Abstract
Limited data exist regarding health care utilization (HCU) in patients receiving allogeneic hematopoietic cell transplantation (alloHCT) for sickle cell disease. Financial data from 2002 to 2011 were analyzed for 26 alloHCT patients and 48 control subjects (referred but without alloHCT). HCU of alloHCT was determined over 3 time periods: pre-alloHCT, during alloHCT (day 0 to day +365), and post-alloHCT. The median total cost per patient during the alloHCT year was $413,000 inpatient and $18,000 outpatient. Post-alloHCT HCU decreased when compared with pre-alloHCT and control subjects. The median cost of post-alloHCT outpatient visits per patient was significantly less when compared with pre-alloHCT (P = .044). The median cost of post-alloHCT inpatient visits per patient approached significance when compared with those pre-alloHCT (P = .079). Sixteen post-alloHCT patients, 19 control subjects, and 14 unaffected siblings were surveyed using Pediatric Quality of Life Inventory and EuroQOL questionnaires; however, the questionnaire scores across all 3 patient groups were not statistically significant (P = .2638). When adjusted for health-related quality of life, the analysis suggested alloHCT has a positive impact on health-related quality of life over control subjects. These pilot data support our hypothesis that alloHCT in children with sickle cell disease reduces HCU compared with control subjects without alloHCT.
Keywords: Transplant, Cost, Outcomes, Quality of life, Pediatric, Sickle cell disease
Introduction
Patients with sickle cell disease (SCD) are plagued with substantial morbidity, decreased life expectancy, and high health care utilization (HCU). The treatment and management of SCD is a substantial public health need. Currently, allogeneic hematopoietic cell transplantation (alloHCT) is the only curative option for patients with SCD and has seen dramatic improvements in outcomes over the past 2 decades. As a result, alloHCT has become more available and more readily recommended for even younger patients and/or those with less severe disease. Therefore, the impact of this shift in management must be analyzed in a way that addresses outcomes, HCU, and health-related quality of life (HRQOL). This analysis is integral for patient decisionmaking and has substantial public policy implications.
SCD affects approximately 100,000 people in the United States, and each year 2000 new diagnoses are detected via newborn screening [1]. Over their lifetime, children with SCD suffer from disease-related complications that have an adverse impact on quality of life and can lead to premature death. These complications directly translate into substantial HCU among this population.
Many studies have examined the clinical outcomes and health care costs of SCD. In a study by Mvundura et al. [2], US children with SCD represented > 1500 visits to the emergency room and >1200 hospitalizations per year. In a subsequent 2010 study, the total pediatric SCD-attributable expenditures in the United States were estimated to be about $335 million per year [3]. The US population of individuals with SCD reflects substantial HCU of up to $1.6 billion per year [4]. In the posthydroxyurea era, life expectancy for sickle cell patients has increased from 30 years to greater than 40 years, but this remains only about half that of individuals without SCD in the United States [5]. With the increase in life expectancy, the total annual hospitalization cost has been documented at over $15 million for adults and $2 million for children treated in the state of Maryland [6]. This difference in annual costs is attributed to the persistence of SCD-related complications and irreversible end-organ damage in adults even after the implementation of hydroxyurea therapy. These figures not only represent the financial impact on our health care system but also the continued medical needs not being met by our current treatment strategies.
Ultimately, these data must be adequately compared with health outcomes and the cost of receiving alloHCT for SCD. A number of studies have investigated curative approaches, including alloHCT [7,8]. The success rate has risen to a 5-year disease-free survival of 85% and an overall survival of 97% with associated lower rates of post-alloHCT morbidity [9]. These outcomes improve further when limited to matched sibling donor (MSD) alloHCT, thus making alloHCT a far more viable option [10].
However, alloHCT carries a significant financial cost in the first year but then subsequently decreases rapidly over time. The reported cost of alloHCT for adults with malignant or nonmalignant conditions in the first year ranges from $96,000 to $204,000 [11]. This cost can vary based on conditioning regimen, allograft type, and donor source. However, limited data exist on the cost associated with receiving alloHCT for children with SCD.
Data on the impact of alloHCT on HRQOL are also limited. It has been shown among pediatric alloHCT recipients that HRQOL is worst immediately post-alloHCT but improves substantially over time [12]. Kelly et al. [13] investigated this among pediatric alloHCT recipients for hemoglobinopathies and found a similar reduction in HRQOL immediately post-alloHCT with return to baseline at 3 months post-alloHCT. To date, no studies evaluate HRQOL in an isolated population of children with SCD beyond 1 year post alloHCT and correlate this with health care costs. In this pilot study, we hypothesize that alloHCT will reduce HCU and cost when compared with SCD control subjects while improving HRQOL.
Methods
Patients and Eligibility
Patients aged 21 years or less were from the New York Presbyterian Morgan Stanley Children's Hospital of Columbia University Medical Center with homozygous hemoglobin S disease, sickle hemoglobin C disease, sickle β+-thalassemia, or sickle β0-thalassemia. Control subjects were children with SCD with documented HLA typing and/or alloHCT consultation during the study period. Cases included eligible recipients of alloHCT within the study period.
Patients received either a myeloablative conditioning regimen, which consisted of busulfan (16 mg/kg), cyclophosphamide (120 to 200 mg/kg) ± rabbit antithymocyte globulin (8 mg/kg) or busulfan (12.8 to 16 mg/kg), fludarabine (180 mg/m2), and alemtuzumab (54 mg/m2), or a reduced-intensity conditioning regimen, which consisted of melphalan (140 mg/m2), fludarabine (180 mg/m2), and alemtuzumab (54 mg/m2). When used, alemtuzumab was administered proximally. Acute graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus ± mycophenolate mofetil. In addition, hospital standard operating practice was used for seizure prophylaxis as well as infection prevention and prophylaxis as published previously [10].
Interrogating the Database
A retrospective review of the internal financial data collected was performed. This database contained cost and HCU information for each patient encounter within the hospital, emergency department, or ambulatory setting and served as the pilot cohort of data. Of note, the database did not include any data on physician fees or cost. To date, our database includes over 200 patients with SCD, and an additional 26 patients received alloHCT for SCD from 2002 to 2012.
Study patients were identified using unique diagnostic codes. The database was first searched to identify disease-specific alloHCT patients using ICD-9 code 282.6 (International Classification of Diseases, Ninth Revision) and alloHCT codes and/or descriptions. The generated patient list was then compared with internal bone marrow transplant database records to ensure data capture and accuracy. Once validated, a complete database search was performed to obtain HCU information using patient medical record numbers from the internal bone marrow transplant database for alloHCT patients and alloHCT referrals.
Costs were recorded in terms of direct, indirect, and total costs from the perspective of the health care institution or hospital; total costs were used as the cost variable for analysis. Data on charges were also provided; however, cost data only were analyzed as charges vary. These cost data included patient care setting (outpatient, inpatient, etc.) as well as inpatient length of stay (LOS), number of inpatient visits, and number of outpatient visits.
Determining Costs and Utilization
The above financial data were analyzed across the 2 groups: the alloHCT case group and the control group. The HCU for the alloHCT group was determined over 3 time periods: pre-alloHCT (before the start of conditioning), during the alloHCT year (start of the conditioning regimen to day +365), and post-alloHCT (beyond day +365). Control patients were analyzed over the duration of care at the institution within the study period of 2002 to 2012.
To provide statistically relevant analysis, patients with less than 6 months of financial data within the pre- or post-alloHCT periods of the study were excluded. This included 5 patients who transferred care to another institution with their primary transplant attending and therefore lacked complete post-alloHCT data. Another 3 patients were referred to our institution for alloHCT and therefore lacked sufficient pre-alloHCT data. An additional 4 patients died during the alloHCT year and therefore lacked post-alloHCT data. Because of the small sample size, HCU data could not be extrapolated for inclusion in the analysis, making these patients unassess-able in the comparative analysis. This resulted in an assessable sample size of 14 patients for HCU. However, all alloHCT patients had assessable data during the alloHCT year and were therefore included in the analysis for this period. The data from this time period of the study were not used for case-control comparative analysis.
Because the amount of data available for alloHCT patients varied based on date of transplant within the study period, all HCU variables were determined in terms of HCU per patient per month. This established a standard or constant data point for comparison between patients and patient groups.
HRQOL
Surviving alloHCT recipients and control subjects were surveyed after HCU data collection using age-appropriate Pediatric Quality of Life Inventory (PedsQL) and EuroQOL (EQ-5D) questionnaires. Post-alloHCT siblings without SCD were also surveyed as the unaffected control subjects. PedsQL queries HRQOL across 4 scales—physical, emotional, social, and school functioning—based on recall over the past month [14]. A total scaled score was then determined using the PedsQL scoring algorithm.
EQ-5D provides a 2-part assessment of 5 dimensions of functioning—mobility, self-care, usual activities, pain/discomfort, and anxiety/depression—as experienced at the time of the survey and an overall self-report of health status in the visual analogue scale (VAS) [15]. Mean HRQOL scores (maximum score of 100 for PedsQL and EQ-5D VAS) were calculated for each of the 3 groups. Utility scores were determined based on EQ-5D responses using established valuation methods and US value sets [16]. Scores were compared using Wilcoxon rank sum ordering to determine significance.
The quality-adjusted life year (QALY) for each patient in the alloHCT year was then determined using the sum of the interval average utilities and time at days +45, +90, +180, and +365 based on utility norms from previously published data [13]. To compare alloHCT patients to control subjects, the incremental cost-effectiveness ratio (ICER), the ratio of the change in costs to incremental benefits of alloHCT, was calculated as the difference in annual costs for the comparison groups divided by the change in QALY as determined using the area under the curve [17].
Statistical Considerations
Most of the HCU analysis was descriptive. The cumulative HCU per patient depended on the follow-up time and can be influenced by terminal event death. Those patients without corresponding inpatient HCU variables to outpatient were assigned 0 value for calculations. The mean and standard deviation (SD) were calculated; median and range were also reported.
The comparison between pre- and post-alloHCT groups was carried out using the generalized linear models with identity link and normal error for the comparison of the cost of inpatients and cost of outpatients and by the generalized linear models with Poisson distribution and log link for the comparison of inpatient visits, outpatient visits, and LOS. HCU during the alloHCT year was compared across various clinical variables by univariate logistic regression on upper quartiles of HCU variables in the alloHCT year only. Multivariate analysis was not performed because of the small sample size and lack of sufficient power for this type of analysis. All outcomes with a P < .05 were considered statistically significant. All analysis was carried out using SAS software (version 9.3; SAS institute, Cary, NC).
Results
Demographics
The alloHCT cohort included 26 patients (mean age at alloHCT, 9.10 years [SD, 6.25]). Twenty-one patients were male and 5 patients were female (Table 1). Most patients had prior vaso-occlusive crisis followed by acute chest syndrome and a small percentage of cerebrovascular accidents. In addition, 62% received chronic treatment with either hydroxyurea or chronic transfusions before alloHCT.
Table 1. Demographic and Disease Characteristics of alloHCT Recipients and Control Subjects.
| Variables | AlloHCT (n=26) | Control Subjects (n = 48) | P |
|---|---|---|---|
| Analysis compared with all alloHCT recipients within the study period | |||
| Mean age, yr | 9.10 ± 6.25 | 4.23 ± 3.74 | .001 |
| Gender | .010 | ||
| Female | 5 (19%) | 24 (50%) | |
| Male | 21 (81%) | 24 (50%) | |
| Prior treatment | 16 (62%) | 26 (54%) | .541 |
| Hydroxyurea | 9 (34.6%) | 22 (46%) | |
| Chronic transfusions | 10 (38%) | 4 (8%) | |
| Sickle cell–related complications | |||
| VOC | 20 (77%) | 41 (85%) | .361 |
| ACS | 16 (61%) | 35 (73%) | .431 |
| CVA | 3 (11%) | 4 (8%) | .691 |
|
| |||
| Variables | Pre/Post- AlloHCT (n = 14) | Control Subjects (n = 48) | P |
|
| |||
| Analysis compared with assessable alloHCT recipients used in HCU comparison | |||
| Mean age, yr | 4.45 ± 4.45 | 4.23 ± 3.74 | .855 |
| Gender | .072 | ||
| Female | 3 (21.4%) | 24 (50%) | |
| Male | 11 (78.6%) | 24 (50%) | |
| Prior treatment | 6 (42.9%) | 26 (54%) | .456 |
| Hydroxyurea | 3 (21.4%) | 22 (46%) | |
| Chronic transfusions | 5 (35.7%) | 4 (8%) | |
| Sickle cell–related complications | |||
| VOC | 10 (71%) | 41 (85%) | .249 |
| ACS | 6 (43%) | 35 (73%) | .054 |
| CVA | 1 (7%) | 4 (8%) | 1.0 |
VOC indicates vaso-occlussive crisis; ACS, acute chest syndrome; CVA, cerebrovascular accident.
Most alloHCTs used either cord blood or bone marrow, with 48% each, and the remainder peripheral blood stem cells. Almost two-thirds of alloHCT recipients had related donor, or MSD, of all donor sources, with the remaining unrelated donor alloHCT consisting of all unrelated cord blood transplant (UCBT) recipients. Most were transplanted with a myeloablative conditioning regimen. Nineteen patients were conditioned with busulfan, fludarabine, and alemtuzumab; 6 with busulfan, cyclophosphamide, ± r-rabbit antithymocyte globulin only; and 1 received melphalan, fludarabine, and alemtuzumab. Cytomegalovirus (CMV) reactivation occurred in 27% of patients. Acute GVHD occurred in 27% of patients in the alloHCT year. Morbidity, defined by development of chronic GVHD, only occurred in 4% of patients (n = 1). This patient since has had complete resolution of GVHD. The 3-year event-free survival rate for patients receiving MSD alloHCT was 100% and for UCBT recipients 55.6%. The latter included 1 graft failure and 4 deaths from graft failure and/or viral infection. As a result, event-free survival was impacted by 15% primary graft failure and overall transplant-related mortality of 15%.
The control cohort included 48 patients (mean age, 4.23 years [SD, 3.74]; P = .001 when compared with pre-alloHCT); this group had an equal gender distribution (50% male, 50% female; P = .010). Most patients had prior vasoocclusive crisis (85%) followed by acute chest syndrome (73%) and cerebrovascular accidents (8%), which did not have a significant difference from the alloHCTgroup (P =.361, .431, and .691, respectively) (Table 1). Of these, 54% received chronic SCD treatment with either hydroxyurea or chronic transfusions (P = .541). All patients were followed at our center and received routine comprehensive care and supportive care according to established, standard guidelines. This group had no documented mortality during the study period.
Utilization
Pre-alloHCT
Fourteen patients were included in the pre-alloHCT analysis (mean age, 4.45 years [SD, 4.45]) (Table 1) over a median of 30 patient care months (range, 7 to 103). A total of 76 inpatient admissions (range, 1 to 16 per patient) with a median LOS of 17 days per patient (range, 4 to 48) were recorded. This represented a median total cost of $42,050 per patient (range, $4551 to $100,619). All patients had outpatient visits totaling to 752 visits (range, 5 to 139 per patient) with a median total cost of $21,176 per patient (range, $4745 to $68,605).
AlloHCT year
For the 26 transplanted patients, the median total cost per patient during the alloHCT year was $413,070 (range, $155,265 to $1,554,690) inpatient and $17,791 (range, $5175 to $88,526) outpatient. This included 94 inpatient admissions (range,1 to 8 per patient) and a median LOS of 67 days per patient (range, 29 to 226). Outpatient data included only 23 patients, because 3 patients died during their initial alloHCT admission. Of these, a total of 1060 visits were recorded (range, 11 to 91 per patient).
HCU of the upper quartiles during this period was compared across various clinical variables using logistic regression (Table 2). A statistically significant increase in inpatient cost and LOS was associated with alloHCT recipients who had a UCBT (odds ratio [OR], 32; P = .006), developed acute GVHD (OR, 7; P = .047), and/or had CMV reactivation (OR, 7; P = .047) compared with those who did not. In addition, patients with acute GVHD had significantly higher numbers of inpatient admissions (P = .047) and outpatient visits (P = .038).
Table 2. Univariate Logistic Regression on Upper Quartiles of HCU Variables in the Transplant Year.
| Utilization Variables | Clinical Variables (reference) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||
| Age | Gender (male) | Donor Source (unrelated) | Graft Type (myeloablative) |
Primary Graft Failure (present) |
GVHD (present) | CMV Reactivation (present) |
|||||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Inpatient | |||||||||||||||||||||
| No. of admissions | .95 | .82-1.10 | .475 | .47 | .06-3.65 | .469 | 1.63 | .27-9.66 | .593 | 1.60 | .15-17.4 | .700 | .89 | .08-10.3 | .925 | 7.11 | 1.02-49.46 | .047 | 2.81 | .44-18.06 | .276 |
| LOS | .82 | .67-1.01 | .062 | .47 | .06-3.65 | .469 | 9.37 | 1.30-67.6 | .027 | 1.60 | .15-17.4 | .700 | 6.38 | .79-51.78 | .083 | 21.3 | 2.36-191.6 | .006 | 7.11 | 1.02-49.46 | .047 |
| Cost | .91 | .78-1.07 | .237 | .16 | .02-1.27 | .083 | 32.0 | 2.76-370.8 | .006 | .47 | .06-3.65 | .469 | 13.5 | 1.1-165.9 | .042 | 7.11 | 1.02-49.46 | .047 | 7.11 | 1.02-49.46 | .047 |
| Outpatient | |||||||||||||||||||||
| No. of visits | 1.02 | .88-1.12 | .827 | .18 | .02-1.45 | .107 | .96 | .14-6.705 | .967 | 1.85 | .17-20.26 | .616 | 2.67 | .14-49.74 | .511 | 10 | 1.22-81.81 | .032 | 3.25 | .46-22.92 | .237 |
| Cost | 1.12 | .96-1.30 | .159 | .35 | .04-2.87 | .330 | .30 | .03-3.07 | .310 | 1.25 | .11-13.92 | .856 | 1.13 | .1-13.44 | .921 | 4.00 | .58-27.8 | .161 | 1.50 | .21-10.82 | .688 |
CI indicates confidence interval.
Age was a continuous variable; all other binary variables were referenced = 1 as listed and the alternative = 0.
Donor source for all patients was analyzed separately; average LOS did not remain significant between UCBT and MSD recipients (127 days versus 70 days per patient; respectively, P = .661). However, average cost remained significantly higher for UCBT recipients when compared with MSD recipients ($705,242 versus $336,607 per patient, respectively; P = .027).
Post-alloHCT
HCU in this period spanned a median of 38 patient care months (range, 9 to 82). Six of 14 assessable patients had 10 admissions (range, 1 to 3 per patient) with a median LOS of 2 days per patient (range,1 to 32). This represented a median total cost of $5170 per patient (range, $744 to $141,587). All patients had outpatient visits totaling to 381 visits (range, 8 to 61 per patient) with a median total cost of $12,738 per patient (range, $6484 to $30,058).
Control subjects
All patients in the control cohort had assessable outpatient and inpatient data, with 544 inpatient admissions during the study period (median, 8 days; range, 1 to 62 per patient) and a median LOS of 21 days per patient (range,1 to 171). This represented a median total cost of $55,414 per patient (range, $3219 to $425,396). Outpatient care consisted of 3870 visits (range, 5 to 281 per patient) at a median total cost of $18,795 per patient (range, $1559 to $148,803). These patients had a median total health care cost of $8245 per year (range, $530 to $63,800).
Case–control comparison
As above, assessable alloHCT patients used for comparison were not significantly different from control subjects in terms of demographic, disease, or treatment-related variables (Table 1). Patients in the post-alloHCT period had significantly fewer inpatient visits per month and lower LOS when compared with pre-alloHCT and with the control group (Figure 1). The associated median post-alloHCT costs per month were not significant ($0 [range, $0 to $2102] versus $730.00 per month [range, $0 to $14,026], P = .079) (Figure 2).
Figure 1.
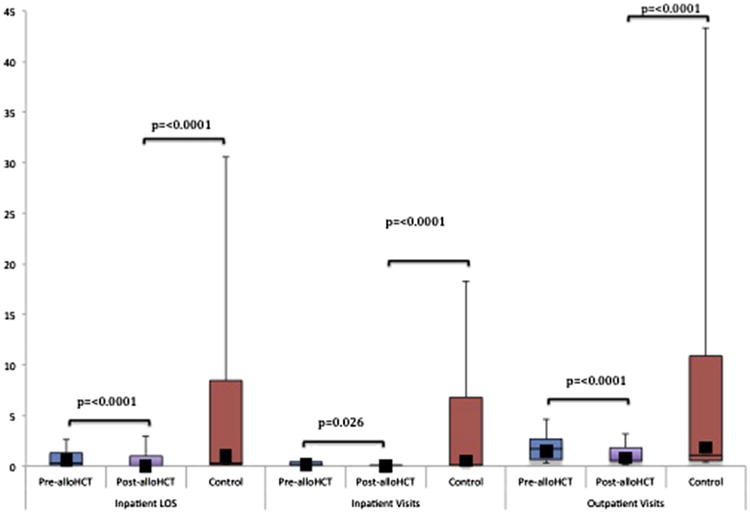
Overall post-alloHCT HCU per patient per month compared with pre-alloHCT and control subjects. The horizontal lines that form the top and bottom of each box are the 75th and 25th percentiles, respectively. The horizontal line that intersects the box is the median. The whiskers represent the minimum and maximum ranges. The solid squares represent the mean values. (See Supplemental Table 1 for exact HCU figures.)
Figure 2.
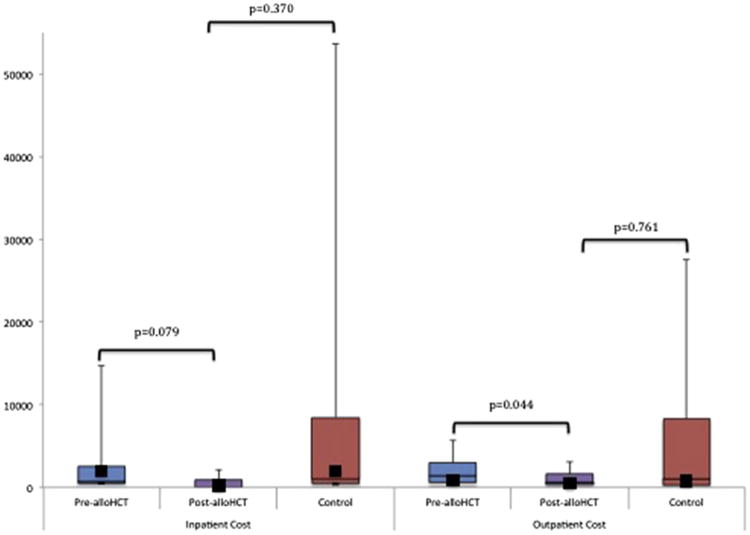
Overall post-alloHCT costs (in US$) per patient per month compared with pre-alloHCT and control subjects. The horizontal lines that form the top and bottom of each box are the 75th and 25th percentiles, respectively. The horizontal line that intersects the box is the median. The whiskers represent the minimum and maximum ranges. The solid squares represent the mean values. (See Supplemental Table 1 for exact cost figures.)
In the outpatient setting, the number of post-alloHCT visits was again significantly lower than when compared with pre-alloHCT and with control subjects (Figure 1). This was associated with a significant reduction in median post-alloHCT cost per patient per month when compared with pre-alloHCT ($295.00 [range, $153 to $1435] versus $781.80 per month [range, $230 to $2709], P = .044) (Figure 2).
HRQOL
Sixteen post-alloHCT patients (mean age,15 years [SD, 5]) and 19 control subjects (mean age, 12 years [SD, 5]) were surveyed. Post-alloHCT patients completed the survey, on average, 6 years post-alloHCT. SCD therapy was documented in terms of treatment pre-alloHCT in the “post-alloHCT” cohort and at any time period greater than 6 months for the control group. SCD therapy included hydroxyurea (post-alloHCT, n = 8; control subjects, n = 10) and chronic transfusions (post-alloHCT, n = 7; control subjects, n = 2). Most patients remained in school (post-alloHCT, n = 12; control subjects, n = 19), whereas the remaining 4 post-alloHCT patients worked full time. Of the post-alloHCT patients, only 1 had a history of chronic GVHD.
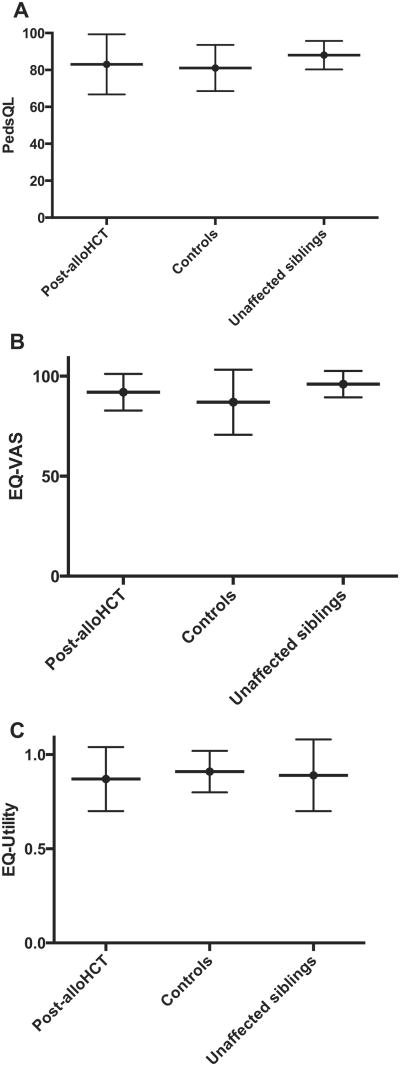
The mean scaled scores were 83 for post-alloHCT patients and 81 for control subjects. Similarly, the mean EQ-5D VAS scores were 92 and 87, respectively. The mean EQ-5D utility scores were .87 and .91, respectively (Figure 3). The lowest mean PedsQL scores for the post-alloHCT group were school and social functioning (76 and 82, respectively), whereas control subjects were lowest in school and physical functioning (77 and 78, respectively). The mean EQ-5D scores when post-alloHCT was compared with control subjects were highest for self-care (1 and 1, respectively) and lowest for pain (.949 and .954, respectively).
Figure 3.
Overall post-alloHCT HRQOL scores for A) PedsQL, B) EQ5D-Visual Analogue Scale, C) EQ5D-Utility compared with control subjects and siblings (P = .2638).
In addition, the post-alloHCT group was compared with a cohort of unaffected sibling donors (mean age, 14 years [SD, 6]). Sibling mean scores were 88 for PedsQL, 96 for EQ-5D VAS, and .89 for EQ-5D utility. The difference in overall or total scores across all 3 groups was not statistically significant (P = .2638).
The established norms of utility for days +45, +90, +180, and +365 were .71, .75, .79, and .84, respectively, from a baseline utility of .75 (personal communications, Susan K. Parsons, Tufts University, 2014) [13]. The alloHCT year has an estimated ICER of about $467,000 per QALY (Table 3). Analysis of the change in HRQOL for alloHCT compared with individuals with SCD performed using the area under the curve showed a QALY of .78 and a loss of .13 occurs in the transplant year when compared with study control subjects [17]. However, when the alloHCT HRQOL was compared with the published utility of adults living with SCD, a QALY gain of .08 occurs [19]. Assuming a life expectancy of 50 years for adults with SCD and lifetime post-alloHCT costs at US per capita expenditures, this translates into an average gain of 10 years of life for post-alloHCT recipients over their lifetime, with an estimated ICER of about $160,000 per QALY (Table 3).
Table 3. QALY Across 3 Patient Groups.
| Day +45 | Day +90 | Day +180 | Day +365 | Total | QALY | Predicted Lifetime | |
|---|---|---|---|---|---|---|---|
| AlloHCT year utility* | .71 | .75 | .79 | .84 | .78 | ||
| AlloHCT year median cost, $ | 204,404 | 87,184 | 38,542 | 11,679 | 341,809 | ||
| ICER, Δ$/QALY | 287,893 | 116,246 | 48,788 | 13,904 | 466,830 | 983,497† | |
| Control utility | .91 | ||||||
| Control median cost, $ | 8245 | ||||||
| ICER, Δ$/QALY | 9060 | ||||||
| Adult SCD cost, $ | 16,000‡ | .7§ | |||||
| ICER, Δ$/QALY | 22,857 | 1,142,857 |
Discussion
To date, this is the first published study analyzing HCU and cost of alloHCT for children with SCD. We demonstrated that despite substantial cost during the alloHCT year, HCU decreases significantly over time post-alloHCT when compared with that pre-alloHCT and with control subjects. More importantly, this change is associated with significant HRQOL improvements.
We describe a median transplant cost of approximately $413,000 per patient during the transplant year. This is substantially higher than published reports of $96,000 to 204,000 for alloHCT in adult recipients for hematologic disorders [11]. However, pediatric alloHCT cost data have been reported at $377,000 to $457,000 during the first year post-alloHCT for pediatric acute leukemia [20], suggesting that factors unique to pediatric populations confer increased costs compared with adults.
Univariate analysis of our cohort identified factors that may uniquely affect HCU in pediatric alloHCT. First, our experience with UCBT and the high mortality are likely contributors to the high inpatient cost. However, this effect could not be studied further because the UCBT arm of the study was closed due to poor clinical outcomes [21].
Second, our data on GVHD are consistent with the findings of Svahn et al. [22], who showed a statistically significant increase in cost with high-grade acute GVHD and unrelated donor transplant. Patients with acute GVHD in our cohort had significantly higher outpatient visits and inpatient HCU including cost. Based on prior publications, the increased cost is presumed due to higher readmission rates for morbidities such as GVHD [23]. This creates a potential source of further investigation in preventing and managing GVHD in our patient population.
Finally, our alloHCT population had a 25% incidence of CMV reactivation. CMV reactivation was associated with increased HCU and higher mortality especially among our UCBT arm. Other published studies have shown similar to higher rates of CMV reactivation and/or viremia among pediatric UCBT recipients for SCD [24]. This is presumably a consequence of our alemtuzumab-based protocols because this risk has previously been identified in the literature [25,26]. As a result, investigation of modified conditioning regimens and prophylactic regimens based on donor source and CMV status is necessary.
At the same time, our analysis supported the lower HCU associated with MSD alloHCT. Previous studies have determined that related donor transplant is less costly than matched unrelated donor or UCBT in the first 100 days after alloHCT [27]. Our study supported this finding during this period and even throughout the remainder of the alloHCT year. This, in conjunction with a 3-year evert-free survival rate of 100%, further suggests that MSD alloHCT is a viable upfront treatment option for children with SCD and an available MSD.
In addition, the morbidity impact on HCU was explored in the context of HRQOL. Our alloHCT patient cohort has similarly been studied and recapitulates the findings of previously published work showing a statistically significant improvement in HRQOL over the first year post-alloHCT when compared with pre-alloHCT [28]. Although the HRQOL analysis in this study was performed at 1 point in time, the findings of our population are representative of published literature in that post-alloHCT patients have HRQOL scores similar to unaffected siblings, indicating HRQOL normalizes post-alloHCT [13,28].
In addition, the control sample of SCD patients also had HRQOL scores similar to unaffected siblings. This may reflect the high hydroxyurea usage at our institution (53% among those who completed HRQOL surveys). The difference in maintenance SCD therapy suggests a difference that when compared with alloHCT recipients was not statistically significant. At the same time, a significant difference in the quality-adjusted cost represents a potential gain in quality life at $60,000 for QALY gained. These factors in tandem suggest that reduced hospitalization post-alloHCT has a significant impact not only on HCU but also on HRQOL.
Our data suggest a cost-to-benefit advantage of alloHCT over standard therapy. This also provides the first analysis of HRQOL as an outcome and the economic impact of alloHCT for pediatric SCD patients. These findings affirm that alloHCT is cost-effective and, more importantly, a beneficial management option for patients.
Limitations of Analysis
This study incorporates single-institution retrospective data that do not allow for complete, prospective analysis of outcomes, quality, and HCU. As such, it did not allow for randomization, and a control group had to be identified based on predictions about the patient population. The use of patients who were HLA typed and/or received alloHCT consultation assumes similarity, yet this introduces bias into the comparison. Specifically, the difference in maintenance SCD therapy suggests a difference in the control subjects when compared with alloHCT recipients that favors patients with more severe SCD including chronic transfusions. However, overall differences in SCD therapy and complications were not statistically significant.
Similarly, this analysis also does not account for the key variables like the impact of duration of survival or LOS. Specifically, this analysis does not allow for post-alloHCT comparison for patients who died during the alloHCT year. Our ability to perform long-term analysis is inherently limited among this group. However, these patients were included in the univariate analysis and likely reflect the increased cost among the unrelated, graft failure, and CMV reactivation groups. Finally, we point out that this study is more of pilot study and lacks a sensitivity analysis on key variables because of a small sample size.
In addition, our analysis of alloHCT does not include assessment of donor or procurement related costs. In a study of children receiving alloHCT from 2004 to 2006, these costs were estimated at around $9000 for matched unrelated donors and $60,000 for UCBT [27]. Therefore, these costs could have substantial implications related to the cost and outcome of UCBT. In addition, it does not clearly delineate pre-alloHCT costs from those associated with evaluation and preparation for alloHCT. In a study by van Agthoven et al. [29], most transplant-associated costs were incurred during the initial alloHCT admission, suggesting that these costs may be minimal in the pre-alloHCT period.
Finally, our database was not comprehensive in inclusion of fees, including physician or provider fees. However, this was deemed negligible at least in the outpatient setting. Ultimately, because no baseline data on this population were available for reporting, the institutional database was selected based on its comprehensiveness on location of service including inpatient, outpatient, and emergency HCU, the latter of which are not available in publicly available financial databases.
Future Studies
This study will serve as the impetus for future research including investigation of specific contributors to HCU such as laboratory, transfusion, and medication costs. In addition, cost-to-benefit analysis will be performed that includes HRQOL assessment over time of not only alloHCT recipients but also of control subjects. Finally, although our sample was reflective of national standards for morbidity and mortality in this patient population, it is not a representative sample. A large multicenter study is required to validate these results. If more representative, national findings are consistent, alloHCT should be accepted as standard of care curative therapy for SCD and therefore reimbursable by public and private insurers.
Supplementary Material
Acknowledgments
The authors thank all the patients, siblings, and families included in this study as well as all faculty and staff in Pediatric Hematology, Oncology, and Stem Cell Transplant at Columbia University Medical Center. Special thanks to those who supported the development of this manuscript and research, specifically Drs. Susan Parsons and Kerice Pinkney as well as Maureen Licursi.
Dr. Arnold was supported in part by a Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program award.
Footnotes
Presented in part at the annual meeting of the American Society of Blood and Marrow Transplantation, Grapevine, Texas, February 27, 2014.
Financial disclosure: The authors declare no competing financial interests.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: S.D.A. and P.S. were integral to and responsible for the study design, concept, and development of the research. S.D.A., S.S., M.B., and P.S. collected and analyzed the data. S.D.A., Z.J., M.B., A.K., and P.S. analyzed and interpreted the data. All authors contributed to the writing and review of the manuscript.
Supplementary Data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbmt.2015.01.010
References
- 1.Hassell KL. Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38:S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Mvundura M, Amendah D, Kavanagh PL, et al. Health care utilization and expenditures for privately and publicly insured children with sickle cell disease in the United States. Pediatr Blood Cancer. 2009;53:642–646. doi: 10.1002/pbc.22069. [DOI] [PubMed] [Google Scholar]
- 3.Amendah DD, Mvundura M, Kavanagh PL, et al. Sickle cell disease-related pediatric medical expenditures in the U.S. Am J Prev Med. 2010;38(4 Suppl):S550–S556. doi: 10.1016/j.amepre.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84:323–327. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- 5.Powars DR, Chan LS, Hiti A, et al. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 6.Lanzkron S, Haywood C, Jr, Segal JB, Dover GJ. Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. Am J Hematol. 2006;81:927–932. doi: 10.1002/ajh.20703. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy S. Has stem cell transplantation come of age in the treatment of sickle cell disease? Bone Marrow Transplant. 2007;40:813–821. doi: 10.1038/sj.bmt.1705779. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh MM, Fitzhugh CD, Tisdale JF. Allogeneic hematopoietic stem cell transplantation for sickle cell disease: the time is now. Blood. 2011;118:1197–1207. doi: 10.1182/blood-2011-01-332510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panepinto JA, Walters MC, Carreras J, et al. Matched-related donor transplantation for sickle cell disease: report from the Center for International Blood and Transplant Research. Br J Haematol. 2007;137:479–485. doi: 10.1111/j.1365-2141.2007.06592.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia M, Jin Z, Baker C, et al. Reduced toxicity, myeloablative conditioning with BU, fludarabine, alemtuzumab and SCT from sibling donors in children with sickle cell disease. Bone Marrow Transplant. 2014;49:913–920. doi: 10.1038/bmt.2014.84. [DOI] [PubMed] [Google Scholar]
- 11.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120:1545–1551. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 12.Felder-Puig R, di Gallo A, Waldenmair M, et al. Health-related quality of life of pediatric patients receiving allogeneic stem cell or bone marrow transplantation: results of a longitudinal, multi-center study. Bone Marrow Transplant. 2006;38:119–126. doi: 10.1038/sj.bmt.1705417. [DOI] [PubMed] [Google Scholar]
- 13.Kelly MJ, Pennarola BW, Rodday AM, Parsons SK. Journeys to Recovery Study H-CS. Health-related quality of life (HRQL) in children with sickle cell disease and thalassemia following hematopoietic stem cell transplant (HSCT) Pediatr Blood Cancer. 2012;59:725–731. doi: 10.1002/pbc.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Gusi N, et al. The EQ-5D health-related quality of life questionnaire Handbook of disease burdens and quality of life measures. New York: Springer; 2010. [Google Scholar]
- 16.Szende A, et al. EQ-5D value sets. Health Econ. 2007;14:21–28. [Google Scholar]
- 17.Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14:487–496. doi: 10.1002/hec.944. [DOI] [PubMed] [Google Scholar]
- 18.Hartman M, Martin AB, Benson J, Catlin A. National Health Expenditure Accounts T. National health spending in 2011: overall growth remains low, but some payers and services show signs of acceleration. Health Aff (Millwood) 2013;32:87–99. doi: 10.1377/hlthaff.2012.1206. [DOI] [PubMed] [Google Scholar]
- 19.Osborne RH, De Abreu Lourenco R, Dalton A, et al. Quality of life related to oral versus subcutaneous iron chelation: a time trade-off study. Value Health. 2007;10:451–456. doi: 10.1111/j.1524-4733.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin YF, Lairson DR, Chan W, et al. The costs and cost-effectiveness of allogeneic peripheral blood stem cell transplantation versus bone marrow transplantation in pediatric patients with acute leukemia. Biol Blood Marrow Transplant. 2010;16:1272–1281. doi: 10.1016/j.bbmt.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radhakrishnan K, Bhatia M, Geyer MB, et al. Busulfan, fludarabine, and alemtuzumab conditioning and unrelated cord blood transplantation in children with sickle cell disease. Biol Blood Marrow Transplant. 2013;19:676–677. doi: 10.1016/j.bbmt.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Svahn BM, Remberger M, Alvin O, et al. Increased costs after allogeneic haematopoietic SCT are associated with major complications and re-transplantation. Bone Marrow Transplant. 2012;47:706–715. doi: 10.1038/bmt.2011.162. [DOI] [PubMed] [Google Scholar]
- 23.Dignan FL, Potter MN, Ethell ME, et al. High readmission rates are associated with a significant economic burden and poor outcome in patients with grade III/IV acute GvHD. Clin Transplant. 2013;27:E56–E63. doi: 10.1111/ctr.12065. [DOI] [PubMed] [Google Scholar]
- 24.Kamani NR, Walters MC, Carter S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol Blood Marrow Transplant. 2012;18:1265–1272. doi: 10.1016/j.bbmt.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12:322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 26.Rustia E, Violago L, Jin Z, et al. Incidence of and risk factors for cyto-megalovirus (CMV), Epstein Barr virus (EBV) and adenovirus (ADV) reactivation in pediatric recipients post allogeneic hematopoietic stem cell transplantation (alloHCT) Biol Blood Marrow Transplant. 2014;20:S84–S85. [Google Scholar]
- 27.Majhail NS, Mothukuri JM, Macmillan ML, et al. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatr Blood Cancer. 2010;54:138–143. doi: 10.1002/pbc.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia M, Kolva E, Cimini L, et al. Health related quality of life following allogeneic hematopoietic stem cell transplantation for sickle cell disease. Pediatr Blood Cancer. 2014;61:S103. doi: 10.1016/j.bbmt.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Agthoven M, Groot MT, Verdonck LF, et al. Cost analysis of HLA-identical sibling and voluntary unrelated allogeneic bone marrow and peripheral blood stem cell transplantation in adults with acute myelocytic leukaemia or acute lymphoblastic leukaemia. Bone Marrow Transplant. 2002;30:243–251. doi: 10.1038/sj.bmt.1703641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.