ABSTRACT
Leptin is a master regulator of energy balance and body adiposity. Additionally, leptin exerts important control on glucose homeostasis, thermogenesis, autonomic nervous system and neuroendocrine axes. In metabolic diseases, such as obesity and diabetes mellitus, leptin signaling may be compromised, indicating the important role of this hormone in the etiology and pathophysiological manifestations of these conditions. In the present manuscript, we reviewed important concepts of leptin signaling, as well as about the effects of leptin on several biologic functions. We also discussed the possible therapeutic use of leptin administration and how our current obesogenic environment contributes to the development of leptin resistance. Our objective was to provide a comprehensive and state-of-the-art review about the importance of leptin to maintain the homeostasis and during pathological conditions.
KEYWORDS: Hypothalamus, food intake, energy balance, obesity, diabetes
Leptin is a 16-kDa peptide hormone produced mainly by adipocytes, although other tissues and organs, such as mammary gland, ovary, skeletal muscle, stomach, pituitary gland and lymphoid tissue may produce lower amounts, possibly for local action.1 Leptin is secreted proportionally to the mass of adipose tissue, thereby representing an important marker of energy storage. The adipocyte-derived leptin secretion displays a circadian profile, with highest levels at night and lowest levels at daytime in humans.2 Leptin secretion also has a marked sexual dimorphism, with higher serum leptin concentration in women at any level of adiposity.3 Several hormones modulate leptin secretion. For example, insulin induces leptin secretion,4,5 whereas cortisol displays an inverse circadian rhythm.6
Remarkably, the physiologic role of leptin started to be studied before the discovery of leptin itself. This unusual situation was possible because spontaneous mutations generated leptin and leptin receptor (LepR; also known as Ob-R) deficient mice decades before the identification of their mutations.7-9 The first description of the leptin deficient mouse occurred in 1950 and this mouse strain was named as ob/ob because of its morbid obese phenotype.10 In 1966, another mutation was described in an inbred strain of mouse that was characterized by a metabolic disturbance resembling diabetes mellitus. Consequently, homozygous mutants were named as db/db because they carried the diabetes gene.9 Years later, Coleman and colleagues through parabiosis experiments came to the conclusion that the obesity of ob/ob mice was likely caused because they lacked a circulating factor, whereas the phenotype of db/db mice was possibly caused by the lack of the receptor for that factor.7,8,11-13 It turned out that Coleman and colleagues were right, and in 1994 Jeffrey Friedman's group discovered that the ob gene encodes the hormone leptin.14 In two separated papers, the Lepr gene was identified and demonstrated that LepR is encoded by the db gene.15,16
Growing literature shows that not only leptin is a master regulator of energy balance, but it also modulates glucose homeostasis, neuroendocrine axes, autonomic nervous system, memory, neural plasticity, and other biologic functions. The objective of this manuscript is to provide a comprehensive review about the effects of leptin on several biologic functions, its mechanisms of action and how leptin is related to several diseases, such as obesity and diabetes mellitus.
The biology of leptin
Leptin receptor and signaling pathways
Leptin acts by binding to its membrane receptor, which is expressed in many tissues. Through an expression cloning strategy, six LepR isoforms were identified (LepRa-f).15 Although all six LepR isoforms share a common extracellular ligand-binding domain at the N-terminus, they differ in their intracellular domain, and therefore in their physiologic role. LepRe lacks the transmembrane domain, representing a soluble LepR isoform. Thus, LepRe is a leptin-binding protein and possibly regulates leptin biologic activity by removing it from circulation.17 LepRa, a short isoform of the receptor, is abundantly expressed in the choroid plexus, and has been hypothesized to be implicated in leptin transport into the central nervous system (CNS) through the blood–brain barrier (BBB).18 Only the long isoform (LepRb or Ob-Rb) contains all intracellular motifs required for complete activation of the intracellular signaling pathways. LepRb is predominantly expressed in the CNS and is essential for leptin action.19-22 Consequently, this isoform is responsible for the main biologic effects of leptin. The obese mouse model known as db/db lacks exclusively the LepRb, and its phenotype is similar to mutants that have no LepR isoforms (db3J mutation).23 LepR belongs to the family of class 1 cytokine receptors with many similarities to the interleukin (IL)-6 receptor. This type of receptor does not contain intrinsic tyrosine enzymatic activity but signals via an associated tyrosine kinase protein from the Janus kinase family (JAK) (Fig. 1). When leptin binds to LepRb, JAK2 is recruited, activated and promotes autophosphorylation and phosphorylation of three tyrosine residues of LepRb (Y985, Y1077 and Y1138). These phosphorylated tyrosine residues act as docking sites for downstream signaling molecules, representing a Src homology 2 (SH2)-binding motif that recruits specific SH2-containing effector proteins to mediate subsequent intracellular signaling.24 The major signaling pathway recruited by leptin is the signal transducer and activator of transcription 3 (STAT3), which depends on the phosphorylation of Y1138.25,26 STAT3 is a transcription factor and after phosphorylation STAT3 migrates to the nucleus where the expression of target genes will be transcriptionally regulated. When the Y985 residue of LepR is phosphorylated, Src homology 2-containing tyrosine phosphatase (Shp2; encoded by Ptpn11 gene) is recruited to LepR, leading to the activation of extracellular signal-regulated kinases (ERK) signaling pathway (Fig. 1).24 Y985 is also a binding site for the suppressor of cytokine signaling 3 (SOCS3), which is a protein that exerts inhibitory effects on leptin signaling. Since SOCS3 transcription is dependent on leptin signaling through STAT3, it exerts its inhibition via a classic negative feedback way. Finally, phosphorylation of Y1077 recruits STAT5. Although the activation of STAT5 is mainly dependent on the Y1077 residue, STAT5 can also be activated by phosphorylation on Y1138.27 STAT5 pathway is more related to metabolic functions controlled by other cytokines like growth hormone (GH) and prolactin.28 However, STAT5 pathway mediates some of the effects of leptin on the regulation of energy balance and reproduction (Fig. 1).28,29
Figure 1.
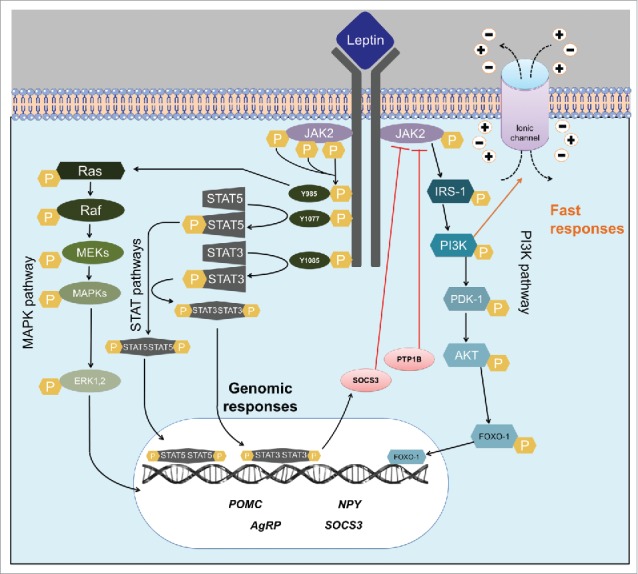
Leptin signaling pathways. Scheme summarizing the main intracellular pathways activated by the long-form of leptin receptor (LepR). Abbreviations: PI3K, phosphatidylinositol-3-kinase; IRS, insulin receptor substrate; JAK2, janus kinase 2; STAT, signal transducer and activator of transcription; SOCS3, suppressor of cytokine signaling-3; PTP1B, phosphotyrosine phosphatase 1B; SHP-2, src-homology-2 containing phosphotyrosine phosphatase 2; MAPK, mitogen-activated-protein-kinase; Raf, raf proto-oncogene serine/threonine-protein kinase; Ras, family of small GTPase; ERK1/2, Extracellular signal-regulated kinases; MEK, mitogen-activated protein kinase kinase; PDK-1, phosphoinositide-dependent kinase-1; AKT, ak strain transforming/protein kinase FOXO-1, forkhead box protein O1.
Another signaling pathway activated by leptin is the phosphatidylinositol 3-kinase (PI3K). PI3K is a major signaling pathway for insulin and other growth factors in peripheral tissues as well as the CNS.30 JAK2 phosphorylates insulin receptor substrates (IRS) leading to PI3K activation (Fig. 1). Unlike the JAK/STAT pathway, which acts through gene expression regulation, PI3K can produce fast cellular responses by promoting changes in ion channels and thereby in cell activity.31
For many years it was challenging to identify leptin-responsive cells. Antibodies against LepR produce poor staining frequently leading to false-positive or false-negative results.32,33 The use of radiolabeled leptin to identify binding sites was not able to identify many brain areas that also contain LepR expression.15 Furthermore, the LepR mRNA levels are commonly low in many brain areas, which makes it difficult the precise identification of LepR-expressing cells.34-39 Due to these limitations, many studies begun to identify leptin-responsive cells via the activation of its signaling pathways. Therefore, STAT3 phosphorylation (pSTAT3) after an acute leptin stimulus became a popular marker to identify leptin-responsive cells (Fig. 2).25,40-42 Additionally, STAT3 phosphorylation is also widely used as a way to evaluate leptin sensitivity.43-46
Figure 2.
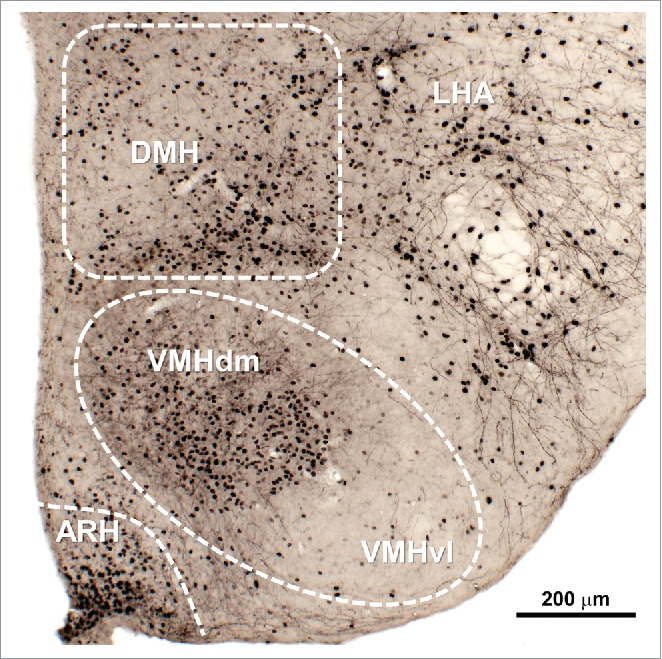
Distribution of leptin responsive neurons in the mouse mediobasal hypothalamus. Leptin-responsive cells could be visualized by the phosphorylation of STAT3 (black nuclear staining) 90 min after an acute peritoneal injection of mouse recombinant leptin (10 µg/g body weight). Abbreviations: ARH, arcuate nucleus of hypothalamus; DMH, dorsomedial nucleus of hypothalamus; LHA, lateral hypothalamic area; VMH, ventromedial nucleus of hypothalamus; VMHdm, dorsomedial part of the VMH; VMHvl, ventrolateral part of the VMH.
The physiologic role of leptin
The word leptin is derived from the Greek root leptós that means thin. The choice of Friedman's group to name the hormone leptin was based on the first studies that infused leptin in mice and observed a significant reduction in their body weight and adiposity.47 Although these earlier studies may indicate that leptin could have an application in the treatment of obesity, latter evidence demonstrated that most cases of obesity are characterized by excess of leptin and leptin resistance.48 Only in rare cases of obesity caused by congenital deficiency (similar to the ob/ob model) leptin treatment was a useful tool to revert obesity.49,50
The deficiency of leptin or LepR produces a very characteristic phenotype of hyperphagia, morbid obesity and insulin resistance.51 As described above, leptin treatment in ob/ob mice reversed their obese phenotype, which led to the conclusion that the physiologic role of leptin is to reduce the body weight and food intake.47 However, over the years, many studies have shown that leptin's role is in fact much more complex, given its participation in many other physiologic functions, from neuronal development and plasticity, memory and cognition, glucose homeostasis, reproduction and metabolic programming, which will be detailed across this review.52,53 For some authors, leptin's biologic role is not to make an animal thin, as originally described, but to act as a signal to the brain to convey the energy status of the body and thereby modulate energy-demanding functions to maintain homeostasis.54 In response to fasting, leptin levels decrease intensively, more than expected by the changes in body adiposity. This rapid reduction in leptin levels coordinates profound alterations in the metabolism and neuroendocrine axes that will save energy to ensure survival during conditions of negative energy balance. Falling in leptin levels can also trigger behaviors that will increase the search for food. When leptin is supplemented in fasted individuals, starvation-induced changes are attenuated.55-57 Therefore, leptin's main physiologic role is likely to promote survival, via neuroendocrine, behavioral and metabolic adaptations to preserve energy and increase food intake in situations of negative energy balance. Because in nature is more common the need of survival mechanisms in situations of undernutrition, leptin elicits more robust physiologic responses when its levels are low (conveying a signal of low levels of energy stocks), differently than when its levels are increased (like in obesity), a rare situation in nature (reviewed in Leibel58).
Food intake
Leptin plays a pivotal role regulating food intake. Ob/ob and db/db mice exhibit a marked hyperphagic behavior.47 Leptin infusion, either peripherally or centrally, produces a significant suppression in food intake in ob/ob and wild-type animals, but not in db/db mice.47,51,59,60 A robust voluntary reduction in energy intake is also achieved in leptin-deficient humans chronically treated with leptin.50,61 Several intracellular signaling pathways mediate the effects of leptin on food intake. While disruption of LepR/STAT3 pathway produces hyperphagia, pharmacological or genetic disruption of the PI3K pathway prevents the suppression of food intake in the 24 h following leptin administration.62,63
Although different neuronal populations are likely responsible to mediate leptin's effects on food intake, the arcuate nucleus of the hypothalamus (ARH) is certainly an important area.64 In the ARH, leptin acts at least in two distinct neuronal populations to affect food intake. Neurons that co-express the proopiomelanocortin (POMC) prohormone and the cocaine and amphetamine regulated transcript (CART) are responsive to leptin.36,65 ARH POMC/CART neurons are activated by leptin and these cells normally inhibit food intake.66-68 This occurs because CART has anorexigenic effects,69 whereas POMC is cleaved in different peptides, including α-melanocyte-stimulating hormone (α-MSH), which is able to activate melanocortin receptors 3 and 4 (MC3R/MC4R) leading to increased satiety.70 MC4R ablation reproduces many of the metabolic aspects observed in db/db mice, including their morbid obesity and hyperphagia.71 Anatomically, POMC neurons are found predominantly in the lateral ARH (Fig. 3A).72 On the other hand, located in the ventromedial ARH (close to the third ventricle and median eminence), neurons that co-express the neuropeptide Y (NPY), the agouti-related protein (AgRP) and the amino acid GABA are inhibited by leptin (Fig. 3A).73 The NPY/AgRP/GABA neurons are potent inducers of food intake due to several reasons.74,75 NPY is one of most powerful orexigenic neuropeptides as demonstrated by the robust feeding produced by intracerebroventricular NPY injections.76 AgRP is an inverse agonist of MC3R/MC4R. Consequently, AgRP blocks the capacity of α-MSH to activate MC3R/MC4R, which also leads to an increase in food intake.70,77 Finally, several pieces of evidence also indicate that the inhibitory GABAergic transmission of these neurons is important to modulate neural circuits involved in feeding behavior.75,78,79 Therefore, via a relationship of antagonism,77 POMC/CART and NPY/AgRP/GABA neurons form the central melanocortin system controlling the activity of second-order neurons that typically express the MC4R. It is worth mentioning though that although leptin exerts important effects on POMC/CART and NPY/AgRP/GABA neurons,80 many pieces of evidence indicate that leptin must act on multiple neuronal populations and neurocircuits to properly regulate food intake and energy balance. For instance, disruption of LepR expression exclusively in POMC/CART or NPY/AgRP/GABA neurons produces relatively modest effects on food intake and energy balance,81,82 which markedly contrasts with the profound impact in the energy homeostasis caused by the complete ablation of NPY/AgRP/GABA neurons (via diphtheria toxin) or by the lack of MC4R.71,83
Figure 3.
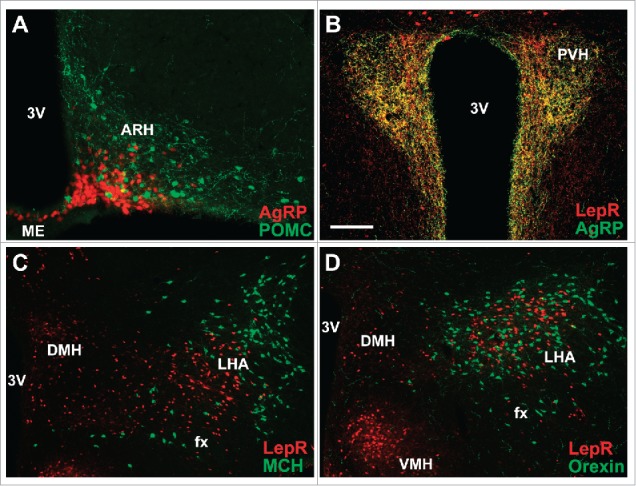
Hypothalamic distribution of key neuronal populations involved in the regulation of energy balance. (A) AgRP neurons in the ARH are located close to the third ventricle and median eminence, whereas POMC neurons are predominantly in the lateral ARH. POMC neurons were visualized by immunostaining β-endorphin peptide, while AgRP neurons were visualized using a reporter mouse that express the tdTomato fluorescent protein under the Agrp promoters. (B) PVH receives dense projections from LepR/AgRP neurons. Green fibers were visualized by immunostaining AgRP peptide, whereas axons from LepR-expressing neurons were visualized using a reporter mouse that express the tdTomato fluorescent protein under the Lepr promoters, as previously shown.86 Note the extensive co-localization (yellow color). (C, D) MCH (C) and orexin (D) neurons represent a segregate neuronal population in the LHA and do not express LepR. MCH and Orexin neurons were immunostained using specific antisera, whereas LepR-expressing neurons were visualized using a reporter mouse that express the tdTomato fluorescent protein under the Lepr promoters. Abbreviations: 3V, third ventricle; ARH, arcuate nucleus of hypothalamus; DMH, dorsomedial nucleus of hypothalamus; fx, fornix; LHA, lateral hypothalamic area; ME, median eminence; PVH, paraventricular nucleus of the hypothalamus; VMH, ventromedial nucleus of hypothalamus. Scale bar: A–B = 100 µm; C–D = 200 µm.
The paraventricular nucleus of the hypothalamus (PVH) is an important brain structure that receives dense projections from either POMC/CART or NPY/AgRP/GABA neurons (Fig. 3B).84-86 Expression of MC4R exclusively in PVH neurons can rescue the increased food intake observed in Mc4r null mice.84 During calorie-restricted conditions, ARH NPY/AgRP/GABA neurons are strongly activated as demonstrated by an increased intrinsic action potential frequency.87 The increased activity of ARH NPY/AgRP/GABA neurons is mediated by the decreased levels of anorexigenic hormones, such as leptin, while the orexigenic hormone ghrelin activates these cells.88 However, recent evidence also indicates that PVH neurons can provide a potent excitatory input to ARH NPY/AgRP/GABA neurons during fasting, representing another way to produce hunger in calorie-restricted conditions.89,90 Consequently, there is a reciprocal regulation between ARH and PVH neurons to control food intake.
Other brain structures also interact directly to ARH neurons to control feeding. For example, NPY/AgRP/GABA neurons project to the parabrachial nucleus (PBN) which in turn regulates neurons in the amygdala to control food intake. This circuit is especially important in conditions when it is unfavorable to eat, such as after severe overfeeding or during illness.78,91,92 The lateral hypothalamic area (LHA) receives projections from different populations of LepR-expressing neurons, and it is also a critical structure that regulates feeding.93 Neurons that express the neuropeptides melanin-concentrating hormone (MCH) or orexin (also known as hypocretin) are found in the LHA and these cells stimulate feeding, although they do not express the LepR (Fig. 3C and D).94 On the other hand, LepR expression in the LHA is found in neurotensin-positive neurons, and LepR ablation in these cells increases food intake.95 Leptin acts in LHA neurotensin neurons leading to an inhibition of orexin neurons.96 Neurotensin receptor-1 disruption promotes hedonic feeding.97
The ability of leptin to regulate feeding depends on several neuronal populations and neurocircuits. Pharmacogenetic activation of LepR-expressing neurons in the median preoptic area (MPO) induces a robust suppression of food intake in mice.98 Dopamine neurons in the ventral tegmental area (VTA) express the LepR as well.99,100 Direct administration of leptin to the VTA decreases food intake.99 Additionally, long-term RNAi-mediated knockdown of LepR in the VTA strengthens the sensitivity to highly palatable food and increases food intake.99 Leptin also modulates the activity of urocortin 1 neurons in the Edinger–Westphal (EW) nucleus.101 Since urocortin 1 produces anorexigenic effects, this particular neuronal population may mediate some of the leptin's effects on food intake.101 LepR expression is also found in brainstem neurons, including cells of the nucleus of the solitary tract (NTS),102-104 which is an important sensory relay from the upper gastrointestinal tract. LepR-expressing cells in the NTS co-express different neurochemical markers including POMC, cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1).105 Selective inactivation of LepR in Phox2b positive cells causes LepR deletion in NTS GLP-1 neurons.106 This genetic manipulation generates mice that display increased food intake after fasting, indicating that leptin action on GLP-1 neurons also controls food intake.106 In line with these findings, RNAi-mediated knockdown of LepR in NTS resulted in hyperphagia for chow, high-fat or high-sucrose diets, leading to an increase in body weight and adiposity.107 Recent evidence also suggests that leptin signaling in non-neuronal cells regulates feeding. Astrocyte-specific LepR deficiency leads to increased food intake.108 Overall, leptin possibly acts in neurons located in the ARH, LHA, MPO, VTA and NTS, as well as in non-neuronal cells to control different aspects of feeding behavior. Fig. 4 summarizes the participation of different brain nuclei in the multiple physiologic functions regulated by leptin signaling.
Figure 4.
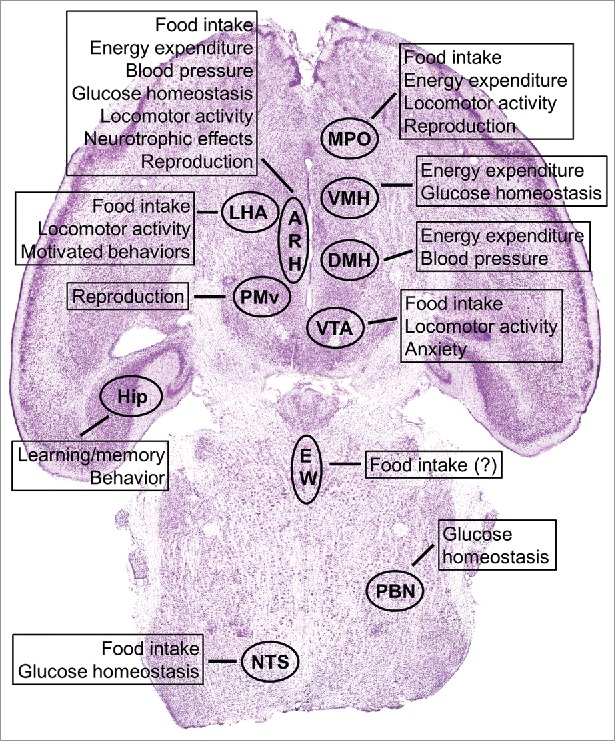
Brain distribution of LepR-expressing neurons and their known biologic functions regarding leptin signaling. Abbreviations: ARH, arcuate nucleus of the hypothalamus; DMH, dorsomedial nucleus of the hypothalamus; EW, Edinger–Westphal nucleus; Hip, hippocampus; LHA, lateral hypothalamic area; MPO, medial preoptic area; NTS, nucleus of the solitary tract; PBN, parabrachial nucleus; PMv, ventral premammillary nucleus; VMH, ventromedial nucleus of the hypothalamus; VTA, ventral tegmental area.
Energy expenditure and thermogenesis
It is well known that leptin affects the energy balance not only through the modulation of food intake, but it also has potent effects on energy expenditure. Ob/ob and db/db mice have high metabolic efficiency because of low energy expenditure.51,109 These defects can be corrected by leptin replacement only in ob/ob mice.51 Similarly to food intake, the regulation of energy expenditure by leptin depends on multiple neuronal populations. Conditional deletion of LepR from POMC cells leads to obesity without affecting food intake.81 Actually, these mutants exhibit a tendency toward low energy expenditure.81 In accordance with these findings, selective expression of LepR only in POMC cells produces no change in food intake, while the low energy expenditure observed in LepR-deficient mice is significantly rescued.110 Ablation of LepR only in AgRP neurons or simultaneously in POMC and AgRP cells does not affect food intake in adult mice, although these animals develop obesity, suggesting an alteration in energy expenditure.82 Accordingly, the low energy expenditure of ob/ob mice is partially improved in NPY-deficient animals.111
The ventromedial nucleus of the hypothalamus (VMH) is also an important structure involved in the energy balance regulation. Since steroidogenic factor-1 (SF1)-positive neurons are found only in the VMH, SF1 became a marker to induce genetic manipulations in this nucleus. Mice carrying a selective deletion of LepR in SF1 cells show a similar degree of obesity compared with POMC specific LepR ablation.112,113 Notably, this obese phenotype occurs in the absence of changes in food intake. However, SF1 specific LepR knockout mice exhibit an attenuated thermogenic response to high-fat diet (HFD).112,113 Several reports have demonstrated that leptin action in the dorsomedial nucleus of the hypothalamus (DMH) increases sympathetic tone to brown adipose tissue (BAT) and interscapular BAT temperature.114-116 Acute cold exposure induces c-Fos in DMH LepR-expressing neurons.115 Disruption of LepR selectively in DMH neurons causes obesity, reduces the energy expenditure and blocks thermogenic responses to leptin, without affecting food intake.116,117 Besides regulating food intake, MPO LepR-expressing neurons are also involved in temperature-dependent body weight homeostasis.98,115 This specific population innervates sympathetic BAT circuits.115 Activation of MPO LepR-expressing neurons suppresses energy expenditure leading to a reduction in body temperature.98
It is worth mentioning that leptin can regulate energy expenditure not only by changes in autonomic nervous system, but also through neuroendocrine mechanisms. Thyrotropin-releasing hormone (TRH) neurons in the PVH are master regulators of the thyroid gland function, whose hormones exert profound impact in the cellular metabolism and consequently in energy expenditure. PVH TRH neurons receive projections from ARH LepR-expressing neurons (Fig. 3B).118,119 During calorie-restricted conditions, the reduction in leptin levels coordinates endocrine and metabolic changes to save energy, which includes suppression of TRH, thyroid-stimulating hormone and thyroid hormones.55,57 Infusion of either α-MSH or CART is able to prevent fasting-induced suppression of TRH mRNA levels in the PVH.118-121 Therefore, leptin signaling in ARH neurons controls neuroendocrine cells of the PVH to regulate thyroid axis according to nutritional status.
Ob/ob mice exhibit a reduced body temperature, and in the past this phenotype was thought to be the result of lower energy expenditure.122-124 However, recent findings indicate that leptin actually acts centrally to modulate thermoregulatory mechanisms raising the defended body temperature and/or reducing thermal conductance.125,126 BAT is the main organ that produces heat by increasing energy expenditure. BAT expresses the uncoupling protein 1 (UCP1), a mitochondrial channel that uncouples the proton motive force of the respiratory chain from ATP production to heat production.127 BAT is innervated by the sympathetic nervous system and the activation of UCP1 is mainly regulated by β3-adrenergic receptors.128 Rats treated with leptin showed increased oxygen consumption and UCP1 mRNA expression, and this response is elevated after fasting.129 In the past, it was believed that BAT's function was critical only in small rodents; however, nowadays we known that BAT is present and functional in adult humans as well and became a potential target for obesity treatment.130 Genetic approaches enhancing leptin and insulin signaling in POMC neurons increase white adipose tissue browning and energy expenditure, conferring a protection against diet-induced obesity (DIO).131 In addition, the absence of hypothalamic insulin and leptin receptors maintains a lower body temperature (at 20–22 °C) than when either of the receptors is removed independently.132
Since endotherms need to maintain their internal body temperature, a process that demands large amounts of energy, during fasting conditions small endotherms may enter a status of daily torpor, thereby suppressing their metabolic rate together with a fall of core temperature lasting for a period of several hours.133 This event can also be present in situations of low energy sources or in in a low temperature ambient.134 Evidence in literature shows that the critical factor that determines the moment of entering torpor is the achievement of a critical low body mass, which drives the decrease in daytime core temperature in both DIO and lean mice facing fasting.135
Since leptin's structure resembles a cytokine, it is not surprising that leptin has an important role in the immune system. Leptin levels increase during infections, inflammations or lipopolysaccharide (LPS) exposure as a part of the host immune response (Fig. 5). This change is mediated by IL1-β.136 Evidence in animal models has demonstrated that systemic inflammation caused by LPS leads to increased circulating leptin,137,138 and the peak of leptin is preceded by an increase in tumor necrosis factor (TNF)-α, which by itself is also capable of increasing leptin circulating levels.139 Systemic inflammation and sepsis are closely related to changes in body temperature, both in animals and humans.140,141 Systemic inflammation is commonly followed by fever; however, in some situations it can evoke hypothermia.142 In animals, the response to LPS depends on the dose given and the environment temperature. In thermoneutral environment, fever is the predominant response. Low doses of LPS induce monophasic fever, which turns into polyphasic fever when the dose is increased.143 However, if a high dose of LPS is given in a low temperature environment, the response changes to hypothermia.144 Evidence shows that fever is a predominant response for mild stimuli, whereas hypothermia is a natural response for stronger inflammatory injuries.145 A major regulator for these responses is TNF-α that, depending on the dose, can be thermogenic or cryogenic. LepR KO rats (Koletsky f/f) have the same fever response to low doses of LPS as wild-type rats, but when treated with high doses of LPS express a severe hypothermia that lasts longer, associated with increased levels of TNF-α. A possible explanation for the TNF-α increase in LepR KO rats is the fact that they are unable to fully activate their hypothalamic-pituitary-adrenal axis which has anti-inflammatory actions in response to LPS, and therefore leptin seems to be important to recover from hypothermia induced by LPS.146 Further evidence for this point is that ob/ob mice do not activate their hypothalamic–pituitary–adrenal axis in response to LPS, compared with wild-type animals.147,148 In leptin responsive animals, stimuli that normally evoke highly febrigenic responses in fed animals result in hypothermia or low fever when the animals are fasted and they show elevated TNF-α levels.149-152 This is suggested to be related to low levels of leptin in fasted animals, because of the similar response observed in leptin-irresponsive rats (Koletsky f/f) (reviewed in refs.153,154).
Figure 5.
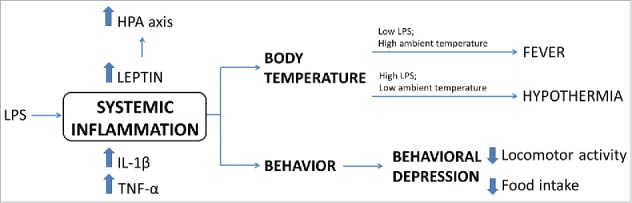
Physiological responses to systemic inflammation. Scheme summarizing changes in body temperature, behavior and secreted factors after LPS-induced inflammation as well as the leptin's role in the recovery of febrigenic or hypothermic processes.
In DIO rats, in which leptin levels were blocked with specific antiserum, the later phases of the fever response were attenuated. Thus, leptin was suggested to play an important role modulating the late phase of the fever response to LPS in obese animals.155 Higher levels of circulating leptin in old rats were associated with enhanced duration and degree of symptoms after LPS injection together with prolonged fever compared with young rats, showing that leptin has a role in age-dependent febrile responses to inflammation.156
Another phenomenon that can alter the regulation of body temperature is the psychological stimulated hyperthermia, both in animals and humans. This condition increases BAT thermogenesis157 and can be diminished by systemic administration of a β3-adrenoceptor antagonist in rats.158 However, the physiologic mechanisms involved in the hyperthermia induced by psychological stress are different from those involved in LPS-induced fever.159
In summary, leptin signaling in the ARH (POMC and AgRP/NPY neurons), VMH, DMH and MPO modulates the autonomic nervous system or endocrine axes leading to changes in energy expenditure and thermogenesis. Therefore, the powerful effects of leptin on the energy balance depend on a simultaneous regulation of food intake and energy expenditure. Furthermore, there is also evidence that leptin regulates immune responses, as well as body temperature through the modulation of central thermoregulatory mechanisms. Fig. 5 summarizes the physiologic responses to systemic inflammation and the role of leptin in the recovery of febrigenic or hypothermic processes.
Regulation of cardiovascular functions via autonomic nervous system
Recent reports indicate that DMH LepR-expressing cells also control blood pressure.160 Interestingly, leptin or LepR deficiency protects against hypertension, despite the morbid obese phenotype caused by these mutations. Additionally, increased leptin levels in DIO promotes increased blood pressure in rodents, which is an effect likely mediated by DMH neurons.114,160 The same protection against obesity-induced hypertension is observed in MC4R deficient mice, which indicates the involvement of the central melanocortin system in this dysfunction.161 Accordingly, MC4R expression in cholinergic neurons, which include sympathetic and parasympathetic preganglionic neurons, restores obesity-associated hypertension in MC4R deficient mice.162 Additionally, exclusive MC4R expression in cholinergic neurons is sufficient to normalize the energy expenditure abnormalities exhibited by MC4R deficiency, without affecting food intake.163 Thus, leptin acts via the melanocortin system and DMH LepR neurons to regulate blood pressure through the modulation of the autonomic nervous system.160,161,164
Glucose homeostasis
Glucose homeostasis is regulated by brain leptin signaling, independently of its effects on adiposity. For example, leptin infusion at low doses that does not affect body weight is able to correct the hyperglycemia and hyperinsulinemia of ob/ob mice.51 Additionally, genetic manipulation that increases leptin sensitivity in LepR-expressing cells does not prevent DIO, but protects mice from obesity-induced insulin resistance.165 Since adiposity plays a major role contributing to insulin resistance, it is sometimes difficult to determine whether leptin signaling in a specific brain structure directly controls glucose homeostasis or if the observed effects are secondary to changes in body weight and adiposity. However, there is solid evidence that some specific neuronal populations are particularly important to mediate leptin's effects on glucose homeostasis. Unilateral LepR reactivation in the ARH of otherwise LepR-deficient mice produces modest effects on body weight and adiposity, although this manipulation causes marked improvements in hyperinsulinemia and blood glucose levels.64 Leptin signaling in POMC neurons probably explains much of these effects, since selective expression of LepR only in POMC cells also produces robust improvements in the hyperinsulinemia and hyperglycemia of LepR-deficient mice, even though it causes small effects on body weight and adiposity.110,166 In accordance with the role of the central melanocortin system controlling glucose homeostasis, Rossi et al.163 found that, compared with MC4R deficient mice, re-expression of MC4R in cholinergic neurons (autonomic sympathetic and parasympathetic preganglionic neurons) produces expressive improvements in glucose control, insulin sensitivity and insulin-mediated suppression of hepatic glucose production. Interestingly, restoration of MC4R expression in the dorsal motor nucleus of the vagus (DMX), which is composed of parasympathetic preganglionic neurons, attenuates the hyperinsulinemia, but does not normalize the hyperglycemia of MC4R deficient mice, suggesting that the melanocortin system probably influences both sympathetic and parasympathetic branches to regulate different aspects of glucose homeostasis.162,163
There is some evidence that leptin signaling in VMH neurons also regulates glucose homeostasis. For example, selective inactivation of SOCS3 in SF1 cells increases leptin sensitivity, leading to an improvement of glucose homeostasis, without affecting body weight.167 VMH is a key area involved in counter-regulatory responses to hypoglycemia.168 Excitatory glutamatergic transmission from VMH neurons is required to prevent fasting-induced hypoglycemia.169 A neurocircuit between brainstem and VMH was recently described to control counter-regulatory responses to hypoglycemia. In this circuit, leptin-responsive cells in the lateral PBN express CCK and are inhibited by glucose. Leptin inhibits PBN CCK neurons and inactivation of LepR in CCK cells enhances counter-regulatory responses to hypoglycemia.170,171 PBN CCK neurons project to and regulate VMH SF1 cells via CCK release, and this neurocircuit is both necessary and sufficient for triggering counter-regulatory responses to hypoglycemia.168,170,171 Thus, several pieces of evidence indicate that leptin controls sympathetic and parasympathetic nervous systems via the melanocortin system to regulate glucose homeostasis. PBN CCK neurons are also directly regulated by leptin to modulate counterregulatory responses to hypoglycemia via connections with VMH SF1 neurons.
Reproduction
Calorie-restricted conditions also suppress the reproductive axis and these effects are leptin dependent.55,57,172,173 Several studies investigated potential brain areas that could mediate leptin's effects on reproduction. Although LepR-expressing cells are located nearby gonadotropin-releasing hormone (GnRH) neurons, there is no direct effect of leptin on these cells.174 Consequently, interneurons are necessary to convey leptin signal to GnRH neurons. Kisspeptin (peptide encoded by the Kiss1 gene) expressing neurons are required to sense estrogen levels to regulate GnRH neurons.175 Although earlier reports identified LepR mRNA expression in Kisspeptin neurons,176 more recent studies found a very limited LepR expression in these cells.177,178 Additionally, selective manipulation of LepR in Kisspeptin cells produced no significant impact on reproduction.177,179 On the other hand, neurons in the ventral premammillary nucleus (PMv) exhibit an abundant expression of LepR and play a critical role mediating leptin's effects on reproduction.180,181 PMv neurons project to GnRH cell bodies and fibers that reach median eminence, exerting an excitatory effect through their glutamatergic transmission.179,182,183 Excitotoxic lesions of the PMv in adult female rats disturb estrous cycle, the activation of GnRH neurons during the proestrus and GnRH and Kiss1 mRNA expression at the night of proestrus.184,185 PMv-lesioned rats also failed to show the stimulatory effects of leptin on luteinizing hormone (LH) secretion during fasting.185 The most substantial evidence that PMv neurons mediate the effects of leptin on reproduction came from studies that induced LepR-expression only in PMv neurons of otherwise LepR-deficient mice. In this study, unilateral re-activation of LepR in the PMv was sufficient to restore fertility, despite a complete absence of changes in body weight and food intake.179
Besides the PMv, MPO LepR-expressing neurons also regulate reproduction. These cells are found close to GnRH neurons and release nitric oxide (NO).103,186 Neuronal NO synthase (nNOS) knockout mice are unable to show the stimulatory effect of leptin on LH secretion, and pharmacological nNOS inhibition in the preoptic area prevents the restoration of fertility in leptin-treated ob/ob female mice.186 NPY/AgRP neurons are also important targets of leptin to regulate reproduction. Db/db mice carrying inactivation in Agrp gene exhibit significant improvements in reproduction, including normal timing of vaginal opening and estrous cycling, as well as recovery in fertility.187 In another study, NPY/AgRP neurons were ablated in ob/ob mice.188 The authors reported a restoration in body weight, food intake and glucose tolerance to levels similar to wild-type mice. Interestingly, this manipulation recovered the fertility of either male or female ob/ob, indicating that under low leptin levels NPY/AgRP neurons exert an important inhibitory effect on reproductive axis.188 Leptin probably acts on POMC/CART neurons to regulate reproduction as well. α-MSH is able to activate 70% of GnRH neurons, whereas approximately 15% of GnRH neurons are excited by CART.189 Additionally, the combined inactivation of LepR and insulin receptor in POMC cells reduces the fertility in mice, elevates serum testosterone levels and causes ovarian abnormalities.190 Therefore, the current evidence in the literature indicates that LepR-expressing neurons in the MPO, PMv and ARH (NPY/AgRP and POMC/CART cells) are able to sense leptin levels to modulate the onset of puberty and reproduction.
Locomotor activity, neuronal plasticity, development and other brain functions
The biologic effects of leptin extend beyond the regulation of energy balance and glucose homeostasis. There is plenty of information indicating that several behaviors and brain functions are regulated by leptin signaling. Voluntary locomotor activity is directly regulated by leptin signaling, independently of body weight changes. For example, ob/ob or LepR-deficient mice show reduced locomotor activity, which is not caused by their morbid obesity. Leptin signaling only in the ARH or specifically in POMC neurons can partially rescue the hypoactivity displayed by LepR-deficient mice.64,110,166 NPY/AgRP neurons also regulate locomotor activity since LepR ablation only in these cells causes decreased ambulatory activity compared with wild-type mice.82 Acute activation of MPO LepR-expressing neurons causes suppression in locomotor activity.98 However, the reduction in body temperature caused by the activation of MPO LepR neurons could impair locomotion.98 Mice carrying LepR ablation in LHA neurotensin neurons exhibit decreased locomotor activity as well.95 These animals show an altered regulation of orexin neurons and mesolimbic dopamine system, since they have lower fasting-induced activation of orexin neurons and reduced orexin expression in the LHA, as well as a blunted amphetamine-induced increase in locomotor activity.95 As previously mentioned, leptin can directly regulate mesolimbic dopamine system through LepR-expressing cells in the VTA.100 Knockdown of LepR in the VTA increases locomotor activity of rats.99 In another study, LepR was selectively inactivated in dopamine neurons.191 These mice display anxiogenic-like behavior in the elevated plus-maze, light-dark box, social interaction and novelty suppressed feeding tests. However, depression-related behaviors were not affected by the lack of leptin signaling in dopamine neurons.191 Therefore, several leptin responsive neural populations are responsible to mediate leptin's effects on locomotor activity, including neurons in the ARH, MPO, LHA and VTA. In addition, the direct (through VTA) or indirect (through LHA) regulation of mesolimbic dopamine system allows leptin to control different behaviors, such as anxiety and preference to palatable foods.
Neuroplasticity is the process through which neural circuits adapt to variations in stimuli coming from the environment. Leptin-deficient mice have reduced brain mass and cortical brain volume,192 and they have an immature expression pattern of synaptic and glial proteins. Some of these defects cannot be reverted by leptin treatment at adulthood.193 However, leptin treatment in adults with missense leptin mutations is able to increase gray matter concentration in the frontal cortex, left inferior parietal lobule and left cerebellum.194
As described above, the most studied role of leptin is related to the regulation of body weight and food intake. However, this function does not begin until the first month of life in rodents. It was shown by Mistry and collaborators that leptin administration peripherally or centrally, even in high doses, has no impact in body weight, food intake or fat deposition until the first month of postnatal life.195 This explains why ob/ob and db/db mice express their obese phenotype only after that period. Therefore, leptin's role is possibly different in very young mice. Leptin is expressed in the fetus, but its production originates from diverse tissues since adipose tissue is minimal during this stage of development.196 The majority of leptin-responsive neurons in adult brain are born in the first 2 weeks of embryonic life.196 Leptin's transport into the brain through the LepRa is finely regulated since embryonic stage,197 and leptin reaches the brain at a very young age.198 Between postnatal days 6 and 14 there is a leptin surge, with concentrations significantly higher than the values exhibited in adult life.199 To understand the physiologic role of this leptin surge, it is important to state that in mice and rats the hypothalamus begins to develop during mid-gestation and continues to develop during the first weeks of postnatal life.200 The development of the projections from ARH neurons to different hypothalamic nuclei coincides with the postnatal leptin surge, although it occurs at different moments, starting with the DMH (established by postnatal day 6, P6), whereas those to the PVH develop later, by P8–10, and finally the projections to the LHA are established by P16.85,201 Previous studies by Bouret and colleagues showed that leptin has an important trophic role in the mouse brain during the first weeks of life by regulating axon growth in specific neuronal populations. Consequently, ob/ob mice have fewer projections from the ARH to other brain regions, including the PVH. This deficiency could be reversed only when leptin was replaced in the first weeks of life, whereas leptin treatment in adult ob/ob mice was unable to restore these projections.85 However, despite this critical neonatal window for leptin's neurotrophic action, leptin treatment is still able to revert the obese phenotype of adult ob/ob mice, indicating that further studies are still necessary to understand the biologic importance of the neurotrophic effect of leptin.179 There is evidence that leptin also influences synaptic plasticity. Ob/ob mice have altered synaptic inputs in NPY/AgRP/GABA and POMC/CART neurons, with marked excitatory inputs on orexigenic neurons, thus stimulating food intake. In contrast to the critical leptin's neonatal window to regulate axon growth, leptin treatment reverses the altered pattern of the synaptic inputs of ob/ob mice within hours.202
Several studies have also identified a role of leptin in cognitive processes through activation of LepR in the hippocampus. It has been reported that leptin regulates hippocampal excitability by modulating large-conductance calcium-activated potassium channels.203 Leptin can reduce hippocampal excitability in an animal model of epilepsy204 or act as pro-convusant, depending on the target population of hippocampal cells.205 The hippocampus is required for learning and memory. The long-term potentiation is a form of synaptic plasticity important for learning/memory and this phenomenon is impaired in db/db mice.206 Additionally, leptin deficiency reduces brain myelination.207 Obese rats also show memory deficits related tasks,208 indicating a possible role of leptin in the formation of memory and learning. Baseline neurocognitive tests in a child with a mutation in the leptin gene indicated a developmental cognitive age lower than expected by their chronological age. Interestingly, treatment with leptin improved their neurocognitive skills.209 LepR-deficient mice also show depression-like and anxiolytic behaviors from a young age, as well as psychosis-like behavior, which is present only in adult animals.210 On the other hand, animals exposed to stress paradigms have a significant decrease in leptin serum levels, and leptin administration into the hippocampus reverted depressive-like behavior in the forced swim test.211,212 In summary, there is evidence indicating that leptin can affect numerous cognitive processes and behaviors.
The role of leptin in disease
Potential therapeutic effects of leptin administration
Obesity
Leptin replacement in leptin-deficient subjects leads to enormous beneficial effects restoring their energy balance, glucose homeostasis, neuroendocrine and cognitive dysfunctions.49,50,61,209 Mutations in the leptin gene can also produce a biologically inactive leptin, in which treatment with recombinant human leptin is able to normalize the eating behavior and cause weight loss.213 However, these mutations are very rare.214 The potential therapeutic effects of leptin administration to treat obese humans, who do not carry any mutations in the Lep gene, were tested in several clinical trials. However, treatment with exogenous leptin resulted in no or only modest effects on body weight,215-220 including obese individuals after gastric bypass surgery.221 Additionally, no significant prevention in calorie restriction-induced neuroendocrine adaptations was achieved with leptin administration.218,222 These results indicate that in obese individuals with already elevated leptin levels, exogenous leptin no further helps in the body weight management. Thus, leptin administration is likely a poor therapy for the obesity treatment. However, the perception of hunger/satiation was significantly improved in leptin-treated patients.223,224 Some reports also indicate that leptin administration induces a robust weight loss in a small percentage of patients,215 suggesting that a subgroup of obese individuals could benefit from leptin treatment.
Lipodystrophy
Since leptin is produced by the adipose tissue, any dysfunction in this organ may affect serum leptin levels. Congenital or acquired generalized lipodystrophy (GL) is a condition characterized by abnormal or degenerative adipose tissue, leading to incapacity to accumulate fat in this tissue that is compensated with lipid deposition in other organs, such as the liver and skeletal muscle. GL can cause severe insulin resistance, hyperglycemia, dyslipidemia and hepatic steatosis. Importantly, patients with GL show low circulating leptin levels. The role of GL-induced leptin deficiency was investigated by studies that treated GL patients with leptin. Leptin administration produced profound improvements in all metabolic defects exhibited by GL patients.225-228 The beneficial effects of leptin therapy were observed not only in congenital GL, but also in HIV-associated lipodystrophy syndrome,229,230 and in type 1 diabetes mellitus (T1DM) associated with acquired GL.231 These findings led the US Food & Drug Administration (FDA) to approve in 2014 Myalept® (metreleptin for injection) as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired GL. This was the first official medical approval of leptin treatment.
Diabetes mellitus
As mentioned earlier, leptin deficiency causes severe insulin resistance, frequently associated with hyperglycemia. Leptin replacement therapy reverses the diabetic phenotype of ob/ob mice or leptin deficient humans.50,51,61 Several studies investigated whether leptin treatment produces beneficial effects on type 2 diabetes mellitus (T2DM) patients.232,233 In a study, metreleptin administration in patients with T2DM only reduced glycated hemoglobin (HbA1c) marginally, without altering body weight or circulating inflammatory markers.233 In other report, recombinant methionyl human leptin for 14 d did not produce any significant effects on insulin sensitivity or body weight of obese people with T2DM.232 Therefore, in accordance with the clinical trials to evaluate the potential efficacy of leptin to treat obesity, the data available indicates that leptin possesses poor anti-diabetic potential when used in hyperleptinemic obese people with T2DM.
In contrast to the minor effects produced in T2DM, leptin therapy in type 1 diabetic non-obese (NOD) mice normalized circulating levels of glucose, HbA1c, free fatty acids, as well as a wide array of hepatic intermediary metabolites.234 Compared to insulin monotherapy, leptin lowers plasma and tissue lipids, and lipogenic and cholesterologenic transcription factors and enzymes.234 The anti-diabetic effects of leptin in T1DM depends on the CNS, since intracerebroventricular infusion of leptin has the same beneficial effects than systemic treatment.235 The mechanisms of action recruited by leptin to produce such dramatic effects resolving the metabolic dysfunctions in T1DM have been investigated. LepR expression in POMC cells or in GABA-positive neurons is sufficient to mediate the lifesaving and antidiabetic actions of leptin in insulin deficiency.236 Additionally, leptin administration improves hyperglucagonemia,234-236 which is a key feature that can lead to hyperglycemia and further complications of T1DM.237-240 Thus, pre-clinical studies in animal models highlight a promising therapeutic potential of leptin to treat T1DM. Regarding T2DM, leptin therapy produces small effects, possibly because of the already elevated serum leptin levels presented by the majority of type 2 diabetic individuals.
Hypothalamic amenorrhea
The reduction in leptin levels during calorie-restricted conditions is a determining factor that leads to suppression of the reproductive axis.55,57 Chronic energy deficiency secondary to strenuous exercise and/or decreased food intake can produce functional hypothalamic amenorrhea, which is a condition characterized by the disruption of GnRH secretion and consequently anovulation and infertility. Therefore, under these conditions, hypothalamic amenorrhea may be caused by the relative leptin deficiency produced by chronic negative energy balance. In an elegant study, eight women with hypothalamic amenorrhea due to strenuous exercise or low body weight received recombinant human leptin for up to 3 months.172 The authors observed that leptin treatment increased mean LH levels and LH pulse frequency after 2 weeks and increased maximal follicular diameter, the number of dominant follicles, ovarian volume and estradiol levels over a period of 3 months. Remarkably, three patients had an ovulatory menstrual cycle and two others had preovulatory follicular development and withdrawal bleeding during treatment.172 Besides improvements in the reproductive axis,172,241 leptin treatment in patients with hypothalamic amenorrhea improved bone mineral density and content, as well as markers of bone metabolism suggestive of bone formation.241,242 Although larger clinical trial studies are still required to better assess the effects of leptin in the hypothalamic amenorrhea, these preliminary findings indicate that leptin treatment may be helpful to improve fertility and bone mineral density in lean hypoleptinemic women.
Leptin resistance and obesity
Definition
As previously mentioned, the high leptin levels and the decreased responsiveness to leptin led the scientific community to hypothesize that obesity is characterized by a condition of leptin resistance. It is worth mentioning that it is believed that leptin triggers more robust biologic responses when its levels decrease, such as during caloric restriction. Therefore, the reduction of leptin levels is a powerful cue to coordinate responses to save energy and increase hunger to increase survival and protect animals from food deprivation. On the other hand, nutrient excess is considered such a rare situation in nature that probably precluded evolution to select mechanisms capable of preventing efficient weight gain. A reduced capacity to respond to chronic high leptin levels is an example of this concept. However, experimental evidence points out possible mechanisms that explain the lower responsiveness to high leptin levels,44 as described in more details in the following section and summarized in Fig. 6.
Figure 6.
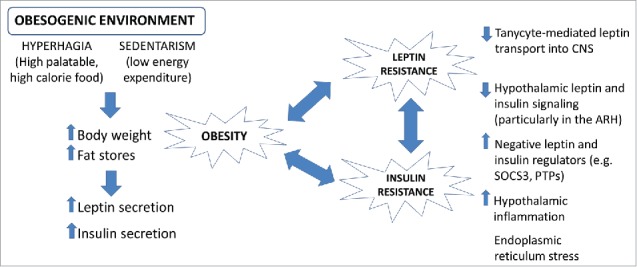
Environmental influences on the development of obesity. Scheme summarizing the mechanisms through which an altered environment can promote obesity and type 2 diabetes mellitus.
Leptin sensitivity in experimental models is normally evaluated by measuring the acute or chronic capacity of exogenous leptin infusion to reduce body weight, adiposity and/or food intake. Leptin sensitivity is also determined by measuring the activation of signaling pathways recruited by LepR after leptin administration (Fig. 2). Using the anorexigenic effects of leptin as readout to determine leptin sensitivity, numerous studies observed that DIO animals showed an attenuated response to leptin.243 However, a recent study questioned the current view of the importance of leptin resistance to induce obesity.244 In this study, LepR antagonist administration surprisingly increased feeding and body weight in both lean and DIO mice. As expected, this antagonist had no effect on leptin or LepR-deficient mice.244 Therefore, although several studies have demonstrated that obesity reduces the anorexigenic effects of leptin, DIO animals still retain some capacity to respond to LepR antagonist.
Regarding the assessment of leptin signaling pathways, Münzberg et al.45 made a very nice observation indicating that some brain regions are more prone to develop leptin resistance compared with others. In this study, an acute peripheral (intraperitoneal) leptin infusion was able to induce an equivalent number of cells expressing pSTAT3 in the VMH, DMH, PMv and NTS of HFD-induced obese mice, compared with lean animals on normal chow diet.45 However, the number of pSTAT3-positive cells in the ARH of obese mice was significantly lower compared with lean animals indicating a nucleus-specific leptin resistance.45 Other studies confirmed these findings114 and further indicated that NPY/AgRP neurons are first affected by DIO (2 d on HFD are enough to cause leptin resistance in these cells), whereas leptin resistance in POMC neurons requires a longer exposition to HFD.245 The reason for this selective leptin resistance may be caused by the anatomic position of LepR-expressing cells in relation to the BBB. NPY/AgRP neurons are very near to median eminence, which is a circumventricular organ. Therefore, median eminence presents extensive vasculature and lack of a normal BBB. Consequently, factors in the systemic circulation can affect more significantly neurons located close to this structure than cells of other brain regions. Therefore, ARH cells, especially NPY/AgRP neurons, are robustly affected by the pro-inflammatory and hyperleptinemic effects induced by HFD consumption.245 Besides HFD consumption, leptin resistance is also observed in other situations, such as pregnancy.104,246-249 As the primary cause of leptin resistance in pregnancy likely differs from what is observed in DIO, the brain nuclei exhibiting leptin resistance in pregnant animals may also be different. In fact, several studies have observed a lower responsiveness to leptin in the VMH of pregnant animals.247-250
The selective leptin resistance may help to explain some metabolic consequences of obesity. While the ability of leptin to regulate the energy balance, body weight and glucose homeostasis is impaired in DIO animals, since ARH is a major area that regulates these functions, others effects of leptin may be upregulated. That seems to be the case of leptin-induced activation of sympathetic nervous system.114,160,164 As obese animals have high leptin levels and brain nuclei that drive leptin-induced activation of sympathetic nervous system maintain leptin sensitivity (e.g., DMH), the overactivation of leptin signaling can, for example, predispose obese individuals to hypertension.160 Thus, leptin resistance is a complex phenomenon that probably affects some neuronal populations and leptin's functions, but not others.
Mechanisms
Some authors have suggested that leptin resistance may be caused by a limited capacity of leptin to enter into the CNS and therefore activate its cognate receptor in different brain areas. This hypothesis is supported by the fact that leptin enters into the CNS through a saturated transport system,251 as well as because the cerebrospinal-fluid/serum leptin ratio is decreased in obesity.252,253 Additionally, some studies have demonstrated that central leptin infusions are more efficient in inducing leptin's anorexigenic effects in obese animals in comparison with peripheral injections, suggesting that leptin transport from systemic circulation to the CNS could represent a limiting factor.60,243 Some pieces of evidence indicate the participation of short forms of LepR, such as the ObRa, in the leptin transport from systemic circulation to the CNS. For example, rats carrying a mutation that prevents the synthesis of short forms of LepR show decreased transport of leptin across the BBB and develop obesity.18,254 In addition, the short form of LepR is abundantly expressed in brain microvessels and choroid plexus, and ObRa is able to mediate a transcellular transport of leptin.39,255 However, other reports presented evidence that leptin transport across the BBB is not mediated by leptin receptors.256 In another study, mice carrying a mutation that prevents the synthesis of the ObRa isoform were studied.257 Although these mutants exhibited a reduced cerebrospinal-fluid/serum leptin ratio, they presented very modest metabolic abnormalities suggesting that ObRa only plays a minor role in mediating leptin's effects.257 More recently, the participation of median eminence tanycytes was described in the leptin transport to the brain.258 Circulating leptin activates ERK signaling pathway in median eminence tanycytes, which allows leptin passage to the cerebrospinal fluid. Interestingly, leptin taken up by tanycytes accumulates in the median eminence of DIO or db/db mice, failing to reach the mediobasal hypothalamus.258 Therefore, although it is not totally clear the exact role of BBB leptin transport as the primary cause of leptin resistance in DIO, defects in this mechanism seem to exert a significant role.
Some authors also suggest that leptin resistance may emerge as a secondary defect caused by the high leptin levels observed in obesity.259 However, studies that artificially produced high leptin levels chronically observed that animals did not defend a higher body weight, even after cessation of leptin administration, suggesting that hyperleptinemia per se does not produce long-term defects to maintain the energy balance and body weight.60,260 On the other hand, the most robust evidence to explain leptin resistance came from studies that identified intracellular proteins that are able to block the activation of leptin signaling cascades. STAT transcription factors robustly regulate the transcription of genes from the SOCS family, which includes eight intracellular proteins, named as SOCS1 to SOCS7, and CIS. These proteins have a C-terminal motif referred to as the SOCS box and a SH2 domain that allows them to bind to other proteins that contain phosphorylated tyrosine residues.261 Therefore, SOCS proteins bind to tyrosine phosphorylated proteins leading them to proteasomal degradation or blocking their capacity to transduce intracellular effects.261 Regarding leptin signaling that conveys its cellular effects through tyrosine phosphorylation and recruits LepR/JAK2/STAT3 pathway, previous studies have identified SOCS3 as the major protein from the SOCS family that is able to inhibit leptin signaling.262 SOCS3 binds to phosphorylated JAK2 and tyrosine residue 985 of LepR, in both cases blocking the capacity of LepR/JAK2 pathway to transmit its intracellular signal (e.g., to recruit downstream proteins such as STAT3).263,264 Interestingly, the activation of LepR/JAK2/STAT3 signaling pathway induces a robust SOCS3 expression, indicating that SOCS3 acts as a negative feedback mechanism to modulate leptin signaling (Fig. 1).262 That is the reason why hyperleptinemic conditions, like obesity, are normally associated with high hypothalamic SOCS3 expression.262 Therefore, the increased SOCS3 levels in LepR-expressing cells could be a potential cause of leptin resistance in obesity. The important role of SOCS3 controlling leptin sensitivity was confirmed by studies that induced selective SOCS3 inactivation72,165,167,173,249,250,264-269 or super-expression.270 For example, brain-specific SOCS3 inactivation increases leptin sensitivity and partially protects against DIO.268 SOCS3 expression in specific neuronal populations including POMC,265 SF1,167, AgRP245 and LepR-expressing165,173,249,250 cells regulates leptin sensitivity and consequently the metabolism. Since SOCS3 also inhibits insulin signaling, and insulin and leptin act together in the CNS to control glucose homeostasis,271 conditional SOCS3 ablation normally produces beneficial effects on glucose control, especially in obese insulin-resistant animals.165,265,268
The SOCS3 capacity to affect leptin signaling and consequently the metabolism is not limited to conditions that simulate pathological states, such as DIO. Recent findings have shown that SOCS3 levels in LepR-expressing cells coordinate the typical metabolic adaptions of pregnancy.249,250 Pregnant animals must increase their food intake, adiposity and develop a certain level of insulin resistance to properly provide nutrients for the developing fetuses, and later for lactation. During pregnancy, changes in hormone levels (e.g., increased prolactin or placental lactogens levels) induce leptin resistance and may increase hypothalamic SOCS3 levels.272-274 Selective inactivation of SOCS3 in LepR-expressing cells prevents leptin resistance and thereby attenuates the increased food intake, adiposity and insulin resistance observed in pregnant mice.249,250 In another study, inactivation of SOCS3 in LepR-expressing cells mitigated post-restriction hyperphagia and weight regain in lean mice by reducing the mRNA levels of hypothalamic orexigenic neuropeptides during fasting.72
Another important group of proteins that regulates leptin sensitivity is composed by protein tyrosine phosphatases (PTPs), which catalyze the dephosphorylation of tyrosine residues. Consequently, these proteins can block leptin signaling through the dephosphorylation of LepR, JAK2 or STAT3.275 The protein tyrosine phosphatase 1B (PTP1B; encoded by Ptpn1 gene), T-cell protein tyrosine phosphatase (TCPTP; encoded by Ptpn2 gene) and protein tyrosine phosphatase epsilon (RPTPε; encoded by Ptpre gene) produce these effects, regulating negatively LepR signaling. Several studies have demonstrated that hypothalamic expression of PTPs is increased in obese animals and selective ablation of these proteins improves leptin sensitivity and partially prevents HFD-induced obesity and insulin resistance.46,131,266,276-280
Leptin signaling can also be positively regulated by adaptor proteins and other enzymes. For example, inactivation of the Shp2 decreases leptin sensitivity and predisposes animals to obesity.281,282 The cytoplasmic adaptor protein SH2B1 increases leptin sensitivity by binding to phospho-Tyr813 of JAK2 which enhances its activity and the activation of downstream leptin-signaling pathways.283 SH2B1 can also bind directly to insulin receptor substrate 1 and 2 (IRS1/IRS2), stimulating leptin-induced activation of PI3K signaling pathway.283 In accordance with these effects, neuron-specific SH2B1 knockout mice exhibit leptin resistance, obesity and hyperglycemia.284 In summary, several intracellular proteins are able to affect either positively or negatively leptin signaling, representing potential candidates to mediate the leptin resistance observed in specific conditions, such as DIO or pregnancy.
Link between environment and obesity
The increasing prevalence of obesity and other metabolic diseases provides a link between the environmental changes that have occurred in the last decades with an impaired capacity to properly regulate the energy balance (Fig. 6). Regarding this, a frequent consumption of high-palatable/high-caloric diets, associated with a sedentary and stressful lifestyle, somehow disturb the functioning of neurocircuits responsible to control the food intake, body weight and blood glucose levels. The intake of high-palatable/high-caloric diets can disrupt the energy balance via several mechanisms.285,286 For example, HFD activates proinflammatory responses in the hypothalamus, which disturb the energy and glucose homeostasis.287 HFD can also lead to suppressed neurogenesis and apoptosis of hypothalamic neurons, especially those that induce satiety.288,289 The proinflammatory and proapoptotic effects of HFD may involve the activation of toll-like receptor 4 (TLR4), since TLR4 is activated by saturated fatty acids.290 Consequently, HFD induces the activation of IKKβ/NF-kappaB signaling pathways in the hypothalamus, which induce the production of proinflammatory cytokines, such as TNF-α.291,292 A high calorie/fat diet can also lead to endoplasmic reticulum (ER) stress.290,291,293 ER stress is caused by the accumulation of misfolded proteins, which activates the unfolded protein response.286 This condition, commonly caused by HFD consumption, plays a central role in the development of leptin resistance.291,294-297
More recently, several studies described the participation of the gut microbiota in the predisposition to obesity and metabolic complications.285,298-303 Differences in our diet can change our gut microbiota, impacting in the risk to develop obesity.300 The gut microbiota can provide short-chain fatty acids or other nutrients after bacterial fermentation, and modulates gastrointestinal permeability and thereby the penetration of bacteria or bacterial products, such as LPS, which can induce intestinal or systemic inflammation.285,298,301 Interestingly, non-caloric artificial sweeteners or dietary emulsifiers, widely used food additives, can change our gut microbiota and promote metabolic syndrome.302,303
Can leptin resistance be treated?
Since leptin resistance is a key feature that predisposes to obesity, the discovery of any treatment capable of preventing or restoring this condition would have a tremendous potential as anti-obesity therapy. Unfortunately, so far there is no efficient method to reestablish leptin sensitivity in obese animals. However, numerous studies have been focused in seeking for potential therapies that can increase leptin sensitivity. Obvious candidates would be compounds that inhibit proteins capable of inducing leptin resistance. Many natural products contain several lignans and flavonoids that exhibit a significant capacity to inhibit PTP1B.304,305 Resveratrol activates SIRT1, a NAD+-dependent protein deacetylase, which modulates leptin and insulin sensitivity.306 Using drug-screening methods, some studies found leptin sensitizer compounds with powerful anti-obesity properties, including the Celastrol, a pentacyclic triterpene, or Withaferin A, a steroidal lactone.307,308 Amylin is a hormone co-secreted with insulin from pancreatic β-cells, which binds specific receptors in the hindbrain. Interestingly, concurrent peripheral administration of amylin and leptin elicits synergistic anorexigenic effects in DIO animals, indicating that amylin agonists restore leptin responsiveness in DIO.309-312 Other studies also observed restoration of leptin responsiveness in DIO mice using an optimized leptin analog in combination with exendin-4, a long-acting GLP-1 receptor agonist, or fibroblast growth factor 21.313
Not all fatty acids are pro-inflammatory. Actually, unsaturated fatty acids can revert diet-induced hypothalamic inflammation,314 suggesting that dietary changes prioritizing the intake of unsaturated fatty acids instead of saturated fatty acids can prevent hypothalamic dysfunctions, leptin resistance and consequently obesity. ω-3 polyunsaturated fatty acids activate the G protein-coupled receptor 120 (GPR120) and produce potent anti-inflammatory and insulin-sensitizing effects.315 Notably, GPR120-deficient mice are prone to develop obesity and GPR120 mutations increase the obesity risk in European populations.316 ω-3 polyunsaturated fatty acids may also promote neurogenesis in POMC-expressing cells of the ARH, indicating the potential of ω-3 polyunsaturated fatty acids to correct obesity-associated hypothalamic neuronal loss.317 Regarding non-pharmacological and non-dietary anti-obesity strategies, physical exercise has a great potential. The beneficial effects of exercise for the energy and glucose homeostasis are well-established. However, much less is known about the molecular mechanisms that makes physical exercise an efficient anti-obesity therapy. Previous studies have shown that both endurance,318,319 and voluntary320-322 exercise improves leptin sensitivity in peripheral tissues or in the hypothalamus of obese animals. Additionally, acute exercise suppresses hypothalamic PTP1B protein levels, leading to a higher activation of insulin and leptin signaling pathways in obese rats.323
Leptin and metabolic programming
Together with the genetic background and the environment where an organism develops, the interaction between mother and fetus during gestation permanently influences the organism's metabolism in adult life, a concept known as metabolic programming.324 Hales and Baker defined the “thrifty phenotype hypothesis,” which suggests that poor nutrition during gestation can permanently program the fetus to develop metabolic disorders such as metabolic syndrome and type 2 diabetes mellitus.325 The consequences of poor nutrition during gestation for the development of obesity were first observed during the Dutch famine of 1944–1945, where exposition to undernutrition during the first half of pregnancy resulted in high obesity rates, whereas exposition during the last trimester or first months after birth resulted in lower obesity rates.326 Therefore, depending on the moment where the exposure to undernutrition occurs, the progeny will be programmed to a higher or lower body mass index.53
A recent study using a mouse model of undernutrition during gestation showed that if food is available during the postnatal period, the undernourished offspring often displays accelerated catch-up growth. This fast recovery seems to be mediated by leptin.327 As mentioned earlier, leptin inhibits NPY/AgRP/GABA neurons in adult animals. However, there is evidence that leptin can activate NPY/AgRP/GABA neurons from postnatal days 13–15 (P13–P15). This opposite effect occurs because of the lack of functional ATP dependent potassium channels in LepR cells at that age. At P21, when pups feed independently, leptin induces depolarization in 41% of the cells, while 25% of neurons are hyperpolarized by leptin. By the fourth week of life, the NPY/AgRP/GABA circuit acquires the adult phenotype.327 In case of undernutrition followed by overnutrition during lactation, the onset of the potassium channels is delayed and instead of inhibiting food intake leptin stimulates it, favoring the accumulation of energy stores until growth and adiposity are similar to controls.328
The opposite scenario, overnutrition, also has severe consequences for the metabolism of the offspring. Several studies have shown that overnutrition before, during and shortly after gestation can induce long-term metabolic disorders in the offspring.329 Female mice fed a HFD during gestation had hyperglycemic pups with higher adiposity.330 If the HFD was extended to lactation, the offspring developed greater risk of becoming obese,331 and the α-MSH and AgRP containing fibers were decreased in the hypothalamus.332 Similarly to what was seen in rodents, children born of obese mothers have higher chances of developing metabolic syndrome.333 Rats treated with leptin antagonist during development presented leptin resistance in adult life, and when submitted to HFD they had higher chances to gain more weight than control rats. At 8 months, rats that received leptin antagonist during development became hyperleptinemic with higher adiposity.334
Human and animal models of retarded intrauterine growth are more susceptible to develop obesity and metabolic syndrome when challenged with HFD in adult life. Leptin levels increase at the end of embryonic development, but are decreased after birth, being lower in babies with retarded intrauterine growth compared with control newborns,335 indicating that lower leptin during development participates in the metabolic programming. In rats, neonatal leptin treatment reverses the developmental programming induced by undernutrition during gestation, showing that the neonatal period can be influenced by serum leptin levels.336
Concluding remarks
Leptin is a master regulator of energy balance and body adiposity. The vast majority of obese individuals present leptin resistance, and recent evidence indicates that our obesogenic environment contributes to this condition. Although leptin administration is a poor therapy to treat obesity, several pathologies, such as lipodystrophy, hypothalamic amenorrhea and T1DM can benefit from leptin treatment. Leptin also has many other important effects throughout life, which includes the regulation of brain development, behaviors, neuroendocrine axes and autonomic nervous system. More than being a hormone that induces weight loss and satiety, leptin coordinates numerous biologic functions to ensure survival, especially during situations of negative energy balance. This myriad of effects depends on the coordinated action of multiple populations of LepR-expressing neurons.
Biographies
Jose Donato Jr., PhD, is an Associate Professor of the Department of Physiology and Biophysics at the University of São Paulo (USP), Brazil. He received his PhD in Morphofunctional Sciences from USP in 2008, followed by a postdoctorate at the University of Texas Southwestern Medical Center, Dallas, TX. Back to Brazil in 2012, Dr. Donato set up his laboratory focused in understanding the importance of hormone signaling in the brain. So far, his group published several papers studying the effects of leptin, prolactin, estradiol and growth hormone on brain functions, especially regarding the regulation of energy balance and glucose homeostasis.

Angela M. Ramos-Lobo, MSc, is a PhD student under Dr. Donato's supervision. She graduated in Biology at the University of Buenos Aires in 2010 and received her MSc in Sciences from USP in 2013. Her PhD focuses on understanding the consequences of the absence of leptin signaling in early life.

Abbreviations
- AgRP
agouti-related protein
- ARH
arcuate nucleus of the hypothalamus
- BAT
brown adipose tissue
- BBB
blood–brain barrier
- CART
cocaine and amphetamine regulated transcript
- CCK
cholecystokinin
- CNS
central nervous system
- DIO
diet-induced obesity
- DMH
dorsomedial nucleus of the hypothalamus
- DMX
dorsal motor nucleus of the vagus
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinases
- GH
growth hormone
- GL
generalized lipodystrophy
- GLP-1
glucagon-like peptide-1
- GnRH
gonadotropin-releasing hormone
- GPR120
G protein-coupled receptor 120
- HFD
high-fat diet
- IL
interleukin
- IRS
insulin receptor substrates
- JAK
Janus kinase
- LepR
leptin receptor
- LH
luteinizing hormone
- LHA
hypothalamic area
- LPS
lipopolysaccharide
- MCH
melanin-concentrating hormone
- MCR
melanocortin receptors
- MPO
median preoptic area
- nNOS
neuronal NO synthase
- NO
nitric oxide
- NOD
non-obese diabetic mice
- NPY
neuropeptide Y
- NTS
nucleus of the solitary tract
- Ob-R
leptin receptor
- PBN
parabrachial nucleus
- PI3K
phosphatidylinositol 3-kinase
- PMv
ventral premammillary nucleus
- POMC
proopiomelanocortin
- PTP
protein tyrosine phosphatases
- PTP1B
protein tyrosine phosphatase 1B
- PVH
paraventricular nucleus of the hypothalamus
- RPTPε
protein tyrosine phosphatase epsilon
- SF1
steroidogenic factor-1
- SH2
Src homology 2
- Shp2
Src homology 2-containing tyrosine phosphatase
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TCPTP
T-cell protein tyrosine phosphatase
- TLR4
toll-like receptor 4
- TNF
tumor necrosis factor
- TRH
thyrotropin-releasing hormone
- UCP1
uncoupling protein 1
- VMH
ventromedial nucleus of the hypothalamus
- VTA
ventral tegmental area
- α-MSH
α-melanocyte-stimulating hormone.
Disclosure of potential conflicts of interest
No potential conflicts of interest are disclosed.
Acknowledgments
The authors would like to thank Ana Maria P. Campos for helping to produce the mouse brain photomicrographs.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP; grants number: 2015/10992-6 to J.D. and 2014/11752-6 to A.M.R.L.).
References
- [1].Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407-1433; PMID:12439643. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- [2].Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344-1347; PMID:8636448. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82:579-584; PMID:9024258.. [DOI] [PubMed] [Google Scholar]
- [4].Tsai M, Asakawa A, Amitani H, Inui A. Stimulation of leptin secretion by insulin. Indian J Endocrinol Metab. 2012;16:S543-S548; PMID:23565488. doi: 10.4103/2230-8210.105570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vital P, Larrieta E, Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol. 2006;190:425-432; PMID:16899575. doi: 10.1677/joe.1.06596. [DOI] [PubMed] [Google Scholar]
- [6].Leal-Cerro A, Soto A, Martinez MA, Dieguez C, Casanueva FF. Influence of cortisol status on leptin secretion. Pituitary. 2001;4:111-116; PMID:11824503. doi: 10.1023/A:1012903330944. [DOI] [PubMed] [Google Scholar]
- [7].Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141-148; PMID:350680. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- [8].Coleman DL. A historical perspective on leptin. Nat Med. 2010;16:1097-1099; PMID:20930752. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- [9].Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127-1128; PMID:5918576. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- [10].Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317-318; PMID:14824537. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- [11].Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294-298; PMID:4767369. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- [12].Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969;217:1298-1304; PMID:5346292. [DOI] [PubMed] [Google Scholar]
- [13].Coleman DL, Hummel KP. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia. 1973;9:287-293; PMID:4588246. doi: 10.1007/BF01221856. [DOI] [PubMed] [Google Scholar]
- [14].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432; PMID:7984236. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [15].Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, et al.. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263-1271; PMID:8548812. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- [16].Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al.. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491-495; PMID:8608603. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- [17].Zhang J, Scarpace PJ. The soluble leptin receptor neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo. Br J Pharmacol. 2009;158:475-482; PMID:19422379. doi: 10.1111/j.1476-5381.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hileman SM, Pierroz DD, Masuzaki H, Bjorbak C, El-Haschimi K, Banks WA, Flier JS. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775-783; PMID:11861497. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- [19].Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686-32695; PMID:9405487. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- [20].Chua SC, Koutras IK, Han L, Liu S-M, Kay J, Young SJ, Chung WK, Leibel RL. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45:264-270; PMID:9344648. doi: 10.1006/geno.1997.4962. [DOI] [PubMed] [Google Scholar]
- [21].Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093-6096; PMID:9102398. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- [22].Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95-97; PMID:8782827. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- [23].Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu S-M, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (Leptin) receptor. Science. 1996;271:994-996; PMID:8584938. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- [24].Banks AS, Davis SM, Bates SH, Myers MG Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563-14572; PMID:10799542. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- [25].Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al.. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856-859; PMID:12594516. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- [26].Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA. 2004;101:4661-4666; PMID:15070774. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, Joost HG, Becker W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109-119; PMID:15634336. doi: 10.1111/j.1432-1033.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- [28].Furigo IC, Ramos-Lobo AM, Frazao R, Donato J Jr. Brain STAT5 signaling and behavioral control. Mol Cell Endocrinol. 2016;438:70-76; PMID:27118133. doi: 10.1016/j.mce.2016.04.019. [DOI] [PubMed] [Google Scholar]
- [29].Patterson CM, Villanueva EC, Greenwald-Yarnell M, Rajala M, Gonzalez IE, Saini N, Jones J, Myers MG Jr. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol Metab. 2012;1:61-69; PMID:24024119. doi: 10.1016/j.molmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG Jr, Seeley RJ, Schwartz MW. Insulin activation of Phosphatidylinositol 3-Kinase in the hypothalamic arcuate nucleus: a key mediator of Insulin-Induced anorexia. Diabetes. 2003;52:227-231; PMID:12540590. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- [31].Donato J Jr, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54:591-602; PMID:21085763. doi: 10.1590/S0004-27302010000700002. [DOI] [PubMed] [Google Scholar]
- [32].Diano S, Kalra SP, Sakamoto H, Horvath TL. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res. 1998;812:256-259; PMID:9813356. doi: 10.1016/S0006-8993(98)00936-6. [DOI] [PubMed] [Google Scholar]
- [33].Roy AF, Benomar Y, Bailleux V, Vacher CM, Aubourg A, Gertler A, Djiane J, Taouis M. Lack of cross-desensitization between leptin and prolactin signaling pathways despite the induction of suppressor of cytokine signaling 3 and PTP-1B. J Endocrinol. 2007;195:341-350; PMID:17951545. doi: 10.1677/JOE-07-0321. [DOI] [PubMed] [Google Scholar]
- [34].Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113-116; PMID:8674530. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- [35].Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101-1106; PMID:8787671. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489-4492; PMID:9322969. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- [37].Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001-7005; PMID:9192681. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH. Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol. 1997;133:1-7; PMID:9359467. doi: 10.1016/S0303-7207(97)00138-X. [DOI] [PubMed] [Google Scholar]
- [39].Bjorbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, Flier JS. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485-3491; PMID:9681499. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- [40].Hubschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci. 2001;21:2413-2424; PMID:11264315.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology. 2002;143:3498-3504; PMID:12193563. doi: 10.1210/en.2002-220077. [DOI] [PubMed] [Google Scholar]
- [42].Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121-2131; PMID:12697721. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- [43].Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci USA. 1998;95:6061-6066; PMID:9600917. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827-1832; PMID:10862798. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Münzberg H, Flier JS, Bjørbæk C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880-4889; PMID:15271881. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- [46].Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, et al.. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489-495; PMID:11970898. doi: 10.1016/S1534-5807(02)00148-X. [DOI] [PubMed] [Google Scholar]
- [47].Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543-546; PMID:7624777. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- [48].Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al.. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292-295; PMID:8532024. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- [49].Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879-884; PMID:10486419. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- [50].Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, et al.. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093-1103; PMID:12393845. doi: 10.1172/JCI0215693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540-543; PMID:7624776. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- [52].Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24-34; PMID:25199978. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zeltser LM. Developmental influences on circuits programming susceptibility to obesity. Front Neuroendocrinol. 2015;39:17-27; PMID:26206662. doi: 10.1016/j.yfrne.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Khan SM, Hamnvik OP, Brinkoetter M, Mantzoros CS. Leptin as a modulator of neuroendocrine function in humans. Yonsei Med J. 2012;53:671-679; PMID:22665330. doi: 10.3349/ymj.2012.53.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250-252; PMID:8717038. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- [56].Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419-3423; PMID:8784108. doi:12727933 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- [57].Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409-1421; PMID:12727933. doi: 10.1172/JCI200317490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60:S15-S19; discussion S68–84, 5–7; PMID:12403079. doi: 10.1301/002966402320634788. [DOI] [PubMed] [Google Scholar]
- [59].Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546-549; PMID:7624778. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- [60].Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878-8883; PMID:9238071. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, et al.. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA. 2004;101:4531-4536; PMID:15070752. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794-795; PMID:11677594. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- [63].Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796-1805; PMID:18382766. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC Jr, et al.. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63-72; PMID:16054045. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [65].Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375-1385; PMID:9883730. doi: 10.1016/S0896-6273(00)80656-X. [DOI] [PubMed] [Google Scholar]
- [66].Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119-2123; PMID:9392508. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- [67].Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063-5066; PMID:9348241. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- [68].Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480-484; PMID:11373681. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- [69].Edwards CM, Abbott CR, Sunter D, Kim M, Dakin CL, Murphy KG, Abusnana S, Taheri S, Rossi M, Bloom SR. Cocaine- and amphetamine-regulated transcript, glucagon-like peptide-1 and corticotrophin releasing factor inhibit feeding via agouti-related protein independent pathways in the rat. Brain Res. 2000;866:128-134; PMID:10825488. doi: 10.1016/S0006-8993(00)02257-5. [DOI] [PubMed] [Google Scholar]
- [70].Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, et al.. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428-4431; PMID:9751529. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- [71].Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al.. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131-141; PMID:9019399. doi: 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- [72].Pedroso JA, Silveira MA, Lima LB, Furigo IC, Zampieri TT, Ramos-Lobo AM, Buonfiglio DC, Teixeira PD, Frazão R, Donato J Jr. Changes in leptin signaling by SOCS3 modulate fasting-induced hyperphagia and weight regain in mice. Endocrinology. 2016;157:3901-3914; PMID:27471877. doi: 10.1210/en.2016-1038. [DOI] [PubMed] [Google Scholar]
- [73].Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271-272; PMID:10195157. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- [74].Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424-1428; PMID:21364278. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al.. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289-1291; PMID:16158063. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- [76].Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology. 1985;117:2435-2442; PMID:3840737. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- [77].Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135-138; PMID:9311920. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- [78].Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225-1234; PMID:19563755. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tong Q, Ye C-P, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998-1000; PMID:19160495. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, Seeley RJ. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci. 1999;19:2362-2367; PMID:10066286.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, et al.. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983-991; PMID:15207242. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [82].van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al.. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773-1785; PMID:18162515. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683-685; PMID:16254186. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- [84].Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al.. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493-505; PMID:16269339. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- [85].Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108-110; PMID:15064420. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- [86].Lima LB, Metzger M, Furigo IC, Donato J Jr. Leptin receptor-positive and leptin receptor-negative proopiomelanocortin neurons innervate an identical set of brain structures. Brain Res. 2016;1646:366-376; PMID:27321158. doi: 10.1016/j.brainres.2016.06.024. [DOI] [PubMed] [Google Scholar]
- [87].Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043-1047; PMID:15591135. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- [88].Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194-198; PMID:11196643. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- [89].Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al.. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238-2342; PMID:24487620. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Liu T, Kong D, Shah Bhavik P, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511-522; PMID:22325203. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594-597; PMID:22419158. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111-114; PMID:24121436. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775-786; PMID:10482243. doi: 10.1016/S0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- [94].Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670-674; PMID:9872314. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- [95].Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, et al.. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313-323; PMID:21907138. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG Jr. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J Neurosci. 2014;34:11405-11415; PMID:25143620. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Opland D, Sutton A, Woodworth H, Brown J, Bugescu R, Garcia A, Christensen L, Rhodes C, Myers M Jr, Leinninger G. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol Metab. 2013;2:423-434; PMID:24327958. doi: 10.1016/j.molmet.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yu S, Qualls-Creekmore E, Rezai-Zadeh K, Jiang Y, Berthoud HR, Morrison CD, Derbenev AV, Zsombok A, Münzberg H. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J Neurosci. 2016;36:5034-5046; PMID:27147656. doi: 10.1523/JNEUROSCI.0213-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hommel JD, Trinko R, Sears RM, Georgescu D, Liu Z-W, Gao X-B, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801-810; PMID:16982424. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- [100].Fulton S, Pissios P, Manchon Ramon P, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811-822; PMID:16982425. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- [101].Xu L, Scheenen WJJM, Leshan RL, Patterson CM, Elias CF, Bouwhuis S, Roubos EW, Myers MG Jr, Kozicz T. Leptin signaling modulates the activity of urocortin 1 neurons in the mouse nonpreganglionic edinger-westphal nucleus. Endocrinology. 2011;152:979-988; PMID:21209012. doi: 10.1210/en.2010-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518-532; PMID:19350671. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Donato J Jr, Frazao R, Fukuda M, Vianna CR, Elias CF. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology. 2010;151:5415-5427; PMID:20881244. doi: 10.1210/en.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nagaishi VS, Cardinali LI, Zampieri TT, Furigo IC, Metzger M, Donato J Jr. Possible crosstalk between leptin and prolactin during pregnancy. Neuroscience. 2014;259:71-83; PMID:24316468. doi: 10.1016/j.neuroscience.2013.11.050. [DOI] [PubMed] [Google Scholar]
- [105].Garfield AS, Patterson C, Skora S, Gribble FM, Reimann F, Evans ML, Myers MG Jr, Heisler LK. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153:4600-4607; PMID:22869346. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest. 2011;121:2413-2421; PMID:21606595. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77-83; PMID:20074530. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti-Buck K, et al.. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908-910; PMID:24880214. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Trayhurn P, Fuller L. The development of obesity in genetically diabetic-obese (db/db) mice pair-fed with lean siblings. The importance of thermoregulatory thermogenesis. Diabetologia. 1980;19:148-153; PMID:7418967. doi: 10.1007/BF00421862. [DOI] [PubMed] [Google Scholar]
- [110].Berglund ED, Vianna CR, Donato J Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, et al.. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000-1009; PMID:22326958. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704-1707; PMID:8939859. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- [112].Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al.. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191-203; PMID:16423694. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- [113].Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138-2148; PMID:18258679. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189-12197; PMID:21865462. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Münzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873-1884; PMID:21289197. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rezai-Zadeh K, Yu S, Jiang Y, Laque A, Schwartzenburg C, Morrison CD, Derbenev AV, Zsombok A, Münzberg H. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab. 2014;3:681-693; PMID:25352997. doi: 10.1016/j.molmet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Dodd GT, Worth AA, Nunn N, Korpal AK, Bechtold DA, Allison MB, Myers MG Jr, Statnick MA, Luckman SM. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab. 2014;20:639-649; PMID:25176149. doi: 10.1016/j.cmet.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000;20:1550-1558; PMID:10662844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Fekete C, Mihaly E, Luo LG, Kelly J, Clausen JT, Mao Q, Rand WM, Moss LG, Kuhar M, Emerson CH, et al.. Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci. 2000;20:9224-9234; PMID:11125000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kim MS, Small CJ, Stanley SA, Morgan DG, Seal LJ, Kong WM, Edwards CM, Abusnana S, Sunter D, Ghatei MA, et al.. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest. 2000;105:1005-1011; PMID:10749579. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, Elmquist JK, Flier JS, Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107:111-120; PMID:11134186. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab. 2011;300:E392-E401; PMID:21062956. doi: 10.1152/ajpendo.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677; PMID:8614460. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- [124].Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG Jr. LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067-3073; PMID:15561935. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- [125].Kaiyala KJ, Ogimoto K, Nelson JT, Muta K, Morton GJ. Physiological role for leptin in the control of thermal conductance. Mol Metab. 2016;5:892-902; PMID:27689002. doi: 10.1016/j.molmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Fischer AW, Hoefig CS, Abreu-Vieira G, de Jong JM, Petrovic N, Mittag J, Cannon B, Nedergaard J. Leptin raises defended body temperature without activating thermogenesis. Cell Rep. 2016;14:1621-1631; PMID:26876182. doi: 10.1016/j.celrep.2016.01.041. [DOI] [PubMed] [Google Scholar]
- [127].Heaton GM, Wagenvoord RJ, Kemp A Jr, Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem. 1978;82:515-521; PMID:624284. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- [128].Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond). 2010;34(Suppl 1):S36-S42; PMID:20935665. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273:E226-E230; PMID:9252501. [DOI] [PubMed] [Google Scholar]
- [130].van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500-1508; PMID:19357405. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- [131].Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Münzberg H, Zhang ZY, Kahn BB, et al.. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88-104; PMID:25594176. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chong AC, Greendyk RA, Zeltser LM. Distinct networks of leptin- and insulin-sensing neurons regulate thermogenic responses to nutritional and cold challenges. Diabetes. 2015;64:137-146; PMID:25125486. doi: 10.2337/db14-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239-274; PMID:14977403. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- [134].Hudson JW, Scott IM. Daily torpor in the laboratory mouse, Mus-Musculus Var Albino. Physiol Zool. 1979;52:205-218. doi: 10.1086/physzool.52.2.30152564. [DOI] [Google Scholar]
- [135].Solymar M, Petervari E, Balasko M, Szelenyi Z. The onset of daily torpor is regulated by the same low body mass in lean mice and in mice with diet-induced obesity. Temperature. 2015;2:129-134. doi: 10.1080/23328940.2015.1014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204-R208; PMID:9458919. [DOI] [PubMed] [Google Scholar]
- [137].Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152-2157; PMID:8621806. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171-175; PMID:8996253. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Finck BN, Kelley KW, Dantzer R, Johnson RW. In vivo and in vitro evidence for the involvement of tumor necrosis factor-alpha in the induction of leptin by lipopolysaccharide. Endocrinology. 1998;139:2278-2283; PMID:9564834. doi: 10.1210/endo.139.5.6012. [DOI] [PubMed] [Google Scholar]
- [140].American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-874; PMID:1597042. doi: 10.1097/00003246-199206000-00025. [DOI] [PubMed] [Google Scholar]
- [141].Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Crit Care Med. 2003;31:1250-1256; PMID:12682500. doi:1395659 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- [142].Clemmer TP, Fisher CJ Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1992;20:1395-1401; PMID:1395659. doi: 10.1097/00003246-199210000-00006. [DOI] [PubMed] [Google Scholar]
- [143].Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1244-R1252; PMID:16081879. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- [144].Steiner AA, Chakravarty S, Robbins JR, Dragic AS, Pan J, Herkenham M, Romanovsky AA. Thermoregulatory responses of rats to conventional preparations of lipopolysaccharide are caused by lipopolysaccharide per se – not by lipoprotein contaminants. Am J Physiol Regul Integr Comp Physiol. 2005;289:R348-R352; PMID:15860647. doi: 10.1152/ajpregu.00223.2005. [DOI] [PubMed] [Google Scholar]
- [145].Romanovsky AA, Szekely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses. 1998;50:219-226; PMID:9578327. doi: 10.1016/S0306-9877(98)90022-6. [DOI] [PubMed] [Google Scholar]
- [146].Steiner AA, Dogan MD, Ivanov AI, Patel S, Rudaya AY, Jennings DH, Orchinik M, Pace TW, O'connor KA, Watkins LR, et al.. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J. 2004;18:1949-1951; PMID:15388670. doi: 10.1096/fj.04-2295fje. [DOI] [PubMed] [Google Scholar]
- [147].Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136-R142; PMID:9887187. [DOI] [PubMed] [Google Scholar]
- [148].Bik W, Wolinska-Witort E, Chmielowska M, Rusiecka-Kuczalek E, Baranowska B. Does leptin modulate immune and endocrine response in the time of LPS-induced acute inflammation?. Neuro Endocrinol Lett. 2001;22:208-214; PMID:11449193. [PubMed] [Google Scholar]
- [149].Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156:1781-1787; PMID:10793089. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Molnar D, Milner RD. Metabolic and hormonal response to endotoxin fever in fed and starved one-week rabbits. Biol Neonate. 1983;44:309-314; PMID:6357299. doi: 10.1159/000241732. [DOI] [PubMed] [Google Scholar]
- [151].Shido O, Nagasaka T, Watanabe T. Blunted febrile response to intravenous endotoxin in starved rats. J Appl Physiol. 1989;67:963-969; PMID:2793726. [DOI] [PubMed] [Google Scholar]
- [152].Inoue W, Luheshi GN. Acute starvation alters lipopolysaccharide-induced fever in leptin-dependent and -independent mechanisms in rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1709-R1719; PMID:20943858. doi: 10.1152/ajpregu.00567.2010. [DOI] [PubMed] [Google Scholar]
- [153].Steiner AA, Romanovsky AA. Leptin: at the crossroads of energy balance and systemic inflammation. Prog Lipid Res. 2007;46:89-107; PMID:17275915. doi: 10.1016/j.plipres.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193-2216; PMID:15970487. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- [155].Pohl J, Woodside B, Luheshi GN. Leptin modulates the late fever response to LPS in diet-induced obese animals. Brain Behav Immun. 2014;42:41-47; PMID:25108212. doi: 10.1016/j.bbi.2014.07.017. [DOI] [PubMed] [Google Scholar]
- [156].Koenig S, Luheshi GN, Wenz T, Gerstberger R, Roth J, Rummel C. Leptin is involved in age-dependent changes in response to systemic inflammation in the rat. Brain Behav Immun. 2014;36:128-138; PMID:24513873. doi: 10.1016/j.bbi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- [157].Timmerman RJ, Thompson J, Noordzij HM, van der Meer JW. Psychogenic periodic fever. Nethe J Med. 1992;41:158-160; PMID:1470287. [PubMed] [Google Scholar]
- [158].Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci. 2011;34:1442-1452; PMID:21978215. doi: 10.1111/j.1460-9568.2011.07863.x. [DOI] [PubMed] [Google Scholar]
- [159].Nakamura K. Neural circuit for psychological stress-induced hyperthermia. Temperature. 2015;2:352-361; PMID:27227049. doi:25480301 10.1080/23328940.2015.1070944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, et al.. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404-1416; PMID:25480301. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326-332; PMID:16027245. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- [162].Sohn J-W, Harris Louise E, Berglund Eric D, Liu T, Vong L, Lowell Bradford B, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612-619; PMID:23374353. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195-204; PMID:21284986. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072-1079; PMID:12668587. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- [165].Pedroso JA, Buonfiglio DC, Cardinali LI, Furigo IC, Ramos-Lobo AM, Tirapegui J, Elias CF, Donato J Jr. Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol Metab. 2014;3:608-618; PMID:25161884. doi: 10.1016/j.molmet.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjørbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537-547; PMID:19490908. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149:5654-5661; PMID:18669597. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Meek TH, Nelson JT, Matsen ME, Dorfman MD, Guyenet SJ, Damian V, Allison MB, Scarlett JM, Nguyen HT, Thaler JP, et al.. Functional identification of a neurocircuit regulating blood glucose. Proc Natl Acad Sci USA. 2016;113:E2073-E2082; PMID:27001850. doi: 10.1073/pnas.1521160113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, et al.. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383-393; PMID:17488640. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG Jr, et al.. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab. 2014;20:1030-1037; PMID:25470549. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Flak JN, Patterson CM, Garfield AS, D'Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JM, Germani M, Jones JC, et al.. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci. 2014;17:1744-1750; PMID:25383904. doi: 10.1038/nn.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987-997; PMID:15342807. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- [173].Bohlen TM, Silveira MA, Zampieri TT, Frazao R, Donato J Jr. Fatness rather than leptin sensitivity determines the timing of puberty in female mice. Mol Cell Endocrinol. 2016;423:11-21; PMID:26762764. doi: 10.1016/j.mce.2015.12.022. [DOI] [PubMed] [Google Scholar]
- [174].Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805-2812; PMID:19179437. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Frazao R, Cravo RM, Donato J Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor alpha. J Neurosci. 2013;33:2807-2820; PMID:23407940. doi: 10.1523/JNEUROSCI.1610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298-303; PMID:16503925. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- [177].Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, Vianna C, Elias CF. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One. 2013;8:e58698; PMID:23505551. doi: 10.1371/journal.pone.0058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, et al.. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37-56; PMID:21093546. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Donato J Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, et al.. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355-368; PMID:21183787. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Donato J Jr, Cravo RM, Frazão R, Elias CF. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology. 2011;93:9-18; PMID:21099209. doi: 10.1159/000322472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Donato J Jr, Elias CF. The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne). 2011;2:57; PMID:22649378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125:735-748; PMID:15099687. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- [183].Leshan RL, Louis GW, Jo Y-H, Rhodes CJ, Munzberg H, Myers MG Jr. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138-3147; PMID:19279251. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Donato J Jr, Lee C, Ratra DV, Franci CR, Canteras NS, Elias CF. Lesions of the ventral premammillary nucleus disrupt the dynamic changes in Kiss1 and GnRH expression characteristic of the proestrus-estrus transition. Neuroscience. 2013;241:67-79; PMID:23518222. doi: 10.1016/j.neuroscience.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Donato J Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci. 2009;29:5240-5250; PMID:19386920. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Bellefontaine N, Chachlaki K, Parkash J, Vanacker C, Colledge W, d'Anglemont de Tassigny X, Garthwaite J, Bouret SG, Prevot V. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J Clin Invest. 2014;124:2550-2559; PMID:24812663. doi: 10.1172/JCI65928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Sheffer-Babila S, Sun Y, Israel DD, Liu SM, Neal-Perry G, Chua SC Jr. Agouti-related peptide plays a critical role in leptin's effects on female puberty and reproduction. Am J Physiol Endocrinol Metab. 2013;305:E1512-E1520; PMID:24169048. doi: 10.1152/ajpendo.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci USA. 2012;109:3155-3160; PMID:22232663. doi: 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012;153:5587-5599; PMID:22948210. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- [190].Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al.. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286-297; PMID:20374961. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Liu J, Perez SM, Zhang W, Lodge DJ, Lu XY. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol Psychiatry. 2011;16:1024-1038; PMID:21483433. doi: 10.1038/mp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Bereiter DA, Jeanrenaud B. Altered neuroanatomical organization in the central nervous system of the genetically obese (ob/ob) mouse. Brain Res. 1979;165:249-260; PMID:421139. doi: 10.1016/0006-8993(79)90557-2. [DOI] [PubMed] [Google Scholar]
- [193].Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755-2762; PMID:10342866. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- [194].Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851-2854; PMID:15713712. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- [195].Mistry AM, Swick A, Romsos DR. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol. 1999;277:R742-R747; PMID:10484491. [DOI] [PubMed] [Google Scholar]
- [196].Ishii Y, Bouret SG. Embryonic birthdate of hypothalamic leptin-activated neurons in mice. Endocrinology. 2012;153:3657-3667; PMID:22621961. doi: 10.1210/en.2012-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008;149:877-885; PMID:18039787. doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Bouret SG. Crossing the border: developmental regulation of leptin transport to the brain. Endocrinology. 2008;149:875-876; PMID:18292198. doi: 10.1210/en.2007-1698. [DOI] [PubMed] [Google Scholar]
- [199].Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020-1027; PMID:9486972. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Markakis EA. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol. 2002;23:257-291; PMID:12127306. doi: 10.1016/S0091-3022(02)00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797-2805; PMID:15028773. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110-115; PMID:15064421. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- [203].O'Malley D, Irving AJ, Harvey J. Leptin-induced dynamic alterations in the actin cytoskeleton mediate the activation and synaptic clustering of BK channels. FASEB J. 2005;19:1917-1919; PMID:16166199. doi: 10.1096/fj.05-4166fje. [DOI] [PubMed] [Google Scholar]
- [204].Shanley LJ, O'Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545:933-944; PMID:12482897. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Ayyildiz M, Yildirim M, Agar E, Baltaci AK. The effect of leptin on penicillin-induced epileptiform activity in rats. Brain Res Bull. 2006;68:374-378; PMID:16377445. doi: 10.1016/j.brainresbull.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [206].Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607-615; PMID:12150780. doi: 10.1016/S0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- [207].Sena A, Sarlieve LL, Rebel G. Brain myelin of genetically obese mice. J Neurol Sci. 1985;68:233-243; PMID:2989440. doi: 10.1016/0022-510X(85)90104-2. [DOI] [PubMed] [Google Scholar]
- [208].Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389-1395; PMID:16300445. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- [209].Paz-Filho GJ, Babikian T, Asarnow R, Delibasi T, Esposito K, Erol HK, Wong M-L, Licinio J. Leptin replacement improves cognitive development. PLoS One. 2008;3:e3098; PMID:18769731. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381-388; PMID:20637218. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643-647; PMID:18024215. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Lu X-Y, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593-1598; PMID:16423896. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Wabitsch M, Funcke JB, Lennerz B, Kuhnle-Krahl U, Lahr G, Debatin KM, Vatter P, Gierschik P, Moepps B, Fischer-Posovszky P. Biologically inactive leptin and early-onset extreme obesity. N Engl J Med. 2015;372:48-54; PMID:25551525. doi: 10.1056/NEJMoa1406653. [DOI] [PubMed] [Google Scholar]
- [214].Farooqi S, O'Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710-718; PMID:17122358. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- [215].Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, et al.. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568-1575; PMID:10546697. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- [216].Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003-4009; PMID:11095423. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- [217].Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26:504-509; PMID:12075577. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- [218].Hukshorn CJ, Menheere PP, Westerterp-Plantenga MS, Saris WH. The effect of pegylated human recombinant leptin (PEG-OB) on neuroendocrine adaptations to semi-starvation in overweight men. Eur J Endocrinol. 2003;148:649-655; PMID:12773137. doi: 10.1530/eje.0.1480649. [DOI] [PubMed] [Google Scholar]
- [219].Hukshorn CJ, Westerterp-Plantenga MS, Saris WH. Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted, overweight men. Am J Clin Nutr. 2003;77:771-776; PMID:12663271. [DOI] [PubMed] [Google Scholar]
- [220].Lejeune MP, Hukshorn CJ, Saris WH, Westerterp-Plantenga MS. Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. Int J Obes Relat Metab Disord. 2003;27:1494-1499; PMID:14634680. doi: 10.1038/sj.ijo.0802431. [DOI] [PubMed] [Google Scholar]
- [221].Korner J, Conroy R, Febres G, McMahon DJ, Conwell I, Karmally W, Aronne LJ. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring). 2013;21:951-956; PMID:23512892. doi: 10.1002/oby.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Shetty GK, Matarese G, Magkos F, Moon HS, Liu X, Brennan AM, Mylvaganam G, Sykoutri D, Depaoli AM, Mantzoros CS. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. Eur J Endocrinol. 2011;165:249-254; PMID:21602313. doi: 10.1530/EJE-11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Kissileff HR, Thornton JC, Torres MI, Pavlovich K, Mayer LS, Kalari V, Leibel RL, Rosenbaum M. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr. 2012;95:309-317; PMID:22237063. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583-2591; PMID:18568078. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73-76; PMID:10485707. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- [226].Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, et al.. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570-578; PMID:11856796. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- [227].Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, et al.. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345-1350; PMID:12021250. doi: 10.1172/JCI0215001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Javor ED, Cochran EK, Musso C, Young JR, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994-2002; PMID:15983199. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- [229].Sekhar RV, Jahoor F, Iyer D, Guthikonda A, Paranilam J, Elhaj F, Coraza I, Balasubramanyam A. Leptin replacement therapy does not improve the abnormal lipid kinetics of hypoleptinemic patients with HIV-associated lipodystrophy syndrome. Metabolism. 2012;61:1395-1403; PMID:22542724. doi: 10.1016/j.metabol.2012.03.013. [DOI] [PubMed] [Google Scholar]
- [230].Magkos F, Brennan A, Sweeney L, Kang ES, Doweiko J, Karchmer AW, Mantzoros CS. Leptin replacement improves postprandial glycemia and insulin sensitivity in human immunodeficiency virus-infected lipoatrophic men treated with pioglitazone: a pilot study. Metabolism. 2011;60:1045-1049; PMID:21081243. doi: 10.1016/j.metabol.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Park JY, Chong AY, Cochran EK, Kleiner DE, Haller MJ, Schatz DA, Gorden P. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93:26-31; PMID:17940115. doi: 10.1210/jc.2007-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474-1477; PMID:21411512. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Moon HS, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG, Mylvaganam GH, Abanni L, Carbone F, Williams CJ, et al.. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647-1656; PMID:21617185. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, et al.. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107:4813-4819; PMID:20194735. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci USA. 2010;107:17391-17396; PMID:20855609. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, et al.. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18:431-444; PMID:24011077. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978;299:433-436; PMID:683275. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- [238].Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391-397; PMID:21270251. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, Burgess SC, Unger RH. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci USA. 2012;109:14972-14976; PMID:22891336. doi: 10.1073/pnas.1205983109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Lee Y, Berglund ED, Yu X, Wang MY, Evans MR, Scherer PE, Holland WL, Charron MJ, Roth MG, Unger RH. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc Natl Acad Sci USA. 2014;111:13217-13222; PMID:25157166. doi: 10.1073/pnas.1409638111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA. 2011;108:6585-6590; PMID:21464293. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60:1211-1221; PMID:21741057. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- [243].Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR Jr. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385-390; PMID:9022070. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D'Alessio DA, Perez-Tilve D. Diet-induced obese mice retain endogenous leptin action. Cell Metab. 2015;21:877-882; PMID:25980347. doi: 10.1016/j.cmet.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci USA. 2013;110:E697-E706; PMID:23386726. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Mounzih K, Qiu J, Ewart-Toland A, Chehab FF. Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology. 1998;139:5259-5262; PMID:9832467. doi: 10.1210/endo.139.12.6523. [DOI] [PubMed] [Google Scholar]
- [247].Ladyman SR, Grattan DR. Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology. 2005;146:3868-3874; PMID:15905318. doi: 10.1210/en.2005-0194. [DOI] [PubMed] [Google Scholar]
- [248].Ladyman SR, Fieldwick DM, Grattan DR. Suppression of leptin-induced hypothalamic JAK/STAT signalling and feeding response during pregnancy in the mouse. Reproduction. 2012;144:83-90; PMID:22580369. doi: 10.1530/REP-12-0112. [DOI] [PubMed] [Google Scholar]
- [249].Zampieri TT, Ramos-Lobo AM, Furigo IC, Pedroso JA, Buonfiglio DC, Donato J Jr. SOCS3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Mol Metab. 2015;4:237-245; PMID:25737950. doi: 10.1016/j.molmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Zampieri TT, da Silva TE, de Paula Romeu D, da Silva Torrao A, Donato J Jr. SOCS3 expression within leptin receptor-expressing cells regulates food intake and leptin sensitivity but does not affect weight gain in pregnant mice consuming a high-fat diet. Physiol Behav. 2016;157:109-115; PMID:26828039. doi: 10.1016/j.physbeh.2016.01.039. [DOI] [PubMed] [Google Scholar]
- [251].Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305-311; PMID:8801538. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- [252].Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159-161; PMID:8684156. doi: 10.1016/S0140-6736(96)03173-X. [DOI] [PubMed] [Google Scholar]
- [253].Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589-593; PMID:8616722. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- [254].Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449-1453; PMID:10698121. doi: 10.1016/S0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- [255].Hileman SM, Tornoe J, Flier JS, Bjorbaek C. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby canine kidney cells. Endocrinology. 2000;141:1955-1961; PMID:10830277. doi: 10.1210/endo.141.6.7450. [DOI] [PubMed] [Google Scholar]
- [256].Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood–brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950:130-136; PMID:12231237. doi: 10.1016/S0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- [257].Li Z, Ceccarini G, Eisenstein M, Tan K, Friedman JM. Phenotypic effects of an induced mutation of the ObRa isoform of the leptin receptor. Mol Metab. 2013;2:364-375; PMID:24327953. doi: 10.1016/j.molmet.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, et al.. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19:293-301; PMID:24506870. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643-651; PMID:20846876. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Ravussin Y, LeDuc CA, Watanabe K, Mueller BR, Skowronski A, Rosenbaum M, Leibel RL. Effects of chronic leptin infusion on subsequent body weight and composition in mice: can body weight set point be reset?. Mol Metab. 2014;3:432-440; PMID:24944902. doi: 10.1016/j.molmet.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cell. 2001;19:378-387; PMID:11553846. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- [262].Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619-625; PMID:9660946. doi: 10.1016/S1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- [263].Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059-30065; PMID:10514492. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- [264].Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG Jr. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354-1360; PMID:17415414. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123-132; PMID:16890540. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [266].Briancon N, McNay DE, Maratos-Flier E, Flier JS. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes. 2010;59:3074-3084; PMID:20876718. doi: 10.2337/db10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734-738; PMID:15220914. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- [268].Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739-743; PMID:15208705. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- [269].Matarazzo V, Schaller F, Nedelec E, Benani A, Penicaud L, Muscatelli F, Moyse E, Bauer S. Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J Neurosci. 2012;32:17097-17107; PMID:23197703. doi: 10.1523/JNEUROSCI.1669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG Jr, Xu AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59:894-906; PMID:20068134. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365-371; PMID:17010638. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [272].Augustine RA, Grattan DR. Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology. 2008;149:1049-1055; PMID:18063686. doi: 10.1210/en.2007-1018. [DOI] [PubMed] [Google Scholar]
- [273].Naef L, Woodside B. Prolactin/leptin interactions in the control of food intake in rats. Endocrinology. 2007;148:5977-5983; PMID:17872372. doi: 10.1210/en.2007-0442. [DOI] [PubMed] [Google Scholar]
- [274].Anderson GM, Beijer P, Bang AS, Fenwick MA, Bunn SJ, Grattan DR. Suppression of prolactin-induced signal transducer and activator of transcription 5b signaling and induction of suppressors of cytokine signaling messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat during late pregnancy and lactation. Endocrinology. 2006;147:4996-5005; PMID:16857756. doi: 10.1210/en.2005-0755. [DOI] [PubMed] [Google Scholar]
- [275].St-Pierre J, Tremblay ML. Modulation of leptin resistance by protein tyrosine phosphatases. Cell Metab. 2012;15:292-297; PMID:22405067. doi: 10.1016/j.cmet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- [276].Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917-924; PMID:16845389. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- [277].Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, Jirousek MR, Trevillyan JM. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195:109-118; PMID:12354677. doi: 10.1016/S0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- [278].Loh K, Fukushima A, Zhang X, Galic S, Briggs D, Enriori PJ, Simonds S, Wiede F, Reichenbach A, Hauser C, et al.. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab. 2011;14:684-699; PMID:22000926. doi: 10.1016/j.cmet.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Rousso-Noori L, Knobler H, Levy-Apter E, Kuperman Y, Neufeld-Cohen A, Keshet Y, Akepati VR, Klinghoffer RA, Chen A, Elson A. Protein tyrosine phosphatase epsilon affects body weight by downregulating leptin signaling in a phosphorylation-dependent manner. Cell Metab. 2011;13:562-572; PMID:21531338. doi: 10.1016/j.cmet.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [280].Tsou RC, Zimmer DJ, De Jonghe BC, Bence KK. Deficiency of PTP1B in leptin receptor-expressing neurons leads to decreased body weight and adiposity in mice. Endocrinology. 2012;153:4227-4237; PMID:22802463. doi: 10.1210/en.2012-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci USA. 2004;101:16064-16069; PMID:15520383. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720-734; PMID:20160350. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247-E1259; PMID:19724019. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397-406; PMID:17235396. doi: 10.1172/JCI29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36:245-271; PMID:25811237. doi: 10.1210/er.2014-1100. [DOI] [PubMed] [Google Scholar]
- [286].Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59-68; PMID:21889406. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- [287].De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192-4199; PMID:16002529. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- [288].Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, et al.. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4:e5045; PMID:19340313. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].McNay DEG, Briancon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012;122:142-152; PMID:22201680. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, et al.. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359-370; PMID:19144836. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61-73; PMID:18854155. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Amaral ME, Barbuio R, Milanski M, Romanatto T, Barbosa HC, Nadruz W, Bertolo MB, Boschero AC, Saad MJ, Franchini KG, et al.. Tumor necrosis factor-alpha activates signal transduction in hypothalamus and modulates the expression of pro-inflammatory proteins and orexigenic/anorexigenic neurotransmitters. J Neurochem. 2006;98:203-212; PMID:16638016. doi: 10.1111/j.1471-4159.2006.03857.x. [DOI] [PubMed] [Google Scholar]
- [293].Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338-348; PMID:20144759. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35-51; PMID:19117545. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- [295].Hosoi T, Sasaki M, Miyahara T, Hashimoto C, Matsuo S, Yoshii M, Ozawa K. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol. 2008;74:1610-1619; PMID:18755873. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- [296].de Git KC, Adan RA. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes Rev. 2015;16:207-224; PMID:25589226. doi: 10.1111/obr.12243. [DOI] [PubMed] [Google Scholar]
- [297].Balland E, Cowley MA. New insights in leptin resistance mechanisms in mice. Front Neuroendocrinol. 2015;39:59-65; PMID:26410445. doi: 10.1016/j.yfrne.2015.09.004. [DOI] [PubMed] [Google Scholar]
- [298].Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031; PMID:17183312. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- [299].Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al.. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178-184; PMID:22797518. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- [300].Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, et al.. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588; PMID:23985875. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- [301].Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673-684; PMID:23415219. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- [302].Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al.. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186; PMID:25231862. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- [303].Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92-96; PMID:25731162. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Donato J., Jr The central nervous system as a promising target to treat diabetes mellitus. Curr Top Med Chem. 2012;12:2070-2081; PMID:23167796. doi: 10.2174/156802612804910214. [DOI] [PubMed] [Google Scholar]
- [305].Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep. 2012;29:580-606; PMID:22491825. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- [306].Sasaki T. Age-associated weight gain, Leptin, and SIRT1. A possible role for hypothalamic SIRT1 in the prevention of weight gain and aging through modulation of leptin sensitivity. Front Endocrinol (Lausanne). 2015;6:109; PMID:26236282. doi: 10.3389/fendo.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Lee J, Liu J, Feng X, Salazar Hernandez MA, Mucka P, Ibi D, Choi JW, Ozcan U. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med. 2016;22:1023-1032; PMID:27479085. doi: 10.1038/nm.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999-1011; PMID:26000480. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257-7262; PMID:18458326. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, Weyer C, Koda J, Baron AD, Parkes DG, et al.. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149:5679-5687; PMID:18669592. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- [311].Trevaskis JL, Lei C, Koda JE, Weyer C, Parkes DG, Roth JD. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity. 2009;18:21-26; PMID:19543217. doi: 10.1038/oby.2009.187. [DOI] [PubMed] [Google Scholar]
- [312].Kusakabe T, Ebihara K, Sakai T, Miyamoto L, Aotani D, Yamamoto Y, Yamamoto-Kataoka S, Aizawa-Abe M, Fujikura J, Hosoda K, et al.. Amylin improves the effect of leptin on insulin sensitivity in leptin-resistant diet-induced obese mice. Am J Physiol Endocrinol Metab. 2012;302:E924-E931; PMID:22275759. doi: 10.1152/ajpendo.00198.2011. [DOI] [PubMed] [Google Scholar]
- [313].Muller TD, Sullivan LM, Habegger K, Yi CX, Kabra D, Grant E, Ottaway N, Krishna R, Holland J, Hembree J, et al.. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J Pept Sci. 2012;18:383-393; PMID:22565812. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- [314].Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, de Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, et al.. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One. 2012;7:e30571; PMID:22279596. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687-698; PMID:20813258. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, et al.. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350-354; PMID:22343897. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- [317].Nascimento LF, Souza GF, Morari J, Barbosa GO, Solon C, Moura RF, Victório SC, Ignácio-Souza LM, Razolli DS, Carvalho HF, et al.. n-3 fatty acids induce neurogenesis of predominantly POMC-Expressing cells in the hypothalamus. Diabetes. 2016;65:673-686; PMID:26512023. doi: 10.2337/db15-0008. [DOI] [PubMed] [Google Scholar]
- [318].Steinberg GR, Smith AC, Wormald S, Malenfant P, Collier C, Dyck DJ. Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E57-E63; PMID:14662513. doi: 10.1152/ajpendo.00302.2003. [DOI] [PubMed] [Google Scholar]
- [319].Kang S, Kim KB, Shin KO. Exercise training improves leptin sensitivity in peripheral tissue of obese rats. Biochem Biophys Res Commun. 2013;435:454-459; PMID:23669042. doi: 10.1016/j.bbrc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [320].Carhuatanta KAK, Demuro G, Tschöp MH, Pfluger PT, Benoit SC, Obici S. Voluntary exercise improves high-fat diet-induced leptin resistance independent of adiposity. Endocrinology. 2011;152:2655-2664; PMID:21586558. doi: 10.1210/en.2010-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Laing BT, Do K, Matsubara T, Wert DW, Avery MJ, Langdon EM, Zheng D, Huang H. Voluntary exercise improves hypothalamic and metabolic function in obese mice. J Endocrinol. 2016;229:109-122; PMID:26931136. doi: 10.1530/JOE-15-0510. [DOI] [PubMed] [Google Scholar]
- [322].Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol. 2009;296:R537-R548; PMID:19158409. doi: 10.1152/ajpregu.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Chiarreotto-Ropelle EC, Pauli LS, Katashima CK, Pimentel GD, Picardi PK, Silva VR, de Souza CT, Prada PO, Cintra DE, Carvalheira JB, et al.. Acute exercise suppresses hypothalamic PTP1B protein level and improves insulin and leptin signaling in obese rats. Am J Physiol Endocrinol Metab. 2013;305:E649-E659; PMID:23880311. doi: 10.1152/ajpendo.00272.2013. [DOI] [PubMed] [Google Scholar]
- [324].McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571-633; PMID:15788706. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- [325].Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20; PMID:11809615. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- [326].Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349-353; PMID:934222. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- [327].Baquero AF, de Solis AJ, Lindsley SR, Kirigiti MA, Smith MS, Cowley MA, Zeltser LM, Grove KL. Developmental switch of leptin signaling in arcuate nucleus neurons. J Neurosci. 2014;34:9982-9994; PMID:25057200. doi: 10.1523/JNEUROSCI.0933-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Juan De Solis A, Baquero AF, Bennett CM, Grove KL, Zeltser LM. Postnatal undernutrition delays a key step in the maturation of hypothalamic feeding circuits. Mol Metab. 2016;5:198-209; PMID:26977392. doi: 10.1016/j.molmet.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Ralevski A, Horvath TL. Developmental programming of hypothalamic neuroendocrine systems. Front Neuroendocrinol. 2015;39:52-58; PMID:26391503. doi: 10.1016/j.yfrne.2015.09.002. [DOI] [PubMed] [Google Scholar]
- [330].Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav. 1995;57:681-686; PMID:7777603. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- [331].Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843-851; PMID:17697422. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- [332].Vogt Merly C, Paeger L, Hess S, Steculorum Sophie M, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, et al.. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156:495-509; PMID:24462248. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290-e296; PMID:15741354. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- [334].Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond). 2008;32:1153-1160; PMID:18379577. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- [335].Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. J Clin Endocrinol Metab. 1998;83:1243-1246; PMID:9543149. doi: 10.1210/jcem.83.4.4731. [DOI] [PubMed] [Google Scholar]
- [336].Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211-4216; PMID:16020474. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]


