Abstract
Background:
Schizophrenia (SZ) is often characterized by cognitive and intellectual impairment. However, there is much heterogeneity across individuals, suggesting different trajectories of the illness. Recent findings have shown brain volume differences across subgroups of individuals with psychosis (SZ and bipolar disorder), such that those with intellectual and cognitive impairments presented evidence of early cerebral disruption, while those with cognitive but not intellectual impairments showed evidence of progressive brain abnormalities. Our aim was to investigate the relations of cognition and intellectual functioning with brain structure abnormalities in a sample of SZ compared to unaffected individuals.
Methods:
92 individuals with SZ and 94 healthy controls part of the Northwestern University Schizophrenia Data and Software Tool (NUSDAST) underwent neuropsychological assessment and structural magnetic resonance imaging (MRI). Individuals with SZ were divided into subgroups according their estimated premorbid crystallized intellectual (ePMC-IQ) and cognitive performance. Brain volumes differences were investigated across groups.
Results:
SZ with ePMC-IQ and cognitive impairments had reduced total brain volume (TBV), intracranial volume (ICV), TBV corrected for ICV, and cortical gray matter volume, as well as reduced cortical thickness, and insula volumes. SZ with cognitive impairment but intact ePMC-IQ showed only reduced cortical gray matter volume and cortical thickness.
Conclusions:
These data provide additional evidence for heterogeneity in SZ. Impairments in cognition associated with reduced ePMC-IQ were related to evidence of broad brain structural alterations, including suggestion of early cerebral disruption. In contrast, impaired cognitive functioning in the context of more intact intellectual functioning was associated with cortical alterations that may reflect neurodegeneration.
Keywords: cognition, IQ, MRI, heterogeneity
Introduction
Cognitive impairments are a key component of schizophrenia (SZ). Individuals diagnosed with SZ show significant deficits across a number of different cognitive domains, such as sustained attention, executive function, working memory, and episodic memory. Further, poor cognitive performance is consistently associated with poorer functional outcomes regardless of age, gender, or illness chronicity.1 Previous studies have suggested some potential common mechanisms that could influence performance across a number of putatively different cognitive domains, such as impairments in structure, function and connectivity of prefrontal, parietal, anterior cingulate, and anterior insula.2 However, while some individuals with SZ show altered cognitive function in the context of reduced premorbid crystallized intellectual functioning (ePMC-IQ), others seem to have more intact ePMC-IQ. This variability seems to suggest potential heterogeneity of neuropathology.3 As such, the goal of the current study was to extend prior work on cognitive function and IQ in SZ by examining the gray and white matter alterations associated with impaired cognition vs impaired ePMC-IQ.
Individuals with SZ, in addition to the cognitive deficits, show on average a medium-sized deficit in ePMC-IQ compared to healthy controls (HC).4,5 Not surprisingly, prior research has shown that individuals with SZ who have preserved premorbid crystallized IQ perform better in overall cognitive scores compared to individuals with either a deterioration in crystallized IQ (eg, normal premorbid IQ, but reduced current IQ) or compromised IQ (eg, both premorbid and current low IQ),6 suggesting that the course of intellectual functioning could play a role in the illness progression. One hypothesis has been that reduced ePMC-IQ could be thought as a marker of early neurodevelopmental abnormality. According to the neurodevelopmental hypothesis, the etiology of SZ is influenced by an interaction of genetic and environmental factors that were present before the known onset of illness, affecting the brain before it approaches its adult anatomical state.7 There is considerable evidence for pathological risk factors that influence early neurodevelopment in SZ, such as both pre- and perinatal (infection, placental pathology, low birth weight), and premorbid (urban environment, childhood trauma, ethnic minority, migrant status) risk factors.8 Individuals with SZ are more likely to have experienced a combination of these early events during development,9 which in turn could influence brain maturation.
There is strong evidence of reduced brain volume in SZ, including total brain, intracranial and gray matter,10 and these reduced volumes have been associated with impaired cognitive performance.11 Previous work by Woodward and Heckers3 showed differences in brain structure among subgroups of individuals with SZ and psychotic bipolar disorder (BD) divided according to ePMC-IQ and current neuropsychological functioning. Cognitively impaired individuals with below-average ePMC-IQ presented evidence of early cerebral hypoplasia demonstrated by reduced intracranial volume (ICV). Conversely, both patients with average ePMC-IQ plus current cognitive deficits, and neuropsychologically intact individuals showed reduced TBV, which is consistent later cerebral dysmaturation or neurodegeneration. These data provided intriguing evidence about the potential differential relationships of ePMC-IQ vs cognitive function to early neural developmental alterations vs potentially later cerebral changes. However, the Woodward and Heckers3 study included individuals with diagnoses of both SZ and BD, with a higher percentage of individuals with BD in the neuropsychological intact (32%) vs impaired (19%) groups. Further, the neuropsychologically normal individuals were significantly younger than the impaired individuals. These issues raise the possibility that some of the effects of neuropsychological function could have reflected either diagnosis or age-related effects.
Therefore, the current study was designed to investigate the relations between cognition and ePMC-IQ with brain structure abnormalities in a sample of individuals with only diagnoses of SZ compared to HC with similar ages across subgroups. Based on the prior work of Woodward and Heckers,3 we hypothesized that participants with SZ presenting both reduced ePMC-IQ and cognitive deficits would have smaller whole brain and ICVs, as well as reduced gray matter volumes compared to HC. In contrast, we hypothesized that individuals with SZ who had impaired cognition in the context of intact ePMC-IQ would show reduced TBV, but only when corrected for ICV. In addition, we were interested in examining whether there might be alterations in cortical thickness or alterations in specific regions related to cognitive performance in SZ in past research, such as hippocampus, anterior cingulate cortex (ACC), dorsolateral prefrontal cortex, and anterior insula.11–14
Methods
Participants
Participants were part of the Northwestern University Schizophrenia Data and Software Tool (NUSDAST), and the data used in preparation of this article were obtained from XNAT Central (https://central.xnat.org/) and SchizConnect database (http://schizconnect.org). Data collection and sharing was approved by the local institutional review board and was funded by NIMH grant 1R01 MH084803 and by NIMH cooperative agreement 1U01 MH097435, and its procedures were extensively described in previous publication.15 For the purpose of this study, we considered only participants with SZ and HC at baseline (some participants had follow-up data) who had available cognitive data.
Ninety-four individuals with SZ and 94 unaffected participants were included. The inclusion criteria were: (1) having a diagnosis of SZ or being a healthy control; (2) completing the neuropsychological battery described below; and (3) participating in a magnetic resonance imaging (MRI) scanning session that included acquisition of a T1-weighted structural scan. Exclusion criteria were: (1) met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for intellectual disability (mild or greater in severity); (2) had a clinically unstable or severe medical disorder, or a medical disorder that confounded the assessment of psychiatric diagnosis or rendered research participation dangerous; (3) had a head injury (past or present) with documented neurological sequelae or loss of consciousness; and (4) met DSM-IV criteria for current substance abuse or dependence (ie, during the month preceding assessment). Informed consent was obtained from each participant after a complete description of the study was given.
Participants with SZ were recruited from local inpatient and outpatient treatment facilities, and were stabilized on antipsychotic medication for at least 2 weeks before participating in the study. Severity of psychotic symptoms was assessed in patients using Scale for the Assessment of Positive Symptoms (SAPS) and Scale for the Assessment of Negative Symptoms (SANS). HC were recruited using local advertisements in the same community, and were required to have no lifetime history of Axis I psychotic or major mood disorders (eg, major depressive disorder and BD) and no first-degree relatives with a psychotic disorder.
Neuropsychological Assessment
Participants completed a neuropsychological battery to evaluate several of the cognitive domains impaired in SZ. The assessment was comprised of the subtests Logical Memory, Family Pictures, Letter-Number Sequencing, Spatial Span, and Digit Span from the Wechsler Memory Scale (WMS-III). The scaled scores were converted into z scores that were subsequently summed to create a composite score of overall cognitive functioning (COG). For an estimate of crystallized knowledge and premorbid crystallized intellectual functioning (ePMC-IQ), we used the Vocabulary subtest from Wechsler Adult Intelligence Scale (WAIS-III).
Participants with SZ were then divided into groups based on their ePMC-IQ and current cognitive functioning. This classification was made considering the patients’ performance above or below the 10th percentile of the healthy control distribution for either the composite cognitive score (COG) or the ePMC-IQ measure, as proposed by Woodward and Heckers.3 The resulted categories were the following: (1) IQ+/COG+: normal ePMC-IQ and not cognitively impaired (n = 25); (2) IQ+/COG−: normal ePMC-IQ and cognitively impaired (n = 31); (3) IQ−/COG−: lower ePMC-IQ and cognitively impaired (n = 36); and (4) IQ−/COG+: lower ePMC-IQ and not cognitively impaired (n = 2). Because the last group had a very small sample size, it was not included in subsequent analyses.
Neuroimaging
NUSDAST provided T1-weighted scans that were acquired on a 1.5 T Vision scanner (Siemens Medical Systems) and collected using an MPRAGE sequence (time to repetition [TR] = 10ms, time to echo [TE] = 4ms, flip angle = 30° ACQ-1, Matrix = 256×256, scanning time = 5.6min) with 1mm × 1mm × 1.25mm isotropic resolution. Scans were then analyzed and processed using FreeSurfer release 3.0.5 with manual editing in accordance to guidelines provided by FreeSurfer. A 2-dimensional smoothing kernel was applied along the cortical surface with a 20-mm full-width/half-maximum window. Spherical maps for each participant were morphed into a common spherical atlas using a nonlinear surface-registration procedure that allows for high-registration, surface-based averaging, and comparison of cortical measurements across participants.16
Estimated total ICV was defined as the sum of gray matter, white matter, and cerebrospinal fluid (CSF). TBV was defined as the sum of gray matter and white matter. The variables considered in this study were TBV, ICV, whole white and gray matter volumes, cortical white and gray volumes, subcortical gray matter volume, CSF volume, cortical thickness, ACC volume, middle frontal gyrus volume, hippocampus volume and anterior insula volume. These regionally specific volumes were taken from the Destrieux parcellation in Freesurfer.17
Data Analysis
Statistical analyses were completed in SPSS v20. To analyze differences between groups in demographic and clinical data, we used 1-way ANOVAs. All brain volumes were analyzed using ANCOVAs with age and gender entered as covariates. For TBV, we repeated the analysis including ICV as an additional covariate. Volumes of specific brain regions were examined also controlling for TBV. Our next level was to compare whole brain volumes for gray and white separately, as well as for cortical and subcortical and cortical thickness. Our last level of analysis was to examine the 4 specific brain volumes of interest. We used FDR to correct for multiple comparisons across all of these comparisons. In addition, when a brain volume variable showed a significant effect of group, we compared the different subgroups in post hoc analyses.
Results
Demographic, cognitive, and clinical data are presented in table 1, and supplementary figures 1 and 2. The groups had similar age, but the gender distribution differed across groups. As expected, HC had more years in school than cognitively impaired SZ (IQ−/COG− and IQ+/COG−; p < .001), and SZ with IQ−/COG− had fewer years in school than SZ with IQ+/COG+ (P = .001). SZ with IQ−/COG− had lower SES and had parents with fewer years in school compared to all of the other groups (P < .005). All patient groups performed worse than HC on the cognition composite (P < .015), even those in the IQ+/COG+ group. The SZ with IQ+/COG+ had fewer negative symptoms than SZ with IQ−/COG− (SANS: P = .016), but all groups of SZ were similar regarding positive symptoms (SAPS). As expected, Vocabulary and Cognition Composite were strongly correlated in both participants with SZ (r = .60, P < .001) and HC (r = .51, P < .001).
Table 1.
Demographic and Clinical Data as a Function of Group
| Variables, Mean (SD) | Healthy Controls, n = 94 | Individuals With Schizophrenia | |||
|---|---|---|---|---|---|
| IQ+/COG+, n = 25 | IQ+/COG−, n = 31 | IQ−/COG−, n = 36 | ANOVAa/ Chi-square | ||
| Age | 32.22 (13.64) | 35.42 (14.09) | 32.20 (12.39) | 38.44 (12.67) | F(3, 177) = 2.02, P = .113 |
| Gender (male/female) | 53/41 | 10/15 | 28/3 | 24/12 | χ2 = 17.25, df = 3, P = .001* |
| Subject education (y) | 14.38 (2.66) | 13.44 (2.62) | 12.38 (1.97) | 11.00 (1.74) | F(3, 178) = 18.94, P < .001* |
| Parental education (y) | 14.13 (2.61) | 14.17 (3.71) | 15.04 (2.59) | 11.50 (2.06) | F(3, 162) = 8.61, P < .001* |
| Personal SES | 3.06 (0.88) | 2.86 (1.11) | 3.28 (1.06) | 4.25 (0.94) | F(3, 131) = 10.33, P < .001* |
| SAPS | — | 18.71 (12.66) | 21.74 (15.16) | 23.15 (18.48) | F(2, 85) = 0.72, P = .490 |
| SANS | — | 24.39 (17.74) | 29.53 (17.58) | 38.43 (17.92) | F(2, 80) = 4.31, P = .017* |
| Cognition Composite | 3.41 (4.22) | 1.03 (2.73) | −6.12 (1.97) | −7.79 (2.85) | F(3, 182) = 119.65, P = .000* |
| Vocabulary (WAIS-III) | 11.71 (3.16) | 11.62 (2.97) | 10.00 (2.21) | 4.44 (1.27) | F(3, 182) = 65.19, P = .000* |
Note: SES, socioeconomic status (higher values indicate lower SES); SAPS, Scale for the Assessment of Positive Symptoms; SANS: Scale for the Assessment of Negative Symptoms; WAIS-III, Wechsler Adult Intelligence Scale.
aANOVA with Bonferroni correction.
*Statistical significance (P < .05).
Whole Brain Volume and Intracranial Volume
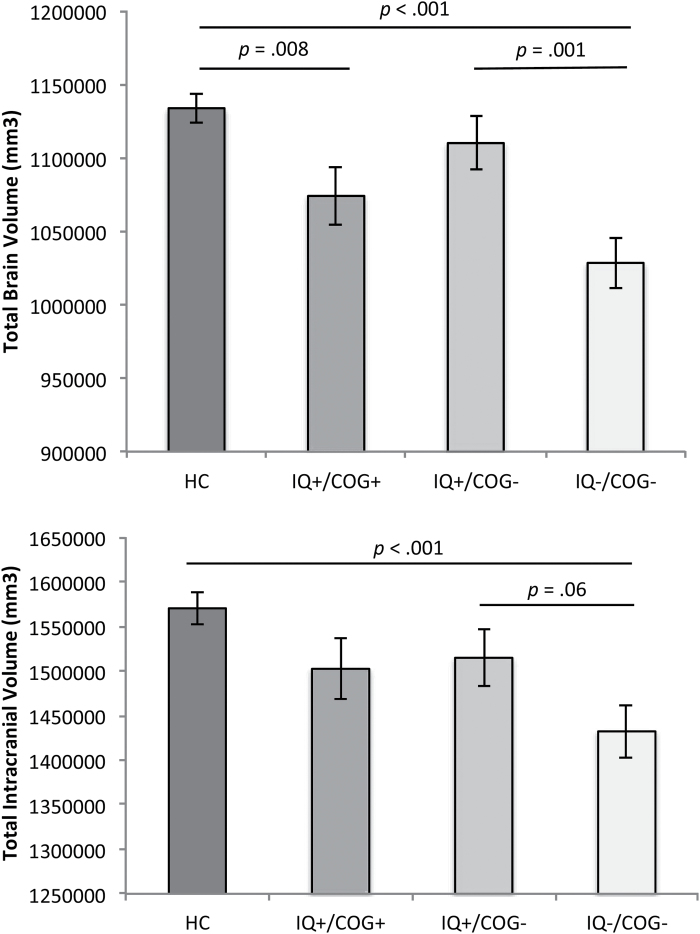
As shown in figure 1 and supplementary figure 3, there were group differences in TBV, ICV, and TBV corrected for ICV (table 2), all of which survived multiple comparison correction. SZ with IQ−/COG− had smaller TBV and ICV than HC and IQ+/COG−. SZ with IQ+/COG+ had also smaller TBV than HC, even when we controlled for ICV (supplementary figure 1). Thus, overall the SZ with impaired ePMC-IQ differed from the other groups on TBV and ICV, but the SZ with impaired cognition did not differ significantly if their ePMC-IQ was relatively intact.
Fig. 1.
Mean differences in total brain volume and estimated total intracranial volume across healthy controls (HC); individuals with schizophrenia (SZ) with normal estimated premorbid crystallized IQ (ePMC-IQ) and not cognitively impaired (IQ+/COG+); individuals with SZ with normal ePMC-IQ and cognitively impaired (IQ+/COG−); and individuals with SZ with lower ePMC-IQ and cognitively impaired (IQ−/COG−). Covariates appearing in the model are evaluated at the following values: age = 33.78, sex = 1.39. Error bars are standard error of the mean (SEM).
Table 2.
Results of MANCOVAS Comparing Group Differences in Brain Structure
| Variables | ANCOVA |
|
FDR-adjusted P-values | HC vs SZ IQ+/ COG+ (Intact) | HC vs SZ IQ+/COG− (Deteriorated) | HC vs SZ IQ−/COG− (Compromised) | SZ IQ+/ COG+ vs SZ IQ+/COG− | SZ IQ+/ COG+ vs SZ IQ−/COG− | SZ IQ+/ COG− vs SZ IQ−/COG− | |
|---|---|---|---|---|---|---|---|---|---|---|
| TBVa | F(3, 175) = 9.95, P < .001 | .146 | .002 | ** | NS | *** | NS | NS | *** | |
| Intracranial brain volume (ICV)a | F(3, 175) = 5.44, P = .001 | .085 | .002 | NS | NS | *** | NS | NS | NS | |
| TBV (corrected)b | F(3, 174) = 4.57, P = .004 | .073 | .006 | * | NS | *** | NS | NS | ** | |
| Whole brain white matter volumec | F(3, 174) = 12.58, P < .001 | .178 | .002 | NS | * | *** | * | *** | ** | |
| Cortical white matter volumec | F(3, 174) = 11.65, P < .001 | .167 | .002 | NS | NS | *** | * | *** | ** | |
| Whole brain gray matter volumec | F(3, 174) = 12.58, P < .001 | .178 | .002 | NS | * | *** | * | *** | ** | |
| Total cortical gray matter volumec | F(3, 174) = 8.60, P < .001 | .129 | .002 | NS | * | *** | NS | *** | * | |
| Subcortical gray matter volumec | F(3, 174) = 1.85, P = .140 | .031 | .19 | NS | NS | NS | NS | NS | NS | |
| Cerebrospinal fluid volumec | F(3, 174) = .06, P = .979 | .001 | .98 | NS | NS | NS | NS | NS | NS | |
| Cortical thicknessc | F(3, 174) = 4.54, P = .004 | .073 | .006 | NS | ** | ** | NS | * | NS | |
| Anterior cingulate cortex volumec | F(3, 174) = 1.80, P = .149 | .030 | .19 | NS | NS | NS | NS | NS | NS | |
| Middle frontal gyrus volumec | F(3, 174) = 1.50, P = .218 | .025 | .25 | NS | NS | NS | NS | NS | NS | |
| Hippocampus volumec | F(3, 174) = .33, P = .804 | .006 | .87 | NS | NS | NS | NS | NS | NS | |
| Anterior insula volumec | F(3, 174) = 5.58, P < .001 | .088 | .002 | NS | * | *** | NS | ** | NS |
Note: HC, healthy controls; SZ, subjects with schizophrenia; TBV, total brain volume; NS, not significant.
aCovariates: age and sex.
bCovariates: age, sex and ICV.
cCovariates: age, sex and TBV.
*P < .05; **P < .01; ***P < .001.
White Matter Volume
There were significant group differences in both whole brain white matter volume and cortical white matter volume (table 2), both of which survived multiple comparison correction. As shown in supplementary figure 4, SZ with IQ−/COG− had significantly larger whole white matter volume and total cortical white matter volume than each of the other 3 groups. Also, SZ with IQ+/COG− had larger white matter volumes than IQ+/COG+ and HC.
Gray Matter Volume and Thickness
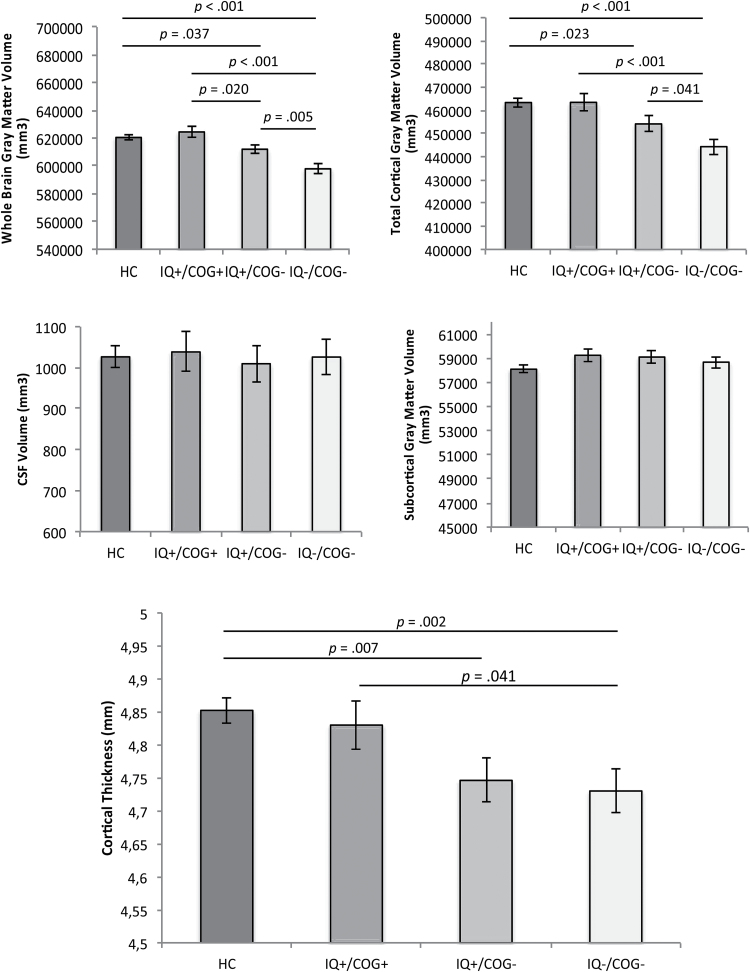
There were significant group differences in whole brain gray matter volume and cortical gray matter volume (table 2), both surviving multiple comparison correction. SZ with IQ−/COG− had smaller total gray matter volume and cortical gray matter volume than each of the other 3 groups (figure 2). SZ with IQ+/COG− had smaller total gray matter volume and cortical gray matter volume than HC, and smaller total gray matter volume than SZ with IQ+/COG+. However, there were no differences between groups in subcortical gray matter volume or CSF (table 2, figure 2). In addition, SZ with impaired cognition whether or not they had impaired ePMC-IQ (IQ−/COG− and IQ+/COG−) had significantly thinner cortex than HC, and SZ with IQ−/COG− showed reduced cortical thickness compared to SZ with IQ+/COG+ (figure 2).
Fig. 2.
Mean differences in gray matter volume, cerebrospinal fluid, and cortical thickness across healthy controls (HC); individuals with schizophrenia (SZ) with normal estimated premorbid crystallized IQ (ePMC-IQ) and not cognitively impaired (IQ+/COG+); individuals with SZ with normal ePMC-IQ and cognitively impaired (IQ+/COG−); and individuals with SZ with lower ePMC-IQ and cognitively impaired (IQ−/COG−). Covariates appearing in the model are evaluated at the following values: age = 33.78, sex = 1.39, total brain volume (TBV) = 1101612.03. Error bars are standard error of the mean (SEM).
Specific Brain Regions
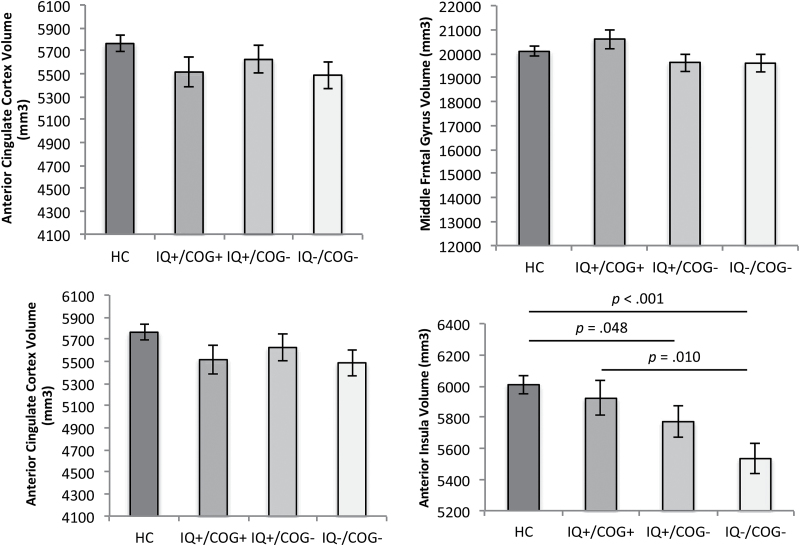
We next examined volumes in specific brain regions that had shown evidence of volume reductions in prior research.10 There were no significant differences between groups in ACC, middle frontal gyrus or hippocampus volumes (table 2). However, there was a significant group difference in anterior insula volume (table 2), which survived multiple comparison correction. Specifically, SZ with impaired cognition (both IQ+/COG− and IQ−/COG−) had smaller anterior insula volume than HC, and SZ with IQ−/COG− also had smaller insula volume than SZ with IQ+/COG+ (figure 3).
Fig. 3.
Mean differences in brain volume across healthy controls (HC); individuals with schizophrenia (SZ) with normal estimated premorbid crystallized IQ (ePMC-IQ) and not cognitively impaired (IQ+/COG+); individuals with SZ with normal ePMC-IQ and cognitively impaired (IQ+/COG−); and individuals with SZ with lower ePMC-IQ and cognitively impaired (IQ−/COG−). Covariates appearing in the model are evaluated at the following values: age = 33.78, sex = 1.39, total brain volume (TBV) = 1101612.03. Error bars are standard error of the mean (SEM).
Discussion
To better understand the relationship between neuropathology and cognitive functioning, we aimed to replicate and extend the work of Woodward and Heckers3 by examining brain structure in individuals with SZ grouped according their ePMC-IQ and current neuropsychological performance. As predicted, we found that individuals with SZ with cognitive deficits and lower estimated premorbid crystallized intellectual functioning (IQ−/COG−) had smaller ICVs than HC. However, we also found that they had reduced total and cortical gray matter volumes and reduced cortical thickness compared to HC. In contrast, individuals with cognitive impairment in the context of intact ePMC-IQ showed only reduced total and cortical gray matter volume, and cortical thickness. When examining specific structures linked to cognitive function in SZ in prior work, individuals with both IQ+/COG− and IQ−/COG− presented reduced volume compared to controls in the anterior insula, but not in hippocampus, ACC or dorsolateral prefrontal cortex.
Similar to what was found in Woodward and Heckers,3 individuals with SZ with IQ−/COG− in the current sample had smaller ICVs compared to HC, while individuals with intact ePMC-IQ, regardless of their cognitive function, did not differ from HC in ICV. In typical development, intracranial and whole brain volumes increase between early childhood and early adolescence. ICV then remains relatively stable across the life course, while TBV starts to reduce during adulthood. As such, changes in ICV vs TBV relative to ICV can be thought as neurodevelopmental and neurodegenerative impairment indexes, respectively.18 Thus, like Woodward and Heckers,3 our results suggest a relationship between impaired ePMC-IQ and an early neurodevelopmental process among individuals with SZ. Interestingly, Craddock and Owen19 hypothesized a model suggesting that the major psychiatric illnesses were part of one spectrum, as they seem to share mechanisms and impairments at the levels of genetic susceptibility, neural systems and cognitive impairment. In this dimensional perspective, SZ would be part of a gradient of psychopathology with neurodevelopmental contribution lesser than intellectual disability and autism, but stronger than BD and major depression. Also, there might be different neurobiological biotypes with unique patterns of neurobiological alterations that each contribute to psychosis. This could explain the heterogeneity seen in the illnesses, and suggests that multiple pathways could lead to similar clinical manifestations of psychosis.20 However, previous studies have shown that high premorbid intelligence reduced the risk of developing SZ, while individuals with IQ between 70 and 85 had double the risk and individuals with IQ below 70 increased 5-fold the risk of being diagnosed with SZ.5 Such data are consistent with the hypothesis IQ may be associated with early risk factors for psychosis. However, unlike Woodward and Heckers,3 we also found that SZ with impaired ePMC-IQ also showed reduced brain volume relative to ICV, as well as reduced cortical gray matter volume. As such, our results are consistent with the finding that both whole brain and ICVs are positively associated with IQ performance in both clinical and nonclinical populations.21 Thus, our data suggest that impaired IQ may also be associated with a neurodegenerative process, as well as a neurodevelopmental process, though as discussed below, this may relate to mechanisms that impair cognitive function in addition to crystallized IQ.
Unlike Woodward and Heckers,3 we did not find that individuals with SZ who had impaired cognition in the context of intact ePMC-IQ (IQ+/COG−) had reduced TBV relative to ICV. However, we did see that these individuals had significantly reduced total and cortical gray matter volume, and cortical thickness compared to HC, as did the individuals with both impaired ePMC-IQ and impaired cognitive function. In SZ, cortical thickness is positively correlated with neuropsychological performance, such as working memory, processing speed, verbal learning, and executive functioning,22–24 which is consistent with our finding that the participants who had cognitive impairment—regardless of ePMC-IQ—had thinner cortices. There seems to be an important association between cognitive impairment and cortical thinning in SZ, in which the participants with SZ that have near-normal neuropsychological performance show near-normal patterns of cortical thickness, while neuropsychologically impaired individuals have overall reduced cortical thickness.16 Interestingly, cortical thickness was shown to progressively and widely decrease in SZ after a 5-year period.25 Nonetheless, there is not yet a consensus as to whether cortical thinning is a progressive pattern in SZ, associated with the duration of illness, or whether it is primarily a neurodevelopmental feature that occurs very early in the disease.26,27 However, abnormal cortical thinning seems to be associated with the development and conversion to psychosis,28 again suggesting evidence for neurodegeneration. As such, our findings of reduced cortical thickness SZ with IQ+/COG−, as well as smaller cortical gray matter volume, are consistent with the hypothesis that processes associated with cognitive impairment and cortical thinning may possibly be a “second hit” among individuals with psychosis, potentially bringing an additional and significant neuroprogressive burden.
In addition to examining whole brain metrics of gray matter, we also examined the volume of specific brain regions shown to be reduced in SZ and/or associated cognitive function in previous studies.10–14 We did not find evidence for reduced volumes of the ACC, hippocampus or middle frontal gyrus among individuals with SZ who had impaired cognition, either with or without impaired ePMC-IQ. However, we did find evidence of significantly reduced anterior insula volume among individuals with both impaired ePMC-IQ and cognition, compared to both HC and SZ with intact ePMC-IQ and cognitive function. Further, we also saw a reduction in the anterior insula volume of SZ individuals with impaired cognition and intact ePMC-IQ compared to controls. Interestingly, in SZ, meta-analyses showed medium-sized significant reductions in insula volume when compared to HC, regardless of other demographic and clinical variables.29 Further, a recent meta-analysis identified gray matter loss in the anterior insula as a transdiagnostic neural abnormality across psychiatric diseases.30 Notably, activation in this area is known to be involved in several processes related to awareness of internal and external emotional and sensory stimuli,31 functions which may be very relevant to understand psychotic processes. Therefore, it is noteworthy that we found reduced anterior insula volume in participants with SZ with impaired cognition, regardless of their ePMC-IQ, and that was present controlling for the differences in TBV.
Our results regarding white matter volumes were unexpected. We found that individuals with impaired cognition and ePMC-IQ had significantly larger white matter volumes than both SZ groups with intact ePMC-IQ, as well as compared to HC. In the literature, findings much more commonly show that individuals with SZ show smaller white matter volumes compared to HC.10 On the other hand, it could be possible that this may reflect a segmentation issue with Freesurfer, such that poor gray/white discrimination leads to underestimates of gray matter volume and overestimates of white matter volume. However, it is not clear why such a problem would lead to a systematic bias in the direction of white matter vs gray matter. Further, we did not see any evidence for alterations in CSF, which might have been expected should this have been a methodological issue (though the CSF vs gray/matter white boundary is starker). In general, the literature on SZ has not provided strong evidence for broad reductions in white matter volume. Interestingly, when we considered the individuals with SZ as a single group, there where no white matter volumes differences compared to HC (P > .145), which is consistent with the broader literature. Nonetheless, our findings in regards to white matter differences in the individuals with SZ who had both impaired cognition and ePMC-IQ were unexpected and require further investigation potentially through examination of diffusion tensor imaging or white matter hypo or hyper intensities, both of which may be more informative of the WM integrity than volumetric MRI measures of WM.32
Regarding the individuals with SZ who had both intact cognitive abilities and ePMC-IQ, we found some slight evidence of reduced TBV relative to ICV when compared to HC, but no other differences. This finding was quite unexpected given that this group had relatively intact ePMC-IQ and cognitive function. However, this would not have survived multiple comparisons testing of the follow-up group comparisons in the analysis of TBV, given that it was not predicted. This subgroup of patients had a relatively higher percentage of females compared to the other 2 groups of SZ (60% vs 10% and 33%, respectively), though all of our analyses controlled for gender. Further, this group had the highest personal SES of any of the 3 patient groups. We did not measure factors such as height, weight and body size that may have contributed to this unexpected finding, which will be important to do in future studies. Conversely, a previous study found that, compared to healthy individuals, individuals with SZ who were relatively neuropsychological normal had smaller gray matter volume than controls across several regions and no white matter volume abnormalities. However, this group of individuals with SZ was older (39.5 y) and their percentage of males was higher (85.7%) than in our sample. Furthermore, in addition to age and gender, this previous study used height instead of TBV to control their analysis of brain structure, what could have contributed to their differential findings.33
One issue that should be mentioned is whether the IQ differences in the groups and consequently their influences to brain structure were due to biological determinants of the disease or to environmental factors. In our sample, subjects with SZ with lower ePMC-IQ (IQ−/COG−) had parents with lower years of education compared to all the other groups. This could either suggest that the environment to which the SZ patients were exposed had an influence on their IQ development, or it could reflect inherited cognitive function that may be disease relevant. Further work that would allow one to dissociate these environmental vs genetic contributions would be needed to address this important question, such as twin or adoption designs.
Our study had several limitations. First, we did not have records of past or current medication use, although all patients were stably medicated. Further, we did not have data on age of onset or illness duration, both of which are factors that could also influence metrics of structural brain integrity in psychosis. In addition, we only presented results of analyses of brain structure, and did not have data on brain function or connectivity among these individuals, which may also show important relationships to IQ and cognitive function. Also, the 1.5T scanner may have limit performance in the acquisition of quantitative anatomical data. Finally, we only reported an estimated measure of premorbid crystallized intellectual functioning, and not an actual assessment of IQ before the disease onset. Also, the cognitive composite was created considering subtests that include many but not all cognitive domains that may be impaired in psychosis, which may limit our generalization of the results. Further, we recognize that there are important relationships between crystallized IQ and cognitive function, and that they are frequently correlated, as they were in our sample. However, they can also be dissociated and are thought to have differential contributions from various factors.
In summary, our results are consistent with the hypothesis that there may be different neurodevelopmental mechanisms that contribute to different patterns of crystallized IQ and cognitive impairment among individuals with SZ, though these mechanisms may converge to alter common aspects of brain structure. This interpretation is in line with the idea of psychosis as the end result of multiple different pathogenetic paths9 possibly deriving from interactions between various genes and environmental insults.7 Intellectual and cognitive performances could also be related to a spectrum of severity of the illness, as our data suggest that that individuals with SZ with both impaired ePMC-IQ and cognitive function had worse brain structure outcomes. In the present study, we saw that ePMC-IQ related in important ways to brain structure in SZ, suggestive of a protective factor for certain types of brain structural abnormalities even in the context of impaired cognition. However, like Woodward and Heckers,3 our results suggest that there are alterations in cortical volume and thickness, as well as insula volume, that are present among individuals with impaired cognition even in the context of relatively intact ePMC-IQ. Future research considering differences in premorbid intellectual and cognitive performances will hopefully advance our comprehension of the different pathways of SZ, improving our understanding of its neuropathology and potential pathways for treatment.
Funding
Brazilian Council of Scientific and Technological Development (CNPq-SWE 233949/2014-3). Data collection and sharing was funded by National Institute of Mental Health (NIMH) grant 1R01 MH084803 and by NIMH cooperative agreement 1U01 MH097435. This study was supported by CNPq/Brazil.
Supplementary Material
Acknowledgments
Data used in preparation of this article were obtained from the NU Schizophrenia Data and Software Tool (NUSDAST) database (http://central.xnat.org/REST/projects/NUDataSharing) and from the SchizConnect database (http://schizconnect.org). As such, the investigators within NUSDAST and SchizConnect contributed to the design and implementation of NUSDAST and SchizConnect and/or provided data but did not participate in analysis or writing of this report. D.M.B. served as a consultant to Amgen, Pfizer, Roche and Takeda.
References
- 1. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 2. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodward ND, Heckers S. Brain structure in neuropsychologically defined subgroups of schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2015;41:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. [DOI] [PubMed] [Google Scholar]
- 5. Khandaker GM, Barnett JH, White IR, Jones PB. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res. 2011;132:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ammari N, Heinrichs RW, Pinnock F, Miles AA, Muharib E, McDermid Vaz S. Preserved, deteriorated, and premorbidly impaired patterns of intellectual ability in schizophrenia. Neuropsychology. 2014;28:353–358. [DOI] [PubMed] [Google Scholar]
- 7. Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. [DOI] [PubMed] [Google Scholar]
- 10. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. [DOI] [PubMed] [Google Scholar]
- 12. Kubota M, van Haren NE, Haijma SV, et al. Association of IQ Changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry. 2015;72:803–812. [DOI] [PubMed] [Google Scholar]
- 13. Sheffield JM, Repovs G, Harms MP, et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sigurdsson T, Duvarci S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front Syst Neurosci. 2015;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Kogan A, Cobia D, et al. Northwestern University Schizophrenia Data and Software Tool (NUSDAST). Front Neuroinform. 2013;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cobia DJ, Csernansky JG, Wang L. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr Res. 2011;133:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. [DOI] [PubMed] [Google Scholar]
- 19. Craddock N, Owen MJ. The Kraepelinian dichotomy - going, going. but still not gone. Br J Psychiatry. 2010;196:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M. Meta-analysis of associations between human brain volume and intelligence differences: how strong are they and what do they mean? Neurosci Biobehav Rev. 2015;57:411–432. [DOI] [PubMed] [Google Scholar]
- 22. Ehrlich S, Brauns S, Yendiki A, et al. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr Bull. 2012;38:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartberg CB, Lawyer G, Nyman H, et al. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 2010;182:123–133. [DOI] [PubMed] [Google Scholar]
- 24. Hartberg CB, Sundet K, Rimol LM, et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J Int Neuropsychol Soc. 2011;17:1080–1093. [DOI] [PubMed] [Google Scholar]
- 25. van Haren NE, Schnack HG, Cahn W, et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–880. [DOI] [PubMed] [Google Scholar]
- 26. Kubota M, Miyata J, Yoshida H, et al. Age-related cortical thinning in schizophrenia. Schizophr Res. 2011;125:21–29. [DOI] [PubMed] [Google Scholar]
- 27. Assunção Leme IB, Gadelha A, Sato JR, et al. Is there an association between cortical thickness, age of onset, and duration of illness in schizophrenia? CNS Spectr. 2013;18:315–321. [DOI] [PubMed] [Google Scholar]
- 28. Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–784. [DOI] [PubMed] [Google Scholar]
- 30. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 32. Kubicki M, Westin CF, McCarley RW, Shenton ME. The application of DTI to investigate white matter abnormalities in schizophrenia. Ann N Y Acad Sci. 2005;1064:134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wexler BE, Zhu H, Bell MD, et al. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am J Psychiatry. 2009;166:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.