Abstract
Objective:
To assess the value of coronal short-tau inversion recovery whole-body MRI (STIR-WBMRI) for screening osteonecrosis in patients with polymyositis (PM)/dermatomyositis (DM).
Methods:
The imaging and medical records of 129 patients with PM/DM who met the Bohan and Peter diagnostic criteria were retrospectively analyzed. STIR-WBMRI was performed in all patients. 18 patients had follow-up STIR-WBMRI. 12 patients underwent regional knee and/or hip MRI while 25 patients underwent radiography of the lower extremities.
Results:
STIR-WBMRI detected osteonecrosis in 15 (11.6%) patients. 38 joints were affected (mean, 2.5 per patient; range, 1–5 joints). Of the 38 joints affected by osteonecrosis, 33 had no clinical symptoms. Among the 12 patients who underwent regional MRI, STIR-WBMRI detected all 10 osteonecrotic sites seen on the regional MRI. The location, shape and size of the osteonecrotic lesions revealed on regional MRI were in accordance with those displayed on STIR-WBMRI. Of the 15 patients with osteonecrosis, 6 performed routine radiography of the affected joints and revealed no osteonecrotic lesions. Follow-up WBMRI detected new osteonecrosis in two patients whose first WBMRI revealed that there was no osteonecrosis in any skeleton.
Conclusion:
In addition to displaying muscle inflammation, STIR-WBMRI can efficiently detect early multifocal osteonecrosis in the whole bodies of patients with PM/DM.
Advances in knowledge:
In patients with PM/DM, WBMRI which takes 12–15 min can display muscular involvement and detect early multisite osteonecrosis in the whole body at the same time. Osteonecrotic lesions revealed by WBMRI are in accordance with those displayed on regional WBMRI.
INTRODUCTION
Polymyositis (PM) and dermatomyositis (DM) belong to the idiopathic inflammatory myopathy group of autoimmune diseases characterized by symmetrical proximal muscle weakness and inflammatory changes of skeletal muscle.1 Conventional MRI is the best modality of imaging to detect skeletal muscle abnormalities. Its values for diagnosing PM/DM, evaluating disease extent, monitoring disease progression or regression and targeting potential sites for biopsy have been widely recognized.2–4 On the other hand, conventional MRI includes limited scans of the proximal lower limb muscle girdle or scans of the proximal upper limb girdle if the patient is symptomatic at this site. Although the inflammation is clinically symmetric and often involves the proximal muscle groups, muscle involvement can be patchy and asymmetric, and the distal muscles of the upper and lower limbs may be involved.5,6 A limitation of conventional MRI is its inability to quickly scan a large volume of muscle; therefore, only part of the symptomatic muscles are imaged.
Reports in recent years have described the use of short-tau inversion recovery (STIR) whole-body MRI (WBMRI) in patients with PM/DM.5–7 STIR-WBMRI scans the entire body in a reasonable amount of time and collects images of the psoas, intercostal and neck muscles, which are not collected using conventional MRI. STIR-WBMRI has the advantage of evaluating entire body muscles as well as subcutaneous fat tissue, thus providing a complete assessment of the total inflammatory burden in patients with PM/DM and helping the clinician choose the appropriate treatment.
PM/DM are potentially treatable myopathies. Corticosteroids are not only the preferred treatment for patients with PM/DM but also the primary cause of non-traumatic osteonecrosis.8 Osteonecrosis is most commonly found in the hip, followed by the knee and shoulder.9 If untreated, 80% of patients with osteonecrosis will suffer articular surface collapse within a few years of disease progression.10 Once articular surface collapse occurs, osteoarthritis becomes inevitable and the function of the joint will be seriously affected, which will eventually lead to artificial joint replacement.11 Although hip replacement is an effective approach for relieving pain and improving hip function, its long-term performance remains unsatisfactory.12 Joint preservation without joint replacement depends on the diagnosis and treatment before subchondral fracture and subchondral bone plate collapse occur. Thus, screening early osteonecrosis in patients with PM/DM has important clinical significance. MRI is the most sensitive imaging tool with high specificity for detecting early osteonecrosis,13 whereas a STIR-WBMRI scan covers the bones of the entire body. Thus, we propose the following hypothesis: STIR-WBMRI can comprehensively display muscle inflammation and efficiently screen for early osteonecrosis in patients with PM/DM.
The imaging and medical records of 129 patients with PM/DM who underwent a STIR-WBMRI scan were retrospectively analyzed in this study. The objective was to assess the value of STIR-WBMRI for screening early osteonecrosis. To our knowledge, there is only one individual case report in the literature on the use of STIR-WBMRI to diagnose osteonecrosis in a patient with PM/DM.14
METHODS AND MATERIALS
Study population
The following were the inclusion criteria: (1) clinically diagnosed with PM/DM; (2) received muscle biopsy; (3) underwent a WBMRI examination; and (4) willingness to participate in the study. The study was approved by China-Japan friendship hospital ethics committee, and all patients signed the informed consent form. In our hospital, patients who were suspected to have PM/DM and who had no contraindications of the MRI examination routine underwent WBMRI. From August 2013 to April 2015, 165 patients underwent WBMRI. Of them, 129 patients met the inclusion criteria and were enrolled in this study, including 30 with PM and 99 with DM. There were 42 males and 87 females with a mean age of 43.3 ± 15.3 years (range, 12–86 years). The mean interval from the PM/DM symptom onset to the time of the first WBMRI examination was 30.8 ± 47.9 months (range, 10 days to 19 years). Of the 129 patients, 66 were newly diagnosed with PM/DM and 63 were previously diagnosed with PM/DM. 13 of the 66 newly diagnosed patients and all 63 of the previously diagnosed patients had a history of corticosteroid use before the first WBMRI examination. In addition, 12 patients underwent regional knee or hip MRI and 25 patients underwent radiography of the lower extremities within 1 week of the WBMRI examination.
MRI examination
All patients underwent a whole-body coronal MRI scan using a Philips-Ingenia 3.0-T MRI machine (Philips Medical Systems, Best, Netherlands) which employs an orthogonal body coil and an automatic moving bed technology. 18 patients underwent WBMRI twice or more. The time interval between the first and second WBMRI was 3–6 months. The WBMRI coronal scan used the STIR sequence with a repetition time (TR) of 3996 ms, echo time (TE) of 70 ms, inversion time of 230 ms, echo-train length of 53, field of view (FOV) of 512 × 320 mm, slice thickness of 7 mm, interslice spacing of 0.7 mm and slice number of 20. A coronal head-to-toe scan was performed in six consecutive segments, and the breath-holding technique was applied in the chest and abdominal scan. The scanning time for each segment was 17.8 s. Coronal WBMRI using STIR sequence took a total of 10–12 min, which included the time taken to position the image, move the bed, shimming etc. After the coronal scan was complete, the built-in Mobi View™ software (Philips Healthcare, Best, Netherland) was used to integrate the six segmental images into coronal whole-body images (Figure 1a).
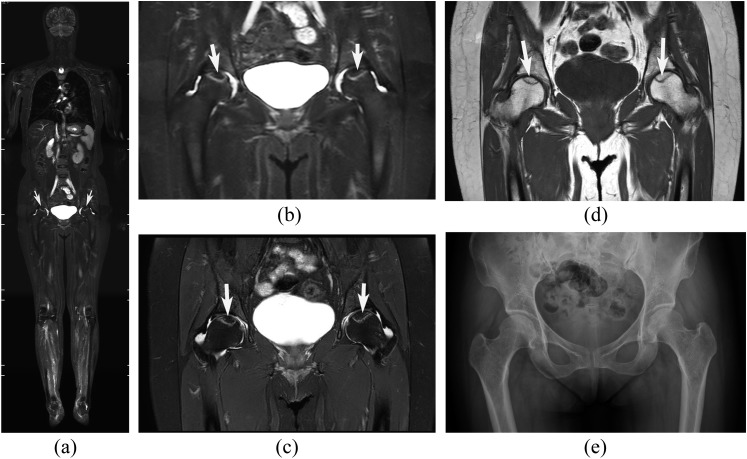
Figure 1.
Osteonecrosis of the bilateral femoral heads in a 50-year-old female with dermatomyositis. (a–e) Short-tau inversion recovery whole-body MRI (STIR-WBMRI) (a), the hip area of coronal STIR-WBMRI (b), corresponding regional hip MR T2 weighted spectral pre-saturation inversion recovery (T2W SPIR) (c), T1 weighted (T1W) images (d) and anteroposterior radiograph (e). The necrotic region (white arrows) is surrounded by a distinct high-intensity rim on STIR-WBMRI, T2W SPIR and is surrounded by a low-signal band on T1W. A high degree of consistency was demonstrated between STIR-WBMRI and regional hip MR on necrotic lesion size, shape and position. Radiography did not detect the osteonecrosis shown on STIR-WBMRI.
12 patients underwent regional knee and/or hip MRI using the same MRI machine within 1 week of the WBMRI examination. For the hips, coronal and sagittal T1 weighted (T1W) and T2 weighted spectral pre-saturation inversion recovery (T2W SPIR) sequences and an axial T2W SPIR sequence were obtained. For the knees, coronal and sagittal T1W and T2W SPIR sequences were used. The T1W sequences were obtained using a TR of 543 ms and TE of 10 ms, whereas the T2W SPIR sequences were obtained using a shortest TR and TE of 60 ms. For the hips, a slice thickness of 3 mm, interslice spacing of 0.3 mm, FOV of 350 × 350 mm and slice number of 22 were used. For the knees, a slice thickness of 4 mm, interslice spacing of 0.5 mm, FOV of 200 × 200 mm and slice number of 24 were used.
Image analysis
Osteonecrosis was defined as circumscribed lesions with a distinct rim of high signal intensity in the normally low-intensity marrow on STIR-WBMRI, T2W SPIR images (Figures 1b,c and 2) and circumscribed lesions with a distinctly low-intensity rim in the normally high-intensity marrow on T1W images (Figure 1d).15,16 Two experienced radiologists blinded to the patients' clinical manifestations and medical history independently judged whether osteonecrosis could be diagnosed on STIR-WBMRI, regional MRI and radiographs. Disagreements were adjudicated by a third radiologist with more than 20 years of experience in musculoskeletal imaging diagnosis. To avoid mutual interference on osteonecrotic diagnosis, the STIR-WBMRI, regional MRI and radiographs were analyzed on different days, and the observers were blinded to other imaging data from the same patients. The kappa test was used to evaluate the agreement between the two observers regarding the diagnosis of osteonecrosis on STIR-WBMRI.
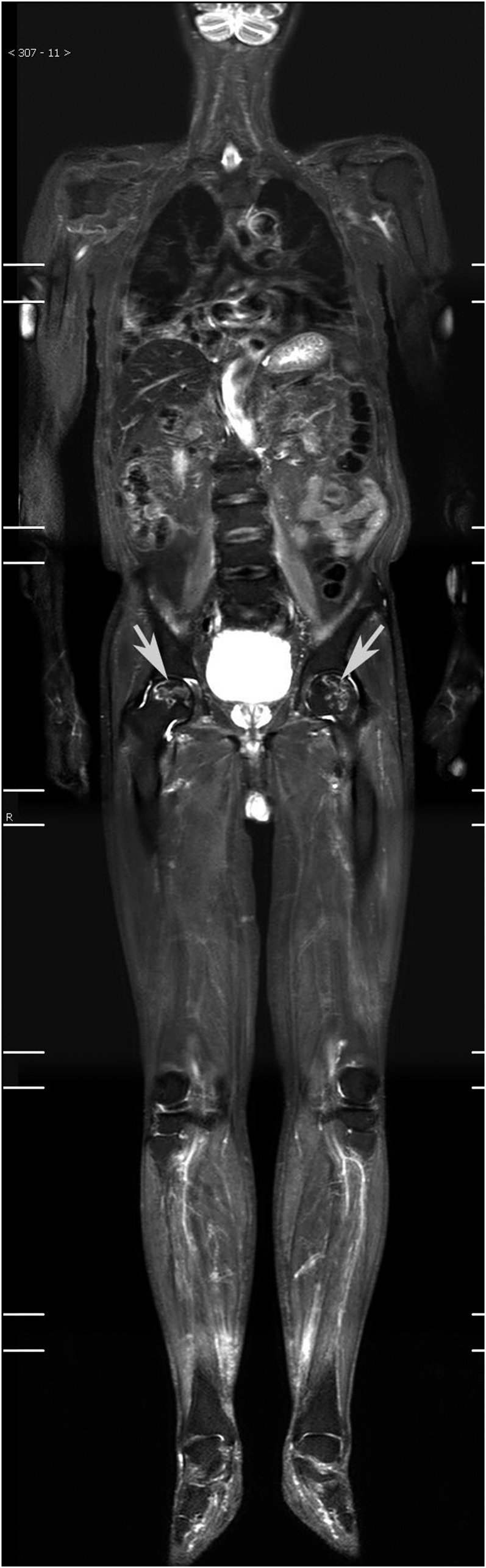
Figure 2.
Osteonecrosis of the bilateral femoral heads in a 43-year-old male with dermatomyositis. A distinct high-intensity rim (white arrows) in the normally low-intensity marrow surrounds the osteonecrotic lesions at the bilateral femoral heads.
The presence or absence of articular surface collapse was observed in all cases of osteonecrosis. Osteonecrotic lesions in the knee area were classified on the basis of whether the medial, lateral femoral condyle and the medial, lateral tibial condyle were involved. Additional characteristics of the lesions located in the medial, lateral femoral condyle and the medial, lateral tibial condyle included certain percentage (<25%, 25–50%, or >50%) of the articular surface was involved. Osteonecrotic lesions affecting the proximal femur were categorized according to the method of Sugano et al,17 which included an analysis of the osteonecrotic lesion affecting the proportion of the weight-bearing volume of the proximal femoral epiphysis.
RESULTS
STIR-WBMRI detected osteonecrosis in 15 (11.6%) patients. Among them, 3 patients were newly diagnosed with PM/DM, and 12 patients were previously diagnosed with PM/DM. The lesion involved the hip or knee in all 15 patients with osteonecrosis, and osteonecrosis involved the hip and knee in 3 patients. All patients with osteonecrosis had a history of using corticosteroid before the WBMRI examination. Until the diagnosis by WBMRI, the total dose of steroid ranged from 3100 to 18,950 mg prednisolone or its equivalent (mean 6354 mg) and the duration of steroid treatment ranged from 1 month to 84 months (mean 23.5 months). A total of 38 joints were affected (mean, 2.5 per patient; range, 1–5) (Table 1). The hip joint was the one most often involved (Figures 1a and 2), followed by the knee (Figures 3, 4b and 5), ankle (Figure 3) and shoulder. Osteonecrotic lesions were detected in both calcanei in one patient who had osteonecrosis of the bilateral knee joints (Figure 4d). 33 of the 38 joints with osteonecrosis had no related clinical symptoms. Among the 38 joints with osteonecrosis, only 1 hip joint showed articular surface collapse.
Table 1.
The distribution of 38 joints affected by osteonecrosis in 15 patients with polymyositis (PM)/dermatomyositis (DM)
| Patients | Number of joints/number of patients |
|||
|---|---|---|---|---|
| Hip | Knee | Ankle | Shoulder | |
| PM | 6/3 | 3/2 | 1/1 | 0/0 |
| DM | 13/8 | 10/5 | 2/1 | 3/2 |
| Total | 19/11 | 13/7 | 3/2 | 3/2 |
Figure 3.
Coronal short-tau inversion recovery whole-body MRI displaying osteonecrosis (white arrows) involving the bilateral distal femurs, proximal tibia and right distal tibia in a 40-year-old female with polymyositis.
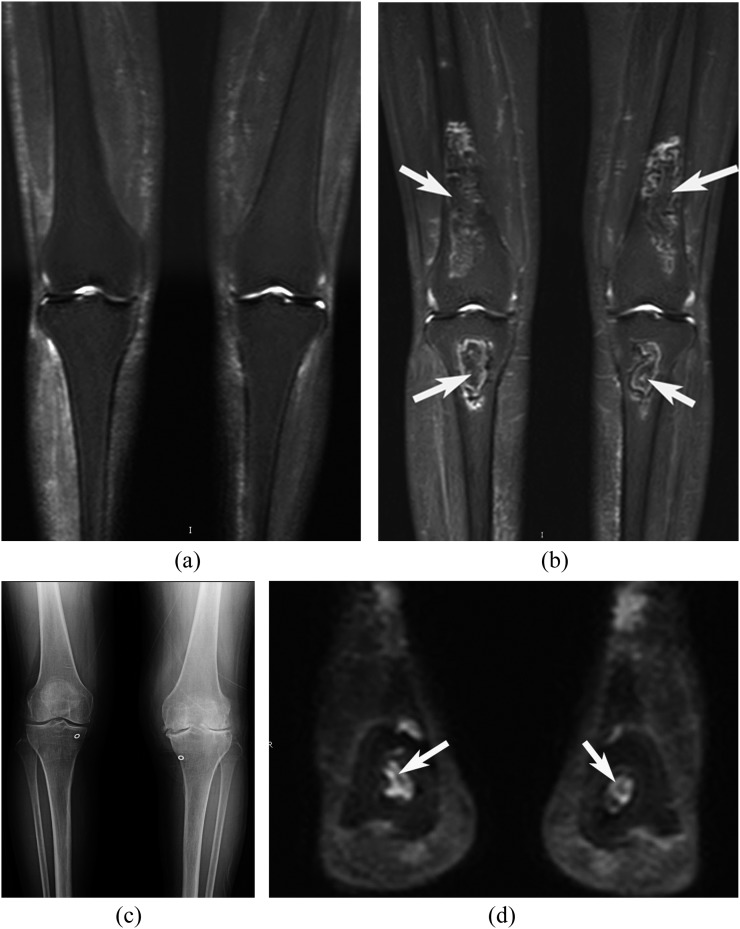
Figure 4.
Osteonecrosis of the bilateral knees and calcanei in a 55-year-old female with dermatomyositis. (a) The knee area of the coronal short-tau inversion recovery whole-body MRI (STIR-WBMRI) before treatment, (b–d) the knee area of the coronal STIR-WBMRI (b), anteroposterior radiograph of the bilateral knees (c) and the ankle area of the coronal STIR-WBMRI images (d) after 6 months of treatment with corticosteroids. The first STIR-WBMRI did not show osteonecrosis in bilateral knees before treatment (a). After 6 months of treatment with corticosteroids, STIR-WBMRI displayed osteonecrosis, but radiography did not show osteonecrosis. Meanwhile, osteonecrotic lesions in both calcanei were displayed on STIR-WBMRI.
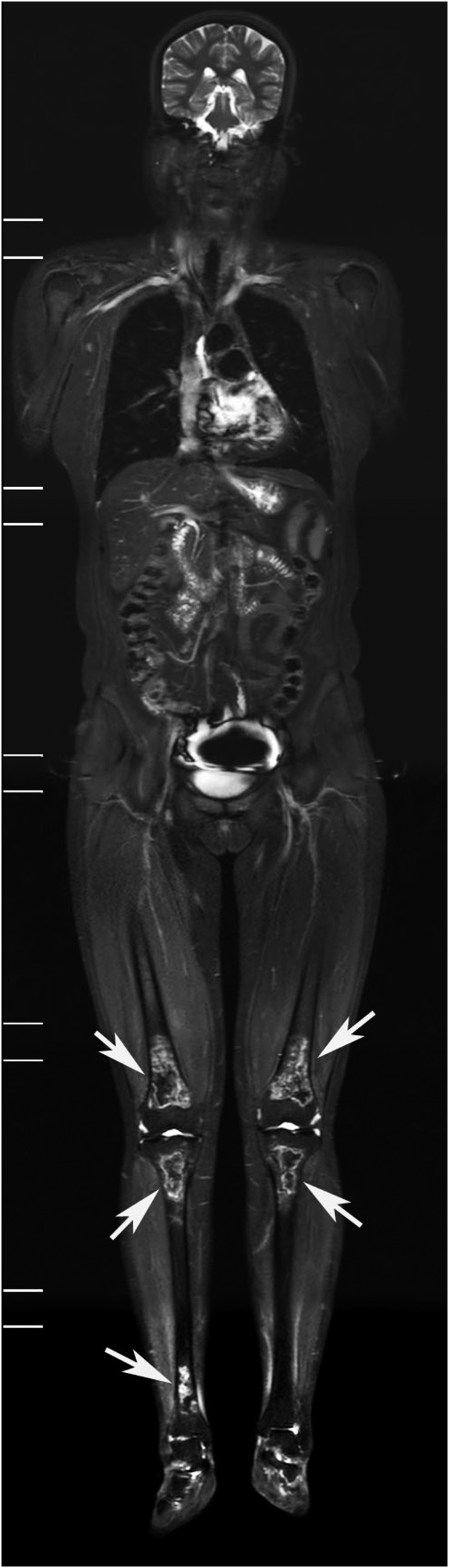
Figure 5.
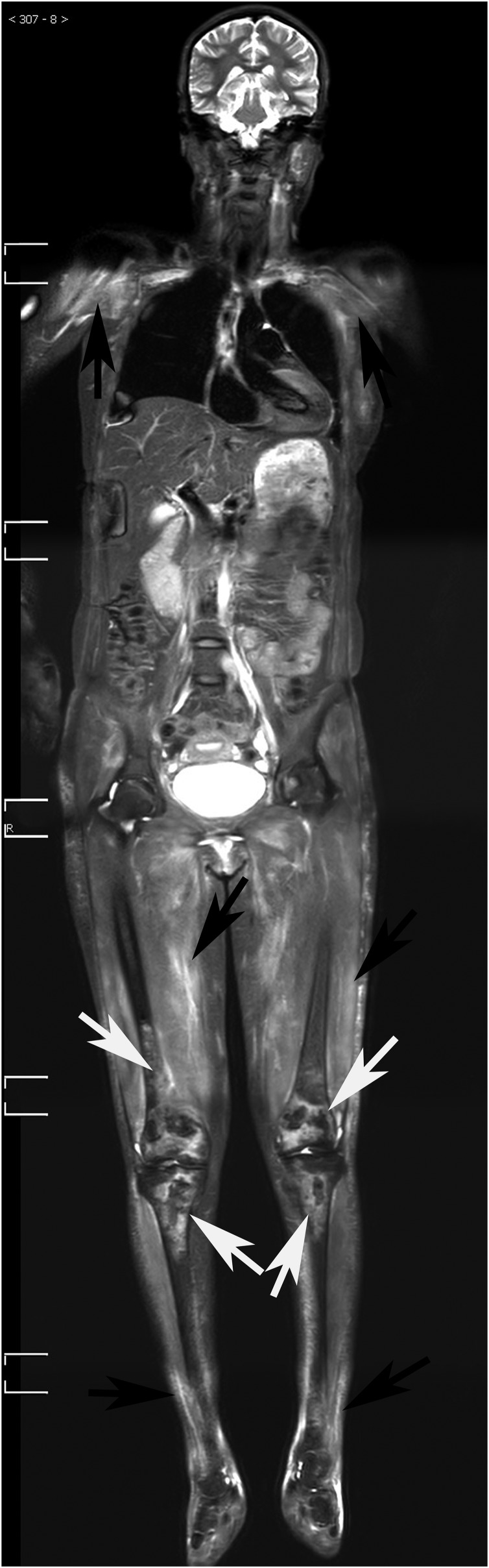
A 24-year-old female patient with dermatomyositis, displaying inflammatory muscular oedema, and osteonecrosis in both knees. Coronal short-tau inversion recovery whole-body MRI (STIR-WBMRI) showed significantly increased muscle signal at both the shoulders, thighs and legs (black arrows), suggesting the presence of inflammatory oedema. Meanwhile, coronal STIR-WBMRI displayed osteonecrosis involving the bilateral distal femurs and proximal tibia (white arrows).
Among the 19 hips with osteonecrosis, the osteonecrotic lesion affecting the proportion of the weight-bearing volume of the femoral head is shown in Table 2. Among the 13 knees with osteonecrosis, the range of lesion locations is shown in Table 3. Lesions affecting >50% of the articular surface were present in three cases of distal femoral osteonecrosis and two cases of proximal tibial osteonecrosis.
Table 2.
The extent of necrotic lesions in 19 hips
| Extent | The proportion of involvement of the weight-bearing area |
Total | ||
|---|---|---|---|---|
| <1/3 | 1/3–2/3 | >2/3 | ||
| Number | 2 | 4 | 13 | 19 |
Table 3.
The location of necrotic lesions in 13 knees
| Location | Simple distal femur | Simple proximal tibia | Distal femur and proximal tibia | Total |
|---|---|---|---|---|
| Number | 0 | 1 | 12 | 13 |
Among the 12 patients who underwent regional MRI, STIR-WBMRI detected all 10 osteonecrotic sites seen on the regional MRI scan. Lesion location, shape and size on regional MRI were comparable to those on STIR-WBMRI (Figure 1). Among the 15 patients with osteonecrosis, routine radiographs of the affected joints were performed in 6 patients (14 joints), and no osteonecrotic lesion was detected on radiographs (Figures 1e and 4c).
Of the 18 patients who underwent follow-up WBMRI, the follow-up WBMRI detected new osteonecrosis in 2 patients whose first WBMRI did not reveal osteonecrosis (Figures 4a,b). The follow-up STIR-WBMRI revealed that the number, shape and size of the osteonecrotic lesion did not change in all five patients whose first STIR-WBMRI displayed osteonecrosis.
The two observers had excellent consistency in determining whether osteonecrosis was present on STIR-WBMRI (kappa = 0.963).
DISCUSSION
Osteonecrosis is a process that occurs due to insufficient blood supply to the subchondral bone that leads to bone cell death. Corticosteroid treatment is a common aetiological factor of osteonecrosis. Corticosteroid-associated osteonecrosis has been observed in various diseases including systemic lupus, rheumatoid arthritis, asthma, inflammatory bowel diseases and organ transplantation. Osteonecrosis was described in various series in 5–25% of patients taking corticosteroids.18
The preferred therapy for PM/DM is corticosteroid. Thus, screening patients with PM/DM for early osteonecrosis has important clinical significance. Conventional regional MRI is the most sensitive imaging method for the diagnosis of early osteonecrosis. It can identify early osteonecrosis that is invisible on radiographs and CT scans.19,20 However, the early stage of osteonecrosis generally lacks clinical symptoms, and steroid-induced osteonecrosis always occurs simultaneously in multiple body sites.21 Regional MRI examinations can image only a single site at a time, therefore multiple regional MRI examinations are required to capture all lesions. STIR-WBMRI is a sensitive, a non-invasive and an efficient imaging method. Previous studies have shown that STIR-WBMRI can provide a comprehensive assessment of muscular involvement in patients with PM/DM.5–7 This study revealed that STIR-WBMRI could accurately display multisite osteonecrosis regardless of its location in the long bones of the lower and upper limbs and the small bones of the feet. STIR-WBMRI is able to screen for osteonecrosis of the entire skeleton within 12–15 min, comparing with conventional regional MRI, which requires 15–20 min per joint.
Osteonecrosis is a potentially disabling disease. Once MRI detects osteonecrosis, appropriate treatments should be taken into account. Conservative management of osteonecrosis includes rest and reduction of weight-bearing. Minimize glucocorticoid dosage as much as possible. During the early stage, an appropriate intervention can prevent or delay progression of the disease. Joint-preserving surgery should be a choice of treatment for young patients.22 Articular surface collapse is the tipping point of disease progression. Once it occurs, lesions enter the irreversible late stage. Being able to accurately predict the risk of articular surface collapse is critical to the selection of appropriate treatment and improvement of prognosis. Studies have found that the osteonecrotic lesion size and location are crucial factors of articular surface collapse and are best assessed by regional MRI.23,24 In this study, STIR-WBMRI detected all osteonecrotic sites seen on regional MRI, and the locations, shapes and sizes of all sites detected on STIR-WBMRI were comparable to those shown on regional MRI. Our study findings suggest that STIR-WBMRI can confirm osteonecrosis in sufficient detail, help predict the eventual osteonecrotic outcome and select appropriate treatments.
There have been a few reports in recent years on the application of STIR-WBMRI in patients with DM/PM, but the number of cases was relatively small (the largest being 41 patients), and the studies examined the performance of STIR-WBMRI for displaying muscle and subcutaneous tissue inflammation.5–7 Here, we performed a large case series of 129 patients to assess the value of STIR-WBMRI for screening early osteonecrosis in patients with PM/DM. Although STIR-WBMRI has been used to detect osteonecrosis in various paediatric disorders such as leukaemia21 and juvenile lupus,25 there was only one individual case report in the literature on the use of STIR-WBMRI to screen for osteonecrosis in patients with PM/DM.14
The present study has a few limitations. First, it was a single-centre retrospective study. Second, several studies have confirmed the value of STIR-WBMRI for detecting muscle inflammation.5–7 The purpose of this study was to evaluate whether STIR-WBMRI can simultaneously display muscle changes and screen for osteonecrosis in patients with PM/DM. Patients who were newly and previously diagnosed with PM/DM were included. Most patients who were newly diagnosed with PM/DM had not used corticosteroids before the STIR-WBMRI examination. Therefore, the number of steroid therapy-related osteonecrosis (15/129) might be underestimated. Third, we used only STIR-WBMRI and regional MRI to diagnose osteonecrosis since bone biopsies were considered invasive and impractical for multisite osteonecrosis. We used a MRI definition of osteonecrosis that is characterized by the presence of the classic band sign and has been validated by histological correlation.20
CONCLUSION
This study demonstrated that a relatively large proportion of patients with PM/DM who were treated with steroids developed multifocal osteonecrosis. Osteonecrosis is most commonly found in the hip, followed by the knee and the shoulder. STIR-WBMRI is a sensitive, a non-invasive and an efficient imaging method. It can not only comprehensively display muscle inflammation but also efficiently screen for early multifocal osteonecrosis (Figure 5), evaluate disease extent and monitor disease progression.
REFERENCES
- 1.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003; 362: 971–82. doi: https://doi.org/10.1016/s0140-6736(03)14368-1 [DOI] [PubMed] [Google Scholar]
- 2.Del Grande F, Carrino JA, Del Grande M, Mammen AL, Christopher Stine L. Magnetic resonance imaging of inflammatory myopathies. Top Magn Reson Imaging 2011; 22: 39–43. doi: https://doi.org/10.1097/rmr.0b013e31825b2c35 [DOI] [PubMed] [Google Scholar]
- 3.Tomasová Studynková J, Charvát F, Jarosová K, Vencovsky J. The role of MRI in the assessment of polymyositis and dermatomyositis. Rheumatology (Oxford) 2007; 46: 1174–9. doi: https://doi.org/10.1093/rheumatology/kem088 [DOI] [PubMed] [Google Scholar]
- 4.Connor A, Stebbings S, Anne Hung N, Hammond-Tooke G, Meikle G, Highton J. STIR MRI to direct muscle biopsy in suspected idiopathic inflammatory myopathy. J Clin Rheumatol 2007; 13: 341–5. doi: https://doi.org/10.1097/rhu.0b013e31815dca0a [DOI] [PubMed] [Google Scholar]
- 5.Cantwell C, Ryan M, O'Connell M, Cunningham P, Brennan D, Costigan D, et al. A comparison of inflammatory myopathies at whole-body turbo STIR MRI. Clin Radiol 2005; 60: 261–7. doi: https://doi.org/10.1016/j.crad.2004.06.027 [DOI] [PubMed] [Google Scholar]
- 6.Ley S, Ley-Zaporozhan J, Schenk JP. Whole-body MRI in the pediatric patient. Eur J Radiol 2009; 70: 442–51. doi: https://doi.org/10.1016/j.ejrad.2009.02.012 [DOI] [PubMed] [Google Scholar]
- 7.O'Connell MJ, Powell T, Brennan D, Lynch T, McCarthy CJ, Eustace SJ. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am J Roentgenol 2002; 179: 967–71. doi: https://doi.org/10.2214/ajr.179.4.1790967 [DOI] [PubMed] [Google Scholar]
- 8.Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol 2007; 63: 16–28. doi: https://doi.org/10.1016/j.ejrad.2007.03.019 [DOI] [PubMed] [Google Scholar]
- 9.Aaron RK, Voisinet A, Racine J, Ali Y, Feller ER. Corticosteroid-associated avascular necrosis: dose relationships and early diagnosis. Ann N Y Acad Sci 2011; 1240: 38–46. doi: https://doi.org/10.1111/j.1749-6632.2011.06218.x [DOI] [PubMed] [Google Scholar]
- 10.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 1995; 77: 459–74. doi: https://doi.org/10.2106/00004623-199503000-00018 [DOI] [PubMed] [Google Scholar]
- 11.Jones LC, Hungerford DS. Osteonecrosis: etiology, diagnosis, and treatment. Curr Opin Rheumatol 2004; 16: 443–9. doi: https://doi.org/10.1097/01.moo.0000127829.34643.fd [DOI] [PubMed] [Google Scholar]
- 12.Babis GC, Soucacos PN. Effectiveness of total hip arthroplasty in the management of hip osteonecrosis. Orthop Clin North Am 2004; 35: 359–64. doi: https://doi.org/10.1016/j.ocl.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 13.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, et al. Osteonecrosis of the hip: Management in the 21st century. Instr Course Lect 2003; 52: 337–55. [PubMed] [Google Scholar]
- 14.Castro TC, Lederman H, Terreri MT, Kaste SC, Hilario MO. Detection of multifocal osteonecrosis in an adolescent with dermatomyositis using whole-body MRI. Pediatr Radiol 2010; 40: 1566–8. doi: https://doi.org/10.1007/s00247-010-1636-4 [DOI] [PubMed] [Google Scholar]
- 15.Cherian SF, Laorr A, Saleh KJ, Kuskowski MA, Bailey RF, Cheng EY. Quantifying the extent of femoral head involvement in osteonecrosis. J Bone Joint Surg Am 2003; 85-A: 309–15. [DOI] [PubMed] [Google Scholar]
- 16.Hu LB, Huang ZG, Wei HY, Wang W, Ren A, Xu YY. Osteonecrosis of the femoral head: using CT, MRI and gross specimen to characterize the location, shape and size of the lesion. Br J Radiol 2015; 88: 20140508. doi: https://doi.org/10.1259/bjr.20140508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res 1994; 305: 190–9. [PubMed] [Google Scholar]
- 18.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med 1992; 326: 1473–9. doi: https://doi.org/10.1056/NEJM199205283262206 [DOI] [PubMed] [Google Scholar]
- 19.Karantanas AH. Accuracy and limitations of diagnostic methods for avascular necrosis of the hip. Expert Opin Med Diagn 2013; 7: 179–87. doi: https://doi.org/10.1517/17530059.2013.757592 [DOI] [PubMed] [Google Scholar]
- 20.Mitchell MD, Kundel HL, Steinberg ME, Kressel HY, Alavi A, Axel L; Avascular necrosis of the hip: comparison of MR, CT, and scintigraphy. AJR Am J Roentgenol 1986; 147: 67–71. [DOI] [PubMed] [Google Scholar]
- 21.Miettunen PM, Lafay-Cousin L, Guilcher GM, Nettel-Aguirre A, Moorjani V. Widespread osteonecrosis in children with leukemia revealed by whole-body MRI. Clin Orthop Relat Res 2012; 470: 3587–95. doi: https://doi.org/10.1007/s11999-012-2579-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee S, Issa K, Pivec R, Kapadia BH, Khanuja HS, Mont MA. Osteonecrosis of the hip: treatment options and outcomes. Orthop Clin North Am 2013; 44: 463–76. doi: https://doi.org/10.1016/j.ocl.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 23.Lieberman JR, Engstrom SM, Meneghini RM, SooHoo NF. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res 2012; 470: 525–34. doi: https://doi.org/10.1007/s11999-011-2050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishii T, Sugano N, Ohzono K, Sakai T, Sato Y, Yoshikawa H. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification using magnetic resonance imaging. J Orthop Res 2002; 20: 130–6. doi: https://doi.org/10.1016/s0736-0266(01)00063-8 [DOI] [PubMed] [Google Scholar]
- 25.Castro TC, Lederman H, Terreri MT, Caldana WI, Kaste SC, Hilário MO. The use of joint-specific and whole-body MRI in osteonecrosis: a study in patients with juvenile systemic lupus erythematosus. Br J Radiol 2011; 84: 621–8. doi: https://doi.org/10.1259/bjr/34972239 [DOI] [PMC free article] [PubMed] [Google Scholar]