Abstract
Objective:
To review the imaging features of invasive lobular carcinoma (ILC) seen on digital breast tomosynthesis (DBT) in comparison with invasive ductal carcinoma (IDC), and to evaluate whether DBT could improve conspicuity and tumour size assessment of ILC in comparison with digital mammography (DM).
Methods:
Institutional review board with waiver of informed consent was obtained for this retrospective study. Patients with ILC or IDC who underwent DBT and DM at the time of diagnosis were included. DM and DBT images were reviewed in consensus by two breast radiologists in order to assess imaging features, conspicuity and maximum tumour diameter of ILC and IDC. Pathology on the surgical specimen was considered the standard of reference for assessment of tumour size.
Results:
43 patients (20 patients with ILC and 23 patients with IDC) were included. On DBT, compared with IDC, ILC presented less frequently as masses (40% vs 78%) (p = 0.01) and more frequently as isolated distortion (20% vs 0%) (p = 0.03). ILC presented more often as asymmetries (60%) than masses (20%) on DM (p = 0.02) but not on DBT (35% vs 40%; p = 1.00). Conspicuity of ILC was significantly higher on DBT than on DM (p = 0.002), while the difference between the two techniques was not significant for IDC (p = 0.2). Regarding ILC, concordance in tumour size measurement between DBT and pathology was fair (intraclass correlation coefficient = 0.24).
Conclusion:
ILC rarely presented as dense masses but frequently demonstrated architectural distortion on DBT. DBT increased lesion conspicuity but failed to accurately assess tumour size of ILC.
Advances in knowledge:
(1) This study describes specific features of ILC on DBT. (2) It shows that DBT can improve conspicuity of ILC.
INTRODUCTION
Invasive lobular carcinoma (ILC) is the second most frequent type of invasive breast cancer, accounting for 5–15% of all breast cancers.1 Pathologically, ILC consists of non-cohesive cells, infiltrating the adjacent breast tissue in a single line. Infiltration typically does not destroy anatomic structures or induce a substantial desmoplastic reaction. Because of these particular pathological features, ILC frequently fails to form a palpable lesion, and clinical detection may be difficult.2 ILC may also present particular characteristics on imaging and is known to be less visible on conventional imaging (mammography and ultrasound) than other types of breast cancer.3,4 As a result, mammograms are less sensitive for the detection of ILC than for invasive ductal carcinoma (IDC): up to 30% of ILCs are not visualized at mammography.5 Therefore, ILC tends to present with a larger size and at a later tumour stage at the time of diagnosis.6 In addition, mammography frequently underestimates tumour size of ILC.7
Digital breast tomosynthesis (DBT) is a recent three-dimensional technique based on digital mammography (DM) that is known to improve breast cancer detection rates and to reduce recall rates.8 On DBT, partial three-dimensional reconstruction of the breast is obtained from a finite number of two-dimensional projections, which are acquired as the X-ray tube moves along an arc. This technology reduces the summation of overlapping breast tissues and may increase detection of architectural distortion.9,10 Since ILC has a higher risk to be hidden by overlapping glandular breast tissue and is often associated with architectural distortion on two-dimensional mammography,11 it has recently been suggested that DBT might be of particular interest for the detection and characterization of ILC.12 Appearance of ILC on DBT has also been described recently.14 However, in this study, features of ILC on DBT have not been assessed in comparison with DM nor compared with those of IDC. Moreover, although accuracy of DBT for tumour size assessment has been previously investigated, it was not been evaluated specifically for ILC.15
In this context, the objectives of our study were (a) to review the imaging features of ILC on DBT in comparison with those of IDC and in comparison with those of ILC on DM according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon, (b) to evaluate whether DBT could improve conspicuity of ILC in comparison with DM and (c) to assess accuracy of DBT for estimation of tumour size of ILC.
METHODS AND MATERIALS
Study population
Patients with ILC diagnosed between March 2013 and October 2015 and who underwent DBT of the affected breast, on at least one view [mediolateral oblique (MLO) or craniocaudal (CC)], and a recent (less than 3 months) digital mammogram at the time of diagnosis were eligible for this retrospective study. Exclusion criteria were as follows: the presence of a post-biopsy marker clip on DM or DBT images and personal history of prior surgery of the ispsilateral breast. In order to compare features of ILC with those of IDC, we aimed to build a case/control study comprising a comparable number of patients with IDC. For that purpose, patients with IDC who underwent DBT within the same time period were identified. From this population, patients were randomly selected among those who met the inclusions criteria, and the same exclusion criteria as described above were applied.
Imaging acquisition
In our institution, DBT is performed as a complementary imaging examination in a diagnostic setting. Therefore, DBT is usually performed in one view (the view showing the abnormality on mammogram). All DBTs of our study were performed in our institution using the Selenia dimensions system (Hologic, Bedford, Mass). Bilateral digital mammograms were performed in two views (CC and MLO) either within or outside our institution. In cases where DM had been performed outside our institution, images were imported into our local picture archiving and communication system (IntelePACS, Intelerad® v. 4.2.4 P443, Canada).
Image analysis
Images were reviewed on dedicated picture archiving and communication system workstations. Two breast radiologists (Ellen Kao and Ann Aldis) with more than 20 years' experience in breast imaging and 3 years' experience in DBT, who were not the investigator, blinded to pathological results (IDC vs ILC) read the examinations in consensus. DM and DBT were read in two separate sessions. In order to reduce potential recall bias, DM was interpreted during a first round and DBT alone was interpreted during a second round 4 weeks later as previously reported. For DM, four views (CC and MLO of both breasts) were displayed. If >1 DBT view was available, the one on which the tumour appeared the most conspicuous was selected beforehand by the investigator. In situations where multiple lesions were present, instructions were given to the readers to assess the most prominent one (index tumour). For DM, lesion features were assessed according to the 2013 edition of the BI-RADS lexicon.16 Description of abnormalities on DBT is not defined in the BI-RADS lexicon. However, as routinely performed in our institution, readers were asked to describe lesions on DBT according to terms used in the fifth edition of the BI-RADS lexicon, as previously reported.17 In particular, the term “mass” was assigned to lesions clearly identified and occupying a volume, whereas “asymmetry” was assigned to lesions that had concave borders, contained fat or demonstrated low conspicuity. Since only one view was available and no previous examination was available for the readers, the terms “focal”, “global” and “developing” were not used for characterization of “asymmetries”.
Readers also assessed lesion conspicuity. Conspicuity was defined as the combination of the confidence in the presence of a lesion with the confidence in decision making based on lesion detectability.18 Conspicuity was assessed with the following four point scale, previously used by Andersson et al17 in a study comparing visibility of breast cancers on DBT and DM—0: no visible findings, 1: low conspicuity, 2: medium conspicuity and 3: high conspicuity. With each technique, readers were instructed to measure the maximum diameter of the index tumour.
Pathology and assessment of tumour size
A breast pathologist with more than 20 years' experience (Atilla Omeroglu) performed all histopathological examinations in our institution. When available, tumour subtype was determined from pathology reports of the final surgical specimen. In patients who underwent neoadjuvant chemotherapy (NAC) or had no surgery in our institution, tumour subtype was determined from reports of the ultrasound-guided 14-G core needle or 9-G stereotactic-guided vacuum-assisted biopsies. For assessment of tumour size, tumour maximum diameter on pathology of the surgical specimen was considered the gold standard. Therefore, patients who received NAC and those who did not undergo surgery were not included in this part of the study. In cases of multifocal breast cancer, the maximum diameter of the largest invasive tumour (index tumour) was assessed.
Statistical analysis
The relative frequency of features of ILC and IDC detected on both DBT and DM was evaluated. Proportions of features were compared between groups using the Fisher exact test. Lesion conspicuity was compared between ILC and IDC using a Mann–Whitney U test and between DM and DBT for each type of cancer using a Wilcoxon matched pairs test.
In order to assess accuracy of DM and DBT for estimation of index tumour size, the maximum diameter of the lesion detected by the readers as measured with each technique was compared with index tumour size on pathology using Wilcoxon matched pairs test. An intraclass correlation coefficient (ICC) was calculated to evaluate concordance in size measurement between imaging and pathology. The ICC is interpreted in the following way: 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, almost perfect agreement.19 In cases where tumours were not visible on imaging, tumour maximum size was reported as 0.
p-values ≤0.05 was considered as statistically significant. Statistical analysis was performed using GraphPad® software v. 5.04 (San Diego, CA).
RESULTS
Study population
Between 21 March 2013 and 15 October 2015, 108 patients with ILC were identified, among whom 27 patients had both DM and at least 1 DBT view at the time of diagnosis. Six patients were excluded because of the presence of a post-biopsy clip on DBT and one because of a history of ipsilateral conservative breast surgery. Within the same time period, 709 patients with IDC were identified. 190 of these patients had both DM and at least one DBT view at the time of diagnosis. Among them, 37 patients were randomly selected using the random function of Excel® (Microsoft, Redmond, WA). Of these, 13 patients had a post-biopsy clip on DBT images and 1 patient had a personal history of ispsilateral conservative surgery and were excluded. Therefore, 43 patients (20 patients with ILC and 23 patients with IDC) comprised the final study group. According to our protocol, one index lesion per patient was evaluated. In our study population, there was no significant difference in age (p = 0.12) between patients with ILC and patients with IDC. 40% (8/20) of ILC and 22% (5/23) of IDC were palpable.
Among these 43 patients, the surgical specimen was not suitable for final pathology assessment in 12 patients (3 patients received NAC and 9 patients did not have surgery in our institution). Accuracy of tumour size assessment on DM and DBT was therefore evaluated in a subgroup of the remaining 31 patients (14 patients with ILC and 17 patients with IDC).
Imaging features
ILC and DBT features on DM and DBT are summarized in Table 1.
Table 1.
Tumour features of invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) on digital mammography (DM) and digital breast tomosynthesis (DBT)
| DM | DBT | |||
|---|---|---|---|---|
| Imaging features | ILC (n = 20) | IDC (n = 23) | ILC (n = 20) | IDC (n = 23) |
| Asymmetry | 12 (60) | 5 (22) | 7 (35) | 3 (13) |
| Mass | 4 (20) | 16 (70) | 8 (40; 8/20) | 18 (78) |
| Shape | ||||
| Round | 0 | 2 (13) | 0 | 1 (6) |
| Oval | 0 | 2 (13) | 0 | 1 (6) |
| Irregular | 4 (100) | 12 (75) | 8 (100, 8/8) | 16 (88) |
| Margins | ||||
| Circumscribed | 0 | 0 | 0 | 1 (6) |
| Microlobulated | 0 | 0 | 0 | 1 (6) |
| Masked | 0 | 0 | 0 | 0 |
| Indistinct | 0 | 8 (50) | 2 (25) | 8 (44) |
| Spiculated | 4 (100) | 8 (50) | 6 (75) | 8 (44) |
| Density | ||||
| Fatty | 0 | 0 | 0 | 0 |
| Not dense | 0 | 4 (25) | 5 (62) | 6 (33) |
| Dense | 4 (100) | 12 (75) | 3 (38) | 12 (67) |
| Distortion | 13 (65) | 10 (43) | 16 (80) | 12 (52) |
| Presence of calcifications | 3 (15) | 6 (26) | 3 (15) | 3 (13) |
| Associated features | 4 (20) | 3 (13) | 4 (20) | 3 (13) |
| Skin retraction | 0 | 1 (33) | 1 (25) | 0 |
| Skin thickening | 0 | 0 | 0 | 1 (33) |
| Nipple retraction | 4 (100) | 2 (67) | 3 (75) | 2 (67) |
Values are given as absolute numbers and percentages (in brackets).
It can be noted that distortion could be described as associated with a mass or asymmetry or as an isolated finding.
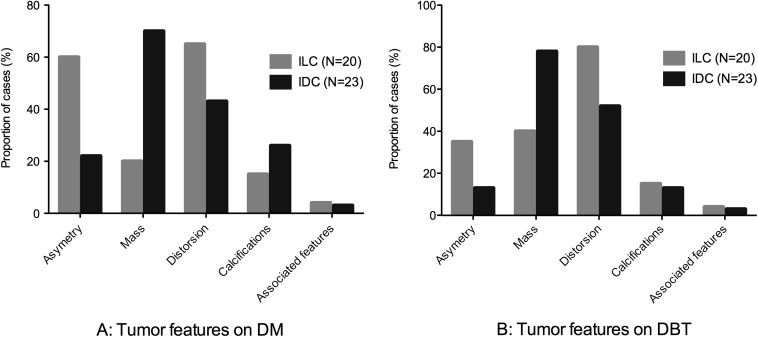
Figure 1 shows comparative proportions of asymmetries, masses, distortions, calcifications and associated features of ILC and IDC on DM (Figure 1a) and DBT (Figure 1b).
Figure 1.
Tumour features: comparative proportions of tumour features are presented for invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) on digital mammography (DM) (a) and digital breast tomosynthesis (DBT) (b). On DBT, compared with IDC, ILC presented less frequently as masses (p = 0.01) and demonstrated a higher proportion of distortion, although this difference did not reach statistical significance (p = 0.1). On DM, ILC presented more often as asymmetries than masses (p = 0.02), whereas on DBT ILC presented a comparable proportion of masses and asymmetries (p = 1.00).
On DM, ILC presented more often as asymmetries (60%, 12/20) than masses (20%, 4/20) (p = 0.02), whereas on DBT, ILC presented a comparable proportion of masses (40%, 8/20) and asymmetries (35%, 7/20) (p = 1.00). On DBT, ILC presented significantly less often as masses than IDC (78%, 18/23) (p = 0.01). When described as a mass on DBT, ILC was rarely described as dense (38%, 3/8) but demonstrated significantly more often spiculated margins (75%, 6/8) than IDC (44%, 8/18) (p = 0.04). ILC also demonstrated a higher proportion of distortion (80%, 16/20) than IDC (52%, 12/23), although this difference did not reach statistical significance (p = 0.1). Moreover, ILC presented significantly more frequently as isolated distortion on DBT (20%, 4/20) than IDC (0%, 0/23) (p = 0.03). In three patients with IDC, calcifications were described on DM but not on DBT. Yet, the rates of calcifications demonstrated no significant difference between tumour types on DBT (p = 1) and between DM and DBT (p = 1 and p = 0.46 for ILC and IDC, respectively).
Conspicuity
Conspicuity of lesions was rated higher on DBT than on DM in 8/20 (40%) ILCs and 6/23 (26%) IDCs and lower in 1/20 (5%) ILC and 3/23 (13%) IDCs.
Conspicuity of ILC was lower than IDC on both DM (p = 0.002) and DBT (p = 0.02).
In comparison with DM, DBT increased significantly conspicuity of ILC (p = 0.002), while this difference was not significant for IDC (p = 0.2).
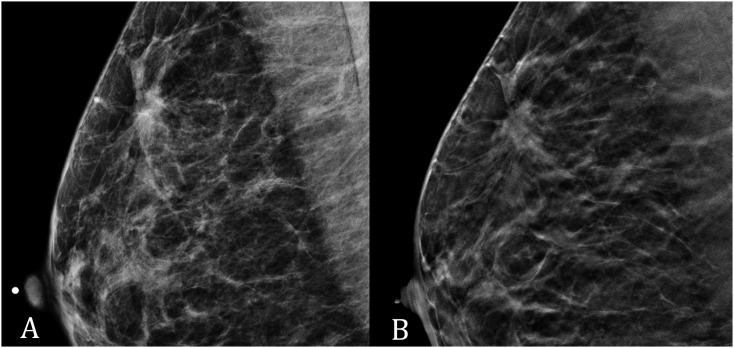
One case of ILC in which conspicuity was rated higher on DBT than on DM is presented in Figure 2.
Figure 2.
(a) Digital mammography (DM) and (b) digital breast tomosynthesis (DBT) in a 58-year-old female with invasive lobular carcinoma of the right breast: conspicuity was rated lower on DM (medium conspicuity) than on DBT (high conspicuity). In particular, architectural distortion was more conspicuous on DBT than on DM.
2 (10%) cases of ILC showed no visible findings on DM. On DBT, these two ILCs were visualized, one case described as an asymmetry with “low visibility” and the other as a dense mass with “medium visibility”. Regarding IDC, all cases were described as visible on DM, while two cases were not visualized on DBT. These two cases were a 27-mm non-dense mass and a 10-mm asymmetry on DM.
Assessment of tumour size
This analysis was performed in a subgroup of 14 patients with ILC and 17 patients with IDC.
Mean maximum tumour diameter on DM, DBT and pathology were 12 mm (9.5–20 mm), 13 mm (9.8–34.8 mm) and 22 mm (15.8–53.8 mm) for ILC and 10 mm (6–26 mm), 13 mm (6–19.5 mm) and 15 mm (8–22 mm) for IDC, respectively.
Size of ILC on pathology was significantly higher than that of IDC (p = 0.028).
Agreement between size as measured on imaging and final pathology was slight with DM (ICC = 0.17) and fair with DBT (ICC = 0.24) for ILC. Both DM and DBT significantly underestimated tumour size of ILC (p = 0.004 and p = 0.017, respectively).
Agreement between size as measured on imaging and final pathology was almost perfect with both techniques for IDC (ICC = 0.89 and ICC = 0.92, respectively).
DISCUSSION
In our study, we reviewed imaging characteristics of ILC on DBT, showing a higher rate of isolated distortion and a lower rate of masses in comparison with IDC. We showed that, in comparison with DM, DBT could increase conspicuity of ILC. Even if not statistically significant, this could be explained at least in part by a lower rate of asymmetries on DBT than on DM. This result supports the hypothesis that DBT has the potential to improve detection of ILC. However, conspicuity of ILC remained lower than that of IDC, which may be explained by the fact that ILC presented rarely as a dense masse on DBT (15% of cases, 3/20). In clinical practice, readers should therefore keep in mind that ILC would still frequently present as a subtle finding on DBT. In addition, considering IDC, two cases were not visualized on DBT, although they were visible on DM. Although DBT was performed only in one view, which could have been the reason, it should be kept in mind that when facing an abnormal DM, negative DBT does not always definitely eliminate breast cancer.
The fact that ILC presented in many cases as distortions, asymmetries or masses of low density could explain our result showing that DBT failed to estimate accurately index tumour size of ILC. We conclude that DBT would not be a good alternative to breast MRI, which is currently the best examination for assessment of tumour size of ILC in the pretreatment work-up.20
Because of its particular histopathological characteristics, it was expected that ILC would present specific features on DBT,12,13 as previously described on mammography and ultrasound.21,22 In particular, our results are in line with previous studies showing that architectural distortion was frequently associated with ILC on mammography11,21 and that DBT has unique strength in detecting distortion.9 They are also supported by the results of the recent study of Grubstein et al,14 showing that DBT could improve detection of ILC by more clearly depicting architectural distortion. In comparison with this work, the interest of our study is to show that features of ILC on DBT are significantly different than those of IDC. Unlike in our study, where the proportion of masses in ILC was lower than in IDC, several articles reported that ILC frequently presented as a mass on mammography.3,11,23 This discrepancy could be explained by the fact that, in our study, findings were described as asymmetries rather than masses as soon as they had concave borders, contained fat or demonstrated low conspicuity, as proposed in the fifth edition of the BI-RADS lexicon.16 This approach is consistent with other studies reporting that the majority of ILCs presented as asymmetries or masses that are of relatively low radiographic opacity.12,24
Our results are consistent with previous studies showing that one-view DBT, alone or in association with DM, could improve visibility of breast cancers.17,25,26 Mariscotti et al13 recently showed that DBT could also improve detection and characterization of ILC. However, in their study, they did not compare ILC with IDC. On the contrary, our results suggest that DBT could improve more conspicuity of ILC than IDC, which confirms what was hypothesized in their review by Johnson et al.12 This observation also correlates with a large multicentre study showing that increase of detection rate by addition of DBT to DM was higher for ILC (+107%) than for IDC (+33%).27 On the contrary, we did not observe a significant increase in conspicuity of IDC in our study. This discrepancy is most likely owing to the facts that conspicuity of IDC was already high on DM and that our population was too small to detect a significant difference.
Few studies have investigated DBT for the estimation of tumour size of breast cancers.15,28,29 They showed that this technique could achieve good performance for this indication, which is consistent with the results we obtained regarding IDC. Yet, unlike these studies, our work investigated this issue according to tumour subtype, showing that as DM, DBT might be accurate for estimation of tumour size for IDC but not for ILC.
There are some limitations to this preliminary study. First, our population is relatively small and, as stated above, the number of cases is probably not sufficient to be able to show a significant increase in conspicuity of IDC with DBT. Moreover, final pathology on surgical specimen could not be evaluated in several patients and performance of DBT for tumour size assessment was only performed in a subpopulation. Second, our population only consists of patients with breast cancer, which may influence the interpretation of the examinations and does not allow assessment of the specificity of the technique. Third, we only interpreted unilateral one-view DBT, which could have limited the performance of the technique and might have had an influence in description of findings, in particular asymmetries. However, it is in line with what has been performed in several recent publications.26,30,31 Moreover, it has recently been shown that two-view DBT might be not necessary for assessment of screen-detected abnormalities.32 The appearance of ILCs or size correlation with pathology might also be different depending on the view acquired. However, the sample in the present study was too small to study any effects with regard to this in more detail. In the future, it would, however, be of great interest to conduct a similar study on bilateral two-view DBT, in larger populations comprising both breast cancers and normal cases.
In conclusion, we showed that ILC rarely presents as a dense mass but frequently demonstrates architectural distortion on DBT. DBT can particularly increase conspicuity of ILC and might therefore be useful in improving detection of this type of cancer. However, DBT failed to estimate accurately index tumour size of ILC, suggesting that it would not be an effective tool for this purpose in the pre-therapeutic work-up.
Acknowledgments
ACKNOWLEDGMENTS
The Société Française de Radiologie (SFR) is acknowledged for its financial support.
Contributor Information
Foucauld Chamming's, Email: fchammings@yahoo.fr.
Ellen Kao, Email: e.kao@sympatico.ca.
Ann Aldis, Email: aealdis@hotmail.com.
Atilla Omeroglu, Email: atilla.omeroglu@muhc.mcgill.ca.
Caroline Reinhold, Email: caroline.reinhold@mcgill.ca.
Benoit Mesurolle, Email: bmesurolle@yahoo.fr.
REFERENCES
- 1.Orvieto E, Maiorano E, Bottiglieri L, Maisonneuve P, Rotmensz N, Galimberti V, et al. Clinicopathologic characteristics of invasive lobular carcinoma of the breast: results of an analysis of 530 cases from a single institution. Cancer 2008; 113: 1511–20. doi: https://doi.org/10.1002/cncr.23811 [DOI] [PubMed] [Google Scholar]
- 2.Tan SM, Behranwala KA, Trott PA, Nasim NA, Moskovic E, Brown G, et al. A retrospective study comparing the individual modalities of triple assessment in the pre-operative diagnosis of invasive lobular breast carcinoma. Eur J Surg Oncol 2002; 28: 203–8. doi: https://doi.org/10.1053/ejso.2001.1236 [DOI] [PubMed] [Google Scholar]
- 3.Le Gal M, Ollivier L, Asselain B, Meunier M, Laurent M, Vielh P, et al. Mammographic features of 455 invasive lobular carcinomas. Radiology 1992; 185: 705–8. doi: https://doi.org/10.1148/radiology.185.3.1438749 [DOI] [PubMed] [Google Scholar]
- 4.Selinko VL, Middleton LP, Dempsey PJ. Role of sonography in diagnosing and staging invasive lobular carcinoma. J Clin Ultrasound 2004; 32: 323–32. doi: https://doi.org/10.1002/jcu.20052 [DOI] [PubMed] [Google Scholar]
- 5.Porter AJ, Evans EB, Foxcroft LM, Simpson PT, Lakhani SR. Mammographic and ultrasound features of invasive lobular carcinoma of the breast. J Med Imaging Radiat Oncol 2014; 58: 1–10. doi: https://doi.org/10.1111/1754-9485.12080 [DOI] [PubMed] [Google Scholar]
- 6.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 2004; 6: R149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boetes C, Veltman J, van Die L, Bult P, Wobbes T, Barentsz JO. The role of MRI in invasive lobular carcinoma. Breast Cancer Res Treat 2004; 86: 31–7. doi: https://doi.org/10.1023/b:brea.0000032921.10481.dc [DOI] [PubMed] [Google Scholar]
- 8.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267: 47–56. doi: https://doi.org/10.1148/radiol.12121373 [DOI] [PubMed] [Google Scholar]
- 9.Partyka L, Lourenco AP, Mainiero MB. Detection of mammographically occult architectural distortion on digital breast tomosynthesis screening: initial clinical experience. AJR Am J Roentgenol 2014; 203: 216–22. doi: https://doi.org/10.2214/ajr.13.11047 [DOI] [PubMed] [Google Scholar]
- 10.Peppard HR, Nicholson BE, Rochman CM, Merchant JK, Mayo RC, Harvey JA. Digital breast tomosynthesis in the diagnostic setting: indications and clinical applications. Radiographics 2015; 35: 975–90. doi: https://doi.org/10.1148/rg.2015140204 [DOI] [PubMed] [Google Scholar]
- 11.Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics 2009; 29: 165–76. doi: https://doi.org/10.1148/rg.291085100 [DOI] [PubMed] [Google Scholar]
- 12.Johnson K, Sarma D, Hwang ES. Lobular breast cancer series: imaging. Breast Cancer Res 2015; 17: 94. doi: https://doi.org/10.1186/s13058-015-0605-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariscotti G, Durando M, Houssami N, Zuiani C, Martincich L, Londero V, et al. Digital breast tomosynthesis as an adjunct to digital mammography for detecting and characterising invasive lobular cancers: a multi-reader study. Clin Radiol 2016; 71: 889–95. doi: https://doi.org/10.1016/j.crad.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Grubstein A, Rapson Y, Morgenstern S, Gadiel I, Haboosheh A, Yerushalmi R, et al. Invasive lobular carcinoma of the breast: appearance on digital breast tomosynthesis. Breast Care (Basel) 2016; 11: 359–62. doi: https://doi.org/10.1159/000450868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Förnvik D, Zackrisson S, Ljungberg O, Svahn T, Timberg P, Tingberg A, et al. Breast tomosynthesis: accuracy of tumor measurement compared with digital mammography and ultrasonography. Acta Radiol 1987; 51: 240–7. [DOI] [PubMed] [Google Scholar]
- 16.D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS®Atlas: breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 17.Andersson I, Ikeda DM, Zackrisson S, Ruschin M, Svahn T, Timberg P, et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol 2008; 18: 2817–25. doi: https://doi.org/10.1007/s00330-008-1076-9 [DOI] [PubMed] [Google Scholar]
- 18.Gennaro G, Toledano A, di Maggio C, Baldan E, Bezzon E, La Grassa M, et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur Radiol 2010; 20: 1545–53. doi: https://doi.org/10.1007/s00330-009-1699-5 [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- 20.van Goethem M, Schelfout K, Dijckmans L, van der Auwera JC, Weyler J, Verslegers I, et al. MR mammography in the pre-operative staging of breast cancer in patients with dense breast tissue: comparison with mammography and ultrasound. Eur Radiol 2004; 14: 809–16. doi: https://doi.org/10.1007/s00330-003-2146-7 [DOI] [PubMed] [Google Scholar]
- 21.Helvie MA, Paramagul C, Oberman HA, Adler DD. Invasive lobular carcinoma. Imaging features and clinical detection. Invest Radiol 1993; 28: 202–7. doi: https://doi.org/10.1097/00004424-199303000-00002 [DOI] [PubMed] [Google Scholar]
- 22.Jones KN, Magut M, Henrichsen TL, Boughey JC, Reynolds C, Glazebrook KN. Pure lobular carcinoma of the breast presenting as a hyperechoic mass: incidence and imaging characteristics. AJR Am J Roentgenol 2013; 201: W765–769. doi: https://doi.org/10.2214/ajr.12.9742 [DOI] [PubMed] [Google Scholar]
- 23.Hilleren DJ, Andersson IT, Lindholm K, Linnell FS. Invasive lobular carcinoma: mammographic findings in a 10-year experience. Radiology 1991; 178: 149–54. doi: https://doi.org/10.1148/radiology.178.1.1984294 [DOI] [PubMed] [Google Scholar]
- 24.Krecke KN, Gisvold JJ. Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am J Roentgenol 1993; 161: 957–60. doi: https://doi.org/10.2214/ajr.161.5.8273634 [DOI] [PubMed] [Google Scholar]
- 25.Gennaro G, Hendrick RE, Ruppel P, Chersevani R, di Maggio C, La Grassa M, et al. Performance comparison of single-view digital breast tomosynthesis plus single-view digital mammography with two-view digital mammography. Eur Radiol 2013; 23: 664–72. doi: https://doi.org/10.1007/s00330-012-2649-1 [DOI] [PubMed] [Google Scholar]
- 26.Gennaro G, Hendrick RE, Toledano A, Paquelet JR, Bezzon E, Chersevani R, et al. Combination of one-view digital breast tomosynthesis with one-view digital mammography versus standard two-view digital mammography: per lesion analysis. Eur Radiol 2013; 23: 2087–94. doi: https://doi.org/10.1007/s00330-013-2831-0 [DOI] [PubMed] [Google Scholar]
- 27.Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311: 2499–507. [DOI] [PubMed] [Google Scholar]
- 28.Luparia A, Mariscotti G, Durando M, Ciatto S, Bosco D, Campanino PP, et al. Accuracy of tumour size assessment in the preoperative staging of breast cancer: comparison of digital mammography, tomosynthesis, ultrasound and MRI. Radiol Med 2013; 118: 1119–36. doi: https://doi.org/10.1007/s11547-013-0941-z [DOI] [PubMed] [Google Scholar]
- 29.Clauser P, Carbonaro LA, Pancot M, Girometti R, Bazzocchi M, Zuiani C, et al. Additional findings at preoperative breast MRI: the value of second-look digital breast tomosynthesis. Eur Radiol 2015; 25: 2830–9. doi: https://doi.org/10.1007/s00330-015-3720-5 [DOI] [PubMed] [Google Scholar]
- 30.Thomassin-Naggara I, Perrot N, Dechoux S, Ribeiro C, Chopier J, de Bazelaire C. Added value of one-view breast tomosynthesis combined with digital mammography according to reader experience. Eur J Radiol 2015; 84: 235–41. [DOI] [PubMed] [Google Scholar]
- 31.Waldherr C, Cerny P, Altermatt HJ, Berclaz G, Ciriolo M, Buser K, et al. Value of one-view breast tomosynthesis versus two-view mammography in diagnostic workup of women with clinical signs and symptoms and in women recalled from screening. AJR Am J Roentgenol 2013; 200: 226–31. doi: https://doi.org/10.2214/ajr.11.8202 [DOI] [PubMed] [Google Scholar]
- 32.Haq R, Lim YY, Maxwell AJ, Hurley E, Beetles U, Bundred S, et al. Digital breast tomosynthesis at screening assessment: are two views always necessary? Br J Radiol 2015; 88: 20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]