Abstract
Control of rodent adventitial infections in biomedical research facilities is of extreme importance in assuring both animal welfare and high-quality research results. Sixty-three U.S. institutions participated in a survey reporting the methods used to detect and control these infections and the prevalence of outbreaks from 1 January 2014 through 31 December 2015. These results were then compared with the results of 2 similar surveys published in 1998 and 2008. The results of the current survey demonstrated that the rate of viral outbreaks in mouse colonies was decreasing, particularly in barrier facilities, whereas the prevalence of parasitic outbreaks has remained constant. These results will help our profession focus its efforts in the control of adventitial rodent disease outbreaks to the areas of the greatest needs.
Abbreviations: DBS, dirty bedding sentinel; EDIM, epizootic diarrhea of infant mice virus; MHV, mouse hepatitis virus; MPV, mouse parvovirus; MVM, minute virus of mice; TMEV, Theiler meningoencephalitis virus
Adventitial infections can have a dramatic influence on rodents used in biomedical research, because the consequent induction of an immune response can have a myriad of physiologic ramifications. Furthermore, given that many mice are intentionally immunosuppressed for either the study of antigen- or pathogen-induced immune response or to allow foreign tissue to be engrafted into the animal, normally benign or subclinical infections can become lethal. Because of these factors, our profession has spent a great deal of time and effort monitoring for and preventing or eliminating many common opportunistic rodent pathogens, with great success.
Focus on the prevention of adventitial infections extends back more than 60 y. The 4 fundamental factors to control such infections involve 1) preventing exposure of rodents to the organisms; 2) early detection of these organisms; 3) prevention of the spread of the organism between cages, rooms, and facilities; and 4) effective management and elimination. Exposure prevention can be addressed by caging and facility design and management, and prevention of transmission by food and water. Prevention of disease exposure by cage design began with the use of filtered cage covers or bonnets in the 1950s30 and progressed to the use of microisolation caging in the early 1980s. Although these protective barriers were effective at preventing the transmission of adventitial organisms from the environment or neighboring cages, they also slowed the detection of these organisms. The next step in this evolution was the move toward IVC in the early 1990s, which provided the further advantage of controlling intracage ammonia and humidity. Prevention of disease through the use of autoclaved or irradiated food and acidified or chlorinated water can be traced back to the early 1960s.7,39,40,59 The use of treated water was implemented to control Pseudomonas and other gram-negative organisms. Interestingly, these disease control methods proved beneficial for mouse colonies even before the recognition and expansion of immunodeficient strains of mice.
Initially, detection of adventitial infections in mouse colonies relied on the presence of clinical signs of illness.45,46 However, widespread use of serology applied to dirty-bedding sentinels (DBS) appears to have begun in earnest in the late 1970s and early 1980s.35 The most important change in the monitoring of mice in research colonies since then was the development of PCR methodology and its implementation in monitoring mouse colonies in the late 1990s and early 2000s.8 Importantly, in many cases, PCR allows for the detection of an organism during active infection, as compared with serology, which reflects only evidence of past exposure to the organism.
Many viral organisms that once wrought mayhem on research facilities have been virtually eliminated from institutions.4 Despite these successes, several viruses, as well as parasites and bacteria, continue to infect research animal colonies, confounding research results and experiments. The continued presence of these organisms necessitates periodic reevaluation of the organisms causing adventitial infections, so that future resources can be directed to the areas of need.
Two types of broad surveys have provided valuable information about the presence of adventitial organisms. The first type of study examines the data from commercial testing centers, which receive hundreds of thousands of samples from North America and Europe, giving a broad view of adventitial organisms.34,41 Secondly, large research institutions have been surveyed periodically, providing an important assessment of the prevalence of most organisms in the face of biosecurity measures used to prevent them.10,11,21 Because approximately a decade has passed since the last survey, we felt that it was appropriate to repeat this evaluation. A second reason to perform this survey was that the use of PCR analysis of colony animals or exhaust manifolds was early in its widespread use as a primary method of disease detection during the past 10 y. With the recent surge in publications reporting the benefits of these techniques, as compared with traditional DBS and serology, we expect a transition toward the use of these techniques in the future.5,19 The timing of the current survey can then serve as a point of comparison for future work to help define the efficacy of these new techniques.
Materials and Methods
Ninety-seven of the top 100 NIH-funded research institutions from 2010 were invited to participate, of which 63 (65%) completed the survey. The survey consisted of 4 sections: 1) characteristics of the mouse research program, including the size, proportion of barrier and nonbarrier facilities, biosecurity measures in both types of facilities, and excluded viral, bacterial and parasitic organisms; 2) mouse sentinel monitoring and quarantine program; 3) outbreaks and treatment methods to address the outbreaks in mouse colonies; and 4) rat disease exclusion list and prevalence of adventitial outbreaks in rats.
The time period reported was 1 January 2014 through 31 December 2015. The person responsible for the quality assurance program at each institution was asked to complete the survey for that institution. One of the goals of the study was to determine the biosecurity standards that institutions use to define their ‘barrier’ or nonbarrier, commonly referred to as ‘conventional’, facilities. Therefore, the survey did not define a barrier and asked each institution to describe the biosecurity measures in both types of facilities. Typically, barrier facilities indicate enhanced biosecurity measures designed to provide extra protection against adventitial infections. Frequently, these measures come at an increased financial cost to the institution (for example, cost of personal protective equipment) and may inhibit the activities of the researchers (that is, the inability to bring animals to their laboratory and then return them to the facility).
Results
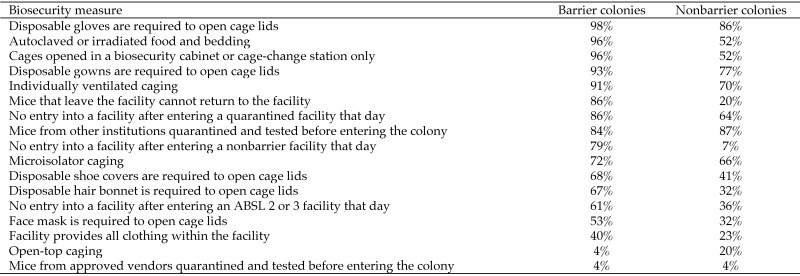
Of the 97 invited institutions, 63 (65%) completed the survey. The characteristics of the institutions are reported in Table 1. Cumulatively, 48% of the facilities were barrier facilities, 38% were nonbarrier facilities, and 14% were combined barrier–nonbarrier facilities. Larger facilities tended to house mice under barrier conditions, with approximately 69% of mice living in barrier housing, 18% living in nonbarrier facilities, and 12% living in facilities with both barrier and nonbarrier housing. There was profound variability between the institutions in the biosecurity practices used in both the barrier and nonbarrier facilities. In fact, no single biosecurity procedure was used in all of the institutions. Figure 1 reports the most commonly used biosecurity procedures in barrier and nonbarrier research facilities. Many institutions had selected facilities with biosecurity procedures that differed (most often with reduced security) from their normal institutional procedures.
Table 1.
The numbers of mouse cages and rats at the institutions participating in the survey
| % of participating institutions | ||
| No. of mouse cages | ||
| <5000 | 17 | |
| 5000–15,000 | 32 | |
| 15,000–30,000 | 29 | |
| 30,000–60,000 | 17 | |
| >60,000 | 5 | |
| No. of rats | ||
| <1000 | 61 | |
| 1000–3000 | 29 | |
| 3000–5000 | 5 | |
| >5000 | 5 | |
Figure 1.
Percentages of institutions using the most common biosecurity measures to protect research mice from adventitial organisms.
Pathogen exclusion.
The exclusion lists for viral and parasitic diseases were quite consistent between institutions. The following organisms were excluded from barrier facilities in greater than 95% of reporting institutions: mouse hepatitis virus (MHV), epizootic diarrhea of infant mice virus (EDIM), mouse parvovirus (MPV), minute virus of mice (MMV), pneumonia virus of mice, Sendai virus, ectromelia virus, reovirus 3, lymphocytic choriomeningitis virus, Theiler meningoencephalitis virus (TMEV), Aspiculuris tetraptera, Syphacia obvelata, Syphacia muris, Myobia musculi, Radfordia spp., and Mycoptes spp. Four viruses (mouse adenovirus 1 and 2, polyoma virus, K virus) and one bacterial organism (Mycoplasma pulmonis) were excluded from 80% to 95% of institutions. Although only 36% of institutions reported excluding Corynebacterium bovis and 36% exclude Demodex musculi, several institutions expressed increased concern for the presence of these organisms. Both organisms are known to cause significant disease in immunosuppressed mice, which are common in most large research facilities.
Our survey paid particular attention to norovirus and Helicobacter spp. In this regard, 13% of institutions completely exclude norovirus from all of their facilities, 11% of institutions maintain entire facilities that are free of norovirus, and 45% of institutions maintain isolated rooms or suites within facilities that are norovirus-free. In addition, 12% of institutions exclude Helicobacter spp. from all facilities, 11% of institutions maintain entire facilities free of Helicobacter spp., and 54% of institutions maintain rooms or suites free of Helicobacter spp. Furthermore, 75% of the institutions that completely exclude norovirus from all facilities also exclude Helicobacter spp. from all facilities.
Although the majority of institutions had no differences in the lists of excluded organisms and sentinel monitoring between their barrier and nonbarrier facilities, 20% of institutions did have significant differences in the exclusion list and sentinel programs in their nonbarrier facilities, including some institutions with no sentinel testing, lower frequency of testing, and tolerance of parasitic infections and several common viral organisms in the nonbarrier facilities.
Detection.
Although interest in the use of PCR analysis and the direct monitoring of colony animals has increased recently, the use of sentinel animals and serology were the mainstay of most institutions’ quality assurance programs during the period surveyed, with 95% of institutions using DBS animals and 98% of institutions using serology. In addition, 28% of institutions used some form of PCR assay for viral monitoring during the period of interest, 88% of institutions used serology as the primary method of detecting norovirus, and 89% used serology for primary detection of MPV. The remaining institutions used PCR analysis for the detection of these viruses. In particular, 50% of institutions used PCR testing of either colony or sentinel animals or exhaust ducts for the identification of fur mites or pinworms, 79% of institutions tested quarterly, and 75% of institutions have either one or 2 sentinel cages per rack. Most (88%) institutions purchase sentinels from approved vendors.
A myriad of techniques are used to monitor for excluded bacterial organisms, including serologic testing of sentinels (58% of institutions), bacterial culture (43%), PCR assay of feces of sentinel animals (32%), PCR analysis on feces of colony animals (20%), and gross and histopathology (20%). All (100%) institutions that test for Helicobacter spp. used fecal PCR testing as the primary means of detection in facilities that have excluded the organism.
The primary detection methods used in the identification of both fur mites and pinworms were surprisingly diverse. For fur mites, the most common techniques used were: PCR analysis of fur swabs from sentinel animals (24% of institutions), fur pluck with microscopic evaluation from sentinel animals (22%), PCR testing of exhaust air (13%), PCR analysis of fur from colony animals (13%), fur pluck from colony animals (10%), and tape tests (10%). For the identification of pinworms, more than 25% of institutions reported testing sentinels by multiple methods. PCR testing of colony or sentinel mice was used in 37% of institutions, direct cecal or colon exam at 32%, tape test at 31%, and fecal floatation followed by microscopic examination at 22%.
Outbreaks.
A total of 77 viral outbreaks were reported during the 2-y period of interest, with MPV accounting for 43 outbreaks at 23 different institutions (37% of institutions reporting an outbreak). EDIM was the next most common, with 16 outbreaks occurring in 9 institutions (14% of institutions), followed by 13 MHV outbreaks in 6 institutions (10%), 4 MVM outbreaks in 3 institutions (5%), and one TMEV outbreak. Although norovirus is considered enzootic in most institutions, 9 institutions that exclude the virus from either all of its facilities or selected facilities, suites, or rooms reported norovirus outbreaks. Despite the fact that approximately 69% of mice live in barrier housing, only 9% of the MHV, EDIM, MVM, and TMEV outbreaks occurred in barrier facilities, with 81% occurring in nonbarrier facilities and 9% occurring in combined barrier and nonbarrier facilities (Table 2).
Table 2.
Relative percentages of mice housed in barrier, nonbarrier, or barrier and nonbarrier housing and the prevalence of outbreaks by housing type
| Facility type | % of total mice in facility type | % of MHV, EDIM, MVM, and TMEV outbreaks | % of MPV outbreaks | % of pinworm and fur mite outbreaks |
| Barrier | 69 | 9 | 38 | 55 |
| Nonbarrier | 18 | 81 | 45 | 32 |
| Barrier and nonbarrier | 12 | 9 | 17 | 13 |
During the 2-y period of the survey, 28 bacterial outbreaks were identified. These were caused by Helicobacter spp. (14), Corynebacterium bovis (5 outbreaks, excluded in 36% of institutions), Pasteurella pneumotropica (4 outbreaks, excluded in 25% of institutions), Klebsiella oxytoca (2 outbreaks, excluded in 16% of institutions), and Pseudomonas aeruginosa, Staphylococcus xylosus, and Staphylococcus spp. (1 each, excluded in 22%, 4%, and 7% of institutions, respectively). Institutions that identified one of these organisms but that do not exclude it from their colonies have not been included in these numbers.
During the 2 y, 34 institutions (56%) reported having fur mite outbreaks and 23 (43%) of institutions reported having pinworm outbreaks. A total of 189 outbreaks were identified. Of these, 55% occurred in barrier facilities, 32% occurred in nonbarrier facilities, and 13% occurred in combined barrier/nonbarrier facilities (Table 2). Most (70%) of the fur mite outbreaks were identified during routine sentinel screening, and 90% of the pinworm outbreaks were identified during routine screening, with the majority of other outbreaks detected by recipient institutions at the time of animal transfer between institutions. In addition, 8% of institutions reported outbreaks of Demodex musculi, which was considered to be an important finding since only 36% of institutions exclude this organism and is likely not tested for in the remaining institutions.
Management of outbreaks.
We pooled the management measures used for MHV, EDIM, MVM, and TMEV due to the small numbers of outbreaks experienced during the targeted 2-y period. MPV, which results in lifelong infection and is therefore resistant to burnout (that is, cessation of breeding while ensuring all of the animals in the room are exposed to the virus), was analyzed separately. For management of the 4 viral diseases, isolation with burnout was the most commonly used technique (41% of the reported management measures), followed by isolation with test and cull (26%) and depopulation (7%). For 26% of the outbreaks, eradication was not instituted. For MPV, test and cull was used in 66% of the outbreaks; eradication was not performed in 30% of the outbreaks.
One third of newly identified Helicobacter spp. outbreaks were addressed by moving positive cages to a facility or room that did not exclude the organism, but no treatment was instituted in one third of the outbreaks. When treatment was instituted, 3 groups rederived the colony, and 2 groups used antibiotic treatment to resolve the infection.
Fur mites were treated with either ivermectin orally (diet or gel) or topically (46% of cases), selemectin (30%), moxidectin (16%), or permethrin (8%). Permethrin was added to one of the other treatments in 13% of cases. Eradication of fur mites was confirmed by either fur pluck or tape testing (43% of the outbreaks) or by PCR analysis (57%). Pinworms were treated most commonly with fenbendazole (70% of reported treatments), ivermectin (13%), or both fenbendazole and ivermectin in food (10%), and test and cull (7%). Eradication of the infection was assessed by using PCR analysis, tape test, or fecal flotation of selected cages in 68% of the outbreaks, with the remainder confirmed by either testing all cages or the exhaust plenum by using PCR analysis.
Quarantine.
Greater than 95% of institutions quarantine incoming mice from other institutions or ‘nonapproved’ vendors, whereas just 4% of institutions quarantine mice coming from ‘approved vendors.’ In addition, 37% of institutions do not test animals upon their arrival to quarantine, whereas 63% of institutions test for endo- and ectoparasites on arrival, and 22% do an initial PCR screen for selected viral organisms. Furthermore, 20% of institutions allow researchers to access mice in quarantine, whereas the remaining 80% do not allow such access. Most (85%) institutions treat animals prophylactically during quarantine, by using fenbendazole (68% of institutions), ivermectin (22%), moxidectin (17%), selamectin (14%), or permethrin (MiteArrest, EcoHealth, Brookline, MA; 7%). Frequently, fenbendazole is used in combination with one of the other products. Prior to release of animals from quarantine, several methods of testing are used, with 38% of institutions using direct-contact sentinels, 65% using DBS, and 90% using direct testing of the principal animals by PCR, fur pluck, tape test, and so forth. When no excluded agents are identified, the average quarantine duration is 5.8 ± 2.3 wk (range, 1 to 12 wk).
Rats.
All of the reporting institutions had rats during the time of interest (Table 1). Greater than 95% of institutions excluded sialodacryoadenitis virus, rat parvovirus, rat virus (Kilham rat virus), Toolan H1 virus, lymphocytic choriomeningitis virus, Sendai virus, pneumonia virus of mice, rat minute virus, Aspiculuris tetraptera, Syphacia obvelata, Syphacia muris, Myobia musculi, Radfordia spp., and Myocoptes spp. In addition, 75% to 95% of institutions excluded rat rotavirus, rat theilovirus, hantavirus, Mycoplasma pulmonis, Clostridium piliforme, Salmonella spp., Streptobacillus moniliformis, cilia-associated respiratory bacillus, Giardia spp., Psorergates simplex, Rodentolepis nana, Hymenolepis diminuta, Taenia taeniformis, Polyplax spinulosa, and Encephalilitozoan cuniculi.
During the 2-y period of interest, 2 institutions reported rat parvovirus outbreaks, 2 reported rat theilovirus outbreaks, one reported rat virus outbreak, 3 reported pinworm outbreaks, 2 reported fur mite outbreaks, and one reported E. cuniculi outbreak. Eight institutions reported having cases of ‘rat respiratory virus.’ and 6 of these reported confirming the presence of Pneumocystis carinii in the affected animals.
Discussion
Controlling adventitial infections is critical in rodent biomedical research studies for a myriad of reasons, including the high prevalence of immunosuppressed mice used, the confounding effects of immune system activation on immune studies, and the effect of immune activation on the different body systems. When compared with the results of the 2 previous surveys of rates of adventitial infections in research rodent colonies, the current survey shows that the prevalence of viral infections has decreased, whereas the prevalence of parasitic infestations has remained essentially constant. Furthermore, although a wide variety of biosecurity measures are used in the barrier facilities of various institutions, the overall low rate of viral infections relative to the total population of mice living in barrier facilities suggests that diverse biosecurity measures are effective at reducing viral outbreaks in murine colonies but apparently are less effective against parasitic pathogens.
Although the 2 previous surveys that examined the prevalence of adventitial infections in biomedical research facilities provide a baseline for comparison with the current results, important differences between the biosecurity classifications were used in the different time frames. In the 1998 survey,21 facilities were defined as either SPF or nonSPF. As with the current study, the previous authors found a wide degree of variability between the definitions and biosecurity procedures employed in SPF colonies at different institutions, making a uniform definition of ‘SPF’ impossible. Although this previous study did not define or quantify the biosecurity measures used in SPF colonies, anecdotal history suggests that SPF biosecurity was somewhat similar to that in current barrier facilities. Moreover, most current nonbarrier facilities use significantly greater biosecurity measures than appear to have been used in nonSPF colonies in the 1990s. This conclusion is supported by the current results, which indicate that the viral and parasitic prevalence in current nonbarrier facilities is more similar to that of the SPF colonies surveyed in 1998. The 2008 survey10 did not divide results into either SPF and nonSPF or barrier and nonbarrier facilities, but the results may have detected a transition from the 1998 nonSPF biosecurity measures toward the current standards. These ‘transitions’ are the result of constant and ongoing attempts to improve the control of adventitial infections. A recent innovation that is growing in use is PCR analysis of samples from pooled colony animals and exhaust plenums of ventilated racks.5,19 However, the use of this method was comparatively low during the current survey's timeframe, thus allowing future comparisons to assess changes in adventitial infections in association with changes in organism detection methods.
Over the course of the 20 y covered by the 3 surveys, the rate of most viral infections in mice has decreased markedly. In the 1998 survey, MHV was present in 12% of SPF colonies and 75% of nonSPF colonies.21 In the 2008 survey, the total dropped to 40% of all facilities experiencing MHV, whereas in the time period of the current survey, only 10% of institutions reported having identified MHV in their barrier or nonbarrier colonies.10 Similar decreases can be noted in several other common viral infections in mice, including EDIM, MVM, and TMEV (Table 3). The 2008 results may represent a midpoint in the transition to the current biosecurity standards and the resultant increased efficacy in disease prevention provided by these standards. The prevalence of the organisms reported in the current study is also consistent with that of infectious agents in mice from a 2009 study from the Charles River Laboratories, which reported the percentages of positive mice submitted for testing.41
Table 3.
Percentages (%) of institutions reporting viral and parasitic outbreaks from the 3 most recent surveys of adventitial outbreaks in biomedical research facilities
| Agent | 1998 survey21 | 2008 survey10 | Current study |
| Mouse parvovirus | 27% SPF; 40% nonSPF | 94% | 37% |
| Epizootic diarrhea of infant mice | 6% SPF; 28% non SPF | 30% | 14% |
| Mouse hepatitis virus | 12% SPF; 75% nonSPF | 40% | 10% |
| Minute virus of mice | not evaluated | 38% | 5% |
| Theiler murine encephalomyelitis virus | 4% SPF; 35% nonSPF | 10% | 1% |
| Pinworms | 32% SPF; 68% nonSPF | 92% | 43% |
| Fur mites | 17% SPF; 38% nonSPF | 55% | 56% |
Interestingly, the reported prevalence of mouse parvovirus has remained essentially unchanged between the 1998 and current surveys, becoming an interesting case history. In the current survey, 37% of institutions report outbreaks, similar to the rate reported in the 1998 survey. This percentage peaked at over 90% of institutions reporting outbreaks in the 2008 survey. This spike in prevalence is likely due to improvements in the MPV assays3,33,42 and the subsequent reduction in prevalence was due to institutions’ efforts to eradicate the organism, in light of its potential effects on the immune system. Unfortunately, reduction in the infection prevalence of this virus to levels seen for other viruses (MHV, EDIM virus, MVM, and so forth) likely will prove difficult due to the biology of the organism. Despite causing a persistent lifelong infection, MPV can be shed intermittently, decreasing the efficacy of detection by fecal PCR assays and the transmission to sentinel animals.22,37,50 Furthermore parvoviruses are extremely resistant to environmental degradation, are readily transmitted by fomites,12,13,37,43,54 and can be transmitted from many different sources.24 In addition, different ages and strains of mice vary in their susceptibility to parvovirus infection.18,20 Finally, conventional attempts to eradicate the virus through cross-fostering to a seronegative dam do not prevent viral transmission or infection. Despite the presence of virus in gametes, embryos, and ovarian tissues, embryo-transfer rederivation has been successfully used to rederive MPV-infected lines of mice.1,6,12,28,37
Despite the fact that 69% of mice live in barrier housing, only 9% of MHV, EDIM, and MVM outbreaks occurred in barrier exclusive housing (Table 2). This finding provides strong evidence that barrier housing biosecurity measures are effective at preventing these viral infections. Because of the low number of these outbreaks, we were unable to determine which biosecurity measures were particularly important in preventing viral outbreaks. However, the 4 biosecurity measures that appeared to be used much more commonly in barrier facilities compared with nonbarrier facilities are 1) once mice leave their housing area, they are not permitted to return (86% of barrier and 20% of nonbarrier colonies), and 2) cage lids are opened only in a biosafety cabinet or cage change station (96% of barrier and 52% of nonbarrier colonies), 3) use of autoclaved food and bedding (96% of barrier and 52% of nonbarrier colonies) and 4) personnel can't enter a facility after having been in a nonbarrier facility (79% of barrier and 7% of nonbarrier colonies). Consistent use of these methods may explain the improved control of viral diseases in barriers. However, the efficacy of other biosecurity measures should not be dismissed, given that the current rates of viral infections in nonbarrier facilities appear to be similar to those that previously were achieved in SPF colonies. Furthermore, biosecurity measures are much more easily implemented and wild mouse populations are more easily controlled in vivaria as compared with laboratories and office spaces across an institution.
For the MHV, EDIM, and MVM outbreaks, 41% were reported to be treated by isolation with burnout, with 33% treated by either test-and-cull or depopulation and 26% of outbreaks not treated. These results were surprising, considering the limitations of the efficacy of burnout in resolving nonrespiratory viral infections.16 Current recommendations for the rapid resolution of viral outbreaks revolve around either depopulation with rederivation for outbreaks with a high percentage of infected cages or test-and-cull eradication when the infection rate is low (typically less than 5% of cages affected).49 Although breeding cessation may appeal to researchers, it is critical that they understand the risks of eradication failure and the costs of potential long-term isolation on their research.
Parasitic infestations remain prevalent in mouse vivaria. Interestingly, while the prevalence of fur mite infections has remained relatively stable over time, the prevalence of pinworm infections has fallen substantially (Table 3). Although some reports indicate that DBS are an effective means to detect fur mites,14,44 the lack of improvement in the rate of fur mite infestations may suggest the relative difficulty in detecting these organisms by conventional methods.31 This difficulty likely results from the long hair cycle in mice and the fact that the eggs are tightly adherent to the hairs, meaning that DBS are unlikely to become exposed to the organism. Parasites may therefore be present in the colony for extended periods of time before diagnosis, allowing an infested cage or cages to serve as a nidus of organisms that can then be spread to other rooms or facilities if proper biosecurity measures are not consistently followed. Because of the difficulty in detecting fur mites by DBS, approximately 50% of institutions have begun using a PCR-based method to detect the organisms.5,19,23,26 Interestingly, the prevalence of both fur mites and pinworm infections in barrier facilities more closely mirrors nonbarrier housed mice (Table 2), suggesting that the biology, transmission, and detection of these organisms collectively decrease the efficacy of the barrier biosecurity methods in controlling parasitic outbreaks as compared with viral outbreaks. Again, with the increased use of PCR testing of racks and plenums, it may be possible to accelerate the diagnosis of parasitic infections, resulting in rapid treatment and the elimination of pockets of infection and thereby decreasing the rate of outbreaks of these organisms.
There was little variability in the organisms excluded among the institutions responding to the survey, with the notable exceptions of norovirus and Helicobacter spp. Currently, few institutions have excluded these organisms from all of their facilities, but more than half of the facilities have rooms, suites, or individual facilities that are norovirus- or Helicobacter-free (or both). The prevalence of norovirus was not addressed in either of the 2 previous surveys.10,21 Because of the potential effect of this virus on the immune system and immune-related studies, we expect a slow increase in the number of facilities achieving and maintaining a norovirus-free status, and rapid, sweeping changes are unlikely due to limited knowledge of the adverse effects of the virus, even in most strains of immunodeficient mice.27,52,57 A recent publication has reported that exhaust plenum PCR testing is a better method for diagnosing MNV infection than the current DBS used by most institutions, provided the rack design is compatible with this form of testing. This finding means that as this technology spreads through the field, the ability to diagnose the infection may occur earlier in the infection, potentially aiding in the elimination of the organism from the colony.5,60
Helicobacter was addressed in both of the 2 previous surveys. In the 1998 survey, the reported prevalence was higher in the SPF mice than nonSPF mice, indicating that many nonSPF colonies likely did not test for the organism.21 In the 2008 survey,10 80% of facilities reported having Helicobacter spp. We suspect that a similar percentage of institutions would report the same results today, because the organism is excluded from only 12% of institutions. Helicobacter is more likely to result in clinical disease in immunosuppressed mice and can have a profound effect on many experimental models of disease.17,56 Embryo transfer has been shown to be effective at eliminating this organism, and cross-fostering mouse pups and oral gavage of amoxicillin, metronidazole, and bismuth are effective in mice, with some failures noted, particularly in genetically manipulated or immunocompromised mice.2,15,25,29,32,36,47,48,53,55,58
A total of 5 Demodex musculi outbreaks were reported in the survey, and several institutions expressed a specific interest in determining the prevalence of this organism within facilities not currently testing for it. This organism has been associated with dermatitis and conjunctivitis in immunosuppressed mice and has been diagnosed by either fur pluck or histopathology. Immunocompetent mice are believed to harbor very low parasitic loads.4,51 Similarly, Corynebacterium bovis, which is an important pathogen of nude mice,4,9 was cited by several institutions as being a growing concern in their immunosuppressed mouse colonies. Recent work suggests that PCR analysis of exhaust manifolds can be used to monitor for this potentially significant pathogen.38
The definition of a ‘barrier’ facility varied greatly between institutions. Compounding this variability is relaxed or increased biosecurity measures in selected facilities at an institution. Our field would benefit from developing standards defining the minimal biosecurity measures necessary for a facility to be called a barrier facility. If additional security measures were implemented, the term barrier would still be appropriate in describing the facility. If the biosecurity procedures were below these minimal standards, institutions would be expected to refrain from describing the facility as a barrier.
The presence of an organism in a vivarium depends on 1) the likelihood of its introduction into the facility; 2) the time required to detect the organism before it has a chance to disseminate to other rooms, suites, or facilities; 3) biosecurity measures to prevent this dissemination; and 4) the ability to eradicate the organism after detection. The biology of the different adventitial organisms will affect these variables and thus the prevalence of their outbreaks. The use of DBS and serology has proven to be effective at detecting even low levels of most viral infections, but these techniques appear to be less effective in the detection of respiratory viruses, pinworms, and fur mites. Improvements in the detection of these organisms appear to be the most practical way to help further minimize their prevalence in mouse facilities, given that the management/treatment of both viral infections and parasitic infestations appear to be effective at eradicating the organisms.
The control of adventitial infections in mice is critical to limit experimental variability in biomedical research. The current study's results show that the prevalence of infectious disease outbreaks has decreased over the last 2 decades and that current barrier security measures greatly reduce the rate of viral infections. Using this information, our field likely will be able to continue to reduce and hopefully eliminate the influence of adventitial infections on biomedical research.
Acknowledgments
We would like to thank the many people from across the country who participated in the survey. In addition, we extend a special thank-you to Anthony Carty and the groups that helped the survey reach its final form by piloting earlier versions.
References
- 1.Agca Y, Bauer BA, Johnson DK, Critser JK, Riley LK. 2007. Detection of mouse parvovirus in Mus musculus gametes, embryos, and ovarian tissues by polymerase chain reaction assay. Comp Med 57:51–56. [PubMed] [Google Scholar]
- 2.Artwohl JE, Purcell JE, Fortman JD. 2008. The use of cross-foster rederivation to eliminate murine norovirus, Helicobacter spp., and murine hepatitis virus from a mouse colony. J Am Assoc Lab Anim Sci 47:19–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Ball-Goodrich LJ, Hansen G, Dhawan R, Paturzo FX, Vivas-Gonzalez BE. 2002. Validation of an enzyme-linked immunosorbent assay for detection of mouse parvovirus infection in laboratory mice. Comp Med 52:160–166. [PubMed] [Google Scholar]
- 4.Barthold SW, Griffey SM, Percy DH. 2016. Pathology of laboratory rodents and rabbits, 4th ed Ames (IA): Wiley–Blackwell. [Google Scholar]
- 5.Bauer BA, Besch-Williford C, Livingston RS, Crim MJ, Riley LK, Myles MH. 2016. Influence of rack design and disease prevalence on detection of rodent pathogens in exhaust debris samples from individually ventilated caging systems. J Am Assoc Lab Anim Sci 55:782–788. [PMC free article] [PubMed] [Google Scholar]
- 6.Besselsen DG, Romero-Aleshire MJ, Munger SJ, Marcus EC, Henderson KS, Wagner AM. 2008. Embryo transfer rederivation of C.B-17/Icr-Prkdcscid mice experimentally infected with mouse parvovirus 1. Comp Med 58:353–359. [PMC free article] [PubMed] [Google Scholar]
- 7.Blabaum CJ, Nichols MS. 1956. Effect of highly chlorinated water on white mice. J Am Water Works Assoc 48:1503–1506. [Google Scholar]
- 8.Blank WA, Henderson KS, White LA. 2004. Virus PCR assay panels: an alternative to the mouse antibody production test. Lab Anim (NY) 33:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burr HN, Wolf FR, Lipman NS. 2012Corynebacterium bovis: epizootiologic features and environmental contamination in an enzootically infected rodent room. J Am Assoc Lab Anim Sci 51:189–198. [PMC free article] [PubMed] [Google Scholar]
- 10.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited..ILAR J 49:272–276. [DOI] [PubMed] [Google Scholar]
- 11.Casebolt DB, Lindsey JR, Cassell GH. 1988. Prevalence rates of infectious agents among commercial breeding populations of rats and mice. Lab Anim Sci 38:327–329. [PubMed] [Google Scholar]
- 12.Compton SR, Paturzo FX, Macy JD. 2012. Transmission of mouse parvovirus to neonatal mice. J Am Assoc Lab Anim Sci 51:797–802. [PMC free article] [PubMed] [Google Scholar]
- 13.Compton SR, Paturzo FX, Smith PC, Macy JD. 2012. Transmission of mouse parvovirus by fomites. J Am Assoc Lab Anim Sci 51:775–780. [PMC free article] [PubMed] [Google Scholar]
- 14.de Bruin WC, van de Ven EM, Hooijmans CR. 2016. Efficacy of soiled bedding transfer for transmission of mouse and rat infections to sentinels: a systematic review. PLoS One 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foltz CJ, Fox JG, Yan L, Shames B. 1996. Evaluation of various oral antimicrobial formulations for eradication of Helicobacter hepaticus. Lab Anim Sci 46:193–197. [PubMed] [Google Scholar]
- 16.Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT. 2015. Laboratory animal medicine, 3rd ed Cambridge (MA): Elsevier. [Google Scholar]
- 17.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. 2010. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol 4:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grove KA, Smith PC, Booth CJ, Compton SR. 2012. Age-associated variability in susceptibility of Swiss Webster mice to MPV and other excluded murine pathogens. J Am Assoc Lab Anim Sci 51:789–796. [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson KS, Perkins CL, Havens RB, Kelly MJ, Francis BC, Dole VS, Shek WR. 2013. Efficacy of direct detection of pathogens in naturally infected mice by using a high-density PCR array. J Am Assoc Lab Anim Sci 52:763–772. [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson KS, Pritchett-Corning KR, Perkins CL, Banu LA, Jennings SM, Francis BC, Shek WR. 2015. A comparison of mouse parvovirus 1 infection in BALB/c and C57BL/6 mice: susceptibility, replication, shedding, and seroconversion. Comp Med 65:5–14. [PMC free article] [PubMed] [Google Scholar]
- 21.Jacoby RO, Lindsey JR. 1998. Risks of infection among laboratory rats and mice at major biomedical research institutions. ILAR J 39:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacoby RO, Smith AL. 2003. Mouse parvovirus: survival of the fittest. Comp Med 53:470–471. [PubMed] [Google Scholar]
- 23.Jensen ES, Allen KP, Henderson KS, Szabo A, Thulin JD. 2013. PCR testing of a ventilated caging system to detect murine fur mites. J Am Assoc Lab Anim Sci 52:28–33. [PMC free article] [PubMed] [Google Scholar]
- 24.Joh J, Proctor ML, Ditslear JL, King WW, Sundberg JP, Jenson AB, Ghim SJ. 2013. Epidemiological and phylogenetic analysis of institutional mouse parvoviruses. Exp Mol Pathol 95:32–37. [DOI] [PubMed] [Google Scholar]
- 25.Karita M, Tsuda M, Okita K, Sugiyama T, Nakazawa T. 1995. Evaluation of a new bismuth-free triple therapy in nude mice and humans. Eur J Gastroenterol Hepatol 7 Suppl 1:S31–S34. [PubMed] [Google Scholar]
- 26.Karlsson EM, Pearson LM, Kuzma KM, Burkholder TH. 2014. Combined evaluation of commonly used techniques, including PCR, for diagnosis of mouse fur mites. J Am Assoc Lab Anim Sci 53:69–73. [PMC free article] [PubMed] [Google Scholar]
- 27.Kastenmayer RJ, Perdue KA, Elkins WR. 2008. Eradication of murine norovirus from a mouse barrier facility. J Am Assoc Lab Anim Sci 47:26–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall LV, Allaband C, Henderson KS. 2016. Prenatal exposure to mouse parvovirus at day 5 and 12 gestation does not induce immune tolerance. PLoS One 11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerton A, Warden P. 2006. Review of successful treatment for Helicobacter species in laboratory mice. Lab Anim 40:115–122. [DOI] [PubMed] [Google Scholar]
- 30.Kraft LM. 1958. Observations on the control and natural history of epidemic diarrhea of infant mice (EDIM). Yale J Biol Med 31:121–137. [PMC free article] [PubMed] [Google Scholar]
- 31.Lindstrom KE, Carbone LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled-bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60. [PMC free article] [PubMed] [Google Scholar]
- 32.Litvinova EA, Kozhevnikova EN, Achasova KM, Kontsevaya GV, Moshkin MP. 2017. Eradication of Helicobacter spp. in mucin 2-deficient mice. Lab anim 51:311–314. [DOI] [PubMed] [Google Scholar]
- 33.Livingston RS, Besselsen DG, Steffen EK, Besch-Williford CL, Franklin CL, Riley LK. 2002. Serodiagnosis of mice minute virus and mouse parvovirus infections in mice by enzyme-linked immunosorbent assay with baculovirus-expressed recombinant VP2 proteins. Clin Diagn Lab Immunol 9:1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livingston RS, Riley LK. 2003. Diagnostic testing of mouse and rat colonies for infectious agents. Lab Anim (NY) 32:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loew FM, Fox JG. 1983. Animal health surveillance and health delivery systems, Chapter 5. p 69–82. In: Foster HL, Small JD, Fox JG. The mouse in biomedical research, vol, 3, normative biology, immunology, and husbandry. New York (NY): Academic Press. [Google Scholar]
- 36.Lofgren JL, Esmail M, Mobley M, McCabe A, Taylor NS, Shen Z, Erdman S, Hewes C, Whary MT, Fox JG. 2012. Prevalence of murine Helicobacter spp. Infection is reduced by restocking research colonies with Helicobacter-free mice. J Am Assoc Lab Anim Sci 51:436–442. [PMC free article] [PubMed] [Google Scholar]
- 37.Macy JD, Cameron GA, Smith PC, Ferguson TA, Compton SR. 2011. Detection and control of mouse parvovirus. J Am Assoc Lab Anim Sci 50:516–522. [PMC free article] [PubMed] [Google Scholar]
- 38.Manuel CA, Pugazhenthi U, Leszczynski JK. 2016. Surveillance of a ventilated rack system for corynebacterium bovis by sampling exhaust-air manifolds. J Am Assoc Lab Anim Sci 55:58–65. [PMC free article] [PubMed] [Google Scholar]
- 39.McPherson CW. 1963. Reduction of Pseudomonas aeruginosa and coliform bacteria in mouse drinking water following treatment with hydrochloric acid or chlorine. Lab Anim Care 13:737–744. [PubMed] [Google Scholar]
- 40.Porter G, Lane-Petter W. 1965. Observations on autoclaved, fumigated and irradiated diets for breeding mice. Br J Nutr 19:295–305. [DOI] [PubMed] [Google Scholar]
- 41.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173. [DOI] [PubMed] [Google Scholar]
- 42.Redig AJ, Besselsen DG. 2001. Detection of rodent parvoviruses by use of fluorogenic nuclease polymerase chain reaction assays. Comp Med 51:326–331. [PubMed] [Google Scholar]
- 43.Reuter JD, Livingston R, Leblanc M. 2011. Management strategies for controlling endemic and seasonal mouse parvovirus infection in a barrier facility. Lab Anim (NY) 40:145–152. [DOI] [PubMed] [Google Scholar]
- 44.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587. [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe WP, Hartley JW, Capps WI. 1963. Mouse hepatitis virus infection as a highly contagious, prevalent, enteric infection of mice. Proc Soc Exp Biol Med 112:161–165. [DOI] [PubMed] [Google Scholar]
- 46.Rowe WP, Hartley JW, Huebner RJ. 1963. Polyoma and other indigenous mouse viruses. Lab Anim Care 13 SUPPL: 166 –175. [PubMed] [Google Scholar]
- 47.Scavizzi F, Raspa M. 2006. Helicobacter typhlonius was detected in the sex organs of 3 mouse strains but did not transmit vertically. Lab Anim 40:70–79. [DOI] [PubMed] [Google Scholar]
- 48.Singletary KB, Kloster CA, Baker DG. 2003. Optimal age at fostering for derivation of Helicobacter hepaticus-free mice. Comp Med 53:259–264. [PubMed] [Google Scholar]
- 49.Smith AL. 2010. Management of rodent viral disease outbreaks: one institutions (r)evolution. ILAR J 51:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith AL, Jacoby RO, Johnson EA, Paturzo F, Bhatt PN. 1993. In vivo studies with an ‘orphan’ parvovirus of mice. Lab Anim Sci 43:175–182. [PubMed] [Google Scholar]
- 51.Smith PC, Zeiss CJ, Beck AP, Scholz JA. 2016. Demodex musculi infestation in genetically immunomodulated mice. Comp Med 66:278–285. [PMC free article] [PubMed] [Google Scholar]
- 52.Tajima M, Kotani Y, Kurosawa T, Miyasaka M. 2013. Pitfalls in mouse norovirus (MNV) detection in fecal samples using RT-PCR and construction of new MNV-specific primers. Exp Anim 62:127–135. [DOI] [PubMed] [Google Scholar]
- 53.Van Keuren ML, Saunders TL. 2004. Rederivation of transgenic and gene-targeted mice by embryo transfer. Transgenic Res 13:363–371. [DOI] [PubMed] [Google Scholar]
- 54.Watson J. 2013. Unsterilized feed as the apparent cause of a mouse parvovirus outbreak. J Am Assoc Lab Anim Sci 52:83–88. [PMC free article] [PubMed] [Google Scholar]
- 55.Watson J, Thompson KN, Feldman SH. 2005. Successful rederivation of contaminated immunocompetent mice using neonatal transfer with iodine immersion. Comp Med 55:465–469. [PubMed] [Google Scholar]
- 56.Whary MT, Fox JG. 2006. Detection, eradication, and research implications of Helicobacter infections in laboratory rodents. Lab Anim (NY) 35:25 –27, 30 –36. [DOI] [PubMed] [Google Scholar]
- 57.Wobus CE, Thackray LB, Virgin HW., 4th 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80:5104–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeom SC, Yu SA, Choi EY, Lee BC, Lee WJ. 2009. Prevalence of Helicobacter hepaticus, murine norovirus, and pneumocystis carinii and eradication efficacy of cross-fostering in genetically engineered mice. Exp Anim 58:497–504. [DOI] [PubMed] [Google Scholar]
- 59.Zimmerman DR, Wostmann BS. 1963. Vitamin stability in diets sterilized for germfree animals. J Nutr 79:318–322. [DOI] [PubMed] [Google Scholar]
- 60.Zorn J, Ritter B, Miller M, Kraus M, Northrup E, Brielmeier M. 2017. Murine norovirus detection in the exhaust air of IVCs is more sensitive than serological analysis of soiled-bedding sentinels. Lab Anim 51:301–310. [DOI] [PubMed] [Google Scholar]