Abstract
Background
Airborne particulate matter (PM) may induce epigenetic changes that potentially lead to chronic diseases. Histone modifications regulate gene expression by influencing chromatin structure that can change gene expression status. We evaluated whether traffic-derived PM exposure is associated with four types of environmentally inducible global histone H3 modifications.
Methods
The Beijing Truck Driver Air Pollution Study included 60 truck drivers and 60 office workers examined twice, 1–2 weeks apart, for ambient PM10 (both day-of and 14-day average exposures), personal PM2.5, black carbon (BC), and elemental components (potassium, sulfur, iron, silicon, aluminum, zinc, calcium, and titanium). For both PM10 measures, we obtained hourly ambient PM10 data for the study period from the Beijing Municipal Environmental Bureau’s 27 representatively distributed monitoring stations. We then calculated a 24 h average for each examination day and a moving average of ambient PM10 measured in the 14 days prior to each examination. Examinations measured global levels of H3 lysine 9 acetylation (H3K9ac), H3 lysine 9 tri-methylation (H3K9me3), H3 lysine 27 tri-methylation (H3K27me3), and H3 lysine 36 tri-methylation (H3K36me3) in blood leukocytes collected after work. We used adjusted linear mixed-effect models to examine percent changes in histone modifications per each μg/m3 increase in PM exposure.
Results
In all participants each μg/m3 increase in 14-day average ambient PM10 exposure was associated with lower H3K27me3 (β=−1.1%, 95% CI: −1.6, −0.6) and H3K36me3 levels (β=−0.8%, 95% CI: −1.4, −0.1). Occupation-stratified analyses showed associations between BC and both H3K9ac and H3K36me3 that were stronger in office workers (β=4.6%, 95% CI: 0.9, 8.4; and β=4.1%, 95% CI: 1.3; 7.0 respectively) than in truck drivers (β=0.1%, 95% CI: −1.3, 1.5; and β=0.9%, 95% CI: −0.9, 2.7, respectively; both pinteraction < 0.05). Sex-stratified analyses showed associations between examination-day PM10 and H3K9ac, and between BC and H3K9me3, were stronger in women (β=10.7%, 95% CI: 5.4, 16.2; and β=7.5%, 95% CI: 1.2, 14.2, respectively) than in men (β=1.4%, 95% CI: −0.9, 3.7; and β=0.9%, 95% CI: −0.9, 2.7, respectively; both pinteraction < 0.05). We observed no associations between personal PM2.5 or elemental components and histone modifications.
Conclusions
Our results suggest a possible role of global histone H3 modifications in effects of traffic-derived PM exposures, particularly BC exposure. Future studies should assess the roles of these modifications in human diseases and as potential mediators of air pollution-induced disease, in particular BC exposure.
Keywords: Histone H3, Epigenetics, Particulate matter, Acetylation, Methylation
1. Introduction
In 2012 an estimated 3.7 million people worldwide died prematurely due to the effects of ambient air particulate matter (PM) pollution (Word Health Organization, 2014). Traffic-derived PM is currently known to make a large contribution to ambient PM, especially in urban areas, and globally it exerts an increasing burden via human disease (Lelieveld et al., 2015; Su et al., 2015). Cohort studies in the United States and Europe have shown associations between exposure to traffic-derived PM and both cardiopulmonary-related diseases and cancer (Kunzli et al., 2000; Raaschou-Nielsen et al., 2011; Tonne et al., 2007; Weuve et al., 2016). Determining biosensors that may reflect individuals’ exposure to traffic-derived pollution is key to developing targeted risk-reduction strategies.
Mechanistic studies have shown that inhaled traffic-derived PM upregulates blood leukocyte oxidative stress and pro-inflammatory responses (Brook et al., 2010; Calderon-Garciduenas et al., 2008; Delfino et al., 2010), two major pathways in the pathogenesis of cardiopulmonary-related diseases and cancer (Brook, 2008; Dominici et al., 2006; Lewtas, 2007). Growing evidence supports a critical role of epigenetic mechanisms in these two pathways (Lepeule et al., 2014; Madrigano et al., 2011). Histone modification is one such mechanism, inducible by oxidative stress (Chervona and Costa, 2012) and certain inflammatory mediators (Bayarsaihan, 2011), which ultimately influences chromatin structure and gene expression (Kouzarides, 2007; Lawrence et al., 2016; Luger et al., 1997). Thus, we hypothesize that histone modifications may serve as a molecular ‘archive’ of an individual’s prior, traffic-derived PM exposure. In particular, modifications to histone H3 are one of the best-characterized in terms of identifying links to changes to gene expression (Henikoff and Shilatifard, 2011). Previous evidence has identified specific histone H3 modifications after exposure to organic chemical compounds (Baccarelli and Bollati, 2009; Bollati and Baccarelli, 2010; Hou et al., 2012b; Zhang et al., 2016) and heavy metals (Arita et al., 2012a, 2012b; Baccarelli and Bollati, 2009; Cantone et al., 2011; Chervona et al., 2012; Ma et al., 2015; Martinez-Zamudio and Ha, 2011; Sun et al., 2009; Zhou et al., 2008). However, few human studies have been conducted to evaluate the effects of ambienttraffic-derived PM on histoneH3 modifications.
Beijing, China is one of the most air-polluted megacities, with 66% of its population potentially exposed to high levels of traffic-derived air pollutants (Su et al., 2015). Traffic emissions and combustion sources are major contributors to PM10 (PM less than 10 μm in diameter) and PM2.5 (PM less than 2.5 μm in diameter) in Beijing (Liu et al., 2015b). In order to test our hypotheses, we measured four specific histone H3 modifications investigated in the above previous environmental studies but as yet unexplored in connection with traffic-related PM exposures: H3 lysine 9 acetylation (H3K9ac), H3 lysine 9 tri-methylation (H3K9me3), H3 lysine 27 tri-methylation (H3K27me3), and H3 lysine 36 tri-methylation (H3K36me3). We conducted our analysis using 240 blood leukocyte samples from 60 truck drivers and 60 indoor workers in Beijing.
2. Materials and methods
2.1. Study population and design
Our Beijing Truck Driver Air Pollution Study was conducted from June 15–July 27, 2008 and included 60 truck drivers and 60 indoor office workers. All study participants worked and lived in the Beijing metropolitan area and had held their current jobs for at least two years. The two study groups had high overall exposure levels and but different component exposures: truck drivers are highly exposed to traffic emissions, particularly from diesel exhausts and road dusts, while office workers are highly exposed to secondary oxidized traffic particles common to the residential areas of Beijing. Both groups were matched on sex, smoking status, education, and age (± 5 years). We collected information on demographics, lifestyle, and other exposures through in-person interviews using a detailed questionnaire. All participants provided written informed consent prior to study enrollment, and Institutional Review Boards at each participating institution approved the study protocol.
2.2. PM exposure data
Hourly monitored ambient PM10 data during the study period were obtained via the Beijing Municipal Environmental Bureau from 27 monitoring stations representatively distributed across the Beijing area. We calculated a 24 h average for each examination day as well as a moving average of ambient PM10 measured 14 days prior to each examination.
Measurements of personal PM2.5 and black carbon (BC) exposure have been previously described (Baccarelli et al., 2014). Briefly, we used personal samplers worn by study participants during eight hours of work to measure average personal PM2.5 and its constituents on two separate examination days 1–2 weeks apart. We found high reproducibility of the PM2.5 measures (r=0.944) in replicate samples from a subset of 24 participants who wore two monitors simultaneously (Baccarelli et al., 2014). BC is a combustion byproduct contained in PM2.5 that has been used as a surrogate measure for PM2.5 from gasoline- and diesel-powered motor vehicles (Kinney et al., 2000). For BC measurement, the blackness of the same filters used to measure PM2.5 was assessed using an EEL Model M43D smoke stain reflectometer, applying the standard black-smoke index calculations of the absorption coefficients based on reflectance (ISO, 1993). We assumed a factor of 1.0 for converting the absorption coefficient to BC mass (Janssen et al., 2003; Kinney et al., 2000), which was then divided by the sampled air volume to calculate average BC concentration (ISO, 1993). Elemental components of PM2.5were analyzed after the gravimetric mass measurement using a XRF PANanalytical Epsilon 5 analyzer (Almelo, The Netherlands). We selected eight elements (potassium, sulfur, iron, silicon, aluminum, zinc, calcium, and titanium) that had previously shown the highest reproducibility (r > 0.75) in replicate samples from the same subset of 24 participants who wore two monitors at the same time (Baccarelli et al., 2014). Higher reproducibility is attributed to the higher concentration of the elemental components collected (Supplemental Table S1).
2.3. Blood sample collection, histone extraction, and global modification detection
We collected 12 mL of blood between 4 and 6 pm (after participants completed a full work day) to eliminate confounding due to diurnal variation. The samples were centrifuged at 2,500 rpm to separate the buffy coat within 2 h of collection. Samples were and stored at −80 °C until histone extraction. Histones were acid-extracted from buffy coats according to the method reported by Shechter et al. (2007) with slight modifications. Briefly, buffy coats were washed with a red blood cell lysis solution for 10 min at room temperature to eliminate erythrocytes. Cells on the pellet were collected by centrifugation at 2,500×g for 15 min and lysed in 500 μL of Triton extraction buffer (1X PBS, 0.5% Triton [v/v], and 2 mM phenylmethylsulfonyl fluoride) supplemented with a protease inhibitor mixture (Roche Applied Sciences, Indianapolis, IN, USA) on ice for 10 min. The pellet was collected by centrifugation at 6,500×g for 10 min at 4 °C, re-suspended in 0.2 N HCl, and kept overnight at 4 °C. The supernatant was collected by centrifugation at 16,000×g for 10 min at 4 °C, and an equal volume of 50% trichloroacetic acid solution was added to precipitate histones. Histones were washed three times with cold acetone and centrifuged at 16,000×g for 20 min. Histones were air-dried for 20 min at room temperature and suspended in 100 μL of ddH2O and stored frozen at −80 °C (Shechter et al., 2007) for approximately 5 months until histone modification quantitation.
Total acid soluble protein in each sample was quantified by bicinchoninic acid protein assay (Smith et al., 1985). Histone modifications were quantified by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described in Arita et al. (2012a), with some modifications. Briefly, polystyrene 96-well microplates (Thermo Fisher Scientific, Pittsburgh, PA, USA) were coated with 100 μL of histone H3 antibody (Abcam ab16061, Cambridge, MA, USA) at a concentration of 1:20,000 in PBS and incubated overnight at 4 °C. Plates were washed with PBST (1X PBS, 0.05% Tween-20) and blocked for 1.5 h at room temperature with 3% milk in PBST. After washing plates with PBST, 100 μL of standard recombinant protein for the standard curve [total H3 (Active Motif 31207), H3K9ac (Active Motif 31253), H3K9me3 (Active Motif 31213), H3K27me3 (Active Motif 31216), or H3K36me3 (Active Motif 31219); Active Motif, Carlsbad, CA, USA] and samples were added per triplicate to plates and incubated at room temperature for 1.5 h with agitation on an orbital shaker at 450 rpm (Titramax 101, Schwabach, Germany). After incubation, wells were washed three times with PBST, and 100 μL diluted primary antibody [total H3, 1:40,000 (Sigma H0164, St. Louis, MO, USA); H3K9ac, 1:500 (Active Motif 39155); H3K9me3, 1:500 (Abcam ab9050); H3K27me3, 1:1,000 (Active Motif 39155); and H3K36me3, 1:1,000 (Abcam ab9050)] in 1% PBST milk was added and incubated at room temperature for 1 h with agitation at 450 rpm. After three washes with TBST, 100 μL diluted secondary antibody (Santa Cruz Biotechnology sc-2004, Santa Cruz, CA, USA) in TBST was added to each well and incubated at room temperature for 1 h without agitation. Wells were washed four times with TBST and 100 μL of 3,3′,5,5′-tetramethylbenzidine solution (Thermo Fisher) was added to each well and incubated at room temperature for 30 min in the dark. The reaction was stopped by adding 100 μL 2 M H2SO4 to each well. All assays were performed in triplicate. Optical density was read at 450 nm using an Infinite M200 PRO reader (TECAN, Männedorf, Switzerland). Relative percent histone modification was derived from standard curves specific to each histone modification, and levels normalized to total histone H3 levels. The respective within- and between-assay coefficients of variation of each assay were 5.28% and 13.37% for total histone H3, 3.49% and 11.88% for H3K9ac, 3.11% and 12.41% for H3K9me3, 6.37% and 8.13% for H3K27me3, and 5.60% and 10.57% for H3K36me3.
2.4. Statistical analysis
For bivariate analysis we employed mixed-effect models to examine differences in histone modifications by participant characteristics. We evaluated associations between ambient PM10, personal PM2.5, BC, and elemental component exposures and histone modifications using multivariable mixed-effect models that included occupation group (office workers vs. truck drivers), sex (male vs. female), age (continuous), BMI (continuous), smoking status (never vs. ever smoked), and day of the week. Number of cigarettes smoked during the examination period was moderately correlated with personal PM2.5 (Supplemental Fig. S1). To account for potential contamination of personal PM monitors, we additionally adjusted for cigarettes smoked during the examination period for analyses of PM2.5 and BC only. Due to lack of variation and deleterious effects on model convergence, we did not include education as most (> 97%) participants reported high school education or less. To optimize power, we conducted primary analyses on the associations of exposure measures and histone modifications by fitting models to all participants combined. Office workers and truck drivers had different occupational environments and lifestyles in our study as described previously (Baccarelli et al., 2014; Hou et al., 2012a, 2013) and evidence suggests differential histone modification can occur in a sex-specific manner (Chervona et al., 2012; Strakovsky et al., 2014; Welstead et al., 2012). Therefore we conducted stratified analyses by occupation group and sex, and examined potential effect modification by testing these as interaction terms with histone H3 modifications. Furthermore, all participants who reported having ever smoked in this study were male. To address this potential source of error in our sex-stratified analyses, we additionally performed a sensitivity analysis to test the effect of combining a product term between each traffic-derived pollutant measure and smoking status for the male stratum. Because temperature and relative humidity could be confounders for ambient PM, we also conducted sensitivity analyses for PM10 on examination day and 14-day average PM10 by including temperature and dew point (daily average data, which were collected at the same locations as ambient PM10) on the examination day and 14-day average, respectively. All tests were two-sided and p < 0.05 was considered statistically significant. Due to data skewness, we log-transformed our histone modification variables prior to analysis and back-transformed them for purposes of reporting our point estimates. For all linear models, we report the average percent histone modification change per one μg/m3 increase in PM exposure. Since the variances of PM10 on the examination day and personal PM2.5 exposure in our sample were substantially greater than those for 14-day average PM10 and BC exposure (Sanchez-Guerra et al., 2015), we report the results of our PM10 on the examination day and PM2.5 models in units per 10 μg/m3 increase and our 14-day average PM10 and BC models in units per 1 μg/m3. For association analysis of the eight elemental components, family-wise error rates (FWER) were calculated from 1,000 permutations in a way that matches the null hypothesis. This permutation method considers dependent multiple tests by controlling FWER, the probability of having at least one false positive among a whole set of comparisons (Rempala and Yang, 2013). All analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
Characteristics of study participants have been described in detail previously (Baccarelli et al., 2014). Briefly truck drivers were older, had higher BMI, and reported more cigarettes smoked during the examination period compared to office workers. We did not observe significant between-group differences in other variables such as day of the week, temperature, and humidity on the examination days. Bivariate analyses of H3K9ac, H3K9me3, H3K27me3, and H3K36me3 levels in blood revealed that only H3K27me3 was significantly associated with any participant characteristics (Table 1). Specifically H3K27me3 was 3.3% higher in men (p=0.03) than in women, 3.0% higher in office workers than in truck drivers (p=0.04), and 3.2% higher in ever smokers compared to never smokers (p=0.03). Similar trends were observed when the same bivariate analyses were conducted within occupation and sex groups (Supplemental Table S2). As reported previously, office workers and truck drivers experienced similar levels of ambient PM10 exposures whereas truck drivers had significantly higher personal levels of PM2.5, BC, and elemental component exposures (Sanchez-Guerra et al., 2015). Our four histone H3 modifications were correlated with one another (r=0.3 to 0.8) (Supplemental Fig. S2) and we also observed weak correlation of histone H3 modification between two examination days (r=0.2 to 0.4) (Supplemental Fig. S3).
Table 1.
H3K9ac, H3K9me3, H3K27me3, and H3K36me3 modification levels by participant characteristics.
| Variable | H3K9ac (%)a
|
H3K9me3 (%)a
|
H3K27me3 (%)a
|
H3K36me3 (%)a
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obsb | Meanc | SEc | p-valued | Obsb | Meanc | SEc | p-valued | Obsb | Meanc | SEc | p-valued | Obsb | Meanc | SEc | p-valued | |
| Occupation | ||||||||||||||||
| Office worker | 103 | 0.23 | 0.02 | 0.29 | 93 | 0.36 | 0.05 | 0.52 | 114 | 27.3 | 1.0 | 0.04 | 113 | 0.56 | 0.04 | 0.40 |
| Truck driver | 114 | 0.20 | 0.02 | 94 | 0.40 | 0.05 | 117 | 24.3 | 1.0 | 115 | 0.51 | 0.04 | ||||
| Sex | ||||||||||||||||
| Female | 76 | 0.24 | 0.02 | 0.15 | 63 | 0.40 | 0.06 | 0.73 | 77 | 23.6 | 1.3 | 0.03 | 75 | 0.53 | 0.05 | 0.84 |
| Male | 141 | 0.20 | 0.02 | 124 | 0.38 | 0.04 | 154 | 26.9 | 0.9 | 153 | 0.54 | 0.03 | ||||
| Age (quartile) | ||||||||||||||||
| Q1 [18–27 years] | 54 | 0.20 | 0.03 | 0.93 | 38 | 0.34 | 0.08 | 0.73 | 57 | 28.5 | 1.5 | 0.16 | 56 | 0.61 | 0.06 | 0.28 |
| Q2 [28–32 years] | 57 | 0.22 | 0.03 | 46 | 0.34 | 0.07 | 60 | 25.8 | 1.4 | 60 | 0.48 | 0.05 | ||||
| Q3 [33–37 years] | 52 | 0.22 | 0.03 | 50 | 0.43 | 0.07 | 55 | 24.5 | 1.5 | 54 | 0.56 | 0.06 | ||||
| Q4 [38–46 years] | 54 | 0.21 | 0.03 | 53 | 0.39 | 0.07 | 59 | 24.3 | 1.4 | 58 | 0.49 | 0.05 | ||||
| BMI | ||||||||||||||||
| 14.8–24.9 kg/m2 | 155 | 0.21 | 0.02 | 0.56 | 126 | 0.39 | 0.04 | 0.41 | 162 | 25.6 | 0.9 | 0.90 | 160 | 0.53 | 0.03 | 0.76 |
| 25–29.9 kg/m2 | 52 | 0.21 | 0.03 | 52 | 0.40 | 0.07 | 58 | 26.2 | 1.5 | 57 | 0.54 | 0.06 | ||||
| 30–32.4 kg/m2 | 10 | 0.28 | 0.06 | 9 | 0.17 | 0.16 | 11 | 26.8 | 3.4 | 11 | 0.62 | 0.13 | ||||
| Smoking status | ||||||||||||||||
| Never smoker | 123 | 0.23 | 0.02 | 0.17 | 108 | 0.39 | 0.05 | 0.79 | 131 | 24.4 | 1.0 | 0.03 | 130 | 0.52 | 0.04 | 0.47 |
| Ever smoker | 94 | 0.19 | 0.02 | 79 | 0.37 | 0.06 | 100 | 27.6 | 1.1 | 98 | 0.56 | 0.04 | ||||
| Cigarettes smoked on examination day | ||||||||||||||||
| 0 | 156 | 0.22 | 0.02 | 0.56 | 137 | 0.37 | 0.04 | 0.82 | 169 | 26.1 | 0.9 | 0.87 | 167 | 0.54 | 0.03 | 0.87 |
| 1–5 | 29 | 0.18 | 0.03 | 23 | 0.42 | 0.12 | 30 | 25.0 | 2.0 | 30 | 0.48 | 0.07 | ||||
| 6–10 | 25 | 0.21 | 0.04 | 21 | 0.44 | 0.09 | 25 | 24.5 | 2.2 | 24 | 0.53 | 0.08 | ||||
| > 10 | 7 | 0.18 | 0.07 | 6 | 0.55 | 0.27 | 7 | 25.2 | 4.1 | 7 | 0.53 | 0.14 | ||||
Percent of H3 modifications over total H3 content.
Number of non-missing H3K27me3, H3K36me3, H3K9ac, or H3K9me3 observations (obs).
Means and standard errors (SE) of H3K9ac, H3K9me3, H3K27me3, or H3K36me3 measured on two examination days and estimated by marginal means and corresponding standard deviations from bivariate analysis using mixed-effect regression models.
p-values were calculated using mixed-effect regression models (age, BMI, and cigarettes smoked on examination day were modeled as continuous variables).
Ambient 14-day average PM10 exposures were significantly associated with H3K27me3 and H3K36me3 levels (Fig. 1A, details in Supplemental Table S3). In all participants combined, each μg/m3 increase in 14-d average PM10 levels was associated with 1.1% lower H3K27me3 (95% CI: −1.6, −0.6; p < 0.01) and 0.8% lower H3K36me3 (95% CI: −1.4, −0.1; p=0.02). We observed a similar association for H3K27me3 in office workers (β=−1.2%; 95% CI: −2.0, −0.5; p < 0.01), although the interaction between 14-d average PM10 and occupation group was not significant (pinteraction=0.31). Similarly we found that each μg/m3 increase in personal BC exposure was associated with 5.8% higher H3K9me3 (95% CI: 0.6, 11.4; p=0.03) in office workers, but this association likewise did not significantly differ by occupation (pinteraction=0.06). However, associations between personal BC exposure and both H3K9ac and H3K36me3 were not only significant among office workers but appeared to be modified by occupation (pinteraction=0.03 and 0.01, respectively). Specifically, each μg/m3 increase in personal BC level was associated with 4.6% higher H3K9ac (95% CI: 0.9, 8.4; p=0.02) and 4.1% higher H3K36me3 (95% CI: 1.3, 7.0; p=0.01) among office workers but only 0.1% higher H3K9ac (95% CI: −1.3, 1.5; p=0.90) and 0.9% higher H3K36me3 (95% CI: −0.9, 2.7; p=0.33) among truck drivers.
Fig. 1.
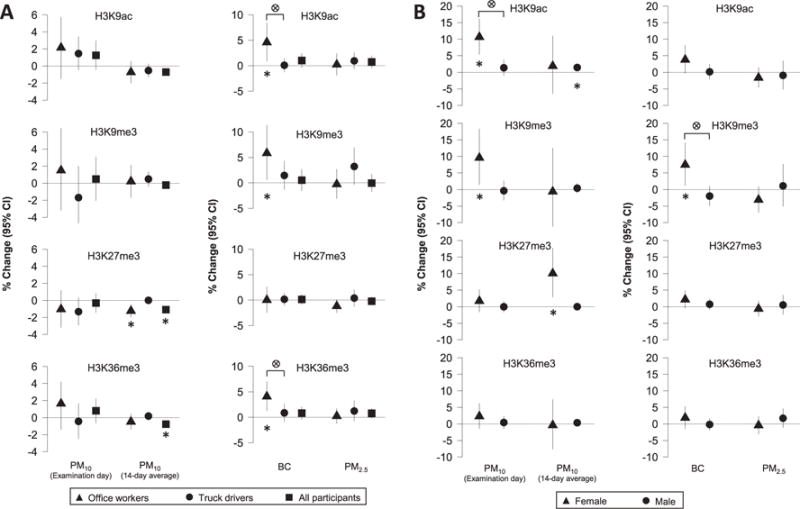
Histone H3 modification changes associated with ambient PM10 and personal BC exposures. A: analyses stratified by occupation group. B: analyses stratified by sex. For PM10 on the examination day and PM2.5, % change was expressed per one-unit increase in exposure. For 14-day moving average PM10 and BC, % change was expressed per 10-unit increase in exposure. Asterisk indicates significant associations between PM exposures (p < 0.05) and circle cross sign indicates an association that is significantly different between groups (pinteraction < 0.05).
We further investigated whether the associations of ambient PM10 and personal BC levels and blood histone modifications were modified by sex (Fig. 1B, details in Supplemental Table S4). Notably, we observed several stronger associations among women. For PM10 exposure each 10 μg/m3 increase in PM10 exposure on the examination day was associated with 9.6% higher H3K9me3 (95% CI: 1.5, 18.3; p=0.03), while each 10-μg/m3 increase in 14-day average PM10 was associated with 10.1% higher H3K27me3 (95% CI: 2.9, 17.7; p=0.01). However, neither of these associations was significantly modified by sex (pinteraction=0.18 and 0.89, respectively). In men, 14-day average PM10 was associated with higher H3K9ac only (β=1.5%; 95% CI: 0.3, 2.6; p=0.01). This association was also not significantly modified by sex (pinteraction=0.11). However we identified two associations, between examination day PM10 exposure and H3K9ac and between BC exposure and H3K9me3, that were both stronger among women and significantly modified by sex (pinteraction=0.01 and 0.03, respectively). For women each 10 μg/m3 increase in PM10 exposure on the examination day was associated with 10.7% higher H3K9ac (95% CI: 5.4, 16.2; p < 0.01), while for personal BC exposure each μg/m3 increase in BC was associated with 7.5% higher H3K9me3 (95% CI: 1.2, 14.2; p=0.03). For men each 10 μg/m3 increase in PM10 exposure on the examination day was associated with 1.4% higher H3K9ac (95% CI: −0.9, 3.7; p=0.25), while for personal BC exposure each μg/m3 increase in BC was associated with 2.0% lower H3K9me3 (95% CI: −4.9, 1.1; p=0.20). Neither personal PM2.5 (Fig. 1A, details in Supplemental Table S3) nor elemental components were associated with any histone modifications after we adjusted for multiple testing (Supplemental Table S5). Our sensitivity analysis suggested that these results did not differ significantly by smoking status, and our association analysis of PM10 was robust for meteorological adjustments when temperature and humidity were included in the models.
4. Discussion
To our knowledge, this is the first human subjects study that has examined the relationship between ambient traffic-derived pollutants and histone H3 modifications. After adjusting for potential confounders we observed that ambient 14-day average PM10 was negatively associated with blood levels of H3K27me3 and H3K36me3 in all participants. Interestingly, occupation and sex appeared to modify the associations between certain traffic-derived PM exposures and H3K9ac/me3; office workers had a stronger positive association with personal BC exposure and women had a stronger positive association with ambient PM10 and personal BC exposures. We found no significant associations between histone H3 modifications and general levels of personal PM2.5 or its elemental components.
H3K27me3 is associated with gene inactivation and primarily maintained by histone methyltrasferases such as zeste 2 (EZH2) (Kuzmichev et al., 2002). In a mouse macrophage cell line Miousse et al. found traffic-derived PM10 concentration was negatively associated with the expression of EZH2 (Miousse et al., 2014), which can consequentially lead to depleted H3K27me3 level (Vire et al., 2006). In addition, inflammation can induce a decrease in H3K27me3 levels, a pre-mark in normal cells during carcinogenesis (Takeshima et al., 2012). Studies have also demonstrated that air pollutants can modify immune response, which in turn increases susceptibility to pathogens and worsens pre-existing diseases (Bauer et al., 2012; Nadeau et al., 2010). Specifically, H3K27me3 has been shown to play a role in the immune response to cancer (Huang et al., 2012). Our study, for the first time, establishes a potential link between traffic-derived PM and H3K27me3 in humans. It is also noteworthy that we observed higher H3K27me3 level among ever-smokers, as H3K27me3 has been positively associated with tobacco smoke exposure in lung cancer cells (Hussain et al., 2009). Our observed inverse association between PM10 and H3K27me3 indicates that exposures to tobacco smoke and PM10 may have distinct biologic effects on H3K27me3 due to their differences in composition. In our study, PM10 exposure is likely derived from traffic-related particles such as fuel and petroleum rather than combustion of plant matter. A significant negative association using 14-day average PM10 exposure rather than exposure on the examination day suggests that H3K27me3 may reflect a temporal delay between PM10 exposure and the epigenetic response to it. This finding suggests that future studies of the epigenetic effects of PM10 should attempt to avoid cross-sectional designs in populations whose exposure to PM could be highly variable over time, as such designs may be unable to account for delayed effects of PM10 exposure.
H3K36me3 facilitates an open chromatin for active transcription (Barski et al., 2007). Relationships between air pollution exposures and H3K36me3 are understudied, however recent studies suggest that this histone fulfills a tumor suppressive function. H3K36me3 facilitates DNA mismatch repair during the cell cycle, and has thus been suggested as a potential early detection biomarker and therapeutic target for cancer (Li et al., 2013; Li, 2013). Loss of H3K36me3 has also been associated with increased risk of death from renal cell (Ho et al., 2016) and liver cancers (Tamagawa et al., 2013). Future studies should confirm H3K36me3′s involvement in environmental carcinogenesis, either as a mediator or an effect modifier, and explore its use as a potential therapeutic or chemopreventive target.
Our findings regarding H3K9ac are consistent with prior studies. In mouse models, traffic-derived air pollution exposure increased global H3K9ac levels in both blood and lung tissues (Ding et al., 2016), indicating that H3K9ac measured in blood could be used as a surrogate tissue. Cumulative exposure to inhaled nickel, arsenic, and iron have also identified associations with increased H3K9ac (Cantone et al., 2011). Moreover, H3K9ac mediates the effects of inhaled PM on blood coagulation (Cantone et al., 2014) suggesting that it may serve as a marker of air pollution-related cardiovascular outcomes, and if confirmed would provide public health officials and researchers with a useful exposure measurement and/or risk assessment tool. Conversely, we found no similar studies explaining a link between ambient air pollution exposure and H3K9me3. However, chromate exposure in human lung A549 cells has been shown to increase the global levels of H3K9me3 (Sun et al., 2009) and it was suggested that this has a strong influence on regional mutation-rate variation in human cancers (Schuster-Bockler and Lehner, 2012). Since H3K9ac activates genes whereas H3K9me3 silences them, future research with larger sample sizes should explore the target genes of these two different modifications of H3K9.
According to antibody manufacturers (http://www.histoneantibodies.com) H3K9me3 has shown slight cross-reactivity with H3K27me3 as both share a similar epitope, which may play a role in the positive correlation observed. However the histone modifications H3K9ac and H3K36me3 do not show cross-reactivity with the other histones in this study. Analyses in embryonic stem cells have suggested that the co-localization of histone modifications might be due to population averaging (Vastenhouw and Schier, 2012), which may be also applicable to leukocyte in our study and partially explain our positive correlation.
The observed weak correlation of histone H3 modification between two examination days could be partially attributed to intrinsic temporal variability of epigenetic markers in blood leukocytes (Feil and Fraga, 2011). In addition, latent measurement errors between the two examination days could also add noise to this variability. As a result, our findings indicate short-term changes in histone H3 modifications of blood leukocytes in response to PM exposures. However the repeated measures and mixed-effect modelling controlled the intra-individual variability, which to some extent controls for false positive findings that are due to transient changes of histone modifications coinciding with PM exposures. Further research is warranted to demonstrate whether exposure-induced changes in global histone modifications are retained after the exposure ceases.
In our study, significant associations between personal level of BC and H3K9ac and H3K9me3 were observed in office workers but not in truck drivers. One possible explanation for this observation is that epigenetic effects of exposure to traffic-derived pollutants may be subject to a ‘ceiling’ effect, where additional exposure at greater levels produces less marginal change in the epigenetic response to it, for instance, via biological adaptation to traffic-derived pollutant exposures (Zhong et al., 2016). Thus, the non-significant findings in truck drivers may be driven, at least in part, by the fact that truck drivers’ exposure level of BC was on average 32% higher than that in office workers across both examination days (Sanchez-Guerra et al., 2015). Similarly, exposures to PM10 and BC in men were on average 17% and 35% higher than those in women. Thus a similar disparity in air pollution exposure between men and women, and biological ‘ceiling’ effect on histone H3 modifications, may also explain our observed sex differences in associations between traffic-derived air pollutants and histone modifications. Alternatively, these findings may be reflective of biological sex differences at the molecular level, which are also worth exploring in future large studies. Additional research is needed to study these possibilities in larger populations with more diverse characteristics, particularly in terms of exposure to air pollutants.
In contrast to ambient PM10, we did not observe any significant associations between histone H3 modifications and personal exposure to PM2.5. We previously observed elevated H3K27ac patterns in individuals from a different population exposed to ambient PM2.5 in Beijing (Liu et al., 2015a). These inconsistent findings reveal differences between personal and citywide PM exposures. The ambient PM2.5 measured in Liu et al.’s study would share more similarities with ambient PM10 measured in our study, as opposed to our personal PM2.5 measures which may not correlate well with citywide ambient measures. Moreover, we measured personal PM2.5 for eight hours during the participants’ work shifts while ambient PM10 was calculated based on citywide daily 24-h data. Therefore, the differences in sampling times could also have contributed to the inconsistencies between the two studies.
Different composition of PM2.5 may also have distinct biologic effects on histone H3 modifications. We did not observe any significant associations between H3 modifications and personal exposure to PM2.5 and its elemental components after adjustment for multiple testing, however personal BC level in PM2.5 (i.e., carbonaceous PM2.5) was associated with three H3 modifications (H3K9ac, H3K9me3, and H3K36me3). These findings underscore the importance of traffic-derived BC in disease etiology. Carbonaceous PM2.5 is believed to be five times more toxic than particles such as crustal material, nitrates, and sulfates (Lelieveld et al., 2015). Further investigation is warranted to provide insights into the histone modification-mediated physiological effects of traffic-derived air pollution.
Our study has several notable strengths. Using blood leukocytes to measure histone modifications may reflect past exposures to air pollution and enable us to study immune cells in their true context, i.e., with all cellular components represented and interacting together, and thus provide a realistic reflection of in vivo processes. Considering potential technical errors in PM measurement we conducted a technical validation of personal PM2.5 and the eight elemental components measured so that only measurements with high reproducibility were included in our data analysis. Our personal-level measurement of PM2.5, BC, and elemental components also provides a more accurate reflection of individual exposures (Baccarelli et al., 2014; Ding et al., 2016; Hou et al., 2016) and allowed us to distinguish the effects of traffic-related pollutants from those of general PM2.5 levels.
Nonetheless our study is subject to limitations. First, we prioritized our focus on PM in this study as it is a dominant air pollutant and a long-standing pollution issue in Beijing due to re-suspended dust from traffic and construction activities resulting from Beijing’s rapid urbanization and motorization in recent decades (Wang et al., 2009a). However, we cannot exclude the possibility that other components of ambient air pollution (e.g., benzo[a]pyrene) may also contribute to histone H3 modification changes (Liang et al., 2012). Second, we utilized citywide measures of ambient PM10 as a proxy of personal exposure to PM10, which potentially subjects our results to the ecological fallacy. However, serial measures of ambient particulate concentrations have been shown to be representative of variations in personal exposure (Wilson and Brauer, 2006), particularly in the presence of high ambient PM levels (Avery et al., 2010). Third, it is possible that the changes in blood histone modification levels were partially due to changes in white blood cell types (Miao et al., 2008; Wang et al., 2009b). However, we previously detected associations of metal-rich PM exposures with histone modifications measured in blood leukocytes and our sensitivity analysis showed no changes in the results across subtypes (Cantone et al., 2011). Unfortunately the current study did not include cell count data, thus we attempted to minimize potential confounding due to short-term changes in blood cell type abundancies (Holgate et al., 2003) by focusing on short-term traffic-derived pollutant exposures coupled with a repeated-measures, matched study design and mixed effect modelling. In addition, we recognize that the ELISA technique for measuring global histone modifications cannot discern the amount of histone modifications at a given locus. Some of the histone modifications studied here (H3K9ac, H3K9me3, and H3K27me3) are within the N-terminal tail of histone 3, which has been reported to be clipped in some human cells (Howe and Gamble, 2015; Zhou et al., 2014). Thus, while our approach could underestimate the amount of histone modifications measured, we would expect that this would only attenuate our observed associations.
5. Conclusion
Our investigation provides evidence that exposures to ambient traffic-derived PM10 and BC are associated with histone H3 modification in human leukocytes. While the direct pathological impact of these findings is presently unclear, the observed occupation- and sex-related differences provide some scientific guidance and evidence for future air pollution studies in humans, particularly of BC exposure. Larger, prospective studies are warranted to validate the present findings and identify specific genes and pathways affected by exposure-related changes in histone modifications, as well as link these observed associations to air pollution-associated disease outcomes.
Supplementary Material
Acknowledgments
Funding Information
This work was supported by the National Institute of Environmental Health Sciences.
[Grant Numbers R21ES020984, R21ES020010], American Heart Association [Grant Number 12GRNT12070254], and the Robert H. Lurie Comprehensive Cancer Center - Rosenberg Family Cancer Research Fund. MSG was financially supported by the Fundación México en Harvard, A.C. and Consejo Nacional de Ciencia y Tecnología [Grant Number CONACYT-Mexico]. JJC was funded by the National Institute of Environmental Health Sciences [Grant Number 1F32ES024068-01].
Abbreviations
- BC
black carbon
- BMI
body mass index
- CI
confidence interval
- ELISA
enzyme-linked immunosorbent assay
- H3
Histone H3
- K
lysine
- H3K9ac
H3 lysine 9 acetylation
- H3K9me3
H3 lysine 9 tri-methylation
- H3K27me3
H3 lysine 27 tri-methylation
- H3K36me3
H3 lysine 36 tri-methylation
- PM
particulate matter
- PM2.5
particulate matter ≤2.5 μm
- PM10
particulate matter ≤10 μm
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2016.11.015.
Footnotes
Competing interests: The authors declare they have no actual or potential competing financial interests.
Authors’ contributions: LH, AAB, SW, DC, PAB, JS, designed the study and supervised study operations. DC, JPM, AD, and XZ prepared study protocols and oversaw their implementation. MSG, COY, and JJC developed and/or supervised experimental analyses. JPM, PK, and CMK were responsible for personal exposure measures. ZZ and YZ performed statistical analyses. YZ, MSG, ZZ, BTJ, JKK, COY, JZ, XZ, AAB, and LH wrote the manuscript. YZ, ZZ, LL, WZ, TG, YC contributed to data interpretation. All authors read and approved the final manuscript.
References
- Arita A, et al. Global levels of histone modifications in peripheral blood mononuclear cells of subjects with exposure to nickel. Environ Health Perspect. 2012a;120:198–203. doi: 10.1289/ehp.1104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita A, et al. The effect of exposure to carcinogenic metals on histone tail modifications and gene expression in human subjects. J Trace Elem Med Biol. 2012b;26:174–178. doi: 10.1016/j.jtemb.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery CL, et al. Estimating error in using ambient PM20.5 concentrations as proxies for personal exposures: a review. Epidemiology. 2010;21:215–223. doi: 10.1097/EDE.0b013e3181cb41f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli AA, et al. Air pollution exposure and lung function in highly exposed subjects in Beijing, China: a repeated-measure study. Part Fibre Toxicol. 2014;11:51. doi: 10.1186/s12989-014-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bauer RN, et al. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129:14–24. doi: 10.1016/j.jaci.2011.11.004. quiz 25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res. 2011;90:9–17. doi: 10.1177/0022034510378683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati V, Baccarelli A. Environmental epigenetics. Heredity. 2010;105:105–112. doi: 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook RD, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- Cantone L, et al. Inhalable metal-rich air particles and histone H3K4 dimethylation and H3K9 acetylation in a cross-sectional study of steel workers. Environ Health Perspect. 2011;119:964–969. doi: 10.1289/ehp.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantone L, et al. Extracellular histones mediate the effects of metal-rich air particles on blood coagulation. Environ Res. 2014;132:76–82. doi: 10.1016/j.envres.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervona Y, et al. Associations between arsenic exposure and global posttranslational histone modifications among adults in Bangladesh. Cancer Epidemiol Biomark Prev. 2012;21:2252–2260. doi: 10.1158/1055-9965.EPI-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervona Y, Costa M. The control of histone methylation and gene expression by oxidative stress, hypoxia, and metals. Free Radic Biol Med. 2012;53:1041–1047. doi: 10.1016/j.freeradbiomed.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, et al. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology. 2010;21:892–902. doi: 10.1097/EDE.0b013e3181f20e6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, et al. H3K9 acetylation change patterns in rats after exposure to traffic-related air pollution. Environ Toxicol Pharmacol. 2016;42:170–175. doi: 10.1016/j.etap.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Dominici F, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2011;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Henikoff S, Shilatifard A. Histone modification: cause or cog? Trends Genet. 2011;27:389–396. doi: 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Ho TH, et al. Loss of histone H3 lysine 36 trimethylation is associated with an increased risk of renal cell carcinoma-specific death. Mod Pathol. 2016;29:34–42. doi: 10.1038/modpathol.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST, et al. Health effects of acute exposure to air pollution. Part I: healthy and asthmatic subjects exposed to diesel exhaust. Res Rep Health Eff Inst. 2003:1–30. discussion 51–67. [PubMed] [Google Scholar]
- Hou L, et al. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ Int. 2012a;48:71–77. doi: 10.1016/j.envint.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, et al. Environmental chemical exposures and human epigenetics. Int J Epidemiol. 2012b;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, et al. Inhalable particulate matter and mitochondrial DNA copy number in highly exposed individuals in Beijing, China: a repeated-measure study. Part Fibre Toxicol. 2013;10:17. doi: 10.1186/1743-8977-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, et al. Particulate air pollution exposure and expression of viral and human MicroRNAs in blood: the Beijing truck driver air pollution study. Environ Health Perspect. 2016;124:344–350. doi: 10.1289/ehp.1408519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CG, Gamble MV. Enzymatic cleavage of histone H3: a new consideration when measuring histone modifications in human samples. Clin Epigenetics. 2015;7:7. doi: 10.1186/s13148-014-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, et al. Global mapping of H3K4me3 and H3K27me3 reveals chromatin state-based regulation of human monocyte-derived dendritic cells in different environments. Genes Immun. 2012;13:311–320. doi: 10.1038/gene.2011.87. [DOI] [PubMed] [Google Scholar]
- Hussain M, et al. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res. 2009;69:3570–3578. doi: 10.1158/0008-5472.CAN-08-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO, ISO 9835. Ambient air – Determination of a black smoke index. International Organization for Standardization; Geneva: 1993. [Google Scholar]
- Janssen NA, et al. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch school children. Environ Health Perspect. 2003;111:1512–1518. doi: 10.1289/ehp.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney PL, et al. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect. 2000;108:213–218. doi: 10.1289/ehp.00108213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kunzli N, et al. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000;356:795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- Kuzmichev A, et al. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M, et al. Lateral thinking: how histone modifications regulate gene expression. Trends Genet. 2016;32:42–56. doi: 10.1016/j.tig.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, et al. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Lepeule J, et al. Epigenetic influences on associations between air pollutants and lung function in elderly men: the normative aging study. Environ Health Perspect. 2014;122:566–572. doi: 10.1289/ehp.1206458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res. 2007;636:95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Li F, et al. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell. 2013;153:590–600. doi: 10.1016/j.cell.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GM. Decoding the histone code: role of H3K36me3 in mismatch repair and implications for cancer susceptibility and therapy. Cancer Res. 2013;73:6379–6383. doi: 10.1158/0008-5472.CAN-13-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, et al. Neonatal exposure to benzo[a]pyrene decreases the levels of serum testosterone and histone H3K14 acetylation of the StAR promoter in the testes of SD rats. Toxicology. 2012;302:285–291. doi: 10.1016/j.tox.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Liu C, et al. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ Health. 2015a;14:65. doi: 10.1186/s12940-015-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. Seasonal and diurnal variation in particulate matter (PM10 and PM25) at an urban site of Beijing: analyses from a 9-year study. Environ Sci Pollut Res Int. 2015b;22:627–642. doi: 10.1007/s11356-014-3347-0. [DOI] [PubMed] [Google Scholar]
- Luger K, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Ma L, et al. Histone methylation in nickel-smelting industrial workers. PLoS One. 2015;10:e0140339. doi: 10.1371/journal.pone.0140339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigano J, et al. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect. 2011;119:977–982. doi: 10.1289/ehp.1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics. 2011;6:820–827. doi: 10.4161/epi.6.7.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao F, et al. Histone methylation patterns are cell-type specific in human monocytes and lymphocytes and well maintained at core genes. J Immunol. 2008;180:2264–2269. doi: 10.4049/jimmunol.180.4.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miousse IR, et al. Epigenetic alterations induced by ambient particulate matter in mouse macrophages. Environ Mol Mutagen. 2014;55:428–435. doi: 10.1002/em.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau K, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–852, e10. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, et al. Lung cancer incidence and long-term exposure to air pollution from traffic. Environ Health Perspect. 2011;119:860–865. doi: 10.1289/ehp.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempala GA, Yang Y. On permutation procedures for strong control in multiple testing with gene expression data. Stat Interface. 2013;6 doi: 10.4310/SII.2013.v6.n1.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guerra M, et al. Effects of particulate matter exposure on blood 5-hydroxymethylation: results from the Beijing truck driver air pollution study. Epigenetics. 2015;10:633–642. doi: 10.1080/15592294.2015.1050174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster-Bockler B, Lehner B. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature. 2012;488:504–507. doi: 10.1038/nature11273. [DOI] [PubMed] [Google Scholar]
- Shechter D, et al. Extraction, purification and analysis of histones. Nat Protoc. 2007;2:1445–1457. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- Smith PK, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, et al. The regulation of hepatic Pon1 by a maternal high-fat diet is gender specific and may occur through promoter histone modifications in neonatal rats. J Nutr Biochem. 2014;25:170–176. doi: 10.1016/j.jnutbio.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JG, et al. Populations potentially exposed to traffic-related air pollution in seven world cities. Environ Int. 2015;78:82–89. doi: 10.1016/j.envint.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Sun H, et al. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol Appl Pharmacol. 2009;237:258–266. doi: 10.1016/j.taap.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H, et al. Induction of aberrant trimethylation of histone H3 lysine 27 by inflammation in mouse colonic epithelial cells. Carcinogenesis. 2012;33:2384–2390. doi: 10.1093/carcin/bgs294. [DOI] [PubMed] [Google Scholar]
- Tamagawa H, et al. Global histone modification of H3K27 correlates with the outcomes in patients with metachronous liver metastasis of colorectal cancer. Eur J Surg Oncol. 2013;39:655–661. doi: 10.1016/j.ejso.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Tonne C, et al. A case-control analysis of exposure to traffic and acute myocardial infarction. Environ Health Perspect. 2007;115:53–57. doi: 10.1289/ehp.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastenhouw NL, Schier AF. Bivalent histone modifications in early embryogenesis. Curr Opin Cell Biol. 2012;24:374–386. doi: 10.1016/j.ceb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vire E, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- Wang W, et al. Atmospheric particulate matter pollution during the 2008 Beijing Olympics. Environ Sci Technol. 2009a;43:5314–5320. doi: 10.1021/es9007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. Characterization of human epigenomes. Curr Opin Genet Dev. 2009b;19:127–134. doi: 10.1016/j.gde.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welstead GG, et al. X-linked H3K27me3 demethylase Utx is required for embryonic development in a sex-specific manner. Proc Natl Acad Sci USA. 2012;109:13004–13009. doi: 10.1073/pnas.1210787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, et al. Exposure to Traffic-Related Air Pollution in Relation to Progression in Physical Disability among Older Adults. Environ Health Perspect. 2016 doi: 10.1289/ehp.1510089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WE, Brauer M. Estimation of ambient and non-ambient components of particulate matter exposure from a personal monitoring panel study. J Expo Sci Environ Epidemiol. 2006;16:264–274. doi: 10.1038/sj.jes.7500483. [DOI] [PubMed] [Google Scholar]
- Word Health Organization. Ambient (outdoor) Air Quality and Health. Fact Sheet N°313 2014 [Google Scholar]
- Zhang Z, et al. Specific histone modifications were associated with PAHs-induced DNA damage response in coke oven workers. Toxicol Res. 2016 doi: 10.1039/c6tx00112b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, et al. Traffic-related air pollution, blood pressure, and adaptive response of mitochondrial abundance. Circulation. 2016;133:378–387. doi: 10.1161/CIRCULATIONAHA.115.018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, et al. Histone cleavage as a mechanism for epigenetic regulation: current insights and perspectives. Curr Mol Med. 2014;14:1164–1172. doi: 10.2174/1566524014666141015155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, et al. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29:1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


