Abstract
Most individuals at ultra-high risk (UHR) for psychosis do not transition to frank illness. Nevertheless, many have poor clinical outcomes and impaired psychosocial functioning. This study used voxel-based morphometry to investigate if baseline grey and white matter brain densities at identification as UHR were associated with functional outcome at medium- to long-term follow-up. Participants were help-seeking UHR individuals (n = 109, 54M:55F) who underwent magnetic resonance imaging at baseline; functional outcome was assessed an average of 9.2 years later. Primary analysis showed that lower baseline grey matter density, but not white matter density, in bilateral frontal and limbic areas, and left cerebellar declive were associated with poorer functional outcome (Social and Occupational Functioning Assessment Scale [SOFAS]). These findings were independent of transition to psychosis or persistence of the at-risk mental state. Similar regions were significantly associated with lower self-reported levels of social functioning and increased negative symptoms at follow-up. Exploratory analyses showed that lower baseline grey matter densities in middle and inferior frontal gyri were significantly associated with decline in Global Assessment of Functioning (GAF) score over follow-up. There was no association between baseline grey matter density and IQ or positive symptoms at follow-up. The current findings provide novel evidence that those with the poorest functional outcomes have the lowest grey matter densities at identification as UHR, regardless of transition status or persistence of the at-risk mental state. Replication and validation of these findings may allow for early identification of poor functional outcome and targeted interventions.
Keywords: grey matter density, functional outcome, psychosis, ultra-high risk, voxel-based morphometry, negative symptoms, clinical high risk
Introduction
For many years, transition to frank psychotic illness has been the outcome measure of interest in research investigating people at ultra-high risk (UHR) for psychosis.1 It is now thought that the structural brain alterations seen in schizophrenia and other psychoses (such as increases in ventricular volume and decreases in grey and white matter volume2–4) may arise during or even before the onset phase of psychosis.5–8 These brain changes have a demonstrated relationship with transition to psychosis5,9–12 and may serve as biomarkers for onset of illness13 and inform intervention efforts.14
However, the majority of UHR individuals do not transition to psychosis15 and despite this, many still show poor psychosocial functioning at follow-up.16–19 Cornblatt et al20 explain this using a model consisting of 2 dimensions. The first dimension represents a period of vulnerability caused by early insult that impacts brain pathology. This vulnerability is manifested in psychosocial problems such as Cognitive deficits, Affective disturbances, Social Isolation, and School failure that are together referred to in abbreviated form as the CASIS model. Presence of basic symptoms21,22 could be associated with this dimension, which may underlie poor functioning and is necessary, but not sufficient, for the development of schizophrenia. The second dimension is characterized by an underlying vulnerability for positive psychotic symptomatology. These symptoms may develop in only a subset of individuals with CASIS vulnerability20 who eventually develop psychosis. Measuring psychosocial outcome, particularly in the early stages of psychosis, is thus important not only for our understanding of psychotic illnesses and their causes19,23–25 but also for our understanding of those who are in the first dimension and show poor functioning, but never transition to frank psychotic illness. Furthermore, functional impairment may be related to the presence or development of nonpsychotic disorders that are common in UHR individuals.26
Research in UHR individuals16,27,28 has shown that reduced neurocognitive performance on measures of verbal learning and memory, processing speed and attention, and verbal fluency predicts poor functional outcome. Functional outcomes also appear to be associated with a history of childhood maltreatment, regardless of transition status,29 and non-resolving attenuated psychotic symptoms.30,31 The limited research on associated brain functioning shows that poorer social functioning as measured by the Social Attainment Survey32 can be predicted by increased activation in anterior cingulate and left inferior frontal gyrus during performance of a reasoning language processing task.33 Likewise, poor functioning as indicated by low Global Assessment of Functioning (GAF34) scores has been predicted by increased left inferior frontal and insula activation during performance of a verbal fluency task and lower thalamic glutamate levels.35 On the structural level, lower baseline fractional anisotropy (FA) in the hippocampus and inferior longitudinal fasciculus in UHR individuals has been shown to predict deterioration in social and role functioning at 15-month follow-up.36 These latter studies have been limited by the small UHR samples and short follow-up period (6–24 mo) for assessment of functional outcome.
The current study aimed to further investigate the structural alterations associated with poor functional outcome in a larger sample of UHR individuals followed over the medium- to long-term. We adopted a voxel-based morphometry approach to investigate whether grey and white matter brain density of individuals at UHR for psychosis could predict functional outcome 2.4 to 12.9 years later, and if this association would be related to transition status. Based on the findings described above, we predicted that lower densities in frontal and temporal regions at baseline would be associated with poorer psychosocial functioning at follow-up.
Methods
Participants
UHR individuals were recruited from the Personal Assessment and Crisis Evaluation (PACE) Clinic at Orygen Youth Health (now Orygen, The National Centre of Excellence in Youth Mental Health), in Melbourne, Australia. They were part of a cohort of UHR patients recruited to participate in research studies between 1993 and 2006. Current data are from participants with both baseline MRI and follow-up functional outcome data (n = 109, 54M:55F). Inclusion criteria were based on the UHR entry criteria for PACE which are the presence of attenuated psychotic symptoms, brief limited intermittent psychotic symptoms (BLIPS), and/or trait vulnerability for psychotic illness (presence of schizotypal personality disorder or a first-degree family history of psychosis), as well as deterioration in functioning or persistent low functioning. Up to 1999, these criteria were established using the Brief Psychiatric Rating Scale (BPRS37)/Comprehensive Assessment of Symptoms and History (CASH38)/GAF34 and the Comprehensive Assessment of At-Risk Mental States (CAARMS39). From 1999, the CAARMS replaced the BPRS/CASH as the means of establishing UHR status. Participants were aged between 15–30 years old and had no history of psychotic illness, organic cause for presentation or past neuroleptic exposure equivalent to a total continuous haloperidol dose of more than 15mg (ie, neuroleptics being taken day after day until the 15mg haloperidol equivalent had been reached). Exclusion from imaging studies were neurological disorder, history of significant head injury, seizures or contraindication for magnetic resonance imaging (MRI). All participants provided written informed consent and the study was approved by the local Research and Ethics Committee (Melbourne Health).
Participants were followed up using the tracking system described in Lin et al.40 More details regarding this long-term follow-up study can be found in Nelson et al15 Follow-up assessments of this sample took place between 2.4 and 12.9 (mean = 9.2, SD = 2.5, median = 9.8) years after study entry at PACE.
Assessments
Participants underwent clinical assessment and MRI at baseline. Clinical assessment included assessment of positive symptoms using the BPRS,37 negative symptoms using the Scale for the Assessment of Negative Symptoms (SANS41), and GAF34 for functioning. During the follow-up assessment, baseline measures were re-administered and the Social and Occupational Functioning Assessment Scale (SOFAS42) and Quality of Life Scale (QLS43) were used as indices of functional outcome. For participants recruited before the year 2000 (n = 73, 67% of the current sample), IQ at follow-up was measured using subtests of the Wechsler Adult Intelligence Scale-Revised (WAIS-R44) proposed by Ward.45 These subtests are information, picture completion, block design, arithmetic, digit span, similarities and digit symbol. For participants recruited from the year 2000 onwards (n = 36, 33% of the current sample), the full Wechsler Abbreviated Scale of Intelligence (WASI46) was used to measure IQ at follow-up. Transition to frank psychosis was established using the CAARMS39 or the state public mental health records when CAARMS data were not available.
MRI Acquisition
Eighty percent (n = 87) of the T1-weighted MRI scans were obtained using a 1.5T GE Signa MR scanner: 124 slices of 1.5mm thickness, repetition time (TR) = 1.43 seconds, echo time (TE) = 3.3ms, flip angle 30°, matrix 256×256, field of view (FOV) 24cm. The remaining 20% (n = 22) of the T1-weighted MRI scans were obtained using a 3T GE LX Horizon Scanner: 124 slices of 2mm thickness, TR = 3.6 seconds, TE = 9ms, flip angle 30°, matrix 410×410, FOV 20cm.
Data Analysis
Behavioral data were analyzed using IBM SPSS Statistics 21 for Windows (IBM Corp). T1-weighted MRI images were automatically processed using the optimized voxel-based morphometry (VBM8) toolbox (http://dbm.neuro.uni-jena.de/vbm/) in statistical parametric mapping software (SPM8, Friston, The Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm). For details on the preprocessing of the data see supplementary material. Regionally specific differences in the association of baseline grey and white matter density (both lower and higher) with functional outcome were assessed using multiple regressions with gender, age, field strength of the scanner, length of the follow-up period and transition status specified as nuisance variables. Threshold-free cluster enhancement (TFCE) is a spatially sensitive statistical inference algorithm that is based on the sensitivity benefits of cluster-based inference but is not dependent on an arbitrary cluster-forming threshold.47 This algorithm was applied to optimize activation in areas that show spatial contiguity and results were considered significant at P < .05 Family-Wise Error (FWE)-corrected.
SOFAS scores were used to investigate the association of baseline grey and white matter densities with psychosocial functioning at follow-up. The strength of the SOFAS is that, in contrast with the GAF, its scores are independent of the overall severity of the individual’s psychological symptoms and are based on the clinician’s judgment of the overall level of functioning. Whilst we argue superiority of the SOFAS over the GAF for use in this study, the GAF is commonly used and data were available for 108 participants at both baseline and follow-up for this measure (in contrast with SOFAS scores which were only available at follow-up). The association between baseline grey matter density and baseline GAF score and change in GAF score over time was therefore also investigated. Change in GAF score was calculated for each participant relative to their baseline score and constituted of a percentage change score and an absolute change score. The SOFAS does not assess social and role functioning separately and therefore the QLS was additionally employed. The QLS is based on self-report but has the advantage that its items can be factored into social functioning, vocational functioning and engagement.48 Data on this measure was available for 108 participants and scores on the social and vocational functioning scales were used to investigate if density associations were more specifically associated with either social- or vocational functioning.
Given that negative symptoms and general cognitive ability are strongly associated with functional outcome,23,27 density associations with negative symptoms and IQ measured at follow-up were additionally examined, along with density associations with positive symptoms at follow-up. Available follow-up data were as follows; SANS n = 107, IQ n = 102, and BPRS positive n = 105.
Results
Characteristics of the sample are presented in table 1. Of the 109 participants, 38 (35%) had transitioned to psychosis by the time of follow-up. The average length of time to conversion was 622 days (SD = 719 d). Of those who had not transitioned to psychosis by the time of follow-up, 27 (38%) met APS or BLIPS criteria. Further details about the sample are presented in the supplementary material and supplementary table A1.
Table 1.
Sample Characteristics
| Measure | Baseline | Follow-up | Baseline vs Follow-up | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | P-value | |
| Age | 109 | 19.5 | 3.6 | 109 | 28.7 | 4.7 | <.001 |
| IQ | 102 | 99.9 | 19.4 | ||||
| BPRS psychotic subscale | 107 | 8.9 | 2.9 | 108 | 6.9 | 3.7 | <.001 |
| SANS total | 108 | 18.7 | 12.6 | 108 | 11.4 | 13.9 | <.001 |
| SOFAS | 109 | 67.2 | 15.8 | ||||
| GAF | 108 | 59.5 | 12.3 | 109 | 64.1 | 15.3 | <.006 |
| QLS social functioning | 108 | 36.6 | 10.6 | ||||
| QLS vocational functioning | 108 | 22.7 | 8.5 | ||||
| Intake criteria | n | % | |||||
| APS only | 53 | 48.6 | |||||
| BLIPS only | 10 | 9.2 | |||||
| Vulnerability only | 19 | 17.4 | |||||
| APS + BLIPS | 6 | 5.5 | |||||
| APS + vulnerability | 17 | 15.6 | |||||
| BLIPS + vulnerability | 1 | 0.9 | |||||
| APS + BLIPS + vulnerability | 3 | 2.8 | |||||
Note: BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; SOFAS, Social and Occupational Functioning Assessment Scale; GAF, Global Assessment of Functioning; QLS, Quality of Life Scale; APS, attenuated psychotic symptoms; BLIPS, brief limited intermittent psychotic symptoms.
Primary Analysis
Lower than average baseline grey matter density was significantly associated with lower SOFAS scores at follow-up. This association was found in large clusters in medial prefrontal cortex, right cingulate gyrus extending into anterior cingulate, left anterior cingulate extending into anterior frontal cortex and subcallosal gyrus, and left cerebellar declive (table 2, figure 1). These areas of association were lower in size when symptom severity (SANS and BPRS psychotic subscale scores at baseline) were additionally added as nuisance variables (supplementary table A2). To determine whether this pattern was being driven by the individuals who had transitioned to psychosis at follow-up (note, transition status at follow-up was entered as a nuisance variable in all analyses), a secondary analysis was conducted. A comparison of the relationship of baseline grey matter with functional outcome in the identified bilateral frontal and limbic areas, and left declive, in those who had transitioned to psychosis at follow-up (n = 37) with those who had not (n = 69) revealed no significant differences. The same comparison for those who had not transitioned to psychosis but met APS or BLIPS criteria at follow-up (n = 27) with those who had not transitioned to psychosis and did not meet APS or BLIPS criteria at follow-up (n = 42) revealed no significant differences either. There was no significant association between higher baseline grey matter density and lower SOFAS scores at follow-up. There was no significant association between baseline white matter density (lower or higher) and SOFAS scores at follow-up and therefore subsequent analyses focused on grey matter density associations only.
Table 2.
Association Between Lower Baseline Grey Matter Density and Poor Functional Outcome (SOFAS, QLS)
| Region | BA | Left/Right | Cluster Size | MNI Coordinates | P-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Association with SOFAS scores | |||||||
| Medial prefrontal cortex | 10 | L | 1952a | −9 | 51 | 16 | .017 |
| Medial prefrontal cortex | 10 | 0 | 50 | 16 | .018 | ||
| Medial prefrontal cortex | 9 | 0 | 44 | 25 | .018 | ||
| Cingulate gyrus | 32 | R | 1352b | 14 | 14 | 28 | .021 |
| Anterior cingulate | 32 | R | 16 | 24 | 25 | .021 | |
| Anterior cingulate | 33 | R | 4 | 8 | 25 | .030 | |
| Anterior cingulate | 32 | L | 945c | −12 | 36 | 9 | .028 |
| Anterior frontal cortex | 10 | L | −12 | 34 | −6 | .031 | |
| Subcallosal gyrus | 25 | L | −9 | 22 | −11 | .034 | |
| Declive | L | 142 | −57 | −58 | −23 | .033 | |
| Association with QLS social functioning scores | |||||||
| Medial prefrontal cortex | 10 | L | 229 | −4 | 51 | 16 | .033 |
| Medial prefrontal cortex | 9 | L | −2 | 45 | 24 | .044 | |
Note: The coordinates represent the loci of the local maxima within each distinct anatomical region in Montréal Neurological Institute (MNI) space. All clusters are Family-Wise Error (FWE)-corrected for multiple comparisons using threshold-free cluster enhancement (TFCE). Cluster sizes are indicated in number of voxels. BA, Brodmann Area.
aThis cluster extended further into BA 47, 32 and 6.
bThis cluster extended further into BA 24.
cThis cluster extended further into BA 9, 24 and the caudate.
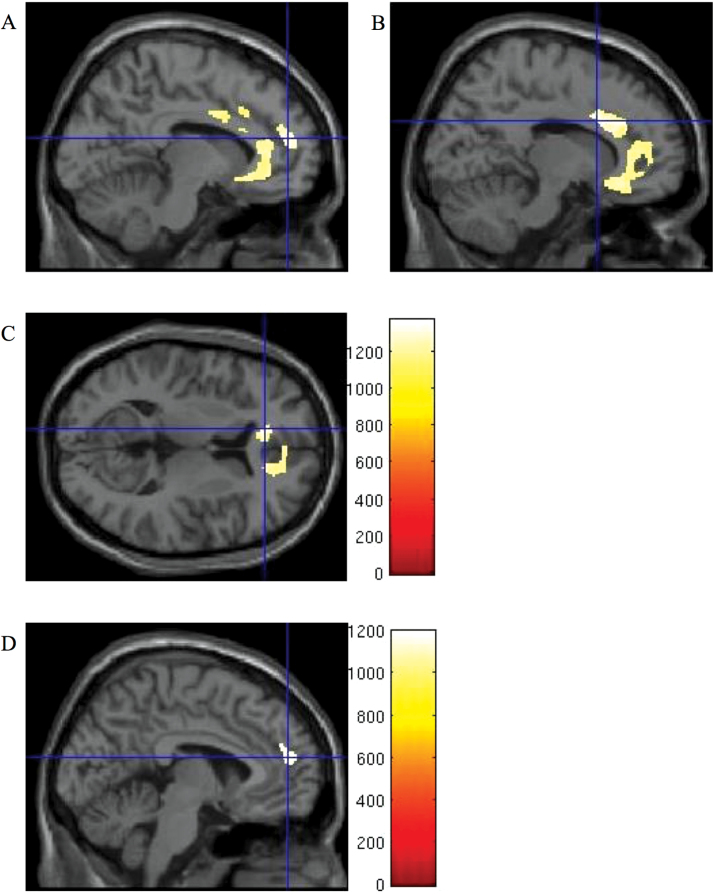
Fig. 1.
Association between lower baseline grey matter density and poor functional outcome. Threshold-free cluster enhancement (TFCE)-enhanced images displaying areas of lower baseline grey matter density associated with poor functional outcome as indicated by lower scores on the Social and Occupational Functioning Assessment Scale (SOFAS) (A, B, C) and social functioning subscale of the Quality of Life Scale (QLS) (D) at follow-up. Threshold at P < .05 Family-Wise Error (FWE)-corrected. Crosshairs at (A) medial prefrontal cortex [−9 51 16], (B) cingulate gyrus [14 14 28], (C) anterior cingulate [−12 36 9], (D) medial prefrontal cortex [−4 51 16]. Color bars show TFCE-enhanced t-statistic. For a color version, see this figure online.
Exploratory Analyses
No significant association was found between baseline grey matter density (lower or higher) and baseline GAF score or percentage change in GAF score. Lower baseline grey matter density in middle and inferior frontal gyri was, however, significantly associated with decline in GAF score over follow-up (supplementary table A3, supplementary figure A1).
Lower baseline grey matter density in left medial prefrontal cortex was significantly associated with lower social functioning scores on the QLS at follow-up (table 2, figure 1). There was no significant association between higher baseline grey matter density and social functioning scores on the QLS at follow-up. No significant association was observed between baseline grey matter density (lower or higher) and vocational functioning scores on the QLS at follow-up.
Significantly lower baseline grey matter density was observed in a large cluster extending from medial prefrontal cortex into cingulate gyrus and anterior cingulate, and clusters in right precentral and cingulate gyrus, left orbitofrontal cortex extending into anterior cingulate, and left anterior cingulate extending into caudate in association with higher SANS scores at follow-up (table 3, figure 2). Further exploratory analyses revealed that this association was not driven by a particular SANS subscale. There was no significant association between higher baseline grey matter density and SANS scores at follow-up.
Table 3.
Association Between Lower Baseline Grey Matter Density and Increased Negative Symptoms (SANS) at Follow-up
| Region | BA | Left/Right | Cluster Size | MNI Coordinates | P-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Medial prefrontal cortex | 9 | 3353a | 0 | 45 | 24 | .010 | |
| Cingulate gyrus | 32 | R | 15 | 15 | 30 | .012 | |
| Anterior cingulate | 32 | R | 16 | 24 | 25 | .012 | |
| Precentral gyrus | 6 | R | 100 | 51 | −3 | 40 | .043 |
| Orbitofrontal cortex | 11 | L | 40 | −9 | 27 | −9 | .048 |
| Anterior cingulate | 32 | L | −10 | 34 | −6 | .048 | |
| Anterior cingulate | 25 | L | 38 | −4 | 14 | −11 | .049 |
| Caudate | L | −3 | 9 | −3 | .049 | ||
| Cingulate gyrus | 23 | R | 3 | 10 | −16 | 33 | .050 |
Note: The coordinates represent the loci of the local maxima within each distinct anatomical region in MNI space. All clusters are FWE-corrected for multiple comparisons using TFCE. Cluster sizes are indicated in number of voxels.
aThis cluster extended further into BA 6 and 8.
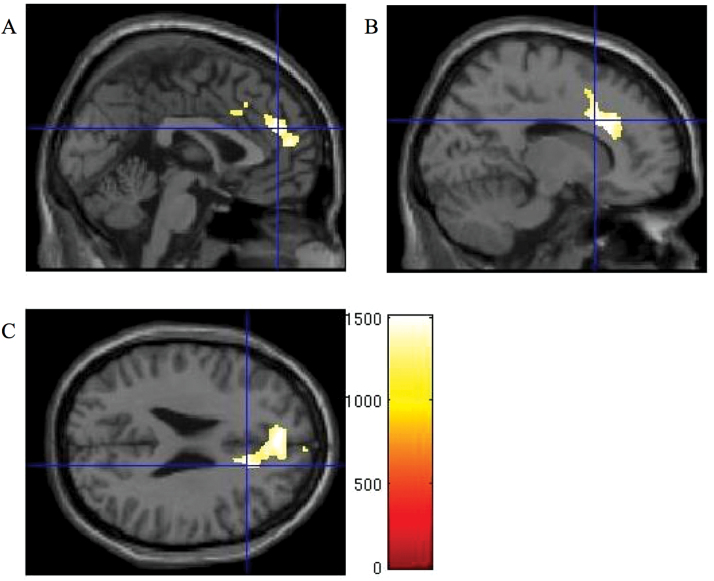
Fig. 2.
Association between lower baseline grey matter density and increased negative symptoms at follow-up. Threshold-free cluster enhancement (TFCE)-enhanced images displaying areas of lower baseline grey matter density associated with increased negative symptoms as indicated by higher scores on the Scale for the Assessment of Negative Symptoms (SANS) at follow-up. Threshold at P < .05 Family-Wise Error (FWE)-corrected. Crosshairs at (A) medial prefrontal cortex [0 45 24], (B) cingulate gyrus [15 15 30], (C) anterior cingulate [16 24 25]. Color bar shows TFCE-enhanced t-statistic. For a color version, see this figure online.
No significant association was observed between baseline grey matter density and IQ at follow-up or between baseline grey matter density and positive symptoms at follow-up.
Discussion
This study investigated the association between baseline grey and white matter density and functional outcome 2.4 to 12.9 years after identification as UHR for psychosis. Lower baseline grey matter density in large clusters in bilateral frontal and limbic regions and left cerebellar declive were associated with poorer functional outcome. These findings were independent of transition status or persistence of the at-risk mental state at follow-up (although in this regard we were only powered to detect a large effect and replication in larger samples is required). We did not observe an association between larger baseline grey matter density and poorer functional outcome, and there was no association between baseline white matter density (lower or higher) and functional outcome. Lower baseline grey matter density in middle and inferior frontal gyri was associated with absolute decline in functioning. When social and vocational functioning were investigated separately, poorer social functioning at follow-up was associated with lower baseline grey matter density in an area of left medial prefrontal cortex that overlapped with the medial prefrontal region observed for the association with functional outcome. Even though both social and vocational functional scores on the QLS showed a strong relationship with functioning as measured by the SOFAS, vocational functioning at follow-up was not associated with baseline grey matter density. Exploration of the association between baseline grey matter density and increased negative symptoms at follow-up revealed areas of lower density in bilateral frontal and limbic regions that partially overlapped with those observed in the association with functional outcome. No association was found between baseline grey matter density and IQ or positive symptoms at follow-up.
The findings regarding QLS social functioning do advocate for a key role for social dysfunction in the poor functional outcomes in this sample. Both the anterior and medial prefrontal regions have been shown to play an important role in emotion processing,49 and in social abilities such as self-referential processing,50,51 empathy, Theory of Mind and perspective taking.52–54 The brain areas observed in the association of baseline grey matter density and SOFAS scores at follow-up support this suggestion as recruitment of the anterior cingulate has been associated with performance of emotional tasks with cognitive demand and emotional recall/imagery.49 Lower baseline grey matter density of the declive was associated with poorer functional outcome but not with increased negative symptoms at follow-up, providing additional support for the suggestion that social dysfunction may underlie poorer functional outcome in the current sample. The cerebellum has been implicated in social-cognitive functioning in that it provides domain-general executive and semantic support,55 consistent with findings of higher cerebellar activity when executive resources are demanded to support mentalizing in contexts with a high level of abstraction.55 It needs noting that the current SOFAS findings represent an overall association between lower baseline grey matter density and poorer psychosocial outcome that includes social and role functioning. While many of the observed areas respond to social cognitive paradigms, they may also have involvement in so called “cold cognition” paradigms in which rational reasoning takes prominence over emotions.56,57
The observed partial overlap in brain areas that showed lower grey matter density at baseline in association with poorer functional outcome and increased negative symptoms illustrates the strong association between negative symptoms and functional outcome on both the conceptual and neural level and is consistent with the manifestation of cognitive deficits, affective disturbances, social isolation and school failure as suggested by the first dimension of vulnerability of the CASIS model.20
The brain areas that show lower grey matter density in association with poorer functional outcome as assessed by the SOFAS do not include the dorsolateral prefrontal cortex, suggesting that changes in baseline density of this brain area and associated cognitive impairments are not associated with poorer functioning in later years. This is consistent with our failure to observe an association between baseline grey matter density and estimated IQ at follow-up as an approximate measure of general cognitive ability. This is also in line with our earlier finding16 which showed that specific neurocognitive domains (verbal learning and memory, processing speed and attention, and verbal fluency), but not global cognitive impairment, predicted poor functional outcome. In contrast, the area observed in association with decline in GAF score in the period between baseline and follow-up does overlap with the dorsolateral prefrontal cortex. Lower densities observed in this association may, however, reflect worse symptomatic outcome rather than cognitive impairment (for further discussion see our recent paper on attenuated psychotic symptoms in this sample30) or general low functioning at follow-up.
The current findings support recent statements that treatment should not only be focused on those who will develop above threshold psychotic symptoms but also on those with poor functional outcome.23,25,58 Moreover and consistent with the current findings, treatments that specifically target social impairments, such as social cognitive remediation,59,60 could be a way of alleviating long-term social disability and distress. Lower grey matter densities as observed in the current study are commonly found in first-episode psychosis6,7,61 and chronic schizophrenia.2,62 Detection of these structural alterations as early as the at-risk mental state supports early intervention approaches in those with a prospective diagnosis of psychosis but also in those with a prospective diagnosis of nonpsychotic disorders that are associated with poor functional outcome. Evidence is emerging that interventions involving exercise or the administration of essential fatty acids alter brain structure63–67 and a recent meta-analysis has shown that these efforts, as well as administration of neuroleptics and cognitive behavioral therapy, may show efficacy in preventing or delaying transition.68 Cognitive enhancement therapy in patients with schizophrenia or schizoaffective disorder has been shown to not only improve neurocognitive functioning,69 but to also have neuroprotective properties as demonstrated by preservation and even increase of grey matter density in limbic areas.70 Early intervention using these techniques may preserve function.21 The next step should therefore involve implementation and evaluation of the efficacy of psychosocial interventions in individuals at UHR for psychosis to reverse the structural alterations that may lead to poorer psychosocial functioning.
A limitation of the current study is the long recruitment period spanning the years 1993–2006. During this period, changes in recruitment, treatment and operationalization of the UHR criteria took place at the PACE clinic. As these changes did not fully account for the decline in transition rates that has been observed over the years, and other factors may have had additional impact,71 they were not controlled for in the current study. A further limitation is the time to follow-up; this varied widely from 2.4 to 12.9 years after baseline assessment and may have had an impact on functional outcome. Participants scanned at the 3T scanner were recruited later, resulting in a shorter follow-up period for this sample than data acquired on the 1.5T scanner. To control for this, length of the follow-up period and field strength of scanner were specified as nuisance variables in all analyses. However, as there are likely to be a number of sample differences over the recruitment periods,71 including over the period where data were obtained at 1.5T, this makes any direct comparison between 1.5T and 3T data far less informative. Finally, only limited information was available regarding participants’ treatment at baseline and during the period between baseline and follow-up making it difficult to control for the potential impact of this factor in our analyses. Future research would do well to record these factors in more detail and control for them in subsequent analyses.
Taken together, the current findings provide novel evidence that those with the poorest functional outcomes have the lowest grey matter densities at identification as UHR, regardless of transition status or persistence of the at-risk mental state. These findings increase our understanding of psychotic illnesses and their causes and once replicated and validated may increase our ability to predict which UHR individuals are at greatest risk of having the poorest functional outcome. This may enable us to target interventions for this group accordingly. Moreover, the current findings provide scope for application in the wider context of mental health, by increasing our understanding of those who show poor functioning but never transition to frank psychotic illness, and may suggest a shift of focus to functioning rather than distinct diagnostic categories.
Funding
This work was supported by National Health and Medical Research Council of Australia (NHMRC) Project (grant number 209062) and Program Grants (grant numbers 350241, 566529), and by the Colonial Foundation. R.L.E.P.R. was supported by a Medical Research Council Research Grant (grant number MR/K013599/1). A.L. and V.L.C. were supported by NHMRC Early Career Fellowships (A.L.: fellowship number 1072593, V.L.C.: fellowship number 628880). A.R.Y. was supported by a NHMRC Senior Research Fellowship (fellowship number 566593). B.N. was supported by an NHMRC Clinical Career Developmental Award (award number 1027532). P.D.M. and C.P. were supported by NHMRC Senior Principal Research Fellowships (SPRF Fellowship IDs: 628386 and 1105825; P.D.M.: fellowship number 1060996; C.P.: fellowship number 628386). C.P. was furthermore supported by a NARSAD Distinguished Investigator Award (award number 18722). S.J.W. was supported by a NHMRC Clinical Career Developmental Award (award number 359223) and a NARSAD Young Investigator Award. The funding sources had no role in the study design, in the collection, analysis and interpretation of the data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Supplementary Material
Acknowledgment
All authors declare that they have no conflicts of interest in relation to the subject of the study.
References
- 1. Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32. [DOI] [PubMed] [Google Scholar]
- 2. Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. [DOI] [PubMed] [Google Scholar]
- 3. Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. [DOI] [PubMed] [Google Scholar]
- 4. De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, Vita A. Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr Pharm Des. 2012;18:486–494. [DOI] [PubMed] [Google Scholar]
- 5. Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. [DOI] [PubMed] [Google Scholar]
- 6. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. [DOI] [PubMed] [Google Scholar]
- 9. Dazzan P, Soulsby B, Mechelli A, et al. Volumetric abnormalities predating the onset of schizophrenia and affective psychoses: an MRI study in subjects at ultrahigh risk of psychosis. Schizophr Bull. 2012;38:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garner B, Pariante CM, Wood SJ, et al. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58:417–423. [DOI] [PubMed] [Google Scholar]
- 11. Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walterfang M, McGuire PK, Yung AR, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193:210–215. [DOI] [PubMed] [Google Scholar]
- 13. Pantelis C, Yücel M, Bora E, et al. Neurobiological markers of illness onset in psychosis and schizophrenia: the search for a moving target. Neuropsychol Rev. 2009;19:385–398. [DOI] [PubMed] [Google Scholar]
- 14. Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelson B, Yuen HP, Wood SJ, et al. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70:793–802. [DOI] [PubMed] [Google Scholar]
- 16. Lin A, Wood SJ, Nelson B, et al. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophr Res. 2011;132:1–7. [DOI] [PubMed] [Google Scholar]
- 17. Addington J, Cornblatt BA, Cadenhead KS, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salokangas RK, Nieman DH, Heinimaa M, et al. Psychosocial outcome in patients at clinical high risk of psychosis: a prospective follow-up. Soc Psychiatry Psychiatr Epidemiol. 2013;48:303–311. [DOI] [PubMed] [Google Scholar]
- 19. Brandizzi M, Valmaggia L, Byrne M, et al. Predictors of functional outcome in individuals at high clinical risk for psychosis at six years follow-up. J Psychiatr Res. 2015;65:115–123. [DOI] [PubMed] [Google Scholar]
- 20. Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29:633–651. [DOI] [PubMed] [Google Scholar]
- 21. Pantelis C, Wannan C, Bartholomeusz CF, Allott K, McGorry PD. Cognitive intervention in eary psychosis - preserving abilities versus remediating deficits. Curr Opin Behav Sci. 2015;4:63–72. [Google Scholar]
- 22. Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009;35:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin A, Wood SJ, Yung AR. Measuring psychosocial outcome is good. Curr Opin Psychiatry. 2013;26:138–143. [DOI] [PubMed] [Google Scholar]
- 24. Lin A, Nelson B, Yung AR. ‘At-risk’ for psychosis research: where are we heading? Epidemiol Psychiatr Sci. 2012;21:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yung AR, Nelson B, Thompson A, Wood SJ. The psychosis threshold in Ultra High Risk (prodromal) research: is it valid? Schizophr Res. 2010;120:1–6. [DOI] [PubMed] [Google Scholar]
- 26. Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry. 2015;172:249–258. [DOI] [PubMed] [Google Scholar]
- 27. Cotter J, Drake RJ, Bucci S, Firth J, Edge D, Yung AR. What drives poor functioning in the at-risk mental state? A systematic review. Schizophr Res. 2014;159:267–277. [DOI] [PubMed] [Google Scholar]
- 28. Carrion RE, McLaughlin D, Goldberg TE, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yung AR, Cotter J, Wood SJ, et al. Childhood maltreatment and transition to psychotic disorder independently predict long-term functioning in young people at ultra-high risk for psychosis. Psychol Med. 2015;45:3453–3465. [DOI] [PubMed] [Google Scholar]
- 30. Cropley VL, Lin A, Nelson B, et al. Baseline grey matter volume of non-transitioned “ultra high risk” for psychosis individuals with and without attenuated psychotic symptoms at long-term follow-up. Schizophr Res. 2016;173:152–158. [DOI] [PubMed] [Google Scholar]
- 31. Kambeitz-Ilankovic L, Meisenzahl EM, Cabral C, et al. Prediction of outcome in the psychosis prodrome using neuroanatomical pattern classification. Schizophr Res. 2016;173:159–165. [DOI] [PubMed] [Google Scholar]
- 32. Goldstein MJ. Further data concerning the relation between premorbid adjustment and paranoid symptomatology. Schizophr Bull. 1978;4:236–243. [DOI] [PubMed] [Google Scholar]
- 33. Sabb FW, van Erp TG, Hardt ME, et al. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr Res. 2010;116:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35. Allen P, Chaddock CA, Egerton A, et al. Functional outcome in people at high risk for psychosis predicted by thalamic glutamate levels and prefronto-striatal activation. Schizophr Bull. 2015;41:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faustman WO. Brief psychiatric rating scale. In: Maurish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Mahwah, NJ: Lawrence Erlbaum Associates; 1994:371–401. [Google Scholar]
- 38. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. [DOI] [PubMed] [Google Scholar]
- 39. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. [DOI] [PubMed] [Google Scholar]
- 40. Lin A, Yung AR, Nelson B, et al. Neurocognitive predictors of transition to psychosis: medium- to long-term findings from a sample at ultra-high risk for psychosis. Psychol Med. 2013;43:2349–2360. [DOI] [PubMed] [Google Scholar]
- 41. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;7:49–58. [PubMed] [Google Scholar]
- 42. Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149:1148–1156. [DOI] [PubMed] [Google Scholar]
- 43. Heinrichs DW, Hanlon TE, Carpenter WT., Jr The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–398. [DOI] [PubMed] [Google Scholar]
- 44. Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 45. Ward LC. Prediction of verbal, performance, and full scale IQs from seven subtests of the WAIS-R. J Clin Psychol. 1990;46:436–440. [DOI] [PubMed] [Google Scholar]
- 46. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 47. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 48. Shtasel DL, Gur RE, Gallacher F, Heimberg C, Cannon T, Gur RC. Phenomenology and functioning in first-episode schizophrenia. Schizophr Bull. 1992;18:449–462. [DOI] [PubMed] [Google Scholar]
- 49. Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. [DOI] [PubMed] [Google Scholar]
- 50. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117:1161–1168. [DOI] [PubMed] [Google Scholar]
- 52. Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Völlm BA, Taylor AN, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. [DOI] [PubMed] [Google Scholar]
- 54. Reniers RL, Völlm BA, Elliott R, Corcoran R. Empathy, ToM, and self-other differentiation: an fMRI study of internal states. Soc Neurosci. 2014;9:50–62. [DOI] [PubMed] [Google Scholar]
- 55. Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–572. [DOI] [PubMed] [Google Scholar]
- 56. Sylvester CY, Wager TD, Lacey SC, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. [DOI] [PubMed] [Google Scholar]
- 57. Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fusar-Poli P, Rocchetti M, Sardella A, et al. Disorder, not just state of risk: meta-analysis of functioning and quality of life in people at high risk of psychosis. Br J Psychiatry. 2015;207:198–206. [DOI] [PubMed] [Google Scholar]
- 59. Isaac C, Januel D. Neural correlates of cognitive improvements following cognitive remediation in schizophrenia: a systematic review of randomized trials. Socioaffect Neurosci Psychol. 2016;6:30054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peyroux E, Franck N. RC2S: a cognitive remediation program to improve social cognition in schizophrenia and related disorders. Front Hum Neurosci. 2014;8:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun D, Stuart GW, Jenkinson M, et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry. 2009;14:976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. [DOI] [PubMed] [Google Scholar]
- 63. Haast RA, Kiliaan AJ. Impact of fatty acids on brain circulation, structure and function. Prostaglandins Leukot Essent Fatty Acids. 2015;92:3–14. [DOI] [PubMed] [Google Scholar]
- 64. Kang JX, Gleason ED. Omega-3 Fatty acids and hippocampal neurogenesis in depression. CNS Neurol Disord Drug Targets. 2013;12:460–465. [DOI] [PubMed] [Google Scholar]
- 65. Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35:S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cell Neurosci. 2014;8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hennebelle M, Champeil-Potokar G, Lavialle M, Vancassel S, Denis I. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr Rev. 2014;72:99–112. [DOI] [PubMed] [Google Scholar]
- 68. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149:56–62. [DOI] [PubMed] [Google Scholar]
- 69. Eack SM, Greenwald DP, Hogarty SS, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eack SM, Hogarty GE, Cho RY, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. 2010;67:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hartmann JA, Yuen HP, McGorry PD, et al. Declining transition rates to psychotic disorder in “ultra-high risk” clients: Investigation of a dilution effect. Schizophr Res. 2016;170:130–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.