Abstract
Purpose
The efficiency of radiation delivery via volumetric modulated arc therapy (VMAT) is indisputable, but outcomes after VMAT for thoracic esophageal carcinoma are largely unknown.
Methods and materials
We retrospectively analyzed 65 patients with thoracic esophageal cancer who received VMAT to 50.4 Gy (range, 45-50.4 Gy) with concurrent chemotherapy from November 2012 to March 2016 at a single tertiary cancer center. We then used propensity score matching to match these 65 patients with 130 other patients treated with step-and-shoot intensity modulated radiation therapy (ssIMRT) and concurrent chemotherapy. Differences in continuous and categorical variables were examined with independent-sample t or Wilcoxon tests and χ2 tests.
Results
Dosimetrically, VMAT had a higher conformity index (87.75 ± 10.70 VMAT vs 83.20 ± 9.42 ssIMRT, P = .003), a higher heart V5, and a lower V50 than ssIMRT, but lung V5-20, heart V30, heart V40, cordmax, and homogeneity index were similar. At median follow-up intervals of 14.3 months (range, 3.8-34.5 months) for VMAT and 31.8 months (range, 1.8-117.2 months) for ssIMRT, overall survival rates were similar between the treatments (93.5% VMAT vs 91.5% ssIMRT at 1 year; 60.0% VMAT and 61.4% ssIMRT at 2 years; P = .868). Recurrence-free survival rates were similar (73.3% VMAT vs 79.5% ssIMRT at 1 year, 59.9% VMAT and 61.8% ssIMRT at 2 years; P = .614), as were pathologic complete response rates (31.2% VMAT vs 23.3% ssIMRT; P = .41) and toxicity and postoperative complications (radiation pneumonitis 9% VMAT vs 15.4% ssIMRT; pericardial effusion 2% VMAT vs 7% ssIMRT; esophageal fistula and stricture 9% VMAT vs 13% ssIMRT; all P > .05).
Conclusion
Compared with ssIMRT, VMAT had better target conformity with similar organ sparing and comparable rates of survival, recurrence, and toxicity. These results suggest that VMAT can be safe and effective for esophageal cancer.
Summary.
Outcomes after volumetric modulated arc therapy (VMAT) for thoracic esophageal carcinoma are largely unknown. We conducted a propensity matched analysis of 65 patients treated with VMAT and concurrent chemotherapy and 130 patients treated with step-and-shoot intensity modulated radiation therapy and concurrent chemotherapy. We found that VMAT had better target conformity with comparable toxicity, response, recurrence, and survival outcomes. VMAT is an efficient, safe, and effective radiation delivery method for the treatment of esophageal cancer.
Introduction
Radiation therapy, whether administered as neoadjuvant or definitive treatment, is effective for localized esophageal cancer and is given most often with concurrent chemotherapy. Preoperative radiation with concurrent chemotherapy followed by surgery is the current standard treatment for operable esophageal cancer, with 5-year overall survival (OS) rates of up to 47%.1, 2, 3 Significant technologic advances have been made in radiation therapy techniques over the past 20 years, and static intensity modulated radiation therapy (IMRT) has become widely used for esophageal cancer because of its dosimetric advantages over 3-dimensional conformal radiation therapy (CRT) in terms of target conformity and homogeneity and its ability to spare the lungs and heart.4, 5, 6 These dosimetric advantages may translate into improvements in the therapeutic ratio and reduced toxicity.6, 7 One retrospective study that compared the long-term outcomes of patients with esophageal cancer after 3-dimensional CRT (n = 413) or IMRT (n = 263) demonstrated that rates of OS, local-regional control, and cardiac death were significantly better after IMRT.6
Volumetric modulated arc therapy (VMAT) is a newer type of IMRT in which IMRT is delivered by combining continuously rotating gantry motion with simultaneous variations in dose rate, gantry speed, and segment shape.8 Compared with static IMRT, VMAT can achieve similar or superior dosimetry in a much shorter delivery time9, 10 and has been shown to be equally effective for head and neck cancer, prostate cancer, and lung cancer.11, 12, 13 However, little is known about the effectiveness of VMAT for carcinoma of the thoracic esophagus. To address this gap, we retrospectively analyzed dosimetric and clinical outcomes after VMAT for patients with carcinoma of the thoracic esophagus treated at a single institution.
Methods and materials
Patient population
We retrospectively reviewed patients with carcinoma of the thoracic esophagus who underwent radiation and concurrent chemotherapy at a single tertiary cancer center. Patients who had an alternative radiation scheme (dose >50.4 Gy or <45 Gy), hematologic metastasis or cervical esophageal carcinoma, and incomplete follow-up information were excluded from the study. Ultimately, we identified 65 patients who were treated with VMAT and concurrent chemotherapy from November 2012 through March 2016 and 487 patients who were treated with step-and-shoot IMRT (ssIMRT) and concurrent chemotherapy from March 2005 through April 2016. Disease staging in all cases was done with full-body positron emission tomography/computed tomography (PET/CT) scanning and esophagogastroduodenoscopy with endoscopic ultrasonography (EGD/EUS). Fine-needle aspiration was used as needed to sample nodes suspected of harboring disease. Clinicopathologic characteristics such as tumor invasion, nodal metastasis, and disease stage were classified on the basis of the TNM classification system by the International Union against Cancer, 7th edition.
Treatment
All cases were discussed by a multidisciplinary team before treatment was initiated, and the plan in all cases was to administer preoperative concurrent chemoradiation therapy. Patients with advanced disease were given induction chemotherapy with the choice of induction and concurrent chemotherapy regimens at the discretion of the treating medical oncologists. Chemotherapy agents were fluorouracil or paclitaxel/docetaxel based. All patients completed concurrent chemoradiation therapy. At 1 month after the concurrent chemoradiation therapy, treatment response was evaluated with PET/CT scans and EGD/EUS. Radiation oncologists, medical oncologists, and thoracic surgeons evaluated these responses in light of patients' performance status, comorbidities, and preferences and decided at that time to proceed with surgery or observation.
Treatment planning for VMAT and ssIMRT
Four-dimensional CT scans (5 mm slice thickness) were obtained for treatment planning with patients positioned supine with their arms above their head and immobilized with a thermoplastic body mask. Gross tumor volume was defined on the basis of PET/CT scans and EGD/EUS and was contoured on the 4-dimensional CT simulation scan. The clinical target volume was defined as the gross tumor volume with 3 to 5 cm superior-inferior margins and 1 cm lateral and anterior-posterior margins and included positive nodes with 1 cm uniform margins.
The planning target volume (PTV) consisted of the clinical target volume with additional 0.5 cm margins. All PTVs and organs at risk (OARs) for both VMAT and ssIMRT were contoured with a Pinnacle treatment planning system, Version 9.10. In principle, the dose prescription was designed to cover 95% of the PTV with 50.4 Gy in 28 fractions or 45 Gy in 25 fractions. For ssIMRT, 4 to 9 fields were used. The manufacturer's direct machine parameter optimization module was used for treatment planning, and 4 to 9 angles were used to evenly separate coplanar fields. For VMAT, 2 to 4 coplanar arcs were used. Daily cone beam CT images were generated before each treatment session for each patient to verify the set-up, and daily corrections were done manually by technicians.
Dosimetric evaluation
All dose-volume histogram (DVH) parameters of targets and OARs were extracted and calculated from the Pinnacle treatment plans. Dosimetric variables were compared by analyzing DVHs from 65 patients with VMAT and 130 patients with ssIMRT. Data are reported as mean doses and volume receiving more than x dose of the various OARs. Dose homogeneity was evaluated with a conformity index (CI) and target conformity to the PTV with a homogeneity index (HI). Higher CI values and lower HI values indicated better conformity and homogeneity of the dose to targets.
Treatment response and toxicity
We evaluated the preliminary treatment results and toxicity, especially pulmonary toxicity and postoperative complications, in patients with thoracic esophageal cancer treated with VMAT or ssIMRT. Tumor responses were classified as complete response, partial response, stable disease, and progression on the basis of the Response Evaluation Criteria in Solid Tumors, Version 1.1. Clinical complete response was defined as no evidence of disease on both PET/CT and EGD/EUS and no residual tumor on biopsy. Treatment-related toxicity was assessed retrospectively from patient records and grades assigned according to the Common Terminology Criteria for Adverse Events, Version 4.0 and the Radiation Therapy Oncology Group criteria. Toxicity was evaluated by physicians once a week during chemoradiation therapy and at every follow-up visit after treatment.
Follow-up
The first follow-up visit was scheduled for 1 month after chemoradiation therapy/surgery, and follow-up PET/CT, EGD/US, and hematologic examinations were performed. Subsequent follow-up visits were scheduled every 4 months for the first year, every 6 months for the second year, and annually thereafter, with the same studies and tests performed, if possible.
Propensity score matching
To reduce bias resulting from the retrospective nature of this study and to enhance comparability between the treatment groups, we used propensity score analysis. Patients were matched by propensity scores based on age, sex, clinical tumor stage, PTV, tumor location and histology, receipt of induction chemotherapy, and receipt of surgery. We ultimately matched the 65 patients who received VMAT with 130 patients who received ssIMRT (1:2 ratio, caliper 0.10). Characteristics of all eligible cases and propensity score–matched pairs are summarized in Table 1.
Table 1.
Characteristics of all eligible cases and propensity-score-matched pairs
| Variables | All Eligible Cases |
P | Propensity Score-Matched Pairs |
P | ||
|---|---|---|---|---|---|---|
| VMAT (n = 65) | ssIMRT (n = 487) | VMAT (n = 65) | ssIMRT (n = 130) | |||
| ECOG performance score | ≤2 | ≤2 | ≤2 | ≤2 | ||
| Age, y, median (range) | 62 (43-84) | 62 (20-86) | .562 | 62 (43-84) | 63 (20-86) | .928 |
| Sex | .841 | .644 | ||||
| Male, n (%) | 56 (86) | 415 (85) | 56 (86) | 115 (88) | ||
| Female, n (%) | 9 (14) | 72 (15) | 9 (14) | 15 (12) | ||
| Disease stage (UICC 7th) | .072 | .209 | ||||
| IA+IB, n (%) | 7 (11) | 22 (4) | 7 (11) | 8 (6) | ||
| IIA+IIB, n (%) | 15 (23) | 160 (33) | 15 (23) | 48 (37) | ||
| IIIA+IIIB+IIIC, n (%) | 38 (58) | 282 (58) | 38 (58) | 67 (52) | ||
| IV, n (%) | 5 (8) | 23 (5) | 5 (8) | 7 (5) | ||
| Tumor histology | .002 | .909 | ||||
| Adenocarcinoma, n (%) | 48 (74) | 429 (88) | 48 (74) | 95 (73) | ||
| Squamous cell cancer, n (%) | 17 (26) | 58 (12) | 17 (26) | 35 (27) | ||
| PTV volume, cm3, mean ± SD | 570 ± 333 | 725 ± 394 | .003 | 570 ± 333 | 585 ± 333 | .764 |
| Location | .005 | .758 | ||||
| Upper, n (%) | 2 (3) | 13 (2) | 2 (3) | 7 (5) | ||
| Middle, n (%) | 14 (22) | 42 (9) | 14 (22) | 26 (20) | ||
| Distal, n (%) | 49 (75) | 432 (89) | 49 (75) | 97 (75) | ||
| Induction chemotherapy | .247 | .912 | ||||
| Yes, n (%) | 19 (29) | 178 (37) | 19 (29) | 39 (30) | ||
| No, n (%) | 46 (71) | 309 (63) | 46 (71) | 91 (60) | ||
| Surgery | .243 | .685 | ||||
| Yes, n (%) | 32 (49) | 277 (57) | 32 (49) | 60 (46) | ||
| No, n (%) | 33 (51) | 210 (43) | 33 (51) | 70 (54) | ||
| Radiation dose, Gy, median (range) | 50.4 (45-50.4) | 50.4 (45-50.4) | .121 | 50.4 (45-50.4) | 50.4 (45-50.4) | .61 |
ECOG, Eastern Cooperative Oncology Group; PTV, planning target volume; ssIMRT, step and shoot intensity-modulated radiotherapy; UICC, International Union Against Cancer; VMAT, volumetric modulated arc therapy.
Statistical analysis
Each variable was compared between the 2 treatment groups with an independent-sample t or Wilcoxon test for continuous variables and χ2 test for categorical variables. Rates of OS, recurrence-free survival (RFS), and progression-free survival (PFS) were calculated with the Kaplan–Meier method with group estimates compared with log-rank tests. OS, RFS, and PFS were measured from the date of initial treatment. P values were two-sided, with a value < .05 indicating statistical significance. SPSS software, Version 23.0 was used for statistical analysis, and R software was used for propensity score matching. GraphPad Prism, Version 5.0 was used to construct the Kaplan-Meier survival curves.
Results
Dose-volume analysis of target coverage and OARs
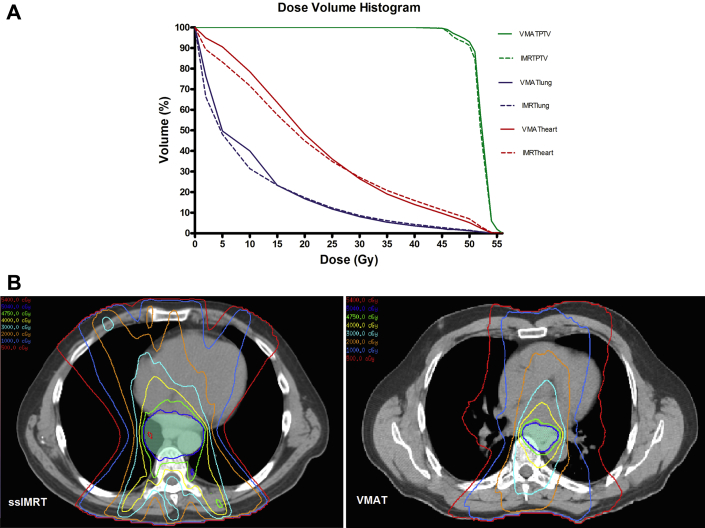
Figure 1A presents a DVH plot of averaged PTVs and OARs from plans of patients who were treated with VMAT (n = 65) and patients who were treated with ssIMRT (n = 130) for esophageal cancer. Figure 1B presents the axial views of the dose distribution. The VMAT plans were slightly superior to ssIMRT in terms of conformity and were similar to ssIMRT in terms of sparing the OARs. Dose distributions for the PTVs and OARs of VMAT (n = 65) and ssIMRT (n = 130) for esophageal cancer are shown in Table 2. These data are presented as the averages over all patients, and errors are shown as standard deviations. This comparison indicates that the CI for VMAT was higher than that for ssIMRT (87.75 ± 10.70 vs 83.20 ± 9.42, respectively; P = .003) and that the heart V5 was higher and the heart V50 was lower for VMAT compared with ssIMRT. Values for lung V5-20, heart V30, heart V40, cordmax, and HI were similar for the 2 treatment groups (all P > .05).
Figure 1.
(A) Cumulative dose-volume histogram indicating the average values for 65 patients treated with volumetric modulated arc therapy and 130 patients treated with step-and-shoot intensity modulated radiation therapy for esophageal cancer, and (B) the axial views of the dose distribution. PTV, planning target volume.
Table 2.
Dosimetric parameters for VMAT and ssIMRT cohorts
| Events | VMAT (n = 65) | ssIMRT (n = 130) | P Value |
|---|---|---|---|
| PTV | |||
| Mean dose, Gy | 52.1 ± 1.2 | 51.8 ± 1.5 | .195 |
| CI, % | 87.75 ± 10.70 | 83.20 ± 9.42 | .003 |
| HI, % | 8.41 ± 5.77 | 9.02 ± 6.44 | .507 |
| Lung (%) | |||
| V5 | 49.7 ± 14.7 | 48.1 ± 13.8 | .45 |
| V10 | 40.0 ± 10.8 | 31.4 ± 9.8 | .733 |
| V15 | 23.3 ± 9.6 | 23.3 ± 7.8 | .97 |
| V20 | 16.8 ± 8.3 | 17.3 ± 6.8 | .661 |
| V25 | 11.8 ± 7.0 | 12.3 ± 5.9 | .603 |
| Mean lung dose, Gy | 9.97 ± 3.38 | 9.79 ± 3.05 | .707 |
| Heart (%) | |||
| V5 | 90.6 ± 20.6 | 83.2 ± 27.1 | .036 |
| V10 | 78.5 ± 22.0 | 71.7 ± 26.7 | .079 |
| V20 | 48.1 ± 22.1 | 44.9 ± 22.5 | .337 |
| V30 | 26.5 ± 15.5 | 27.2 ± 17.7 | .798 |
| V40 | 14.0 ± 9.3 | 15.9 ± 11.2 | .239 |
| V50 | 5.2 ± 4.4 | 7.0 ± 5.7 | .016 |
| Mean heart dose, Gy | 22.0 ± 7.1 | 21.0 ± 8.4 | .420 |
| Cordmax, Gy | 38.2 ± 5.8 | 38.9 ± 6.3 | .448 |
CI, conformity index; Cordmax, maximum (point) dose to the spinal cord; HI, homogeneity index; PTV, planning target volume; ssIMRT, step and shoot intensity-modulated radiotherapy; VMAT, volumetric modulated arc therapy.
Survival
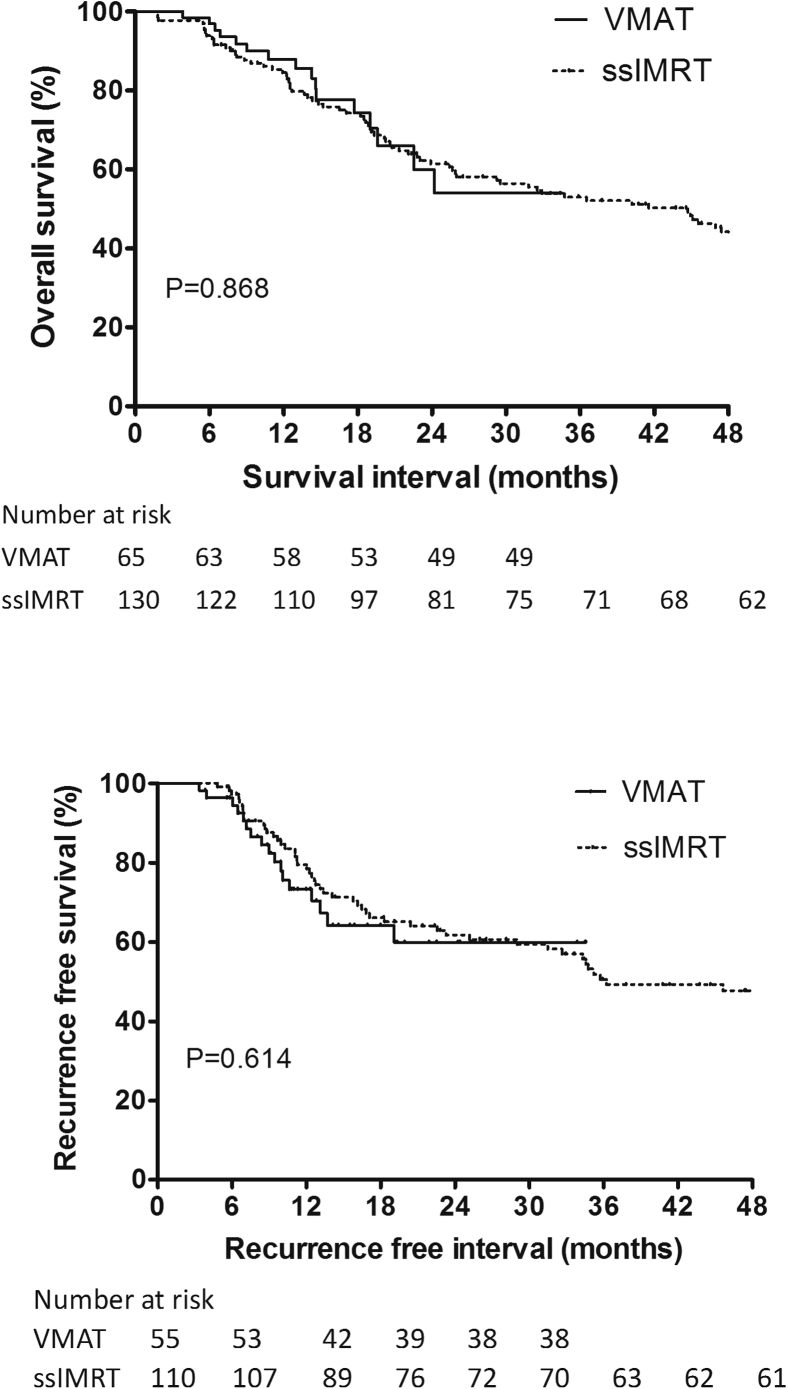
The median follow-up interval was 14.3 months (range, 3.8-34.5 months) for patients treated with VMAT and 31.8 months (range, 1.8-117.2 months) for patients treated with ssIMRT. The 2 groups showed similar OS rates at 1 year and 2 years (93.5% VMAT vs 91.5% ssIMRT at 1 year and 60.0% VMAT vs 61.4% ssIMRT at 2 years; P = .868) and similar RFS (73.3% VMAT vs 79.5% ssIMRT at 1 year and 59.9% VMAT vs 61.8% ssIMRT at 2 years, P = .614) and PFS rates (61.7% VMAT vs 68.4% ssIMRT at 1 year and 50.5% VMAT vs 53.8% ssIMRT at 2 years; P = .471). The OS and RFS curves are shown in Figure 2.
Figure 2.
Overall survival (top) and recurrence-free survival (bottom) curves for patients treated with volumetric modulated arc therapy or step-and-shoot intensity modulated radiation therapy (ssIMRT).
Treatment response
Response rates were also no different in the 2 treatment groups. The clinical complete response rates were 53.8% for the VMAT group and 45.5% for the ssIMRT group (P = .278). Among the 32 patients who underwent surgery after VMAT with concurrent chemotherapy, the pathologic complete response (pCR) was 31.2%. The pCR rate among the 60 patients who underwent surgery after ssIMRT with concurrent chemotherapy was 23.3% (P = 0.41).
Toxicity
The incidence and severity of chemoradiation therapy–induced toxicity are shown in Table 3. The types of toxicity experienced were similar for VMAT and ssIMRT, with radiation pneumonitis rates of 9.2% in the VMAT group and 15.4% in the ssIMRT group (P = .233). No patients in the VMAT group experienced grade ≥3 radiation pneumonitis, but the ssIMRT group had 2 grade 5, 1 grade 4, and 1 grade 3 episodes of radiation pneumonitis. Surgical complications (eg, pneumonia, respiratory insufficiency, atrial fibrillation, anastomotic leak, and wound infection) were also comparable between the groups (Table 4) as were the length of hospital stay and number of postoperative deaths within 30 days (P > .05).
Table 3.
Chemoradiotherapy-induced toxicity in the propensity-matched VMAT and ssIMRT cohorts
| Events | VMAT Group (n = 65) |
ssIMRT Group (n = 130) |
P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of events (%) | Toxicity |
Grade |
No. of events (%) | Toxicity |
Grade |
||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||||
| Radiation pneumonitis | 6 (9.2) | 2 | 4 | 0 | 0 | 0 | 20 (15.4) | 10 | 6 | 1 | 1 | 2 | .233 |
| Pulmonary fibrosis | 0 | 0 | 0 | 0 | 0 | 0 | 7 (5.4) | 7 | 0 | 0 | 0 | 0 | .057 |
| Pleural effusion | 5 (7.7) | 4 | 1 | 0 | 0 | 0 | 20 (15.4) | 18 | 1 | 1 | 0 | 0 | .057 |
| Arrhythmia | 1 (1.5) | 1 | 0 | 0 | 0 | 0 | 2 (1.5) | 0 | 1 | 1 | 0 | 0 | 1.000 |
| Pericardial effusion | 1 (1.5) | 0 | 1 | 0 | 0 | 0 | 9 (6.9) | 0 | 8 | 1 | 0 | 0 | .108 |
| Esophagitis | 57 (87.7) | 16 | 33 | 8 | 0 | 0 | 73 (56.1) | 12 | 44 | 17 | 0 | 0 | 0 |
| Fistula | 1 (1.5) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .156 |
| Esophageal stricture | 8 (12.3) | 3 | 2 | 3 | 0 | 0 | 13 (10.0) | 1 | 4 | 8 | 0 | 0 | .624 |
| Feeding tube | 15 (23.1) | 32 (24.6) | .813 | ||||||||||
ssIMRT, step and shoot intensity-modulated radiotherapy; VMAT, volumetric modulated arc therapy.
Table 4.
Surgical complications after VMAT vs after ssIMRT
| Events | VMAT (n = 32) | ssIMRT (n = 60) | P Value |
|---|---|---|---|
| Pulmonary complication, n (%) | 7 (22) | 13 (22) | .982 |
| Pneumonia | 7 (22) | 7 (12) | .194 |
| Respiratory insufficiency | 1 (3) | 2 (3) | .957 |
| Acute respiratory distress syndrome | 0 | 2 (3) | .296 |
| Pulmonary embolism | 0 | 1 (2) | .463 |
| Cardiac complication, n (%) | 6 (19) | 5 (8) | .142 |
| Atrial fibrillation | 4 (13) | 5 (8) | .522 |
| GI complication, n (%) | 6 (19) | 16 (27) | .397 |
| Ileus | 3 (9) | 1 (2) | .084 |
| Fistula | 2 (6) | 2 (3) | .514 |
| Obstruction | 0 | 1 (2) | .463 |
| Bowel necrosis | 1 (3) | 0 | .169 |
| Anastomotic leak | 3 (9) | 10 (17) | .339 |
| Anastomotic stricture | 1 (3) | 5 (8) | .335 |
| Wound infection, n (%) | 2 (6) | 7 (12) | .405 |
| Hospital length, d, mean | 11.8 ± 11.5 | 11.3 ± 8.3 | .788 |
| Readmissions, n (%) | 5 (16) | 7 (12) | .591 |
| Death within 30 d, n (%) | 1 (3) | 2 (3) | .957 |
GI, gastrointestinal; ssIMRT, step and shoot intensity-modulated radiotherapy; VMAT, volumetric modulated arc therapy.
Discussion
To our knowledge, this is the first report of dosimetric variables and clinical outcomes after VMAT with concurrent chemotherapy for patients with thoracic esophageal cancer. To reduce bias associated with the retrospective nature of this study and to explore potential dosimetric and clinical advantages of VMAT over ssIMRT, we matched and compared patients who had undergone VMAT with those who had undergone ssIMRT. Our findings suggest that VMAT for thoracic esophageal cancer could provide slightly better target conformity but similar homogeneity and sparing of normal tissue. Furthermore, VMAT resulted in encouraging and comparable rates of survival, recurrence, and pCR without increasing chemoradiation therapy–related toxicity or surgical complications compared with ssIMRT.
Although static IMRT has largely replaced 3-dimensional CRT as the standard radiation technique for the treatment of a variety of cancers, VMAT is a novel type of IMRT that is becoming increasingly popular because of the speed at which the radiation can be delivered. Delivery times reportedly can be reduced by 50% to 70% with VMAT compared with static IMRT.9, 10, 14, 15 VMAT resulted in similar or better target conformity and homogeneity, with slightly higher V5 but reduced high-dose radiation of the lung and heart compared with static IMRT.9, 10, 16 Specifically, we found that PTV homogeneity and OAR sparing were similar for the 2 techniques but that VMAT delivered better PTV conformity.
In our study, VMAT did not increase the risk of radiation pneumonitis, pulmonary fibrosis, or pleural effusion compared with ssIMRT. VMAT also seemed to confer a lower risk of radiation pneumonitis (9.2% vs 15.4% for ssIMRT), although this apparent difference was not statistically significant (P = .233). It is tempting to speculate that the apparent reduction in lung V20-V25 with VMAT may have reduced the corresponding risk of radiation pneumonitis; however, the small number of patients in our study precluded our ability to test this speculation. Future larger studies are needed to address this point.
Cardiac complications have been reported to be related to heart V30 and higher as well as mean heart dose.17, 18 A study of 101 patients with inoperable esophageal cancer who were treated with chemoradiation therapy showed a significant increase in the risk of pericardial effusion when the mean pericardial dose exceeded 26.1 Gy (73% vs 13%, P = .002).18 Thus, reducing the radiation dose to the heart may reduce morbidity and even possibly long-term non-cancer-related mortality. In our study, VMAT led to smaller amounts of the heart being exposed to high-dose radiation (V50), which might be expected to reduce the risk of radiation-induced heart disease.
VMAT has been used to treat several kinds of cancers, particularly those of the head and neck and prostate12, 13; however, only a few studies, all with a small number of patients, have reported the clinical outcomes after VMAT. This study is among the first to report detailed treatment outcomes after VMAT for esophageal cancer. In brief, the OS, RFS, and PFS rates in our study were similar or more favorable compared with those of other published studies. Our estimated OS rates for 65 patients consecutively treated with VMAT of 93.5% at 1 year and 60% at 2 years compare well with those of our ssIMRT cohort and with other recent studies showing OS rates to be above 80% at 1 year and 42.8% to 61.4% at 2 years.19, 20, 21 However, the follow-up interval for the ssIMRT cohort was much longer than that of the VMAT cohort, which may result in biases.
pCR is an independent favorable prognostic factor for survival and recurrence among patients who received neoadjuvant chemoradiation therapy for esophageal cancer.22, 23, 24 Our observed pCR rate of 31.2% after VMAT is in line with the pCR rate for our ssIMRT cohort and with those of previously published studies (18%-43%).3, 25, 26
Our study further showed that toxicity associated with VMAT, given with concurrent therapy, was largely low grade and tolerable. The most common form of toxicity in both groups was esophagitis, which did not exceed grade 3 in either group. The most common late side effect was esophageal stricture,20, 27 which was observed in 12.3% of patients in the VMAT group and 10% of patients in the ssIMRT group. The rates of esophageal stricture and fistula in our study did not differ between the 2 treatment groups. Moreover, VMAT did not lead to a higher incidence of feeding tube placement (23.1% VMAT vs 24.6% ssIMRT), and those rates were lower in both groups than what has been reported elsewhere (40%).21
The occurrence of severe postoperative complications has been associated elsewhere with both worse survival (hazard ratio, 2.099; 95% confidence interval, 1.137-3.878; P = .018) and increased risk of recurrence (odds ratio, 2.100; 95% confidence interval, 1.008-4.366; P = .048).28 We found no difference between treatment techniques in terms of total postoperative complications, 30-day mortality, or length of hospital stay, and the rates of these complications were similar to those from other studies. Postoperative complications have been experienced by 22.8% to 49% of patients with esophageal cancer treated with preoperative chemoradiation therapy followed by surgery29, 30, 31, 32, 33 and included pulmonary complications (5.7%-48.3%),1, 3, 26, 29, 32, 33 respiratory failure (5.7%-8.3%),1, 29 pneumonia (20.8%),1 cardiac complications (5.7%-24.1%),3, 26, 29, 32, 33 and anastomotic leakage (2.9%-22%).1, 3, 26, 29, 32 Whereas our postoperative 30-day mortality rate was 3%, the rates in other studies have ranged from 2.5% to 24%.3, 26, 29, 30, 31, 32, 33 Our mean length of hospital stay (11.3-11.8 days) was also no longer than the median of 11.5 to 27 days reported by others.1, 30 Collectively, these results indicate that toxicity in our study was better (or at least no worse) than that in previously published studies. These encouraging results show the feasibility of VMAT as a component of multimodality treatment for esophageal cancer.
Our study had several limitations. Chief among them were the relatively small patient numbers and short follow-up period. Nevertheless, we found that VMAT, compared with ssIMRT, produced slightly superior plans and similar organ sparing. Our findings further suggest that VMAT can be a safe and effective treatment for locally advanced esophageal cancer in terms of survival and recurrence with acceptable complications, despite the small number of patients. Prospective studies with larger numbers of patients are needed to extend these results and establish VMAT as an effective component of treatment for esophageal cancer.
Footnotes
Conflicts of interest: SHL received grant funding from Hitachi Chemical Inc., Peregrine Pharmaceuticals, STCube Pharmaceuticals, and Genentech, as well as an honorarium from AstraZeneca.
References
- 1.Tepper J., Krasna M.J., Niedzwiecki D. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lv J., Cao X.F., Zhu B., Ji L., Tao L., Wang D.D. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16:1649–1654. doi: 10.3748/wjg.v16.i13.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P., Hulshof M.C., van Lanschot J.J. Preoperative chemoradiotherapy for esophageal or junctional cancer. New Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Chandra A., Guerrero T.M., Liu H.H. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol. 2005;77:247–253. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Kole T.P., Aghayere O., Kwah J., Yorke E.D., Goodman K.A. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1580–1586. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 6.Lin S.H., Wang L., Myles B. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin S.H., Zhang N., Godby J. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer. 2016;122:917–928. doi: 10.1002/cncr.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangaraj D., Oddiraju S., Sun B. Fundamental properties of the delivery of volumetric modulated arc therapy (VMAT) to static patient anatomy. Med Phys. 2010;37:4056–4067. doi: 10.1118/1.3453575. [DOI] [PubMed] [Google Scholar]
- 9.Lin C.Y., Huang W.Y., Jen Y.M. Dosimetric and efficiency comparison of high-dose radiotherapy for esophageal cancer: Volumetric modulated arc therapy versus fixed-field intensity-modulated radiotherapy. Dis Esophagus. 2014;27:585–590. doi: 10.1111/dote.12144. [DOI] [PubMed] [Google Scholar]
- 10.Van Benthuysen L., Hales L., Podgorsak M.B. Volumetric modulated arc therapy vs. IMRT for the treatment of distal esophageal cancer. Med Dosim. 2011;36:404–409. doi: 10.1016/j.meddos.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita H., Haga A., Takahashi W. Volumetric modulated arc therapy for lung stereotactic radiation therapy can achieve high local control rates. Radiat Oncol. 2014;9:243. doi: 10.1186/s13014-014-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonteyne V., Lumen N., Ost P. Hypofractionated intensity-modulated arc therapy for lymph node metastasized prostate cancer: early late toxicity and 3-year clinical outcome. Radiother Oncol. 2013;109:229–234. doi: 10.1016/j.radonc.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Guo R., Tang L.L., Mao Y.P. Clinical outcomes of volume-modulated arc therapy in 205 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. PLoS One. 2015;10:e0129679. doi: 10.1371/journal.pone.0129679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt A., van Vliet-Vroegindeweij C., Mans A., Belderbos J.S., Damen E.M. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: A comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys. 2011;81:1560–1567. doi: 10.1016/j.ijrobp.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Holt A., Van Gestel D., Arends M.P. Multi-institutional comparison of volumetric modulated arc therapy vs. intensity-modulated radiation therapy for head-and-neck cancer: A planning study. Radiat Oncol. 2013;8:26. doi: 10.1186/1748-717X-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolini G., Ghosh-Laskar S., Shrivastava S.K. Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry IMRT and 3D conformal radiotherapy for advanced esophageal cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2012;84:553–560. doi: 10.1016/j.ijrobp.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Gayed I.W., Liu H.H., Yusuf S.W. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47:1756–1762. [PubMed] [Google Scholar]
- 18.Wei X., Liu H.H., Tucker S.L. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 19.Tu L., Sun L., Xu Y. Paclitaxel and cisplatin combined with intensity-modulated radiotherapy for upper esophageal carcinoma. Radiat Oncol. 2013;8:75. doi: 10.1186/1748-717X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roeder F., Nicolay N.H., Nguyen T. Intensity modulated radiotherapy (IMRT) with concurrent chemotherapy as definitive treatment of locally advanced esophageal cancer. Radiat Oncol. 2014;9:191. doi: 10.1186/1748-717X-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La T.H., Minn A.Y., Su Z. Multimodality treatment with intensity modulated radiation therapy for esophageal cancer. Dis Esophagus. 2010;23:300–308. doi: 10.1111/j.1442-2050.2009.01004.x. [DOI] [PubMed] [Google Scholar]
- 22.Swisher S.G., Hofstetter W., Wu T.T. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT) Ann Surg. 2005;241:810–817. doi: 10.1097/01.sla.0000161983.82345.85. discussion 817-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger A.C., Farma J., Scott W.J. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Meredith K.L., Weber J.M., Turaga K.K. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159–1167. doi: 10.1245/s10434-009-0862-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.L., Park S.I., Kim S.B. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15:947–954. doi: 10.1093/annonc/mdh219. [DOI] [PubMed] [Google Scholar]
- 26.Walsh T.N., Noonan N., Hollywood D., Kelly A., Keeling N., Hennessy T.P. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. New Eng J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 27.Ajani J.A., Winter K., Komaki R. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 2008;26:4551–4556. doi: 10.1200/JCO.2008.16.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luc G., Gronnier C., Lebreton G. Predictive factors of recurrence in patients with pathological complete response after esophagectomy following neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter study. Ann Surg Oncol. 2015;22:S1357–S1364. doi: 10.1245/s10434-015-4619-8. [DOI] [PubMed] [Google Scholar]
- 29.Apinop C., Puttisak P., Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41:391–393. [PubMed] [Google Scholar]
- 30.Le Prise E., Etienne P.L., Meunier B. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779–1784. doi: 10.1002/1097-0142(19940401)73:7<1779::aid-cncr2820730702>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Bosset J.F., Gignoux M., Triboulet J.P. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. New Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 32.Burmeister B.H., Smithers B.M., Gebski V. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Wei C., Tucker S.L. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;86:885–891. doi: 10.1016/j.ijrobp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]