Abstract
Progressive neurovasculopathy in children with sickle cell disease (SCD) results in decreased cognitive function and quality of life (QoL). Hematopoietic cell transplantation (HCT) is believed to halt progression of neurovasculopathy. Quantitative analysis of T2-weighted fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) for white matter hyperintensity (WMH) burden provides a meaningful estimate of small vessel cerebrovascular disease. We asked if quantitative analysis of WMH could complement standardized clinical assessment of MRI/magnetic resonance angiography (MRA) for assessing SCD central nervous system vasculopathy before and after HCT. Retrospective longitudinal clinical examination of scheduled annual MRI/MRA and quantitative analysis of WMH were performed before and 1 to 7 years after HCT at scheduled annual intervals, along with QoL measurements, in children who had engrafted after HCT. Of 18 patients alive and persistently engrafted (median age, 9.1 years), pretransplantation MRI demonstrated that 9 and 5 had sickle-related stroke and/or small infarcts, respectively. Patients were divided into WMH severity tertiles based on pretransplantation WMH volumes. MRI and WMH were assessed 1 to 7 years after HCT. MRI/MRA and WMH volume were stable or slightly better in 17 of 18 patients. By parent- and self-report, post-HCT QoL improved for children in the lowest WMH tertile significantly more than in the other groups. Based on this single-institution retrospective sample, we report that WMH appears to quantitatively support MRI-based findings that HCT stabilizes long-term small and large vessel cerebrovascular changes and is associated with the degree of improved QoL. While confirmation in larger prospective studies and evaluation by neurocognitive testing are needed, these findings suggest that WMH is a useful biomarker of neurovasculopathy after transplantation for SCD.
Keywords: Sickle cell disease, Hematopoietic cell, transplantation, White matter hyperintensity, Quality of life, Magnetic resonance imaging brain
INTRODUCTION
Sickle cell disease (SCD) is the most common cause of stroke in children. Strokes and “clinically silent” infarcts are caused by progressive sickle vasculopathy in large and small vessels [1–4], leading to SCD brain vasculopathy. Both stroke types can affect neurological and cognitive function starting early in childhood [1,2,5–9]. Stroke or stroke risk is a frequent indication for pediatric SCD transplantation [10–12]. Based on several studies using brain magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA), hematopoietic stem cell transplantation (HCT) is believed to halt progression of vasculopathy [10,12–16]. However, at least 1 case series reported progressive damage soon after HCT [17].
Standard MRI and MRA scans for neuroradiologic assessment of sickle vasculopathy visualize abnormalities in brain parenchyma and large and medium cerebral arteries, respectively. These scans typically provide clinical assessment of occurrence, evolution, or resolution of large or small infarcts over time using standardized approaches [18]. However, small vessel damage is not typically assessed quantitatively in the clinical review of these scans [18]. Moreover, clinical reads can be compared but typically do not provide standardized information to track change over time or to compare between individuals.
Quantitative analysis of T2-weighted fluid attenuated inversion recovery (FLAIR) MRI for white matter hyperintensity (WMH) burden can provide a meaningful estimate of small vessel cerebrovascular disease [19,20]. In adult non-SCD populations, increased WMH is a risk factor for stroke and also can track longitudinal changes in subclinical disease [20]. In children with SCD, larger volume of WMH is associated with decreased cognitive function [8]. Analysis of WMH burden may complement clinical MRI evaluation by providing quantitative information on amount of small vessel involvement, which may not have obvious clinical correlation but could provide insight into the degree of brain involvement and risk for future events.
Using a retrospective sample, we asked whether quantitative WMH analysis could add to the clinical MRI/MRA evaluation of central nervous system (CNS) vasculopathy over time, starting from before transplantation and at subsequent annual intervals. The purposes of this study were to: (1) examine CNS involvement in SCD transplantation patients by considering both standard and quantitative WMH analysis of MRI scans; (2) explore whether analysis of WMH supports the MRI-based perspective that HCT halts long-term progression of sickle vasculopathy [13,14,21]; and (3) assess a relationship between WMH and post-HCT changes in quality of life (QoL), the latter as a marker of patient-reported function [22].
METHODS
This study was approved by Columbia University’s institutional review board.
A single-site retrospective analysis examined pretransplantation WMH and standard MRI/MRA clinical read to sequential analyses from 1 to 7 years after HCT, accompanied by a prospective functional assessment. Eligible patients included all of those younger than 22 years who underwent HCT for symptomatic sickle hemoglobinopathy, HbSS or HbS-B0 thalassemia, at our site between 2003 and April 2014 and who were stably engrafted more than 1 year after HCT. Required observations included a standard clinical head MRI/MRA within 2 months before HCT, including a T2-weighted FLAIR sequence, and annually after HCT for as long as patients returned for follow-up assessment. For the current analysis, study inclusion required at least 1 annual post-HCT scan available for review at the study site. Patients with graft loss were not included. Only annual MRI/MRA scans were included in the analysis reported, including the most recent scheduled MRI, even if interim scans had been performed for acute clinical indications. MRIs included the most recent follow-up scan for each patient. The same 1.5-Tesla scanner was used for all scans analyzed in this sample. Transplantation outcomes of 10 of the 18 subjects have been published previously [23], but details of outcomes of neurologic imaging had previously not been described.
MRI/MRA Interpretation
Each scan underwent 2 independent reviews by board-certified neuroradiologists: 1 for the initial clinical interpretation and 1 performed for this study (A.L.). Discrepancies in interpretation, if any, were addressed by the study neuroradiologist. By T-2–weighted FLAIR, infarct sizes were graded per the SWiTCH trial classification for parenchymal thrombosis [18]: small (<5 mm), medium (5–15 mm), or large (>15 mm). By MRA, stenosis was noted if ≥2 mm. Extracranial internal carotid arteries were not examined.
WMH volume was estimated following a 3-step operator-driven approach described previously [24]. First, a scan-specific intensity threshold was applied to the FLAIR images to define the range of hyperintense values. Second, gross regions-of-interest were manually drawn that included hyperintense voxels but excluded nonparenchymal artifacts (eg, dermal fat). Third, the thresholded image was concatenated with the manual regions-of-interest and the intersecting regions defined the hyperintensities. A single volumetric value for each patient was obtained by multiplying the number of labeled voxels by voxel dimensions [24,25]. For descriptive purposes, participants were divided into 3 (tertiles) based on the range of their WMH severity before HCT. By definition, tertile 1 had little or no WMH. The z-scores were calculated to assess the standardized change (ie, mean, 0; SD, 1) in WMH volume from one year to the next. For example, a patient with a z = .16 increase in WMH volume from year 1 to year 2 was .16 SD greater than the mean of the total sample.
Age-Specific QoL
Data were collected using the PedsQL 4.0 Generic Core Scale [22,26]. The scale exists in 2 versions, a self-report format for children ages 5 to 18 years and a proxy report for parents of children ages 2 to 18 years. The scale contains 23 items that assess overall QoL, which consists of physical (8 items), social (5 items), psychological (5 items), and school functioning (5 items). Scores range from 0 to 100, with higher scores indicating higher QoL. Data were collected from patients and their caregiver before transplantation and were requested annually. Completion at annual intervals varied; the most recently completed follow-up QoL was used here to maximize subject data. Participants and their caregivers independently completed either the English or Spanish version of the PedsQL 4.0 at each time point. Participants’ QoL data were previously published [27]; in all cases, from earlier in the post-HCT period.
Statistical Analysis
Demographic comparisons between study subgroups were calculated using unpaired students t-test and paired signed rank test.
Mixed-effect linear modeling with restricted maximum likelihood estimation was used to examine WMH volume over time. For this analysis, time (in years) was considered a fixed factor and intercept and subject were considered random factors. For descriptive purposes, participants were divided into 3 tertiles based on the range of their WMH severity before HCT. By definition, tertile 1 had little or no WMH. We calculated z-scores for annualized rates of WMH volume change from baseline values. For these calculations, the difference of WMH volume at the second and third MRI scan and WMH at baseline and transformed these difference scores into a z-score distribution (ie, with mean = 0 and SD = 1).
Descriptive statistics for overall QoL variables were calculated by parent-and self-report at baseline (ie, before transplantation) and the most recent annual follow-up QoL form for 2 groups, tertile 1 (little or no WMH) and tertiles 2 and 3 (abnormal) combined based upon their baseline MRI FLAIR results. The change in QoL over time was defined as the difference in QoL measure between the most recent follow-up and baseline. The comparison was analyzed with the Wilcoxon 2-sample test for 2 groups and with the Kruskal-Wallis test for the 3 groups.
RESULTS
All 18 eligible patients with a ≥ 1-year follow-up MRI were included in this analysis (Table 1). The median age of our sample at HCT was 9.2 years (range, 2.2 to 20.2); the ratio of male to female was 13:5. Of the 18, 13 had HbSS and 5 had HbS-B0thalassemia. The primary clinical indication for HCT in 6 patients was CNS pathology: stroke (n = 5) and multiple silent cerebral infarcts (SCI) (n = 1). Transplantation indications for the remaining patients are listed as SCD complications not affecting the CNS (Table 1), even if MRI abnormalities were revealed upon pretransplantation MRI evaluation by the study neuroradiologist (A.L.). Several patients had more than 1 indication for HCT. Before HCT, 4 patients had been on chronic transfusion for CNS or other severe SCD complications.
Table 1.
Patient Characteristics
| Patient No. |
Transplantation Year |
Diagnosis Gender/Age, yr |
GVHD Conditioning |
Stem Cell Source |
GVHD Prophylaxis |
Post-Transplantation Neurologic Complications |
Years after HCT |
MRI Lesions | MRA Stenosis, mm |
WMH Baseline (est vol.), mm3 |
WMH tertile |
WMH z-score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| (Years 2 to 1) | (Years 3 to 1) | ||||||||||||
| 1 | 2003 | HbSS | Myeloablative | RCB | Tac/MMF | None | 0 | 2 Medium, 2 small | 727.5 | 3 | |||
| Female/2.2 | 7 | 2 Small | −.11 | ||||||||||
| 2 | 2006 | HbSS | Myeloablative | RCB | Tac/MMF | None | 0 | 1 Small | 0 | 1 | |||
| Male/5.3 | 2 | No lesions | .18 | ||||||||||
| 6 | “ | .32 | |||||||||||
| 3 | 2007 | HbS-Beta0 Thal | Myeloablative | UCB | Tac/MMF | None | 0 | No lesions | 0 | 1 | |||
| Male/4.5 | 1 | “ | .18 | ||||||||||
| 2 | “ | .30 | |||||||||||
| 4 | 2008 | HbS-Beta0 Thal | Myeloablative | RBM | Tac/MMF | None | 0 | 1 Medium, 2 small | 2 mm | 299.1 | 2 | ||
| Male/16.3 | 1 | Stable | Stable | .21 | |||||||||
| 5 | 2008 | HbSS | Myeloablative | UCB | Tac/MMF | None | 0 | No lesions | 207.5 | 2 | |||
| Male/2.4 | 2 | “ | .11 | ||||||||||
| 6 | 2010 | HbSS | Myeloablative | RBM | Tac/MMF | PRES | 0 | 2 Small | 18.4 | 1 | |||
| Male/12.4 | 1 | No lesions | .16 | ||||||||||
| 2 | “ | .22 | |||||||||||
| 3 | “ | ||||||||||||
| 7 | 2011 | HbSS | RIC | RBM | TAC, MMF, Prednisone | Hemorrhagic stroke at HCT | 0 | 2 Small | 94.6 | 1 | |||
| Male/8.3 | 1 | 3 Small | 1.80 | ||||||||||
| 2 | Stable | 2.63 | |||||||||||
| 3 | Stable | ||||||||||||
| 8 | 2011 | HbSS | RIC | MUD BM | TAC, Mtx, Methylpred | None | 0 | 3 Medium, 5 small | 723.4 | 3 | |||
| Female/6.4 | 1 | Stable | .08 | ||||||||||
| 2 | Stable | .21 | |||||||||||
| 9 | 2011 | HbS-Beta0 Thal | Myeloablative | RBM | TAC/MMF | None | 0 | 2 Medium, 6 small | 19769.1 | 3 | |||
| Female/20.2 | 1 | Stable | −2.21 | ||||||||||
| 10 | 2011 | HbSS | RIC | MUD BM | TAC, Mtx, Methylpred | None | 0 | 1 Medium, 1 small | 763.1 | 3 | |||
| Male/15.8 | 1 | Stable | .02 | ||||||||||
| 2 | Stable | −.16 | |||||||||||
| 11 | 2012 | HbSS | Myeloablative | RCB | Tac/MMF | None | 0 | 3 Small | 393.7 | 2 | |||
| Male/3.3 | 1 | Stable | .12 | ||||||||||
| 2 | Stable | .21 | |||||||||||
| 3 | Stable | ||||||||||||
| 12 | 2012 | HbSS | Myeloablative | RBM | Tac/MMF | None | 0 | 3 Medium, 1 small | 2 mm | 67.1 | 1 | ||
| Male/2.3 | 1 | Stable | Stable | .01 | |||||||||
| 13 | 2013 | HbSS | RIC | MUD BM | TAC, Mtx, Methylpred | None | 0 | 2 Large, 2 med, 1 small | 2093.8 | 3 | |||
| Male/3.8 | 1 | Stable | .47 | ||||||||||
| 2 | Stable | −1.33 | |||||||||||
| 14 | 2013 | HbSS | Myeloablative | RBM | Tac/MMF | None | 0 | 1 Small | 494.8 | 2 | |||
| Male/13.3 | 1 | Stable | .07 | ||||||||||
| 2 | Stable | .06 | |||||||||||
| 15 | 2008 | HbSS | Myeloablative | RBM | Tac/MMF | None | 0 | No lesions | 3 mm | ||||
| Female/14.7 | 1 | “ | Stable | 1 | |||||||||
| 2 | “ | Stable | |||||||||||
| 16 | 2008 | HbS-Beta0 Thal | Myeloablative | UCB | Tac/MMF | None | 0 | No lesions | 173.9 | 2 | |||
| Male/9.9 | 1 | “ | .15 | ||||||||||
| 17 | 2010 | HbS-Beta0 Thal | Myeloablative | RBM | Tac/MMF | None | 0 | 4 Medium, 3 small | 386.7 | 2 | |||
| Male/19.2 | 1 | Stable | .16 | .12 | |||||||||
| 2 | Stable | ||||||||||||
| 18 | 2014 | HbSS | Myeloablative | MUD BM | Tac/MMF | None | 0 | 3 Medium, 2 small | 814.9 | 3 | |||
| Female/12.6 | 1 | Stable | 1.02 | ||||||||||
RCB indicates related cord blood; Tac, tacrolimus; MMF, mycophenolate mofetil; UCB, unrelated cord blood; RBM, related bone marrow; MUD BM, matched unrelated bone marrow; PRES, posterior reversible encephalopathic syndrome; RIC, reduced-intensity conditioning; MTX, methotrexate; Methylpred, methylprednisone.
Differing conditioning regimens, stem cell sources, and graft-versus-host disease (GVHD) prophylaxis regimens were used, according to institutional review board–approved transplantation protocols [23]. Stem cell sources were related bone marrow [8] or cord blood [3] or unrelated marrow [4] or cord blood [3]. Conditioning regimens were either myeloablative (n = 14) with busulfan (12.8 mg/kg to 16 mg/kg), fludarabine (180 mg/m2), and alemtuzumab (54 mg/m2) or reduced intensity (n = 4) with fludarabine (150 mg/m2), melphalan (140 mg/m2), and alemtuzumab (48 mg). GVHD prophylaxis included tacrolimus and mycophenolate mofetil (n = 14); tacrolimus, mycophenolate mofetil, and prednisone (n = 1); or tacrolimus, methotrexate, and methylprednisone (n = 3).
After transplantation, neurologic complications included hemorrhagic stroke at 0 months after transplantation (n = 1) or posterior reversible encephalopathic syndrome (n = 1). Incidences of acute and chronic GVHD were 11% and 17%, respectively. Duration of follow-up (in years) was 1 (n = 4), 2 (n = 9), >2 (n = 5).
Clinical Assessment of MRI/MRA
Before HCT
A normal scan (lacking cerebrovasculopathy) was seen in 4 of the 18 patients (Table 1), median age 7.2 (range, 2.4 to 14.7). The other 14 patients had MRI scans indicative of sickle neurovasculopathy, 9 with overt stroke and 5 with SCI (median age, 10.4; range, 2.2 to 20.2). Infarcts were measured and rated as small, medium, or large (Table 1) [18]. An abnormal MRA was seen in 3 subjects, each of whom also had an abnormal MRI. No stenoses were ≥5 mm.
At One to Seven years after HCT
MRI scans were reviewed 1 to 7 years after transplantation, with a median of 2.5 years. Scans from all 4 patients with a normal pretransplantation MRI scan were unchanged (Table 1). Two of the 3 patients with 1 or 2 SCI had a normalized MRI 1 year after transplantation (no infarcts seen) and continued to be normal in 1 or more subsequent annual scans (Table 1, patient numbers 2 and 6). One patient [7] with an abnormal pretransplantation MRI (2 SCI) and low WMH suffered a hemorrhagic stroke during the HCT procedure; this child had the only worsened MRI after transplantation among the 18 patients. This event may have been the result of pre-existing vasculopathy, as he had pre-HCT SCIs.
Quantitative WMH Analysis
Before HCT
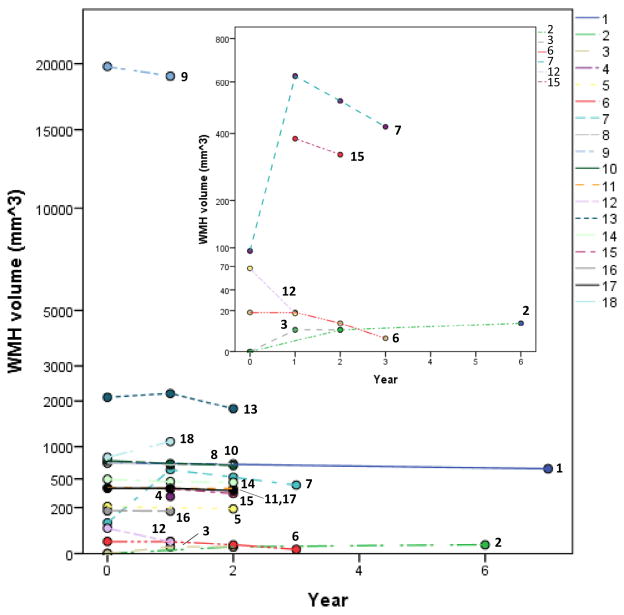
As a group of 18, notable variability was seen between patients (Table 1, Figure 1). Based on the distribution of the patients’ pretransplantation WMH assessed by FLAIR, tertile 1 was considered as normal or only mildly abnormal WMH, with volume <100 mm3. Tertiles 2 and 3 represent FLAIR measurements with abnormal (elevated) WMH. For 15 of 18, the assigned tertile by WMH was consistent with abnormalities detected by clinical evaluation of the MRI: patients with little or no pathology noted on clinical evaluation had little or no measured WMH (ie, tertile 1). Other patients with pathology on clinical MRI of differing degrees were generally associated with tertiles 2 or 3 (Figure 2). In contrast, 3 patients (numbers 5, 14, and 16) with little or minimal visible pathology (ie, 0 or 1 SCI) had more pretransplantation WMH than expected. Each of these 3 patients had WMH values within the second tertile and had no detectable post-transplantation progression.
Figure 1.
WMH before HSCT and annually (1 to 7 years). Shown are the 3 ranges of white mater hyperintensity (WMH) selected: little or none, medium, high. The assigned categories are based on the distribution of the 18 patients’ pretransplantation WMH assessed by FLAIR. Tertile 1 was considered as normal or only mildly abnormal WMH with volume <100 mm3. Tertiles 2 and 3 depict FLAIR measurements with abnormal (elevated) MWH. WMH date are numbered for each subject.
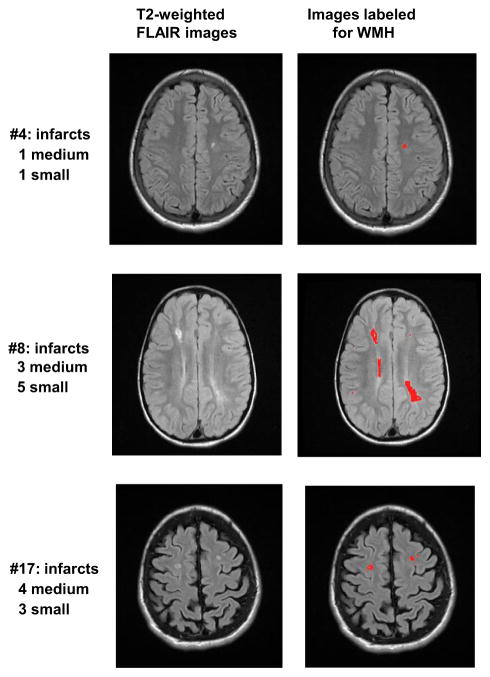
Figure 2.
T2-weighted FLAIR raw images (left) and labeled for WMH (right). Note that each image represents on a single slice from the scan, so not all pathology is shown here.
At One to Seven Years after HCT
Table 1 and Figure 2 demonstrate that WMH volume generally did not change over the 3 MRI scans (main effect of time, F(1, 12.7) = .815, P = .383). Both patients with 1 or 2 SCIs before transplantation had FLAIR examinations with no or minimal WMH (tertile 1) and no WMH progression after transplantation. Descriptively, WMH remained stable in 15 of 18 patients over subsequent annual assessments. In the 2 patients with markedly elevated WMH before HCT (patients 13 and 18), values decreased at follow-up but were still high, corresponding to changes attributable to a recent infarction before HCT. Patient 7, with a peri-transplantation hemorrhage, also had a large increase in subsequent WMH, as expected. The z-scores (Table 1) reflect year-by-year changes in volume of WMH, reflecting changes from as much as −2.21 (less volume) in patient number 9 and +1.80 (greater volume) in patient number 7, to as little (stable) as .01 in patient number 12.
QoL Scores
Pretransplantation and post-transplantation QoL assessments were generally performed within a time frame similar to those for MRIs. At baseline (ie, before HCT), patients reported a median overall QoL of 64.1 (quartile [Q] 1 = 61.1 to Q3 = 88.3), while parents reported a median overall QoL for their child of 63.0 (Q1 = 54.2 to Q3 = 79.4). Both of these values are below the at-risk cutoff score for impaired QoL, defined as ≥1 standard deviation below the mean total QoL score of the healthy reference population (ie, a score ≤69.7) [22]. Overall, scores were obtained during the most recent follow-up at median of 3 years (range, 1 to 7). QoL assessment for 3 patients were performed 1 to 2 years beyond the time frame of MRI scans. At most recent follow-up, QoL increased for patients’ self-report (median, 83.7; Q1 = 66.3 to Q3 = 97.8), with a within-subject median increase of 9.4 points and for parents (median, 82.6; Q1 = 68.5 to Q3 = 98.6), with a within-subject median increase of 12.6 points.
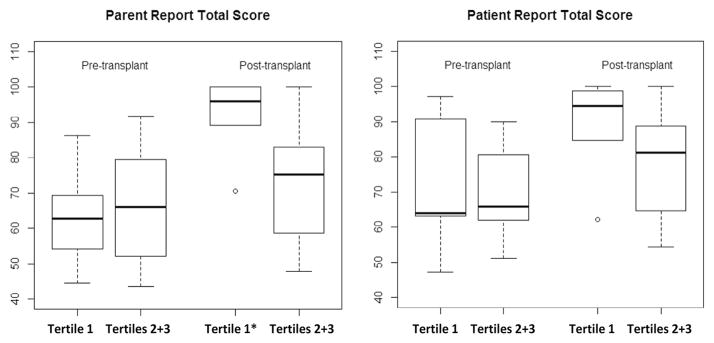
QoL data were subsequently compared across patient groups defined by baseline MRI FLAIR: tertile 1 (N = 6 versus tertiles 2 and 3 combined (N = 12). At baseline, patients in tertile 1 reported an overall baseline QoL of median 63.9 (Q1 = 63.0 to Q3 = 90.6). Parents reported a median overall baseline QoL of 62.8 (Q1 = 54.2 to Q3 = 69.4) (Figure 3). Also at baseline, patients in tertiles 2 and 3 (abnormal FLAIR) reported an overall QoL of 65.9 (Q1 = 59.7 to Q3 = 88.3) and their parents reported a baseline of 66.0 (Q1 = 52.1 to Q3 = 79.4). These scores for tertile 1 scores were not significantly different from scores in tertiles 2 and 3 reported by patients (P = .10) or by parents (P = .70).
Figure 3.
Pre- and post-transplantation quality of life (QoL) scores, by parent- and self-report. Shown are scores and median values at baseline and at most recent follow-up (average duration, 3 years). Tertiles 1 versus 2 and 3 were defined as normal or abnormal white matter hyperintensity at pretransplantation MRI evaluation, respectively. (n = 6 for tertile 1 and 12 for tertiles 2 + 3.) By Wilcoxon signed rank test compared to baseline, tertile 1 QoL scores improved by parents’ (P = .027) but not by patients’ report.
At the most recent follow-up, patients in tertile 1 reported that their QoL increased 19 points to a median QoL score of 90.9 (Q1 = 84.7 to Q3 = 98.8) (Figure 3), with a within-subject median increase 9.4 points. Their parents reported a median QoL of 96.0 (Q1 = 89.1 to Q3 = 100), with a within-subject median increase of 30 points. In contrast, patient-reported median overall QoL for tertiles 2 and 3 were a median of 81.1 (Q1 = 64.7 to Q3 = 88.7), a within-subject median increase of 10.9 points. At the same time, parents reported a median of 75.2 (Q1 = 58.7 to Q3 = 83.0), a within-subject median increase of 3.8 points. Wilcoxon signed rank test comparing in tertile 1 (n = 6) to tertiles 2 and 3 (n = 10) showed that QoL scores improved significantly compared to baseline by parent report (P value = .027) but not by patient report (P value = 1.00).
DISCUSSION
In this retrospective single-site analysis of 18 patients who underwent successful HCT for sickle hemoglobinopathy, we examined data from MRI/MRA using standard clinical neuroradiologic evaluation and quantitative analysis of WMH before and 1 to 7 years after transplantation. The majority of children had MRI abnormalities before HCT, some as the rationale for transplantation referral and others uncovered during the pretransplantation evaluation. Three had abnormal MRA before transplantation. All but 1 patient had stable or modestly improved MRI/MRA and WMH after HCT, despite posterior reversible encephalopathic syndrome and other potential CNS complications related to transplantation, immune suppression, or to presumed progression of pre-existing sickle vasculopathy [16]. HCT appears to have provided protection from progressive vasculopathy and SCI detected by these methods for all but 1 of the patients in this sample. These findings compare favorably to the rate of new SCI with transfusion support [2].
WMH were measureable and reproducible at multiple annual time points. There was considerable variability in WMH volume at baseline, but WMH volume appeared to remain stable after HCT in almost all patients. In some patients with recent pretransplantation infarct, WMH volume declined, reflecting radiological transition from acute to chronic lesions. There was general consistency between quantitative analysis of WMH and evaluation of the clinical MRI: patients with greater WMH tended to have more clinical MRI findings, and WMH increased in the 1 patient with an acute stroke. Discrepancies between clinical reads and quantitative WMH were found in the 3 patients with higher WMH than expected from their minimal (0 or 1 SCI) or no MRI findings. This observation highlights challenges in discerning small infarcts from normal brain architecture in clinical reads [28] and in comparing clinical assessment to independent quantitative approaches. Analysis of WMH may provide a measurement of subtle subclinical small vessel disease manifesting even in the absence of clinical or clinical MRI-based focal injury [29,30]. It is also important to note that quantitative analysis of WMH excluded obvious artifacts that appear as hyperintense signal, such as dermal fat, but did not systematically exclude areas sometimes thought to reflect normal developmental variability, such as the terminal zones of myelination. Potential discrepancies based these different MRI-based analyses need to be validated in a larger prospective study.
While not neurocognitive testing, QoL evaluation provided serial assessment and comparison of self-reported functional performance before and after transplantation for physical, emotional, social functioning, and school performance from parents and from their children [22,26]. Before HCT, low QoL scores for patients were reported by parents and by self-report. Long-term parent- and self-reported QoL in these patients indicated considerable improvement from baseline after HCT to most recent follow-up. Normal baseline MRI FLAIR was associated with improved QoL after transplantation to levels markedly higher than for the children with abnormal pretransplantation FLAIR. These findings suggest that baseline QoL may be mediated by medical burden, while persistent brain abnormalities may contribute to post-transplantation QoL. This hypothesis also will require testing in a larger prospective analysis.
Primary limitations to this study are the small sample size, varying duration of follow-up, heterogeneity of transplantation conditioning regimen, stem cell source and donor type, and retrospective approach. Subject number was insufficient to assess the rate of primary or secondary large vessel stroke [31] or differences in QoL subindices such as school performance. For a few patients, follow-up QoL was obtained somewhat further from transplantation than the most recent MRI scans. SCD-specific QoL was not available for most pretransplantation patients; hence, it was not used here [32]. Cognitive testing, if performed, could have provided prospective assessment of intellectual and developmental function for children with SCD who have undergone transplantation. Potential limitations also include the possible impact of anemia or other SCD-related damage on WMH that could not be assessed, especially as some of our patients with known strokes and other severe complications presented to transplantation while on chronic transfusion therapy. White matter abnormalities after HCT have been reported in association with cyclosporine immune suppression [33], although our patients received the similar tacrolimus. SCD patients who have received multiple transplantations or those who had not undergone transplantation could not be compared for lack of annualized MRI/MRA assessment. Prospective natural history data by sequential MRIs of children with HbSS from the Cooperative Study of SCD (mean age, 8.3 years) over a mean period of 2 years revealed that one-quarter of children with SCIs had new or enlarging lesions on follow-up MRI [1]. Our sample was too small to provide comparison, although the post-HCT evaluations suggest long-term protection.
As in other CNS vasculopathies [32], analysis of WMH may prove to be a sensitive approach to complement clinical MRI/MRA in assessing and longitudinal tracking of the extent and volume of brain damage from stroke associated with sickle vasculopathy [8]. Clinical significance, if any, of focal small vessel disease in SCD detected by WMH but not seen on clinical MRI/MRA remains to be determined. White matter abnormalities from sickle vasculopathy have been reported in children with SCD despite lack of infarct observed on standard clinical MRI scans. Some uncertainty may be clarified with the shift to more powerful scanner magnets and/or use of additional MRI-based sequences and analytic methodologies [34–36].
Our findings of stability in 17 of 18 children who had undergone transplantation support previous MRI/MRA-based data that sickle vasculopathy stabilizes after transplantation [21], possibly including small vessel involvement. HCT may also normalize cerebral blood flow of large vessels in children with abnormally high velocities, even for those with persistent abnormalities despite chronic transfusions [37]. To extend our work, future transplantation protocols will include larger sample size, and prospective formal neuropsychological assessment. Enhanced data for assessing sickle neurovasculopathy using quantitative analysis of WMH and other MRI-based approaches may be useful when considering HCT to limit damage and preserve neurocognitive function.
Supplementary Material
Acknowledgments
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
References
- 1.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014–3018. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 2.DeBaun MR, Kirkham FJ. Central nervous system complications and management in sickle cell disease. Blood. 2016;127:829–838. doi: 10.1182/blood-2015-09-618579. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009;146:300–305. doi: 10.1111/j.1365-2141.2009.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 5.DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119:4587–4596. doi: 10.1182/blood-2011-02-272682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang WC, Pavlakis SG, Helton KJ, et al. MRI abnormalities of the brain in one-year-old children with sickle cell anemia. Pediatr Blood Cancer. 2008;51:643–646. doi: 10.1002/pbc.21612. [DOI] [PubMed] [Google Scholar]
- 7.Quinn CT, McKinstry RC, Dowling MM, et al. Acute silent cerebral ischemic events in children with sickle cell anemia. JAMA Neurol. 2013;70:58–65. doi: 10.1001/jamaneurol.2013.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Land V, Hijmans CT, de Ruiter M, et al. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br J Haematol. 2015;168:553–556. doi: 10.1111/bjh.13179. [DOI] [PubMed] [Google Scholar]
- 9.Schatz J, White DA, Moinuddin A, Armstrong M, DeBaun MR. Lesion burden and cognitive morbidity in children with sickle cell disease. J Child Neurol. 2002;17:891–895. [PubMed] [Google Scholar]
- 10.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. NEJM. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 11.Angelucci E, Matthes-Martin S, Baronciani D, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99:811–820. doi: 10.3324/haematol.2013.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–2756. doi: 10.1182/blood-2007-03-079665. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41:109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 14.Walters MC, Storb R, Patience M, et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Multicenter investigation of bone marrow transplantation for sickle cell disease. Blood. 2000;95:1918–1924. [PubMed] [Google Scholar]
- 15.Walters MC, Sullivan KM, Bernaudin F, et al. Neurologic complications after allogeneic marrow transplantation for sickle cell anemia. Blood. 1995;85:879–884. [PubMed] [Google Scholar]
- 16.Bodas P, Rotz S. Cerebral vascular abnormalities in pediatric patients with sickle cell disease after hematopoietic cell transplant. J Pediatr Hematol Oncol. 2014;36:190–193. doi: 10.1097/MPH.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 17.Woodard P, Helton KJ, Khan RB, et al. Brain parenchymal damage after haematopoietic stem cell transplantation for severe sickle cell disease. Br J Haematol. 2005;129:550–552. doi: 10.1111/j.1365-2141.2005.05491.x. [DOI] [PubMed] [Google Scholar]
- 18.Helton KJ, Adams RJ, Kesler KL, et al. Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood. 2014;124:891–898. doi: 10.1182/blood-2013-12-545186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilling S, DeStefano AL, Sachdev PS, et al. APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology. 2013;81:292–300. doi: 10.1212/WNL.0b013e31829bfda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters MC, Hardy K, Edwards S, et al. Pulmonary, gonadal, and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2010;16:263–272. doi: 10.1016/j.bbmt.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia M, Jin Z, Baker C, et al. Reduced toxicity, myeloablative conditioning with BU, fludarabine, alemtuzumab and SCT from sibling donors in children with sickle cell disease. Bone Marrow Transplant. 2014;49:913–920. doi: 10.1038/bmt.2014.84. [DOI] [PubMed] [Google Scholar]
- 24.Brickman AM, Sneed JR, Provenzano FA, et al. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Res. 2011;193:101–106. doi: 10.1016/j.pscychresns.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia M, Kolva E, Cimini L, et al. Health-related quality of life after allogeneic hematopoietic stem cell transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2015;21:666–672. doi: 10.1016/j.bbmt.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liem RI, Liu J, Gordon MO, et al. Reproducibility of detecting silent cerebral infarcts in pediatric sickle cell anemia. J Child Neurol. 2014;29:1685–1691. doi: 10.1177/0883073813506491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BJ, Lee SH. Prognostic impact of cerebral small vessel disease on stroke outcome. J Stroke. 2015;17:101–110. doi: 10.5853/jos.2015.17.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldeweg T, Hogan AM, Saunders DE, et al. Detecting white matter injury in sickle cell disease using voxel-based morphometry. Ann Neurol. 2006;59:662–672. doi: 10.1002/ana.20790. [DOI] [PubMed] [Google Scholar]
- 31.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117:772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panepinto JA, Torres S, Bendo CB, et al. PedsQL™ Multidimensional Fatigue Scale in sickle cell disease: feasibility, reliability, and validity. Pediatr Blood Cancer. 2014;61:171–177. doi: 10.1002/pbc.24776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trullemans F, Grignard F, Van Camp B, Schots R. Clinical findings and magnetic resonance imaging in severe cyclosporine-related neurotoxicity after allogeneic bone marrow transplantation. Eur J Haematol. 2001;67:94–99. doi: 10.1034/j.1600-0609.2001.t01-1-00440.x. [DOI] [PubMed] [Google Scholar]
- 34.Kawadler JM, Kirkham FJ, Clayden JD, et al. White matter damage relates to oxygen saturation in children with sickle cell anemia without silent cerebral infarcts. Stroke. 2015;46:1793–1799. doi: 10.1161/STROKEAHA.115.008721. [DOI] [PubMed] [Google Scholar]
- 35.Sun B, Brown RC, Hayes L, et al. White matter damage in asymptomatic patients with sickle cell anemia: screening with diffusion tensor imaging. AJNR Am J Neuroradiol. 2012;33:2043–2049. doi: 10.3174/ajnr.A3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helton KJ, Glass JO, Reddick WE, et al. Comparing segmented ASL perfusion of vascular territories using manual versus semiautomated techniques in children with sickle cell anemia. J Magn Reson Imaging. 2015;41:439–446. doi: 10.1002/jmri.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernaudin F, Verlhac S, Arnaud C, et al. Long-term treatment follow-up of children with sickle cell disease monitored with abnormal transcranial Doppler velocities. Blood. 2016;127:1814–1822. doi: 10.1182/blood-2015-10-675231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.