Abstract
Objective:
To test using the facial nerve as a reference for assessment of the cochlear nerve size in patients with acquired long-standing sensorineural hearing loss (SNHL) using MRI multiplanar reconstruction.
Methods:
The study was retrospectively performed on 86 patients. Group 1 (study group, n = 53) with bilateral long-standing SNHL. Group 2 (control group, n = 33) without hearing loss. The nerve size was measured by drawing a region of interest around the cross-sectional circumference of the nerve in multiplanar reconstruction images.
Results:
No significant correlation was noted between the cochlear nerve and facial nerve size, and the patient's age, gender and weight (p > 0.05). In Group 1, the mean ratio of the cochlear to facial nerve size was 0.99 ± 0.30 (range: 0.52–1.86) and 1.12 ± 0.35 (range: 0.34–2.3) for the right and left sides, respectively. In Group 2, it was 1.18 ± 0.23 (range: 0.78–1.71) and 1.25 ± 0.25 (range: 0.85–1.94) for the right and left sides, respectively. The cochlear nerve size was statistically (p = 0.0004) smaller in Group 1 than in Group 2.
Conclusion:
The cochlear nerve size and the cochlear to facial nerve size ratio are significantly smaller in patients with acquired long-standing SNHL.
Advances in knowledge:
The facial nerve can be used as a reference for assessment of the cochlear nerve in patients with acquired long-standing SNHL.
INTRODUCTION
Hearing loss represents the most common form of sensory impairment in an ageing population. It starts physiologically by the third decade of life and affects mainly the high frequencies.1 It starts to affect the speech frequencies during the fifth decade of life.2,3 When young, acquired sensorineural hearing loss (SNHL) could be idiopathic or as a result of trauma or infection, or be drug induced.4 MRI plays an important role in the assessment of patients with SNHL, especially before cochlear implantation. It is used to assess the fluid content of the cochlea and its morphology, to exclude other causes of hearing loss and neoplastic processes, especially vestibular Schwannoma, and to assess the content of the internal auditory canal. The size (diameter) of the cochlear nerve is significantly strongly correlated with the total spiral ganglion cell count (p < 0.001).5 The residual spiral ganglion cell count is a very important parameter, and the size (diameter) of the cochlear nerve is significantly strongly correlated with the total spiral ganglion cell count, for patient assessment before cochlear implantation.6
Previously published work emphasized the change in size of the cochlear nerve in patients with long-standing hearing loss, both in children7 and in adults.8 Cochlear nerve deficiency has been described both in patients with congenital and acquired SNHL.9 The facial nerve has been subjectively used as a reference for assessment of the size of the cochlear nerve in children with congenital unilateral SNHL in a previous study.10 Although most studies described a form of cochlear nerve size reduction associated with SNHL,9,11 none of the previously published studies took into consideration possible constitutional differences or attempted to compare the size of the cochlear nerve to the size of other structures to neutralize such differences in an objective study in patients suffering from acquired long-standing SNHL.
On the basis of this, the current study was formulated to assess the size of the cochlear nerve in patients with acquired long-standing SNHL while taking into consideration the possible constitutional differences by comparing the size of the cochlear nerve to the adjacent facial nerve in an objective study.
METHODS AND MATERIALS
Patients
The ethical committee approved the current retrospective study, and informed consent was obtained from all patients before MRI examination. The study included 86 patients divided into 2 groups. Group 1 (study group, n = 53, 28 females and 25 males) with acquired bilateral long-standing hearing loss (more than 2 years). The mean duration of SNHL was 27.4 years (standard deviation: 13.5, range: 4–62 years). Group 2 (control group, n = 33, 11 females and 22 males) not suffering from hearing loss and referred for MRI examination of the inner ear for other reasons, including suspected vestibular Schwannoma (n = 15, none of them had Schwannoma), tinnitus (n = 8), vertigo (n = 5), otalgia (n = 3) and suspected glomus tumour (n = 2). In Group 2 patients, all MRI examinations showed no abnormalities of the cochlear or facial nerve.
Inclusion and exclusion criteria
Study group (Group 1)
The following were the inclusion criteria: patients with acquired bilateral long-standing SNHL who received high-resolution MRI of the Petrous bone for planning possible cochlear implantation between January 2011 and April 2012. The following were the exclusion criteria: patients with conductive hearing loss, patients with vestibular Schwannoma or other tumours of the internal auditory canal, patients with congenital hearing loss and patients with general contraindications to MRI examination, e.g. due to pacemaker or non-MRI-compatible implants.
Control group (Group 2)
The following were the inclusion criteria: patients without hearing loss who received high-resolution MRI of the Petrous bone between January 2011 and April 2012 and who were referred for MRI examination due to other abnormalities of the ear. The following were the exclusion criteria: patients with vestibular Schwannoma or other tumours of the internal auditory canal, patients with facial nerve abnormalities or suspected inflammation of the facial nerve and patients with general contraindications to MRI examination, e.g. due to pacemaker or non-MRI-compatible implants.
MRI examination and evaluation
All patients received an MRI examination including the high-resolution isotropic T2 weighted MRI sequence. All patients were examined using a 1.5-T MRI system (Magnetom® Avanto™ or Espree™; Siemens Medical Solutions, Forchheim, Germany) using a head coil in the head-first supine position.
For the current study evaluations, we used a T2 weighted three-dimensional (3D) variable-flip-angle turbo spin echo Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) isotropic sequence in the axial orientation covering the Petrous bone and planned parallel to the long axis of the Petrous bone (duration: 4 min and 36 s, orientation: transverse, repetition time/echo time: 1200/264, field of view: 200, matrix: 324 × 320, flip angle: 150°, slice thickness: 0.6 mm and voxel size: 0.6 × 0.6 × 0.6).
All multiplanar reconstructions (MPRs) were performed by a single radiologist with more than 12 years of experience in MRI of the head and neck. The reconstructions were performed using the 3D application of the syngo® workstation (Siemens Medical Solutions). All reconstructions were performed using the isotropic T2 weighted SPACE sequence of the Petrous bone (Figure 1). First, the images were loaded in the 3D application, and MPR was performed in the parasagittal orientation perpendicular to the long axis of the internal auditory canal (perpendicular to the long axis of the cochlear nerve). To ensure homogeneous and accurate estimation in all patients, no change in the display parameters (window or centre) was allowed and the display parameters were kept constant in all images.
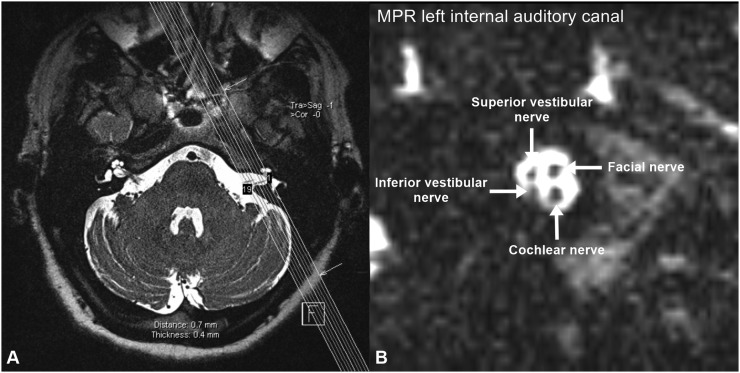
Figure 1.
Axial high-resolution T2 weighted MRI at the level of the internal auditory canal showing the orientation of the parasagittal multiplanar reconstruction (MPR) of the internal auditory canal (a) and the corresponding reconstructed image (b) showing the level of assessment where the four nerves (cochlear, facial, and superior and inferior vestibular nerves) are seen separately at the mid-point of the internal auditory canal.
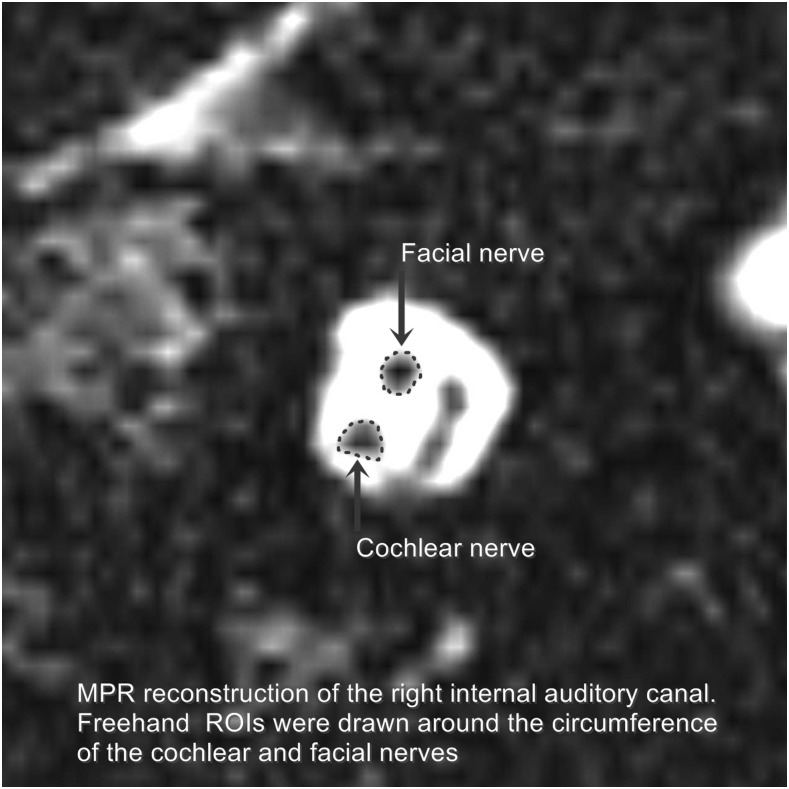
Image evaluation was performed by two radiologists with more than 12 and 11 years of experience in MRI of the head and neck in consensus. The reading radiologists were blinded to the clinical data of the patient. For evaluation, the MPR images were scrolled till the level where the four nerves (namely the facial, cochlear, superior vestibular and inferior vestibular nerves) were clearly separate from each other at the mid-point of the internal auditory canal (Figure 1). At this level, a region of interest (ROI) was carefully drawn along the perimeter of the cochlear nerve and facial nerve separately (Figure 2). The obtained cross-sectional surface area of each nerve was reported for each side separately. All surface areas were reported in square millimetres. The results of MPR image evaluations were tabulated to facilitate their analysis. For image analysis, the mean, standard deviation and range of the cochlear and facial nerve cross-sectional surface areas were calculated. The ratio of the cochlear nerve to facial nerve size was calculated by dividing the cross-sectional surface area of the cochlear nerve by the cross-sectional surface area of the ipsilateral facial nerve.
Figure 2.
Schematic representation of how the size of the cochlear nerve and facial nerve was measured by manually drawing a region of interest (ROI) around the nerve circumference in the parasagittal reconstructed T2 weighted MRI. MPR, multiplanar reconstruction.
Statistical analysis
The difference in cross-sectional surface area of the cochlear and facial nerves and the difference between the cochlear nerve to facial nerve size between the study and control groups were tested for statistical significance using the Wilcoxon Mann–Whitney U test. The correlation between the cochlear nerve and facial nerve size on one hand and the patient's weight and age on the other hand was tested for statistical significance using the Spearman rank correlation test. The sex differences regarding the size of the nerves were tested using Wilcoxon Mann–Whitney U test. Statistical analyses were performed using BiAS for Windows® (Epsilon Verlag, Darmstadt, Germany). p-values <0.05 were considered statistically significant.
RESULTS
The mean age of the patients in Group 1 was 51.3 ± 17.1 years (range: 14–81 years). The mean weight of the patients in Group 1 was 76.9 ± 14.8 kg (range: 50–110 kg). The mean age of the patients in Group 2 was 47.8 ± 17.1 years (range: 17–75 years). The mean weight of patients in Group 2 was 79.1 ± 10.8 kg (range: 58–98 kg). There was no statistically significant difference between both the groups regarding the age (p = 0.304) or weight (p = 0.396) of the patients.
Table 1 summarizes the results (p-values) of the statistical analysis regarding the correlation between the patient's age, gender and weight on one side and the size of the cochlear nerve and facial nerve on the other side. There was no statistically significant correlation between the patient's age, weight and gender and the size of the nerves on both sides (all p-values >0.05).
Table 1.
Correlation between patient's age, gender and weight and the size of the cochlear and facial nerves in both groups and on both sides
| Group variable | Cochlear nerve | Facial nerve |
|---|---|---|
| Group 1 | ||
| Patient's age | 0.280 | 0.746 |
| Patient's gender | 0.784 | 0.222 |
| Patient's weight | 0.828 | 0.589 |
| Group 2 | ||
| Patient's age | 0.151 | 0.188 |
| Patient's gender | 0.241 | 0.077 |
| Patient's weight | 0.112 | 0.449 |
Results are presented as p-values.
Cochlear nerve size
In Group 1, the mean cross-sectional surface area of the cochlear nerve on the right side was 1.29 ± 0.39 mm2 (range: 0.38–2.1 mm2), and it was 1.29 ± 0.33 mm2 (range: 0.57–2.2 mm2) on the left side.
In Group 2, the mean cross-sectional surface area of the cochlear nerve on the right side was 1.51 ± 0.35 mm2 (range: 1.00–2.3 mm2), and it was 1.53 ± 0.32 mm2 (range: 1.00–2.5 mm2) on the left side.
A statistically significant difference (p = 0.0004) was noted between both the groups regarding the cross-sectional surface area (size) of the cochlear nerve. There was no statistically significant difference between the right and left sides regarding the size of the cochlear nerve both in Group 1 (p = 0.50) and in Group 2 (p = 0.53).
Facial nerve size
In Group 1, the mean cross-sectional surface area of the facial nerve on the right side was 1.34 ± 0.39 mm2 (range: 0.66–2.3 mm2), and it was 1.21 ± 0.33 mm2 (range: 0.55–2.1 mm2) on the left side.
In Group 2, the mean cross-sectional surface area of the facial nerve on the right side was 1.33 ± 0.41 mm2 (range: 0.70–2.5 mm2), and it was 1.27 ± 0.34 mm2 (range: 0.67–2.3 mm2) on the left side.
No statistically significant difference (p = 0.735) was noted between both groups regarding the cross-sectional surface area (size) of the facial nerve. There was no statistically significant difference between the right and left sides regarding the size of the facial nerve both in Group 1 (p = 0.41) and in Group 2 (p = 0.48).
Cochlear nerve to facial nerve size ratio
In Group 1, the mean cochlear nerve to facial nerve size ratio on the right side was 0.99 ± 0.30 (range: 0.52–1.86), and it was 1.12 ± 0.35 (range: 0.34–2.3) on the left side.
In Group 2, the mean cochlear nerve to facial nerve size ratio on the right side was 1.18 ± 0.23 (range: 0.78–1.71), and it was 1.25 ± 0.25 (range: 0.85–1.94) on the left side.
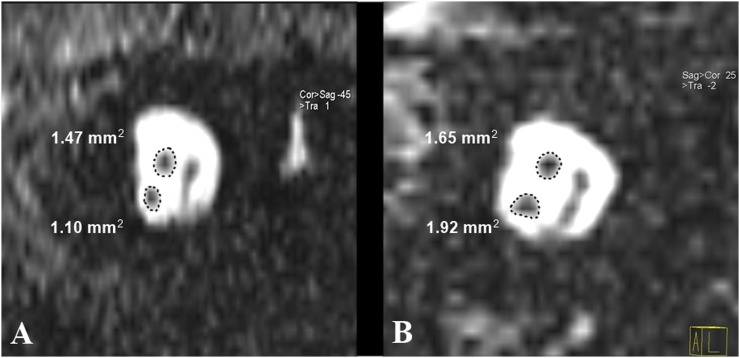
A statistically significant difference (p = 0.0001) was noted between both the groups regarding the cochlear nerve to facial nerve size ratio (Figure 3).
Figure 3.
Parasagittal reconstructed T2 weighted MRI from two patients (ROIs are schematically represented): one patient (a) from Group 1 with long-standing sensorineural hearing loss showing the small-sized cochlear nerve compared with the facial nerve and one patient (b) from Group 2 with cochlear nerve size larger than that of the facial nerve. cor, coronal; sag, sagital.
DISCUSSION
One of the most common indications for MRI examination of the temporal bone is hearing loss. The available high-resolution MRI sequences can provide a detailed description of the inner ear structures, including the neural structures, cochlea and vestibular system.12–14 Cochlear nerve changes in patients with long-standing hearing loss have been previously described in medical literature.7,15–17 Several studies described a decrease in cochlear nerve size in association with hearing loss,18 yet none of these studies kept into consideration the possible constitutional differences regarding the size of the cochlear nerve. The idea of relative size assessment of the nerves within the internal auditory canal has been previously described in literature both in normal subjects19 and in those with congenital nerve anomalies.20
The current study was performed to test the possible changes in the cochlear nerve size in association with long-standing SNHL while keeping in consideration the possible constitutional differences regarding the nerve size. Obvious constitutional differences such as patient's age, gender and weight were tested directly against the size of the nerve while other factors that might affect the nerve size were tested through comparison of the cochlear nerve size with the adjacently located facial nerve size. Furthermore, we tested the possibility of using the facial nerve as a reference to evaluate the size of the cochlear nerve by calculating the cochlear nerve to facial nerve size ratio. The choice of the facial nerve was based on the fact that the facial nerve is anatomically located in a very close proximity to the cochlear nerve, hence it will be included in the MRI examination of the cochlear nerve without the need to examine additional anatomical regions. The second reason is that the facial nerve (contrary to the vestibular nerve) is sharply delineated enabling its accurate size assessment. In addition, the facial nerve has been previously used in a similar study to comparatively assess the cochlear nerve size.10 The size assessment was performed at the mid-point of the internal auditory canal because, at this level, the four nerves can be clearly separated from each other.7
The results of the current study showed that patients with profound (planned for cochlear implantation) long-standing (more than 2 years) SNHL have a statistically significant smaller size of the cochlear nerve than subjects with normal hearing. The facial nerve size was similar in both the groups, and the ratio of the cochlear nerve to facial nerve size was statistically significantly lower in the study group than in the control group. The exact reason for the small-sized cochlear nerve in patients with long-standing SNHL is not clear; in fact, it is not clear whether the small size of the nerve is the cause of the hearing loss or whether it is the result of hearing loss as a form of disuse atrophy of the nerve for example. In their study, Clemmens et al18 referred to cochlear nerve deficiency as a cause and not as a result of SNHL in children with unilateral SNHL.
Interestingly, a case report presented by Furuta et al15 showed that the cochlear nerve size on the side with SNHL in a patient with unilateral hearing loss was smaller than in a patient with the normal hearing side. The described results agree with our findings of the reduced size of the cochlear nerve in association with SNHL and that these changes are not related to constitutional differences. A study performed by McClay et al10 used the facial nerve as a reference to assess the size of the cochlear nerve in children suffering from unilateral SNHL. Although the principle of evaluation is similar to our current study, there were several differences between both studies. Firstly, the authors used the facial nerve to subjectively assess the cochlear nerve and divide it into normal in size or deficient, where normal referred to cochlear nerves which were equal to or larger than the adjacent facial nerve and deficient cochlear nerves referred to those nerves smaller than the facial nerve. In the current study, we performed an objective size evaluation of the size of the facial and cochlear nerves. Secondly, the authors included patients with congenital unilateral SNHL, hence they included also cases with congenitally deficient or even absent cochlear nerves. In the current study, we addressed the changes that happened in patients with acquired bilateral SNHL in a more advanced age group of patients.
The current study results also agree with what was previously reported regarding the lack of correlation between the cochlear nerve size and the age of the patient.8,21 Similarly, the described lack of sex differences regarding the size of the nerves agrees with what was previously published in literature.17,22 A similar study by Kang et al22 concluded that the size of the facial nerve is not affected by the sex of the patient or age for adults. Regarding the correlation of the patient's weight with the size of the cochlear and facial nerves, we did not find comparable studies in the medical literature.
A similar study to the current study by Sildiroglu et al17 concluded that there is no effect of SNHL on the size of the cochlear nerve. The current study results do not agree with what was reported by Sildiroglu et al.17 Reasons of such discrepancy include: firstly, the small number of patients included in their study, where only 10 patients were compared with 14 volunteers. Secondly, it is not clear whether the study group of 10 patients had long-standing hearing loss or whether they included cases with recent hearing loss too. Thirdly, the selected patients had a speech discrimination score <90%, and this means that the study group included patients with hearing loss that was not profound; in our study, we included only patients with profound long-standing hearing loss necessitating cochlear implantation. Finally, the used method for assessment of the nerve size was based on special software to assess the perimeter and cross-sectional surface area of the cochlear nerve; in our study, we performed measurements using manual freehand ROI drawing and measurement.
In their study, Nakamichi et al23 reported the size of the normal facial and cochlear nerves in patients with normal hearing. The reported cross-sectional surface area is relatively smaller than the current study measurements, where the authors reported a size of 1.07 and 0.83 mm2 for the cochlear and facial nerves, respectively, compared with 1.51–1.53 mm2 for the cochlear nerve and 1.27–1.33 mm2 for the facial nerve for patients with normal hearing in the current study. The reason for such a discrepancy is probably related to the difference in measurement methods where Nakamichi et al assessed the surface area based on a two-dimensional calculation of the surface area using a mathematical equation, whereas the current study assessed the surface area using a ROI drawn around the nerve circumference.
Limitations of the current study include the retrospective nature of the study and the fact that we included patients with bilateral profound hearing loss to study the effect of hearing loss on cochlear nerve size. An optimal study design has to neutralize all constitutional factors by performing the study in patients with pure unilateral hearing loss and use the normal side for comparison. Although this was our idea in the beginning, it turned out to be very difficult to find such patients in an adequate sample size to obtain reliable results because all patients with apparent unilateral affection turned out to have some sort of hearing impairment on the apparently normal side (apart from congenital cases). Furthermore, we neutralized the constitutional differences using the facial nerve as a reference for changes in the size of the cochlear nerve.
In conclusion, patients with long-standing SNHL have a significantly smaller cross-sectional surface area of the cochlear nerve than patients with normal hearing. The facial nerve can be used as a reference to assess the size of the cochlear nerve in patients with SNHL with the ratio of cochlear to facial nerve size significantly lower in patients with hearing loss.
Contributor Information
Nagy N N Naguib, Email: nagynnn@yahoo.com.
Constanze Hey, Email: constanze.hey@kgu.de.
Mohamed S Shaaban, Email: mohamed.shaban@gmail.com.
Amr M Elabd, Email: amr_elabdmz@yahoo.com.
Hebatallah H M Hassan, Email: hebahassan13@gmail.com.
Tatjana Gruber-Rouh, Email: tgruberrouh@googlemail.com.
Benjamin Kaltenbach, Email: benjamin.kaltenbach@outlook.de.
Marc Harth, Email: marc.harth@gmx.de.
Hanns Ackermann, Email: ackermann@add.uni-frankfurt.de.
Timo Stöver, Email: timo.stoever@kgu.de.
Thomas J Vogl, Email: t.vogl@em.uni-frankfurt.de.
Nour-Eldin A Nour-Eldin, Email: nour410@hotmail.com.
REFERENCES
- 1.Roth TN, Hanebuth D, Probst R. Prevalence of age-related hearing loss in Europe: a review. Eur Arch Otorhinolaryngol 2011; 268: 1101–17. doi: https://doi.org/10.1007/s00405-011-1597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson EG, Hinojosa R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope 2006; 116: 1–12. doi: https://doi.org/10.1097/01.mlg.0000236089.44566.62 [DOI] [PubMed] [Google Scholar]
- 3.Soucek S, Michaels L, Frohlich A. Evidence for hair cell degeneration as the primary lesion in hearing loss of the elderly. J Otolaryngol 1986; 15: 175–83. [PubMed] [Google Scholar]
- 4.Tharpe AM, Sladen DP. Causation of permanent unilateral and mild bilateral hearing loss in children. Trends Amplif 2008; 12: 17–25. doi: https://doi.org/10.1177/1084713807313085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadol JB, Jr. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg 1997; 117(3 Pt 1): 220–8. [DOI] [PubMed] [Google Scholar]
- 6.Seyyedi M, Viana LM, Nadol JB, Jr. Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol Neurotol 2014; 35: 1446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo EE, Manolidis S, Morriss MC. Cochlear nerve size evaluation in children with sensorineural hearing loss by high-resolution magnetic resonance imaging. Am J Otolaryngol 2006; 27: 166–72. doi: https://doi.org/10.1016/j.amjoto.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 8.Kim BG, Chung HJ, Park JJ, Park S, Kim SH, Choi JY. Correlation of cochlear nerve size and auditory performance after cochlear implantation in postlingually deaf patients. JAMA Otolaryngol Head Neck Surg 2013; 139: 604–9. doi: https://doi.org/10.1001/jamaoto.2013.3195 [DOI] [PubMed] [Google Scholar]
- 9.Glastonbury CM, Davidson HC, Harnsberger HR, Butler J, Kertesz TR, Shelton C. Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol 2002; 23: 635–43. [PMC free article] [PubMed] [Google Scholar]
- 10.McClay JE, Booth TN, Parry DA, Johnson R, Roland P. Evaluation of pediatric sensorineural hearing loss with magnetic resonance imaging. Arch Otolaryngol Head Neck Surg 2008; 134: 945–52. [DOI] [PubMed] [Google Scholar]
- 11.Ylikoski J, Collan Y, Palva T. Pathologic features of the cochlear nerve in profound deafness. Arch Otolaryngol 1978; 104: 202–7. doi: https://doi.org/10.1001/archotol.1978.00790040024005 [DOI] [PubMed] [Google Scholar]
- 12.Kojima S, Suzuki K, Hirata M, Shinohara H, Ueno E. Depicting the semicircular canals with inner-ear MRI: a comparison of the SPACE and TrueFISP sequences. J Magn Reson Imaging 2013; 37: 652–9. doi: https://doi.org/10.1002/jmri.23863 [DOI] [PubMed] [Google Scholar]
- 13.Silver RD, Djalilian HR, Levine SC, Rimell FL. High-resolution magnetic resonance imaging of human cochlea. Laryngoscope 2002; 112: 1737–41. doi: https://doi.org/10.1097/00005537-200210000-00005 [DOI] [PubMed] [Google Scholar]
- 14.Jaryszak EM, Patel NA, Camp M, Mancuso AA, Antonelli PJ. Cochlear nerve diameter in normal hearing ears using high-resolution magnetic resonance imaging. Laryngoscope 2009; 119: 2042–5. doi: https://doi.org/10.1002/lary.20516 [DOI] [PubMed] [Google Scholar]
- 15.Furuta S, Ogura M, Higano S, Takahashi S, Kawase T. Reduced size of the cochlear branch of the vestibulocochlear nerve in a child with sensorineural hearing loss. AJNR Am J Neuroradiol 2000; 21: 328–30. [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano A, Arimoto Y, Matsunaga T. Cochlear nerve deficiency and associated clinical features in patients with bilateral and unilateral hearing loss. Otol Neurotol 2013; 34: 554–8. doi: https://doi.org/10.1097/mao.0b013e3182804b31 [DOI] [PubMed] [Google Scholar]
- 17.Sildiroglu O, Cincik H, Sonmez G, Ozturk E, Mutlu H, Gocgeldi E, et al. Evaluation of cochlear nerve size by magnetic resonance imaging in elderly patients with sensorineural hearing loss. Radiol Med 2010; 115: 483–7. doi: https://doi.org/10.1007/s11547-009-0440-4 [DOI] [PubMed] [Google Scholar]
- 18.Clemmens CS, Guidi J, Caroff A, Cohn SJ, Brant JA, Laury AM, et al. Unilateral cochlear nerve deficiency in children. Otolaryngol Head Neck Surg 2013; 149: 318–25. doi: https://doi.org/10.1177/0194599813487681 [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Kim DI, Chung IH, Lee WS, Kim KY. Topographical relationship of the facial and vestibulocochlear nerves in the subarachnoid space and internal auditory canal. AJNR Am J Neuroradiol 1998; 19: 1155–61. [PMC free article] [PubMed] [Google Scholar]
- 20.Casselman JW, Offeciers FE, Govaerts PJ, Kuhweide R, Geldof H, Somers T, et al. Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology 1997; 202: 773–81. doi: https://doi.org/10.1148/radiology.202.3.9051033 [DOI] [PubMed] [Google Scholar]
- 21.Lou J, Gong WX, Wang GB. Cochlear nerve diameters on multipoint measurements and effects of aging in normal-hearing children using 3.0-T magnetic resonance imaging. Int J Pediatr Otorhinolaryngol 2015; 79: 1077–80. doi: https://doi.org/10.1016/j.ijporl.2015.04.033 [DOI] [PubMed] [Google Scholar]
- 22.Kang WS, Hyun SM, Lim HK, Shim BS, Cho JH, Lee KS. Normative diameters and effects of aging on the cochlear and facial nerves in normal-hearing Korean ears using 3.0-tesla magnetic resonance imaging. Laryngoscope 2012; 122: 1109–14. doi: https://doi.org/10.1002/lary.23184 [DOI] [PubMed] [Google Scholar]
- 23.Nakamichi R, Yamazaki M, Ikeda M, Isoda H, Kawai H, Sone M, et al. Establishing normal diameter range of the cochlear and facial nerves with 3D-CISS at 3T. Magn Reson Med Sci 2013; 12: 241–7. doi: https://doi.org/10.2463/mrms.2013-0004 [DOI] [PubMed] [Google Scholar]