Abstract
Background:
Context processing may reflect a specific cognitive impairment in schizophrenia. Whether impaired context processing is observed across psychotic disorders or among relatives of affected individuals, and whether it is a deficit that is independent from the generalized neuropsychological deficits seen in psychotic disorders, are less established.
Methods:
Schizophrenia, schizoaffective, and psychotic bipolar probands (n = 660), their first-degree relatives (n = 741), and healthy individuals (n = 308) studied by the Bipolar-Schizophrenia Network on Intermediate Phenotypes consortium performed an expectancy task requiring use of contextual information to overcome a pre-potent response. Sensitivity for target detection and false alarm rates on trials requiring inhibition or goal maintenance were measured.
Results:
Proband groups and relatives with psychosis spectrum personality traits demonstrated reduced target sensitivity and elevated false alarm rates. False alarm rate was higher under inhibition vs goal maintenance conditions although this difference was attenuated in schizophrenia and schizoaffective proband groups. After accounting for global neuropsychological impairment, as reflected by the composite score from the Brief Assessment of Cognition in Schizophrenia neuropsychological battery, deficits in schizophrenia and bipolar proband groups were no longer significant. Performance measures were moderately familial.
Conclusion:
Reduced target detection, but not a specific deficit in context processing, is observed across psychotic disorders. Impairments in both goal maintenance and response inhibition appear to contribute comparably to deficits in schizophrenia and schizoaffective disorder, whereas greater difficulty with response inhibition underlies deficits in bipolar disorder. Yet, these deficits are not independent from the generalized neurocognitive impairment observed in schizophrenia and psychotic bipolar disorder.
Introduction
Context processing is an executive ability that refers to the adaptive control of behavior through use of prior contextual information that must be mentally represented and maintained to support context-appropriate behavior.1 Context processing thus depends on several cognitive processes including selective attention, working memory, cognitive control, and response inhibition, and it is believed to be subserved primarily by dorsolateral prefrontal cortex.2–4 Experimentally, context processing has been studied using expectancy continuous performance tasks (CPT2,5,6) in which the representation and maintenance of antecedent contextual information relevant to an immediate goal is needed to overcome an established automatic or prepotent response.
Deficits in context processing have been reported in schizophrenia and they appear stable across the course of illness without significant impact by antipsychotic medication.2,7–11 Findings of impaired context processing among unaffected relatives9,12,13 and among those with schizoptypal personality disorder have also been reported,14 suggesting that impairment in this ability may represent an endophenotype for schizophrenia.
Studies of context processing in bipolar disorder are fewer than those for schizophrenia, and have reported impaired performance relative to controls albeit not to the same extent as that observed in schizophrenia15,16; although, this may depend upon consideration of a history of psychosis.17 Indeed, psychotic bipolar disorder patients typically have a pattern of cognitive impairment, including impaired working memory and inhibitory control, that is qualitatively similar to schizophrenia.18–21 The occurrence of context processing deficits among relatives of bipolar probands has not yet been systematically investigated.
Recent data suggest that a deficit in goal maintenance, ie, the ability to activate task related goals and keep them represented in working memory to guide behavior, may underlie impaired context processing in schizophrenia, as evidenced by greater impairment among patients under conditions requiring intact goal maintenance rather than response inhibition.11,22 Whether impaired context processing, and deficient goal maintenance in particular, is present across psychotic disorders is not established. Further, it is not clear if impaired context processing reflects a specific deficit independent from the generalized neuropsychological impairment observed in psychotic disorders.
The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) is a multisite consortium designed to characterize intermediate phenotypes across schizophrenia, schizoaffective, and psychotic bipolar diagnoses. Here we report findings from the BSNIP consortium on the Dot Probe Expectancy (DPX) task, a variant of the AX-CPT that allows for examination of differential performance deficits attributable to impairment in context processing and loss of goal maintenance.11,22 We evaluated sensitivity for target detection and evidence for any specific deficit in context processing across psychotic probands and relatives. Familiality estimates of task performance were calculated as part of the consideration of this ability as a possible endophenotype for psychosis. Finally, to determine whether target detection or context processing deficits account for additional variance beyond that attributable to general impairments in cognitive function, we examined DPX performance after accounting for broad neuropsychological impairment.
Methods
Participants
Participants included probands with schizophrenia (n = 274), schizoaffective disorder (n = 164), and psychotic bipolar disorder (n = 222), their first-degree relatives (ns = 303, 187, 251, respectively), and healthy controls (n = 308) who were enrolled in the B-SNIP consortium (table 1; see Tamminga et al23 for details of ascertainment of samples). All participants completed the DPX task22 and the Brief Assessment of Cognition in Schizophrenia test battery (BACS),24 a measure of global neuropsychological functioning.
Table 1.
Demographic Characteristics of Proband, Relative, and Control Groups
| Probands | Relatives | |||||||
|---|---|---|---|---|---|---|---|---|
| Controla (N = 308) | Sczb (N = 274) | SczAc (N = 164) | BPd (N = 222) | Scze (N = 303) | SczAf (N = 187) | BPg (N = 251) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Post hoc† | |
| Age | 37.7 (12.6) | 35.6 (12.7) | 36.8 (12.0) | 36.3 (12.7) | 43.1 (14.9) | 40.6 (16.3) | 40.6 (15.8) | e>a,b,c,d; f,g>b,d |
| WRAT-IV Reading | 103.7 (13.8) | 94.5 (15.6) | 96.9 (14.8) | 101.6 (13.8) | 97.6 (14.6) | 100.1 (15.8) | 103.4 (13.9) | b<a,d,f,g; c<a,d,g; e<a,d,g |
| BACS Composite (z-score) | 0.01 (1.15) | −1.82 (1.35) | −1.45 (1.32) | −0.89 (1.26) | −0.62 (1.22) | −0.54 (1.37) | −0.20 (1.21) | a>b,c,d,e,f; b,c<d,e,f,g; d<g; e<g |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | Post hoc† | |
| Male sex | 137 (44.5) | 185 (67.5) | 65 (39.6) | 86 (38.7) | 92 (30.4) | 57 (30.5) | 85 (33.9) | b>a,c,d,e,f,g; a,c,d>e,f,g |
| Race | ||||||||
| Caucasian | 196 (63.6) | 120 (43.8) | 87 (53.0) | 167 (75.2) | 162 (53.5) | 117 (62.6) | 199 (79.3) | b<a,d,f,g; d>c,e; g>a,c,e,f |
| African American | 84 (27.3) | 133 (48.5) | 67 (40.9) | 43 (19.4) | 123 (40.6) | 60 (32.1) | 41 (16.3) | b>a,d,f,g; e>d; g<a,b,c,e,f |
| Asian | 16 (5.2) | 8 (2.9) | 3 (1.8) | 6 (2.7) | 4 (1.3) | 2 (1.1) | 5 (2.0) | — |
| Other | 12 (3.9) | 13 (4.7) | 7 (4.3) | 6 (2.7) | 14 (4.6) | 8 (4.3) | 6 (2.4) | — |
Note: Scz, Sczhizophrenia; SczA, Schizoaffective; BP, Psychotic Bipolar; BACS, Brief Assessment of Cognition in Schizophrenia test battery; WRAT-IV, Wide Range Achievement Test Fourth Edition.
†Hochberg test for post hoc comparison from univariate analysis for continuous variables; Z-test for post hoc comparison from chi-square analysis for categorical variables.
Diagnostic interviews for all subjects were completed using the Structured Clinical Interview for DSM-IV (SCID-IV).25 Relatives with no history of psychosis and controls were also administered the Structured Interview for DSM-IV Personality (SID-P).26 Individuals within 1 criterion of a Cluster A personality disorder diagnosis were considered to have elevated psychosis spectrum personality traits (table 2). Diagnoses were determined by a consensus process led by a senior clinician that included reviews of the structured interviews, psychiatric and medical histories, and available medical records. Assessment of current symptoms was obtained using the Positive and Negative Syndrome Scale (PANSS),27 the Montgomery Asberg Depression Rating Scale (MADRS),28 and the Young Mania Rating Scale (YMRS)29 (table 2).
Table 2.
Clinical Characteristics of Proband and Relative Groups
| Probands | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sczb (N = 274) | SczAc (N = 164) | BPd (N = 222) | ||||||
| Medication Treatment | N (%) | N (%) | N (%) | Post hoc† | ||||
| First Gen. Antipsychotic | 34 (12.4) | 16 (9.7) | 13 (5.9) | b>d | ||||
| Second Gen. Antipsychotic | 214 (78.1) | 129 (77.7) | 148 (66.7) | b,c>d | ||||
| Mood Stabilizer | 40 (14.6) | 73 (44.5) | 101 (45.5) | b<c,d | ||||
| Lithium | 15 (5.5) | 16 (9.8) | 56 (25.2) | b,c<d | ||||
| Antidepressant | 106 (38.7) | 93 (56.7) | 97 (43.7) | b,d<c | ||||
| Stimulant | 13 (4.7) | 8 (4.9) | 25 (11.3) | b,c<d | ||||
| Sedative/ Hypnotic | 58 (21.7) | 50 (30.5) | 72 (32.4) | b<d | ||||
| Anticholinergic | 50 (18.2) | 21 (12.8) | 21 (9.5) | b>d | ||||
| Probands | ||||||||
| Sczb (N = 274) | SczAc (N = 164) | BPd (N = 222) | ||||||
| Clinical symptom ratings | Mean (SD) | Mean (SD) | Mean (SD) | Post hoc† | ||||
| PANSS | ||||||||
| Positive | 16.6 (5.6) | 17.9 (5.2) | 12.7 (4.4) | b>d; c>b,d | ||||
| Negative | 16.5 (5.8) | 16.1 (5.2) | 11.9 (3.9) | b,c>d | ||||
| Total | 65.1 (17.0) | 68.8 (15.9) | 53.0 (13.8) | b,c>d | ||||
| YMRS | 5.5 (5.7) | 7.1 (6.4) | 5.6 (6.4) | b<c | ||||
| MADRS | 8.3 (7.9) | 14.7 (10.2) | 9.7 (9.1) | c>b,d | ||||
| Relatives | ||||||||
| Scze (N = 303) | SczAf (N = 187) | BPg (N = 251) | ||||||
| Clinically affected relatives | N (%) | N (%) | N (%) | Post hoc† | ||||
| Psychotic disorders | 34 (11.2) | 27 (14.4) | 22 (8.8) | — | ||||
| Elevated psychosis spectrum personality traits | 40 (13.2) | 25 (13.3) | 30 (12.0) | — | ||||
Note: PANSS, Positive and Negative Syndrome Scale; MADRS, Montgomery Asberg Depression Rating Scale.
†Hochberg test for post hoc comparison from univariate analysis for continuous variables; Z-test for post hoc comparison from chi-square analysis for categorical variables.
All subjects met the following inclusion criteria: (1) age 15–65; (2) age-corrected Wide Range Achievement Test Fourth Edition (WRAT-IV) Reading Standard Score ≥65; (3) no history of neurologic disorder or significant head injury; (4) no history of substance abuse within the last month or substance dependence within the last 6 months; and (5) negative urine toxicology for drugs of abuse on the day of testing. Control subjects met additional inclusion criteria: (1) no personal or first-degree family history of any psychotic, bipolar or recurrent mood disorder; (2) no lifetime history of substance dependence; and (3) no history of significant psychosis spectrum personality traits as defined above. Institutional review boards at each site approved the study and written informed consent was obtained from all subjects.
Testing Procedures
The DPX task22 is a variant of expectancy CPT designed to assess the ability to represent and maintain local contextual information relevant to an immediate goal. Similar to other expectancy paradigms, the DPX task includes sequences of cue and probe stimuli in which a target or nontarget response is made to each stimulus. Rather than use letter stimuli, the DPX task uses novel dot pattern stimuli on which subjects are trained to identify valid cues and target stimuli. “A” is a valid cue, and “X” a valid probe only when preceded by “A.” Other cues and probes are invalid and referred to as “B” and “Y,” respectively. By increasing the frequency of AX trials, a pre-potent response bias is established because an “X” stimulus typically follows an “A” stimulus. It is expected that individuals with established goal maintenance, but difficulty with response inhibition, will make a greater proportion of errors (false alarms) on AY trials relative to BX trials. Those with poor goal maintenance are expected to make more errors on BX trials because they are less able to maintain representation of “B” cues and incorrectly respond to the subsequent invalid “X” probe. Therefore, performance on AY trials relative to BX trials provides an evaluation of the specific capacity for goal maintenance.
The DPX task consisted of 2 blocks of 40 trials, for which “Target” or “Nontarget” responses were made for each stimulus presentation. Cue length was 1000ms, probe length was 500ms, inter-stimulus interval was 4000ms, and inter-trial interval was 1200ms. Seventy percent of trials were AX trials; 12.5% of trials were BX trials; 12.5% of trials were AY trials; and 5% of trials were BY trials.
Sensitivity for target detection was indexed by d′context which was derived using the AX hit rate and BX false alarm rate to emphasize maintenance of the “A” context for correct performance.22 Data were screened for subjects who performed poorly characterized by error rates equal to or greater than: (1) 56% on AX trials; (2) 100% on AY or BX trials; or (3) 50% on BY trials.22 Cases excluded due to these criteria included 4 controls, 24 schizophrenia, 12 schizoaffective, and 12 bipolar probands and 10 schizophrenia, 5 schizoaffective, and 6 bipolar relatives of probands.
Statistical Analyses
Linear mixed effects models using SPSS were used to test for group differences, with family membership treated as a random effect. Comparison of d′context between groups was used to evaluate overall performance (ie, sensitivity for target detection). Comparison of false alarm rate between AY and BX trials, with trial type treated as a repeated factor, evaluated whether there was a specific deficit in goal maintenance (ie, whether false alarm rates were higher on BX relative to AY trials for which elevated false alarm rate reflects problems with inhibition). In primary analyses of relative groups, those relatives with psychosis were included. In secondary analyses evaluating effects of psychosis spectrum personality traits among nonpsychotic relatives and generalized impairment as measured by the BACS composite score, mixed effects modeling was repeated with inclusion of these variables. Age, sex, site, and race were evaluated as potential covariates in all models; those with a significant correlation with dependent measures (P < .05) were retained. Post hoc analyses were corrected using the Sidak method. Proband and relative groups’ deficits relative to controls are reported as effect sizes computed using Glass’s delta (d). This is conceptually similar to Cohen’s d and expresses effect size deficits relative to the control group SD, thereby facilitating direct comparison of effect sizes across proband and relative groups. Partial correlational analyses with adjustment for potential covariates examined the association between DPX performance and symptom ratings, medication effects (ie, current treatment with a class of medication), and daily chlorpromazine equivalents of antipsychotic treatment.30 Familiality was estimated using a maximum likelihood method in the Sequential Oligogenic Linkage Analysis Routines (SOLAR) software.31 Significance of familiality was determined using a maximum likelihood ratio test of a model in which phenotypic variation explained by family membership was compared to one in which it was not.
Results
Table 3 contains raw mean d′context and error rates across proband, relative, and control groups.
Table 3.
Mean (SD) Dot Probe Expectancy (DPX) Performance Measures for Proband, Relative, and Control Groups
| Probands | Relatives | ||||||
|---|---|---|---|---|---|---|---|
| Control (N = 304) | Scz (N = 250) | SczA (N = 152) | BP (N = 210) | Scz (N = 293) | SczA (N = 182) | BP (N = 246) | |
| Error Rate by Trial Type | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| AX | 0.05 (0.06) | 0.09 (0.10) | 0.09 (0.09) | 0.06 (0.07) | 0.06 (0.07) | 0.06 (0.08) | 0.05 (0.06) |
| AY | 0.13 (0.15) | 0.19 (0.18) | 0.23 (0.20) | 0.19 (0.18) | 0.14 (0.16) | 0.18 (0.15) | 0.14 (0.15) |
| BX | 0.09 (0.15) | 0.17 (0.20) | 0.21 (0.23) | 0.12 (0.16) | 0.11 (0.16) | 0.11 (0.18) | 0.09 (0.15) |
| BY | 0.02 (0.06) | 0.03 (0.06) | 0.04 (0.10) | 0.03 (0.09) | 0.02 (0.07) | 0.02 (0.08) | 0.02 (0.07) |
| d′context | |||||||
| 2.90 (0.66) | 2.42 (0.85) | 2.29 (0.94) | 2.68 (.73) | 2.78 (0.69) | 2.73 (0.80) | 2.88 (0.64) | |
Findings in Probands
Sensitivity for Target Detection.
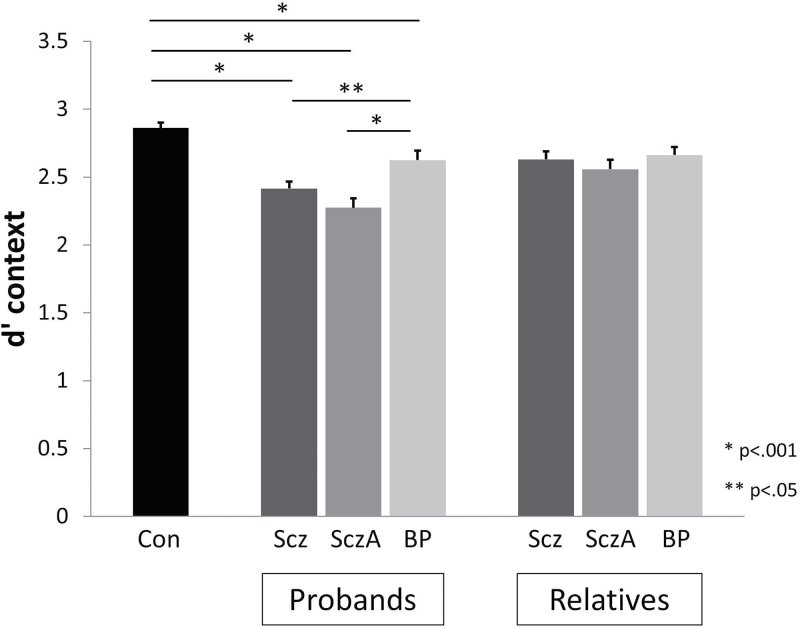
The mixed effects analysis of target sensitivity as measured by d′context revealed significant main effects of proband group (F(3,909) = 24.09, P < .0001). Post hoc analysis revealed that compared to controls, all proband groups had lower d′context values (schizophrenia d = −0.43, P < .0001; schizoaffective d = −0.56, P < .0001; bipolar d = −0.23, P = .004). Schizophrenia and schizoaffective probands had significantly lower d′context compared to bipolar probands (P = .04, P < .001, respectively) but did not differ from each other (figure 1).
Fig. 1.
Mean (SE) d′context for control, proband and relative groups. Proband groups had reduced d′context compared to controls and schizophrenia and schizoaffective disorder probands had reduced accuracy compared to psychotic bipolar probands. Relatives did not differ in d′context from controls or one another. Scz = Sczhizophrenia; SczA = Schizoaffective; BP = Psychotic Bipolar.
Specificity of Goal Maintenance vs Inhibition Deficit.
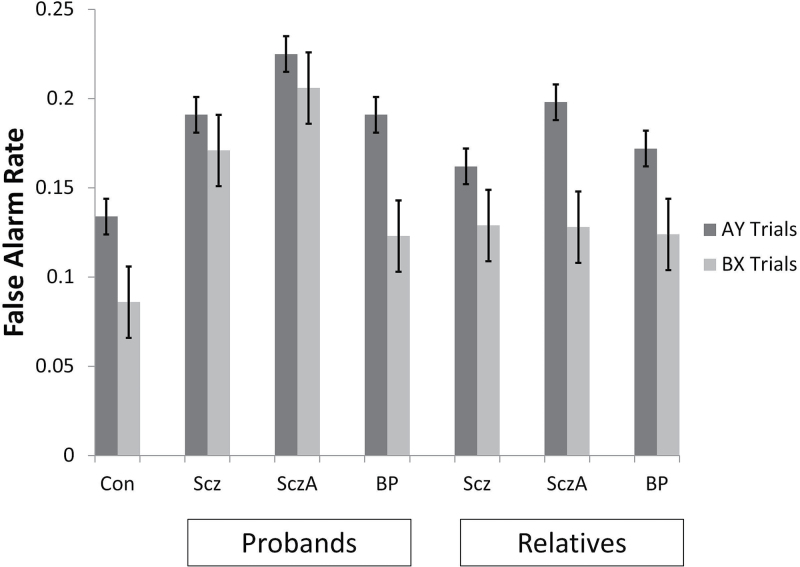
The mixed effect analysis comparing false alarm rate of BX (goal maintenance) relative to AY (inhibition) trials, revealed significant effects for group (F(3,912) = 25.14, P < .0001) and trial type (F(1,912) = 23.46, P < .0001) with a trend towards the interaction between these terms (F(3,912) = 2.15, P = .09). All proband groups had elevated false alarm rates overall compared to controls (schizophrenia d = 0.51, P < .0001; schizoaffective d = 0.75, P < .0001; bipolar d = 0.34, P = .001), and schizoaffective probands had higher false alarm rates than bipolar probands (P < .0001). False alarm rates were significantly higher on AY relative to BX trials. Post hoc inspection of false alarm rates for each trial type revealed that false alarm rates on AY and BX trials were comparable for schizophrenia and schizoaffective probands whereas AY false alarm rate was significantly higher than that observed in BX trials for bipolar and control groups (figure 2).
Fig. 2.
Mean (SE) false alarm rate on AY and BX trials for control, proband, and relative groups. Proband groups had comparably elevated false alarm rates overall compared to controls, and schizoaffective probands had modestly elevated false alarm rates compared to bipolar probands. Relative groups’ false alarm rates did not differ from controls or one another. False alarm rates were higher on AY (response inhibition) relative to BX (goal maintenance) trials for all groups except for schizophrenia and schizoaffective probands who had comparable false alarm rates on both trial types. Scz = Sczhizophrenia; SczA = Schizoaffective; BP = Psychotic Bipolar.
DPX Performance Associations With Clinical Ratings and Medication.
Associations between proband symptom ratings and DPX performance measures were minimal (r values between −.16 and .16); none remained significant after correction for multiple comparisons. Treatment with anticholinergic medications was associated with lower d′context (r = −.21, P = .001) and higher BX false alarm rate (r = −.21, P = .001) among schizophrenia probands. No other significant associations between medication exposure including daily antipsychotic dose and DPX performance measures were observed.
Findings in Relatives
Sensitivity for Target Detection.
The mixed effects analysis of d′context revealed a trend effect for relative group (F(3,637.52) = 2.17, P = .09; figure 1). We observed a significant effect of psychosis spectrum personality traits among nonpsychotic relatives (F(2,799.431) = 3.20, P = .04). Only relatives with elevated psychosis spectrum traits had lower d′context compared to controls (d = −0.20, P = .04); those without these traits did not (d = −0.03, P = .84).
Specificity of Goal Maintenance vs Inhibition Deficit.
The mixed effects analysis comparing false alarm rate of BX relative to AY trials among relatives, revealed a significant main effect of trial type (F(1,1021) = 56.81, P < .0001), with a trend effect for relative group (F(3,614.44) = 2.31, P = .08) and no interaction between group and trial type (figure 2). Secondary analysis of the relative data indicated a trend effect of psychosis spectrum personality traits among relatives on false alarm rate across both BX and AY trials (F(2,822.91) = 2.83, P = .06). Those relatives with elevated spectrum traits trended towards higher false alarm rate overall compared to controls (d = 0.19. P = .06), while those relatives without such trait elevations did not (d = 0.05, P = .73).
Familiality Estimated of DPX Performance
Estimates of familiality for DPX performance measures for each family group are provided in table 4. d′context and BX false alarm rate were modestly familial across pedigrees, whereas AY false alarm rate were modestly familial only among schizoaffective pedigrees.
Table 4.
Estimates of Familiality for DPX Performance Measures
| d′context | AY False Alarm Rate | BX False Alarm Rate | |
|---|---|---|---|
| h 2 (SE) | h 2 (SE) | h 2 (SE) | |
| Schizophrenia | 0.37 (0.10) P = .00007 | 0.08 (0.12) P = .24 | 0.14 (0.11) P = .10 |
| Schizoaffective | 0.47 (0.11) P = .00001 | 0.19 (0.10) P = .03 | 0.37 (0.11) P = .0006 |
| Bipolar | 0.39 (0.11) P = .0003 | 0.19 (0.10) P = .02 | 0.28 (0.12) P = .01 |
DPX Performance and Global Neuropsychological Functioning
Table 5 contains the correlations among DPX performance measures and BACS composite score among proband and control groups.
Table 5.
Correlations Among DPX Performance Measures and BACS Composite Score in Psychosis Probands and Controls
| d′context | AY False Alarm Rate | BX False Alarm Rate | |
|---|---|---|---|
| Healthy Controls | 0.41** | −0.14* | −0.29* |
| Schizophrenia | 0.51** | −0.18** | −0.41** |
| Schizoaffective | 0.40** | −0.25** | −0.30** |
| Bipolar | 0.46** | −0.22** | −0.35** |
Note: *P < .05, ** P < .01 (uncorrected).
Sensitivity for Target Detection.
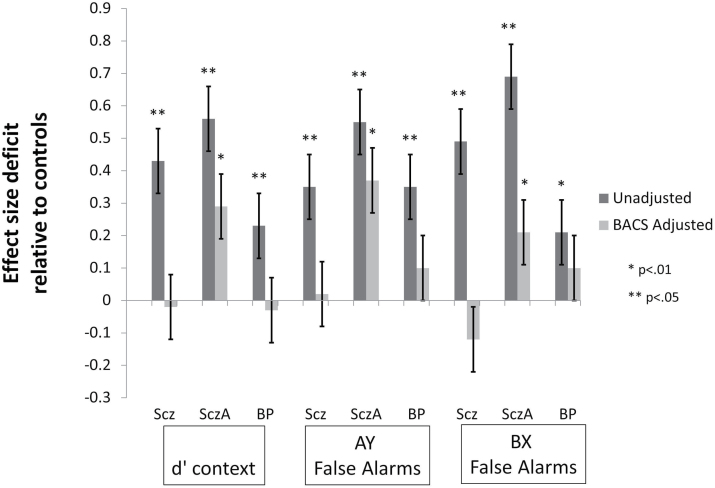
There were significant effects of the BACS composite score (F(1,911) = 229.81, P < .0001) and proband group (F(3,911) = 4.56, P = .004) on d′context. Post hoc tests for group differences indicated that only the schizoaffective proband group’s d′context remained significantly lower than controls (d = −0.29, P = .02; schizophrenia d = 0.02, P > .99; bipolar d = 0.03, P > .99) after accounting for global neuropsychological impairment (figure 3). Schizoaffective probands had lower d′context compared to schizophrenia (P = .01) and bipolar (P = .01) proband groups after accounting for BACS performance.
Fig. 3.
Mean (SE) effect size deficit of Dot Probe Expectancy (DPX) performance measures in proband groups relative to controls before (dark bars) and after (light bars) adjustment for generalized impairment as indexed by the BACS composite score. Performance deficits were reduced after accounting for generalized neuropsychological impairment and no longer significantly different from controls in schizophrenia and psychotic bipolar disorder proband groups. Only schizoaffective disorder probands had reduced d′context and elevated false alarm rates after accounting for BACS performance. Scz = Sczhizophrenia; SczA = Schizoaffective; BP = Psychotic Bipolar; BACS = Brief Assessment of Cognition in Schizophrenia test battery.
Specificity of Goal Maintenance vs Inhibition Deficit.
Analysis of false alarm rates on AY and BX trials accounting for generalized neuropsychological impairment revealed significant effects of trial type (F(3,912) = 23.46, P < .0001), BACS composite (F(1,911) = 134.71, P < .0001), and group (F(3,911) = 6.35, P < .0001), and a trend for the interaction between group and trial type (F(3,912) = 2.15, P = .09). Post hoc analysis of the group effect revealed that, after accounting for generalized neuropsychological functioning, only the schizoaffective proband group had a significantly elevated false alarm rate compared to controls (schizoaffective d = 0.37, P = .001; schizophrenia d = 0.02, P > .99; bipolar d = 0.10, P = .78; figure 3). Schizoaffective probands continued to have higher false alarm rates on AY and BX trials compared to schizophrenia (P = .001) and bipolar (P = .03) proband groups after accounting for BACS performance.
Discussion
We evaluated the executive ability to adaptively control behavior across a psychosis spectrum in a large sample that included schizophrenia, schizoaffective disorder and psychotic bipolar probands and their first-degree relatives using an expectancy task designed to assess the ability to represent and maintain contextual information relevant to an immediate goal. Accurate target detection was reduced among all proband groups and this effect was greater among schizophrenia and schizoaffective disorder probands compared to those with psychotic bipolar disorder. Elevated false alarm rate on conditions requiring goal maintenance (BX) or response inhibition (AY) were observed among all proband groups. While relative groups overall did not differ in expectancy performance compared to controls, those with psychosis spectrum personality traits had mildly reduced target detection and increased false alarm rates, and these measures were moderately heritable across pedigrees. After accounting for global neuropsychological impairment as indexed by the composite score from the BACS neuropsychological battery, the performance of schizophrenia and bipolar disorder probands no longer differed from controls. This suggests that the deficits identified by the DPX task in these proband groups are captured by a neuropsychological index of generalized cognitive impairment.
Context Processing in Psychotic Probands
Our findings of moderate to large effect size deficits in target detection among schizophrenia, schizoaffective disorder, and psychotic proband groups are consistent with those from prior studies of schizophrenia spectrum and bipolar disorders using variants of the DPX and other expectancy tasks.6,11,16,17,22,32 The current finding of worse target detection among schizophrenia and schizoaffective disorder probands relative to bipolar probands is consistent with prior studies that demonstrated less impairment among bipolar patients.15 Indeed, our findings using other cognitive measures with this same cohort demonstrate the extent of impairment across these groups is qualitatively similar but varies in degree.19–21
We did not observe greater false alarm rates on BX trials vs AY trials which would have supported a specific deficit with context processing and goal maintenance as has been observed by others in studies of schizophrenia probands.2,5,7–9,11,13,22 Rather, we observed that the schizophrenia and schizoaffective disorder groups had comparable false alarm rates on BX and AY trials, while bipolar proband and control groups demonstrated higher false alarm rate on AY trials. These findings suggest that impaired performance on expectancy tasks in schizophrenia and schizoaffective disorder are not attributable to impaired context processing per se, and that patients with bipolar disorder have greater difficulty on expectancy tasks due to deficits in response inhibition, consistent with findings of inhibitory deficits among this group.19
Given the size of our sample, it is unlikely that our findings are due to insufficient power to detect differential performance on certain trials among schizophrenia and schizoaffective probands or among relatives of psychotic patients. It is possible that disproportionate impairment in goal maintenance could have been observed under different trial conditions such as lower expectancies or longer inter-stimulus intervals which have been demonstrated to influence BX errors.10,22
Context Processing in Relatives and Familiality
Unlike findings among probands, sensitivity for target detection was unimpaired among first-degree relatives and there was no evidence for specific impairment in context processing which is in contrast to some9,12,13 but not all prior studies33 using expectancy tasks. In exploratory follow-up analyses, relatives with elevated psychosis spectrum personality traits differed, albeit mildly, from controls and those relatives without such traits on sensitivity to detect targets. This finding is consistent with studies demonstrating impaired performance on expectancy tasks among individuals with schizotypal personality disorder,14 suggesting that this may track with clinical expression along the psychosis spectrum. While performance measures were moderately familial across all 3 psychosis pedigrees, the modest deficits observed only among those relatives with elevated psychosis spectrum personality traits provide limited support for context processing as an endophenotype for psychosis.
Context Processing and Global Neuropsychological Functioning
Among proband and control groups, DPX performance was moderately correlated with the BACS composite score, consistent with prior findings demonstrating the associations between DPX performance and other measures of neuropsychological functioning.34 These findings indicate a degree of common variance shared between these measures as is routinely observed in neuropsychological studies. When proband group differences were evaluated accounting for global neuropsychological functioning as indexed by the BACS composite, the moderate deficits in both sensitivity for target detection and false alarm rate observed in schizophrenia and psychotic bipolar probands were no longer significant. This suggests that DPX deficits in these patient groups overlap considerably with impairments detected using the BACS index of generalized cognitive deficit. Only the schizoaffective proband group continued to demonstrate residual impairment in context processing after accounting for BACS performance, suggesting there may be a degree of cognitive impairment in this clinical group that extends beyond the general impairment. In a prior report from the BSNIP cohort, only the schizoaffective proband group demonstrated greater residual impairment on the WMS Spatial Span (backward span) task after accounting for BACS performance.35 To the extent that both the WMS Spatial Span and DPX tasks require working memory abilities, it is possible that the combined presence of schizophrenia-spectrum and affective disturbance may disrupt executive cognitive functions in a way that extends beyond the global impairment observed across the psychosis spectrum.
Limitations and Conclusions
Our findings must be considered within certain limitations to our study. The generalizability of our findings may be limited by certain inclusion criteria. Probands were required to have no current substance abuse or significant lifetime dependence and had to have a family member willing and able to participate, and both needed to be able to cooperate with the rigorous demands of the BSNIP protocol. Our controls were restricted to those without a personal or family history of psychosis, bipolar disorder, and recurrent major depressive disorder, and no elevated psychosis spectrum personality traits. This allowed for comparison of our proband and relative samples with a control group without illness-related characteristics and thus a healthy comparison group that is healthier than the general population. Probands were only required to have at least 1 family member to participate and many pedigrees consisted of a proband and single relative. As such, our calculations may underestimate the familiality of these measures. Finally, it is important to note that this study does not address the utility of the DPX task, and other tasks developed from the cognitive neurosciences, in other contexts such as evaluating the functioning of specific neural systems.
The results of the present evaluation indicate that decreased sensitivity for target detection and elevated false alarm rate on conditions requiring goal maintenance and response inhibition, are present across schizophrenia, schizoaffective disorder, and psychotic bipolar diagnostic categories. We observed comparable impairments in goal maintenance and response inhibition among schizophrenia and schizoaffective probands, whereas impairment in bipolar probands was more attributable to difficulty with response inhibition. While these measures were modestly familial, deficits were not observed among unaffected relatives of affected individuals (albeit some were abnormal in relatives with features of schizophrenia spectrum personality disorders). As such, these measures of context processing do not appear to be particularly promising as cognitive endophenotypes for psychosis, especially given that the deficits were largely attributable to the global neuropsychological impairment observed in psychotic disorders.
Funding
National Institutes of Health (MH083126 to J.L.R., MH077851 to C.A.T., MH078113 to M.S.K., MH077945 to G.P., MH077852 to G.T., MH077862 to J.A.S.)
Acknowledgments
We express gratitude to the patients and families who participated in this study. We also thank Angus MacDonald, PhD, for providing the version of DPX task used, and Gunvant Thaker, MD, for his scientific contributions to the BSNIP consortium. C.A.T. has received support from Intracellular Therapies (ITI, Inc.), PureTech Ventrues, Eli Lilly Pharmaceuticals, Sunovion, Astellas, Merck (ad hoc consulting), International Congress on Schizophrenia Research (unpaid volunteer), NAMI (unpaid volunteer), American Psychiatric Association (Deputy Editor), and Finnegan Henderson Farabow Garrett & Dunner, LLP. J.L.R. has received investigator initiated support from Naurex, Inc. R.S.E.K. has received investigator initiated support from the Department of Veteran’s Affair, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, Psychogenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. R.S.E.K. has received honoraria, served as a consultant, or advisory board member for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biomarin, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, EnVivo, Helicon, Lundbeck, Merck, Mitsubishi, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. R.S.E.K. is a shareholder in Sengenix and NeuroCog Trials, Inc. and receives royalties from the BACS testing battery and the MATRICS Battery (BACS Symbol Coding). Dr Bishop has received research support from Ortho-McNeil Janssen. M.S.K. has received support from Sunovion and GlaxoSmithKline. J.A.S. has received support from Takeda, BMS, Roche, and Eli Lilly and research funding from Janssen. The other authors have nothing to disclose.
References
- 1. Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99:45–77. [DOI] [PubMed] [Google Scholar]
- 2. Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. [DOI] [PubMed] [Google Scholar]
- 3. MacDonald AW, Carter CS, Kerns JG, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. [DOI] [PubMed] [Google Scholar]
- 4. Miller EK. The prefontral cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. [DOI] [PubMed] [Google Scholar]
- 5. Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic performance in a variant of the CPT-AX: a test of theoretical predictions concerning the processing of context. Arch Gen Psychiatry. 1996;53:1105–1113. [DOI] [PubMed] [Google Scholar]
- 6. MacDonald AW. Building a clinically relevant cognitive task: case study of the AX paradigm. Schizophr Bull. 2008;34:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barch DM, Carter CS, MacDonald AW, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- 8. MacDonald AW, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol. 2003;112:689–697. [DOI] [PubMed] [Google Scholar]
- 9. MacDonald AW, III, Pogue-Geile MF, Johnson MK, Carter CS. A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry. 2003;60:57–65. [DOI] [PubMed] [Google Scholar]
- 10. Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biol Psychiatry. 2008;63:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones JAH, Sponheim SR, MacDonald AW. The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess. 2010;22:131–141. [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Garcia P, Young Espinoza L, Molero Santos P, Marin J, Ortuño Sanchez-Pedreño F. Impact of COMT genotype on cognition in schizophrenia spectrum patients and their relatives. Psychiatry Res. 2013;208:118–124. [DOI] [PubMed] [Google Scholar]
- 13. Richard AE, Carter CS, Cohen JD, Cho RY. Persistence, diagnostic specificity and genetic liability for context-processing deficits in schizophrenia. Schizophr Res. 2013;147:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McClure MM, Barch DM, Flory JD, Harvey PD, Siever LJ. Context processing in schizotypal personality disorder: evidence of specificity of impairment to the schizophrenia spectrum. J Abnorm Psychol. 2008;117:342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brambilla P, MacDonald AW, Sassi RB, et al. Context processing performance in bipolar disorder patients. Bipolar Disord. 2007;9:230–237. [DOI] [PubMed] [Google Scholar]
- 16. Robinson LJ, Thompson JM, Gallagher P, Gray JM, Young AH, Ferrier IN. Performance monitoring and executive control of attention in euthymic bipolar disorder: employing the CPT-AX paradigm. Psychiatry Res. 2013;210:457–464. [DOI] [PubMed] [Google Scholar]
- 17. Frydecka D, Eissa AM, Hewedi DH, et al. Impairments of working memory in schizophrenia and bipolar disorder: the effect of history of psychotic symptoms and different aspects of cognitive task demands. Front Behav Neurosci. 2014;8:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bora E, Yücel M, Pantelis C. Neurocognitive markers of psychosis in bipolar disorder: a meta-analytic study. J Affect Disord. 2010;127:1–9. [DOI] [PubMed] [Google Scholar]
- 19. Ethridge LE, Soilleux M, Nakonezny PA, et al. Behavioral response inhibition in psychotic disorders: diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr Res. 2014;159:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reilly JL, Frankovich K, Hill S, et al. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull. 2014;40:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hill SK, Reilly JL, Keefe RSE, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. Am J Psychiatry. 2013;170:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henderson D, Poppe AB, Barch DM, et al. Optimization of a goal maintenance task for use in clinical applications. Schizophr Bull. 2011;38:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1263–1274. [DOI] [PubMed] [Google Scholar]
- 24. Keefe R, Harvey P, Goldberg T, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102:108–115. [DOI] [PubMed] [Google Scholar]
- 25. First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0). New York, NY: Biometrics Research; 1995. [Google Scholar]
- 26. Pfhol B, Blum M, Zimmerman M. Structured Interview for DSM-IV Personality Disorders (SIDP-IV). Iowa City, IA: University of Iowa; 1995. [Google Scholar]
- 27. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 28. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 29. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 30. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strauss ME, McLouth CJ, Barch DM, et al. Temporal stability and moderating effects of age and sex on CNTRaCS task performance. Schizophr Bull. 2014;40:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chkonia E, Roinishvili M, Herzog MH, Brand A. First-order relatives of schizophrenic patients are not impaired in the Continuous Performance Test. J Clin Exp Neuropsychol. 2010;32:481–486. [DOI] [PubMed] [Google Scholar]
- 34. Sheffield JM, Gold JM, Strauss ME, et al. Common and specific cognitive deficits in schizophrenia: relationships to function. Cogn Affect Behav Neurosci. 2014;14:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hill S, Buchholz A, Amsbaugh H, et al. Working memory impairment in probands with schizoaffective disorder and first degree relatives of schizophrenia probands extend beyond deficits predicted by generalized neuropsychological impairment. Schizophr Res. 2015;166:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]