A, Ohashi T, Kunieda E, Sanuki N, Enomoto T, Takeda T, et al. Comparison of clinical, tumour-related and dosimetric factors in grade 0-1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br J Radiol 2012; 85: 636–42. doi: https://doi.org/10.1259/bjr/71635286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara R, Itami J, Komiyama T, Katoh D, Kondo T. Serum levels of KL-6 for predicting the occurrence of radiation pneumonitis after stereotactic radiotherapy for lung tumors. Chest 2004; 125: 340–4. doi: https://doi.org/10.1378/chest.125.1.340 [DOI] [PubMed] [Google Scholar]
- 37.Kanemoto A, Matsumoto Y, Sugita T. Timing and characteristics of radiation pneumonitis after stereotactic body radiotherapy for peripherally located stage I lung cancer. Int J Clin Oncol 2015; 20: 680–5. doi: https://doi.org/10.1007/s10147-014-0766-3 [DOI] [PubMed] [Google Scholar]
- 38.Alite F, Balasubramanian N, Adams W, Surucu M, Mescioglu I, Harkenrider MM. Decreased risk of radiation pneumonitis with coincident concurrent use of angiotensin-converting enzyme inhibitors in patients receiving lung stereotactic body radiation therapy. Am J Clin Oncol. 2016. Epub ahead of print. doi: https://doi.org/10.1097/coc.0000000000000324 [DOI] [PubMed] [Google Scholar]
- 39.Chang JY, Liu H, Balter P, Komaki R, Liao Z, Welsh J, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiat Oncol 2012; 7: 152. doi: https://doi.org/10.1186/1748-717x-7-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue T, Katoh N, Onimaru R, Shimizu S, Tsuchiya K, Suzuki R, et al. Stereotactic body radiotherapy using gated radiotherapy with real-time tumor-tracking for stage I non-small cell lung cancer. Radiat Oncol 2013; 8: 69. doi: https://doi.org/10.1186/1748-717X-8-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aibe N, Yamazaki H, Nakamura S, Tsubokura T, Kobayashi K, Kodani N, et al. Outcome and toxicity of stereotactic body radiotherapy with helical tomotherapy for inoperable lung tumor: analysis of Grade 5 radiation pneumonitis. J Radiat Res 2014; 55: 575–82. doi: https://doi.org/10.1093/jrr/rrt146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J, Yorke ED, Li L, Kavanagh BD, Li XA, Das S, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys 2016; 95: 1357–66. doi: https://doi.org/10.1016/j.ijrobp.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys 1995; 31: 361–9. doi: https://doi.org/10.1016/0360-3016(94)00477-3 [DOI] [PubMed] [Google Scholar]
- 44.Roberts CM, Foulcher E, Zaunders JJ, Bryant DH, Freund J, Cairns D, et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med 1993; 118: 696–700. doi: https://doi.org/10.7326/0003-4819-118-9-199305010-00006 [DOI] [PubMed] [Google Scholar]