Abstract
Research suggests that childhood trauma is associated with cognitive alterations, but it is not known whether the cognitive alterations observed in patients with psychotic disorder, and their relatives, is trauma-related. Patients with a schizophrenia-spectrum diagnosis (n = 1119), siblings of patients (n = 1059) and healthy comparison subjects (HCS; n = 586) were interviewed 3 times over a period of 6 years. Repeated measures of IQ were analyzed as a function of childhood trauma and group, controlling for confounders. There were significant differences in the impact of childhood trauma on IQ across the 3 groups. Exposure in HCS was associated with a nearly 5-point reduction in IQ (−4.85; 95% confidence interval [CI]: −7.98 to −1.73, P = .002), a lesser reduction in siblings (−2.58; 95% CI: −4.69 to −0.46, P = .017) and no significant reduction in patients (−0.84; 95% CI: −2.78 to 1.10, P = .398). One-fourth of the sibling-control difference in IQ was reducible to childhood trauma, whereas for patients this was only 5%. Over the 6-year follow-up, those with trauma exposure showed significantly less learning effects with repeated cognitive assessments (b = 1.36, 95% CI: 0.80‒1.92, P < .001) than the nonexposed (b = 2.31, 95% CI: 1.92‒2.71, P < .001; P interaction = .001). Although childhood trauma impacts cognitive ability and learning in non-ill people at low and high genetic risk, its effect on the observed cognitive alterations in psychotic disorder may be minor. Twin and family studies on cognitive alterations in psychotic disorder need to take into account the differential impact of trauma on cognition across ill and non-ill, at risk groups.
Keywords: psychosis, trauma, cognition, genetics
Introduction
Childhood abuse and neglect has been found to impact many aspects of (social) cognition1–8; impairments that have been suggested to persist into adulthood.9–11
Both a history of childhood trauma and cognitive impairments are highly prevalent in patients with psychotic disorders.12,13 Cognitive alterations are highly relevant clinically, impacting on community functioning, which may be mediated particularly by alterations in social cognition.14 Although it has been suggested that childhood trauma may contribute to the neurocognitive impairments observed in patients with nonaffective psychotic disorders, findings have been inconsistent across cognitive domains, diagnostic, demographic, and genotype subgroups, and relatively small samples.15–21 Sideli et al22 reported an association between trauma and cognition in the controls, but not in patients, suggestive of a floor effect in patients, whose cognition already is substantially lower compared to controls. No studies to date had access to measures of cognition over time, in order to study not only any association between childhood trauma and cross-sectional measures of cognition, but also between childhood trauma and change of cognition over time.
In the current study, we attempted to study the link between childhood trauma and cognition taking into account the following issues: (1) adequate sample size to study main effects and test for moderation by group; (2) focus on schizophrenia spectrum diagnosis; (3) inclusion of endophenotypic measures of cognition in siblings of patients, in order to tease apart effects of aetiological and disease-related factors; and (4) repeated measures of the cognitive outcome over time.
We hypothesized (1) a main effect of childhood trauma on cross-sectional cognitive outcome, conform the findings by Sideli et al.,22 and (2) moderation by group (healthy comparison subjects [HCS], patients, siblings of patients). In addition, we hypothesized that those with exposure to childhood trauma would show less prominent learning effects with repeated cognitive assessments over time. Given evidence that (1) most of the overall effect of a schizophrenia diagnosis on cognitive performance is mediated through a single common factor, indicating that a generalized cognitive deficit is a core underlying feature,23 and (2) the effect of trauma on cognitive function is driven by deficits in general cognitive functioning17 the analyses focused on IQ as a summary measure of cognitive performance.
Methods
GROUP Study
Full details of the GROUP study have been presented elsewhere.24,25 In representative geographical areas in the Netherlands and Belgium, patients were identified through clinicians working in regional psychotic disorder services, whose caseload was screened for inclusion criteria. Subsequently, a group of patients presenting at these services either as out-patients or in-patients were recruited for the study. HCSs were selected through random mailings to addresses in the catchment areas of the cases. The GROUP study was not conducted in a geographically well-defined small area, as it in fact included the majority of mental health services in the Netherlands, and a substantial part of mental health services in Dutch-speaking Belgium. HCSs could not be representative in all aspects, as an exclusion criterion was absence of a family history of psychotic disorder. The goal was to collect a group of HCSs that (1) was collected from the same geographical area as the case in the relevant mental health service, (2) was sufficiently large to allow for chance variation, (3) was frequency-matched in age- and sex distribution to the siblings, and (4) had absence of family history of psychotic disorder.
Sample.
The full GROUP sample at baseline consisted of 1119 patients with nonaffective psychotic disorder, 1059 siblings of these patients, 920 parents of the patients, and 586 unrelated HCSs. Inclusion criteria were: (1) age range 16–50 years and (2) good command of Dutch language. For patients, an additional inclusion criterion was the presence of a clinical diagnosis of nonaffective psychotic disorder. HCSs status was confirmed by using the Family Interview for Genetic studies26 with the HCS as informant, to establish absence of first degree relatives with a psychotic disorder. Diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV) criteria,27 assessed with the Comprehensive Assessment of Symptoms and History (CASH) interview28 or Schedules for Clinical Assessment for Neuropsychiatry (SCAN 2.1).29 The majority of patients had a DSM-IV diagnosis of schizophrenia (DSM-IV 295.x; n = 940, 84%). In the sibling and HCS groups, there were respectively, 154 (14%) and 59 (10%) participants with a history of a common mental disorder at baseline, the majority of whom had a mood disorder (DSM-IV 296.x).
The study was approved by the standing ethics committee, and all the subjects gave written informed consent in accordance with the committee’s guidelines.
Follow-up.
Patients, HCSs, and siblings were eligible for follow-up. Of the 586 HCSs and 1059 siblings at baseline, 78% (n = 1275) were assessed at 3-year follow-up (HCSs: 79%, n = 460; siblings: 77%, n = 815) and 67% (n = 1104) at 6-year follow-up (HCSs: 67%, n = 394; siblings: 67%, n = 710). Of the 1119 patients at baseline, 811 (73%) participated at 3-year follow-up and 662 (59%) at 6-year follow-up. Ratings of CASH, SCAN, and Community Assessment of Psychic Experiences (CAPE)30 at follow-up reflected the period between baseline and first follow-up, and between first and second follow-up, respectively. Mean time to first follow-up was 3.3 years (SD = 0.4) and mean between first and second follow-up was 3.1 years (SD = 0.4).
Measures
CAPE.
The Community Assessment of Psychic Experiences (CAPE; www.cape42.homestead.com) was developed in order to rate self-reports of lifetime psychotic experiences.30 Items are modelled on patient experiences as contained in the PSE-9,31 schedules assessing negative symptoms such as the Scale for the Assessment of Negative Symptoms (SANS)32 and the Subjective Experience of Negative Symptoms (SENS),33 and scales assessing depressive symptoms such as the Calgary Depression Scale.34 Items are scored on a 4-point scale. In the current analyses, CAPE dimensions of frequency of positive experiences (20 items), negative experiences (14 items) and depressive experiences (8 items) were included (measured at baseline and 3-year follow-up), representing the person’s perceived psychosis load over the lifetime (at baseline) or in the past 3 years (follow-up). A total score representing the mean of all items was calculated for each dimension.
IQ.
At baseline and 3-year follow-up, IQ was estimated based on the 4-subtest version (Information, Block Design, Digit Symbol Coding and Arithmetic)35 of the Wechsler Adult Intelligence Scale (WAIS-III).36 At 6-year follow-up, IQ was estimated based on a short version of the WAIS-III short form: the Digit Symbol Coding subtest, uneven items of the Arithmetic subtest, uneven items of the Block Design subtest, every third item of the Information subtest.37 Change in IQ over the follow-up period (hereafter: delta IQ) was defined as change in IQ from baseline (T0) to T2 at 6 years or, in case T2 was missing, to T1 at 3 years.
Cannabis Use.
Substance use was assessed repeatedly at baseline, 3-year follow-up (use over interval baseline and 3-year follow-up) and 6-year follow-up (use over interval 3- and 6-year follow-up), using the Composite International Diagnostic Interview (CIDI).38 CIDI cannabis pattern of use during the lifetime period of heaviest use (hereafter: cannabis frequency use) was used in the analyses as the exposure variable, in agreement with previous work in this sample, and was scored as: none (0), less than weekly (1), weekly (2), and daily (3),39,40 dichotomised in the analyses as “no use” (0) vs “any use” (1).
Childhood Trauma.
Childhood trauma was assessed with the Dutch version of the Childhood Trauma Questionnaire (CTQ) 25-item Short Form,41 with items rated on a 5-point Likert scale (1 = never true to 5 = very often true). Emotional, physical, and general abuse, and emotional and physical neglect were assessed, 5 items covering each trauma type.41 The total trauma score represents the mean score of all 25 items. Childhood trauma was analyzed both as a continuous variable and as a dichotomous variable, around a cut-off representing the control group 80th percentile, conform previous analyses in this sample.42
Statistical Analyses
GROUP database version 5.0 was used for all analyses. In order to examine cross-sectional associations between childhood trauma and IQ, and differences herein as a function of group (controls, siblings, patients), random intercept multilevel regression models (given clustering of individuals within families as well as clustering of repeated measures within subjects) with IQ as dependent variable were fitted using the MIXED routine in the Stata program, version 14.43 Independent variables were CTQ score, group and the interaction between CTQ and group. Analyses were corrected a priori for age, sex, ethnic group (white European vs other), educational level (continuous variable ranging from 0 [no education], 3–5 [school diploma] to 8 [university degree]), CAPE total score and binary cannabis use (hereafter: fully adjusted model).
In order to examine associations between childhood trauma and change in IQ, and differences in this association across group, random intercept multilevel regression models with IQ as dependent variable were fitted with CTQ, time (baseline, 3- and 6-year follow-up), group and the CTQ × time × group interaction. Analyses were corrected a priori for age, sex, ethnic group, educational level, CAPE total score, and binary cannabis use (hereafter: fully adjusted model).
Interactions between childhood trauma and group were fitted in the models of IQ and delta IQ, yielding 2 interaction terms (1 for siblings and 1 for patients); in the case of significant interaction, stratified values for HCSs, siblings, and patients were calculated from the model containing the interaction using the Stata MARGINS routine. Marginal effects and post-estimation contrasts were similarly calculated with the Stata MARGINS routine.
Associations are expressed as regression coefficients (b; change in y with one unit increase in x) from the multilevel random regression model.
Results
Sample Characteristics
Sample characteristics are displayed in table 1. The 3 groups were comparable in age, patients more often were of male sex. As reported before, siblings had values that were intermediate to HCSs and patients with regard to childhood trauma,44 cannabis use,45 and IQ.46 All groups displayed increases in IQ over the 6-year follow-up.
Table 1.
Sample Demographics and Measures of Childhood Trauma and IQ at Baseline (With the Exception of Delta IQ)
| Age | Educationa | CTQ (Continuous) | Urbanicity Birthb | IQ | Delta IQc | % CTQ Binary Exposured | % Cannabis Usee | % Female | % Ethnic Minority | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCS | Mean | 30.42 | 2.92 | 1.34 | 2.64 | 109.82 | 4.32 | 0.19 | 0.28 | 0.54 | 0.10 |
| SD | 10.58 | 1.28 | 0.36 | 1.69 | 15.00 | 10.08 | |||||
| N | 584 | 583 | 492 | 549 | 573 | 428 | 492 | 579 | 586 | 586 | |
| Sibling | Mean | 27.83 | 2.56 | 1.41 | 2.66 | 102.87 | 6.44 | 0.25 | 0.38 | 0.54 | 0.17 |
| SD | 8.27 | 1.47 | 0.41 | 1.67 | 15.49 | 9.52 | |||||
| N | 1059 | 1039 | 816 | 969 | 1012 | 761 | 816 | 1047 | 1059 | 1059 | |
| Patient | Mean | 27.57 | 1.95 | 1.61 | 2.76 | 96.08 | 3.72 | 0.44 | 0.63 | 0.24 | 0.23 |
| SD | 7.95 | 1.36 | 0.50 | 1.68 | 15.32 | 10.20 | |||||
| N | 1107 | 1086 | 755 | 981 | 1006 | 708 | 755 | 1094 | 1119 | 1119 | |
| Total | Mean | 28.28 | 2.40 | 1.47 | 2.70 | 101.77 | 4.95 | 0.31 | 0.46 | 0.42 | 0.18 |
| SD | 8.76 | 1.44 | 0.45 | 1.68 | 16.18 | 9.98 | |||||
| N | 2750 | 2708 | 2063 | 2499 | 2591 | 1897 | 2063 | 2720 | 2764 | 2764 | |
CTQ, childhood trauma questionnaire; HCS, healthy comparison subjects.
aEducation (Verhage): range 0 (no education), 3–5 (school diploma), and 8 (university degree).
bUrbanicity: 1 ≤ 500/km2; 2 = 500–1000/km2; 3 = 1000–1500/km2; 4 = 1500–2500/km2; 5 = 2500+/km2.
cDefined as change from baseline (T0) to T2 at 6 years or, in case T2 was missing, to T1 at 3 years.
dExposure defined as CTQ value > 80th percentile of control CTQ value.
eDefined as CIDI cannabis pattern of use during the lifetime period of heaviest use, dichotomised as “no use” (0) vs “any use” (1).
Childhood Trauma and IQ
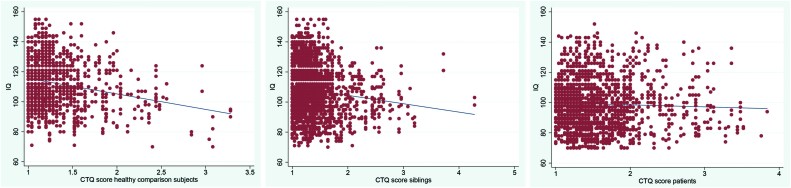
Table 2 displays IQ values at the 3 time points as a function of binary trauma exposure. Plotting IQ and continuous childhood trauma in the 3 groups suggested a negative association in HCSs, a weaker negative association in siblings and no associations in patients (figure 1). This was confirmed in the multilevel random regression analyses, which revealed a significant interaction between continuous childhood trauma and group in the model of IQ (unadjusted: siblings P = .027, patients P < .001; adjusted for age, sex, education and ethnic group: siblings P = .007, patients P < .001; adjusted for age, sex, education, ethnic group, CAPE total score and cannabis use: siblings P = .005, patients P < .001). The association between IQ and childhood trauma, stratified by group, calculated from the fully adjusted model was −8.09 (95% CI: −11.50 to −4.68, P < .001; HCSs) −2.27 (−4.57 to 0.02, P = .052; siblings) and −0.18 (−2.17 to 1.82, P = .863; patients). Using the binary trauma variable in the fully adjusted model, stratified effect sizes for trauma exposure across the 3 groups were: −4.85 for HCSs (95% CI: −7.98 to −1.73, P < .001); −2.58 for siblings (95% CI: −4.69 to −0.46, P = .017) and −0.84 for patients (95% CI: −2.78 to 1.10, P = .398).
Table 2.
IQ as a Function of Trauma Exposure at Baseline, 3-Year and 6-Year Follow-up
| Baseline | 3-Year Follow-up | 6-Year Follow-up | Total | ||
|---|---|---|---|---|---|
| No trauma exposurea | |||||
| Healthy comparison subject | Mean | 111.20 | 113.10 | 116.76 | 113.42 |
| SD | 14.87 | 15.89 | 16.89 | 15.95 | |
| N | 395 | 222 | 284 | 901 | |
| Sibling | Mean | 105.44 | 109.17 | 113.92 | 109.14 |
| SD | 15.53 | 17.03 | 17.51 | 16.96 | |
| N | 591 | 425 | 455 | 1,471 | |
| Patient | Mean | 97.47 | 99.76 | 102.96 | 99.84 |
| SD | 15.00 | 16.26 | 17.34 | 16.27 | |
| N | 393 | 295 | 305 | 993 | |
| Total | Mean | 104.82 | 107.15 | 111.49 | 107.54 |
| SD | 16.06 | 17.32 | 18.17 | 17.31 | |
| N | 1,379 | 942 | 1,044 | 3,365 | |
| Trauma exposurea | |||||
| Healthy comparison subject | Mean | 105.52 | 105.30 | 106.85 | 105.88 |
| SD | 14.16 | 16.49 | 16.62 | 15.50 | |
| N | 90 | 53 | 65 | 208 | |
| Sibling | Mean | 100.61 | 102.84 | 107.22 | 103.26 |
| SD | 14.63 | 14.14 | 17.46 | 15.67 | |
| N | 197 | 120 | 144 | 461 | |
| Patient | Mean | 96.84 | 97.96 | 99.98 | 98.06 |
| SD | 15.90 | 16.54 | 16.42 | 16.26 | |
| N | 305 | 206 | 205 | 716 | |
| Total | Mean | 99.42 | 100.53 | 103.58 | 100.96 |
| SD | 15.52 | 16.04 | 17.15 | 16.25 | |
| N | 592 | 379 | 414 | 1,385 | |
aDefined dichotomously as CTQ value > 80th percentile of control CTQ value.
Fig. 1.
Scatterplots with linear regression line of childhood trauma questionnaire (CTQ) score one the one hand, and IQ on the other in healthy comparison subjects, siblings, and patients.
In the fully adjusted model, the association between group and IQ, before entering continuous childhood trauma in the model, was −3.07 for siblings (95% CI: −4.57 to −1.57) and −9.66 for patients (95% CI: −11.24 to −8.07); after adding continuous childhood trauma to the model, this became −2.33 for siblings (95% CI: −3.97 to −0.69) and −9.15 for patients (95% CI: −10.93 to −7.38). Thus, in siblings, 24% of the sibling-HCS difference in IQ was thus reducible to childhood trauma, whereas for patients this was 5%.
Childhood Trauma and Delta IQ
Table 3 shows delta IQ for the 3 groups as a function of binary childhood trauma exposure. The results of the fully adjusted model with the 3-way interaction between time, binary childhood trauma and group revealed a marginal effect of time (linear effect b = 2.03, 95% CI: 1.67‒2.39, P < .001) which differed as a function of binary childhood trauma (P interaction = .0013), exposed individuals showing less of an increase in IQ over time (b = 1.36, 95% CI: 0.80‒1.92, P < .001) than the nonexposed (b = 2.31, 95% CI: 1.92‒2.71, P < .001). The 3-way interaction between time, childhood trauma and group was not significant (siblings: P = .393; patients: P = .820), indicating that the moderating effect of childhood trauma on the effect of time on IQ did not differ between HCSs, siblings, and patients.
Table 3.
Change in IQ (Positive Value Indicates Increase Over Time), Defined as Change From Baseline (T0) to T2 at 6 Years or, in Case T2 was Missing, to T1 at 3 Years, as a Function of Group and Trauma Exposure
| Mean Delta IQ | SD | N | |
|---|---|---|---|
| No trauma exposure | |||
| Healthy comparison subject | 4.76 | 10.33 | 335 |
| Sibling | 6.86 | 9.51 | 514 |
| Patient | 4.34 | 10.19 | 344 |
| Total | 5.54 | 10.00 | 1,193 |
| Trauma exposure | |||
| Healthy comparison subject | 2.59 | 9.00 | 69 |
| Sibling | 5.09 | 9.71 | 161 |
| Patient | 2.33 | 9.51 | 245 |
| Total | 3.31 | 9.57 | 475 |
Discussion
We found that childhood trauma impacted IQ in groups whose cognitive ability was not already compromised (HCSs) or only partially compromised (siblings of patients). Around a fourth of the sibling-HCS difference was attributable to childhood trauma, compared to only 5% of the case-healthy comparison difference. In addition, childhood trauma impacted on the course of IQ over time, in the sense of showing less learning effects of repeated cognitive assessments over time, regardless of group. The results suggest that childhood trauma impacts cognitive abilities and impedes learning; however, its impact on the observed cognitive alterations in psychotic disorder may be relatively small given smaller impact of childhood trauma on cognitive ability with progressively greater genetic risk for psychotic disorder.
Previous work indicates that the cognitive alterations observed in psychotic disorder are attributable to genetic liability underlying psychotic outcomes.47 However, the findings suggest that the observation of familial clustering of cognitive alterations in patients with psychotic disorder and their siblings is confounded by the fact that the source of these alterations is different across groups. In siblings, 24% of the alterations of cognition respective to the healthy comparison group was reducible to childhood trauma, whereas in patients this was only 5%. This suggests that sibling and twin studies on cognition of schizophrenia focusing on genetic factors need to take into account the influence of childhood trauma for accurate results.
Given the large sample size, diagnostic homogeneity, adjustment for confounders, and the availability of repeated cognitive assessments over time, it is unlikely that the findings are due to chance or confounding. Several previous studies reported absence of association between childhood trauma main effects and cognitive outcomes in patients16,19,21,22 and there is a body of work showing association between childhood trauma and cognitive outcome in non-ill groups.1–11 Sideli et al.22 reported findings that resembled the current report, in that an association between childhood trauma and cognitive outcomes was found in the control, but not the patient group. One likely explanation for the absence of an association between trauma and cognition in the patient group is a floor effect—ie, the impact of trauma is “trumped” by other sources that impact on cognitive alterations, eg, genetic effects,47 the effects of current adversity,48 or altered motivation to engage in neuropsychological testing.49 Although childhood trauma did not show main effects, it can still interact with any of these factors to impact cognition; future work is required to investigate this issue. The fact that childhood trauma impacted learning, regardless of group, does suggest that childhood trauma is not cognition-neutral in the patient group.
There is evidence that, contrary to the current findings on IQ—considered an indicator of altered neurodevelopment in psychotic disorder50—that psychopathology, particularly psychotic symptoms, is associated with childhood trauma in both patients and individuals in the general population.12,51 Taken together, the results suggest that different aetiological influences may impact different psychopathological and neurodevelopmental domains in psychotic disorder. The focus of treatment related to exposure to childhood trauma may be more in the realm of treating the psychological effects of trauma rather than cognitive remediation of trauma-related cognitive alterations.52–54
Childhood trauma was associated with a decrease in the learning effect associated with repeated cognitive assessments. This impact did not differ between the different groups, including the patient group, suggesting that exposure to childhood trauma may have clinical relevance by moderating learning outcomes in patients with psychotic disorder. Although the effect was small, it may nevertheless be relevant in settings of rehabilitation and the application of cognitive remediation therapy.
Methodological Issues
Although the IQ assessment at second follow-up differed slightly, this cannot have impacted differential impact of childhood trauma across groups. Illness duration may be a factor in the patient group, potentially obscuring associations with childhood trauma. However, when modelling IQ in the patient group as a function of childhood trauma and the interaction with illness duration (years between baseline assessment and onset of first psychotic symptom), no interaction was present (P = .110), suggesting illness duration is not relevant for the association between IQ and childhood trauma. This finding is in agreement with the literature showing cognitive alterations in psychotic disorder are stable and not progressive.55
Funding
The infrastructure for the GROUP study is funded through the Geestkracht programme of the Dutch Health Research Council (ZON-MW, grant number 10-000-1001), and matching funds from participating industry (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag), universities and mental health care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Center and the mental health institutions: GGZ Ingeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Centre, GGZ Noord Holland Noord. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGZ Eindhoven en De Kempen, GGZ Breburg, GGZ Oost-Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan, Zuyderland, MetGGZ, Riagg-Virenze Maastricht, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem. Groningen: University Medical Center Groningen and the mental health institutions: Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht and Parnassia psycho-medical center The Hague. Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal, Riagg Amersfoort and Delta). This study was funded in part by the European Community’s Seventh Framework Program under grant agreement No. HEALTH-F2-2009–241909 (Project EU-GEI).
Acknowledgments
We are grateful for the generosity, in terms of time and effort, shown by the patients, their families and the HCSs, and for the continuing collaboration with all the researchers who make the GROUP project possible. Furthermore, we would like to thank all research personnel involved in the GROUP project, in particular: Joyce van Baaren, Erwin Veermans, Ger Driessen, Truda Driesen, Karin Pos, Erna van ‘t Hag, Jessica de Nijs, Atiqul Islam, Wendy Beuken and Debora Op ‘t Eijnde.
References
- 1. Cicchetti D, Rogosch FA, Maughan A, Toth SL, Bruce J. False belief understanding in maltreated children. Dev Psychopathol. 2003;15:1067–1091. [DOI] [PubMed] [Google Scholar]
- 2. Pears KC, Fisher PA. Emotion understanding and theory of mind among maltreated children in foster care: evidence of deficits. Dev Psychopathol. 2005;17:47–65. [DOI] [PubMed] [Google Scholar]
- 3. Colvert E, Rutter M, Kreppner J, et al. Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation? Findings from the English and Romanian adoptees study. J Abnorm Child Psychol. 2008;36:1057–1068. [DOI] [PubMed] [Google Scholar]
- 4. Fonagy P, Gergely G, Target M. The parent-infant dyad and the construction of the subjective self. J Child Psychol Psychiatry. 2007;48:288–328. [DOI] [PubMed] [Google Scholar]
- 5. Hoffman-Plotkin D, Twentyman CT. A multimodal assessment of behavioral and cognitive deficits in abused and neglected preschoolers. Child Dev. 1984;55:794–802. [PubMed] [Google Scholar]
- 6. Perna RB, Kiefner M. Long-term cognitive sequelae: abused children without PTSD. Appl Neuropsychol Child. 2013;2:1–5. [DOI] [PubMed] [Google Scholar]
- 7. Bucker J, Kapczinski F, Post R, Cereser KM, Szobot C, Yatham LN, Kapczinski NS, Kauer-Sant’Anna M. Cognitive impairment in school-aged children with early trauma. Compr Psychiatry. 2012;53:758–764. [DOI] [PubMed] [Google Scholar]
- 8. De Bellis MD, Hooper SR, Woolley DP, Shenk CE. Demographic, maltreatment, and neurobiological correlates of PTSD symptoms in children and adolescents. J Pediatr Psychol. 2010;35:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Majer M, Nater UM, Lin JM, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 2010;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res. 2012;46:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Philip NS, Sweet LH, Tyrka AR, et al. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging Behav. 2016;10:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195:475–482. [DOI] [PubMed] [Google Scholar]
- 14. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 15. Lysaker PH, Meyer P, Evans JD, Marks KA. Neurocognitive and symptom correlates of self-reported childhood sexual abuse in schizophrenia spectrum disorders. Ann Clin Psychiatry. 2001;13:89–92. [DOI] [PubMed] [Google Scholar]
- 16. Aas M, Dazzan P, Fisher HL, et al. Childhood trauma and cognitive function in first-episode affective and non-affective psychosis. Schizophr Res. 2011;129:12–19. [DOI] [PubMed] [Google Scholar]
- 17. Aas M, Steen NE, Agartz I, et al. Is cognitive impairment following early life stress in severe mental disorders based on specific or general cognitive functioning? Psychiatry Res. 2012;198:495–500. [DOI] [PubMed] [Google Scholar]
- 18. Shannon C, Douse K, McCusker C, Feeney L, Barrett S, Mulholland C. The association between childhood trauma and memory functioning in schizophrenia. Schizophr Bull. 2011;37:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCabe KL, Maloney EA, Stain HJ, Loughland CM, Carr VJ. Relationship between childhood adversity and clinical and cognitive features in schizophrenia. J Psychiatr Res. 2012;46:600–607. [DOI] [PubMed] [Google Scholar]
- 20. Schenkel LS, Spaulding WD, DiLillo D, Silverstein SM. Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr Res. 2005;76:273–286. [DOI] [PubMed] [Google Scholar]
- 21. Green MJ, Chia TY, Cairns MJ, et al. , Australian Schizophrenia Research B. Catechol-O-methyltransferase (COMT) genotype moderates the effects of childhood trauma on cognition and symptoms in schizophrenia. J Psychiatr Res. 2014;49:43–50. [DOI] [PubMed] [Google Scholar]
- 22. Sideli L, Fisher HL, Russo M, et al. Failure to find association between childhood abuse and cognition in first-episode psychosis patients. Eur Psychiatry. 2014;29:32–35. [DOI] [PubMed] [Google Scholar]
- 23. Dickinson D, Iannone VN, Wilk CM, Gold JM. General and specific cognitive deficits in schizophrenia. Biol Psychiatry. 2004;55:826–833. [DOI] [PubMed] [Google Scholar]
- 24. Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L. Genetic Risk and Outcome of Psychosis (GROUP), a multi site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res. 2012;21:205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. G.R.O.U.P. Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry. 2011;68:138–147. [DOI] [PubMed] [Google Scholar]
- 26. NIMH Genetics Initiative. Family Interview for Genetic Studies (FIGS). Rockville, MD: National Institute of Mental Health; 1992. [Google Scholar]
- 27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev. ed. Washington, DC: APA; 2000. [Google Scholar]
- 28. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. [DOI] [PubMed] [Google Scholar]
- 29. Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. [DOI] [PubMed] [Google Scholar]
- 30. Konings M, Bak M, Hanssen M, van Os J, Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand. 2006;114:55–61. [DOI] [PubMed] [Google Scholar]
- 31. Wing JK, Cooper JE, Sartorius N. The Measurement and Classification of Psychiatric Symptoms. London: Cambridge University Press; 1974. [Google Scholar]
- 32. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. [DOI] [PubMed] [Google Scholar]
- 33. Selten JP, Sijben NE, van den Bosch RJ, Omloo Visser J, Warmerdam H. The subjective experience of negative symptoms: a self-rating scale. Compr Psychiatry. 1993;34:192–197. [DOI] [PubMed] [Google Scholar]
- 34. Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;22:39–44. [PubMed] [Google Scholar]
- 35. Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46:209–215. [DOI] [PubMed] [Google Scholar]
- 36. Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale (3rd ed.) Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 37. Velthorst E, Levine SZ, Henquet C, de Haan L, van Os J, Myin-Germeys I, Reichenberg A. To cut a short test even shorter: reliability and validity of a brief assessment of intellectual ability in schizophrenia—a control-case family study. Cogn Neuropsychiatry. 2013;18:574–593. [DOI] [PubMed] [Google Scholar]
- 38. World Health Organisation. Composite International Diagnostic Interview (CIDI) Version 1.0. Geneva, Switzerland: World Health Organisation; 1990. [Google Scholar]
- 39. van Winkel R, Genetic Risk and Outcome of Psychosis (GROUP) Investigators Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry. 2011;68:148–157. [DOI] [PubMed] [Google Scholar]
- 40. van Winkel R, van Beveren JM; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. . AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology. 2011;36:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–348. [DOI] [PubMed] [Google Scholar]
- 42. van Nierop M, Janssens M; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. . Evidence that transition from health to psychotic disorder can be traced to semi-ubiquitous environmental effects operating against background genetic risk. PLoS One. 2013;8:e76690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. StataCorp. STATA Statistical Software: Release 14. TX: College Station; 2015. [Google Scholar]
- 44. Heins M, Simons C, Lataster T, et al. Childhood trauma and psychosis: a case-control and case-sibling comparison across different levels of genetic liability, psychopathology, and type of trauma. Am J Psychiatry. 2011;168:1286–1294. [DOI] [PubMed] [Google Scholar]
- 45. Genetic Risk and Outcome in Psychosis Investigators. Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry. 2011;68:138–147. [DOI] [PubMed] [Google Scholar]
- 46. Verweij KH, Derks EM; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. . The association between intelligence scores and family history of psychiatric disorder in schizophrenia patients, their siblings and healthy controls. PLoS One. 2013;8:e77215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toulopoulou T, Picchioni M, Rijsdijk F, et al. Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch Gen Psychiatry. 2007;64:1348–1355. [DOI] [PubMed] [Google Scholar]
- 48. Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341:976–980. [DOI] [PubMed] [Google Scholar]
- 49. Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry. 2014;71:1058–1065. [DOI] [PubMed] [Google Scholar]
- 50. Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. [DOI] [PubMed] [Google Scholar]
- 51. van Dam DS, van Nierop M, Viechtbauer W, et al. Childhood abuse and neglect in relation to the presence and persistence of psychotic and depressive symptomatology. Psychol Med. 2015;45:1363–1377. [DOI] [PubMed] [Google Scholar]
- 52. Mueser KT, Goodman LB, Trumbetta SL, et al. Trauma and posttraumatic stress disorder in severe mental illness. J Consult Clin Psychol. 1998;66:493–499. [DOI] [PubMed] [Google Scholar]
- 53. Frueh BC, Grubaugh AL, Cusack KJ, Elhai JD. Disseminating evidence-based practices for adults with PTSD and severe mental illness in public-sector mental health agencies. Behav Modif. 2009;33:66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frueh BC, Grubaugh AL, Cusack KJ, Kimble MO, Elhai JD, Knapp RG. Exposure-based cognitive-behavioral treatment of PTSD in adults with schizophrenia or schizoaffective disorder: a pilot study. J Anxiety Disord. 2009;23:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]