Abstract
Purpose
In patients with esophageal cancer (EC), intensity modulated radiation therapy (IMRT) improves dose sparing to the heart and lung, with some evidence showing clinical benefit. Herein, we report our cumulative clinical experience with the use of IMRT for EC.
Methods and materials
This is a retrospective analysis of 587 patients with nonmetastatic EC who were treated consecutively with IMRT from January 2004 to June 30, 2013. All patients with stage I-IVA (American Joint Committee on Cancer 2002) received concurrent chemoradiation therapy either preoperatively or definitively. The Kaplan-Meier method was used to compute overall survival (OS) and locoregional recurrence-free survival and disease-free survival. The Common Terminology Criteria for Adverse Events, Version 4.0 were used to grade acute and subacute complications.
Results
The median radiation dose was 50.4 Gy in 28 daily fractions. As of July 2015, the median follow-up was 31.4 months (range, 2.9-130.7 months) for all patients and 61.8 months (range, 7.7-130.7 months) for survivors. The median OS was 38.9 months, and the 1-, 3-, and 5-year OS rates were 86.7%, 51.8%, and 41.2%, respectively. The 1-, 3-, and 5-year locoregional recurrence-free survival rates were 77.6%, 68.2%, and 66.1%, respectively, and the 1-, 3-, and 5-year disease-free survival rates were 58.6%, 43.7%, and 41.4%, respectively. Outcomes for both trimodality and bimodality treated patients were better than the outcomes reported in the literature. Eight patients (1.4%) experienced grade ≥3 pneumonitis, and 74 patients (13%) developed grade ≥3 esophagitis. For patients who underwent surgery, the most common postoperative complications were pneumonia (9.6%), anastomotic leakage (11.1%), and atrial fibrillation (12.5%).
Conclusions
This is the largest, single institutional study to date on the long-term outcomes of treatment with IMRT for EC. For photon-based radiation therapy, IMRT yields excellent outcomes and should be considered for the treatment of EC.
Summary.
Radiation therapy is an integral part of the management of esophageal cancer. Although the current standard is 3-dimensional conformal radiation therapy, intensity modulated radiation therapy (IMRT) affords improved organ sparing effects over the 3-dimensional technique. However, the reported clinical experience with IMRT is sparse. Herein, we report the largest, single institutional experience using IMRT, demonstrating excellent toxicity and survival outcomes relative to historical controls for patients who were treated with neoadjuvant or definitive chemoradiation therapy.
Introduction
Esophageal carcinoma (EC) has a poor prognosis, with an estimated 482,300 new cases and 406,800 deaths worldwide each year.1 In the United States, 16,980 new cases and 15,590 deaths were estimated in 2015.2 For localized EC, although concurrent chemoradiation therapy (CCRT) followed by surgery is the standard of care in the United States, some patients are not eligible for or refuse surgery.3, 4 Patients who receive definitive chemoradiation therapy without surgery have a 5-year survival rate of 20%.5 Radiation therapy can cause significant toxicities that are largely determined by the radiation quality and techniques used. Three-dimensional conformal radiation therapy (3D-CRT), which is still commonly used today, delivers excessive radiation doses to the lungs and heart, which may result in increased risk of toxicities such as postoperative complications and radiation pneumonitis.
Intensity modulated radiation therapy (IMRT) is an advanced radiation technique that has significant dosimetric advantages over 3D-CRT in terms of conformity and dose homogeneity to the target and normal tissue sparing.6, 7, 8 Do the dosimetric advantages translate into clinical benefits? Published reports have demonstrated that the use of IMRT may be associated with better survival and reduced toxicities compared with 3D-CRT; however, these studies were mostly retrospective, single institutional studies that involved smaller cohorts of patients.7, 8
In this report, we present our cumulative experience of patients with EC who were treated with concurrent chemotherapy and IMRT with or without surgery at a single institution over the course of nearly a decade. We report the long-term survival outcomes and toxicities that were associated with this treatment. Our favorable long-term results may have implications for the utility of this radiation therapy technique.
Methods and materials
Study cohort
This is a single institutional retrospective analysis of consecutive patients with EC who had localized disease treated with CCRT with IMRT between 2004 and June 30, 2013. Patients were excluded if they initially presented with metastatic disease (n = 7) or disease at an unknown stage (n = 15). All patients had baseline staging that included imaging studies (computed tomography [CT] or fluorodeoxyglucose [FDG] positron emission tomography [PET]-CT) and T-staging was assessed with esophagogastroduodenoscopy (EGD) with endoscopic ultrasonography. Nodal (N) staging was a combination of fine-needle aspiration–positive nodal disease that was assessed during the endoscopic ultrasonography procedure and by PET-CT imaging if the nodes were not assessable due to location in the peritumoral region or were inaccessible by needle biopsies. Clinical staging was determined with the 6th edition (2002) of the American Joint Committee on Cancer TNM staging system.9 The institutional review board approved this analysis.
Treatment and radiation planning
Concurrent chemotherapy consisted of fluoropyrimidine (intravenous or oral) or a platinum compound (cisplatin, carboplatin, or oxaliplatin) and a taxane, as described previously.10, 11 Induction chemotherapy with taxane and fluoropyrimidine (intravenous or oral) with platinum-based compounds was given to some patients either during the clinical trial or at the clinical discretion of the medical oncologists. At 5 to 6 weeks after CCRT completion, most patients underwent restaging using CT or FDG-PET/CT and EGD with a biopsy of the primary disease site and were discussed at the weekly multidisciplinary esophageal tumor board. If surgery was an option, it was usually performed 4 to 8 weeks after the follow-up visit. The most common esophagectomy procedure was Ivor Lewis. A few patients also underwent transhiatal, total (three-field technique), or minimally invasive esophagectomy.
All patients were treated with IMRT to the dose of 41.4 to 66 Gy in 23 to 33 fractions (range, 1.8-2 Gy per fraction) given 5 days per week. Radiation therapy was planned using 4-dimensional CT simulation. Gross tumor volume was contoured on the basis of the results of the EGD, PET/CT, or CT scans (when PET/CT was not obtained) and the maximum-intensity projected phase image of the 4-dimensional CT simulation. The clinical target volume was defined as the gross tumor volume with 3- to 4-cm superior-inferior margins and 1-cm lateral and anterior-posterior margins and positive nodes regions. The planning target volume consisted of the clinical target volume with additional 0.5-cm margins. All plans were compliant with the dose-volume constraints and similar to the guidelines set forth by the National Comprehensive Cancer Network.12 All patients were treated using step-and-shoot IMRT with posteriorly positioned beam arrangements that were optimized to reduce cardiac dose exposure.13
Outcome measures, toxicity, and perioperative complications assessment
Follow-up investigations included blood tests, EGD with biopsies (first follow-up after chemoradiation therapy and selectively at interval follow-up visits at the discretion of the treating physician), and imaging studies including CT and/or FDG-PET/CT scans (every visit). The follow-up schedule was typically every 3 to 4 months for the first year, every 6 months for the 2 following years, and then once per year for the next 2 years for a total of 5 years of visits after CCRT.
The pathologic response after CCRT was graded into 4 categories, as done on the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) study: grade 1 (no evidence of residual tumor cells), grade 2 (<10% residual tumor cells), grade 3 (10%-50% residual tumor cells), and grade 4 (>50% residual tumor cells).4 R1 resection was defined as tumor cells present at 1 mm or less from the resection margin without evidence of re-excision. R0 resection is complete resection without evidence of tumor cells present at least >1 mm from the resection margin.
Recurrence of any type (locoregional recurrence [LRR], distant recurrence) was diagnosed pathologically (by EGD or CT guidance) or clinically (CT or PET/CT scans) in accordance with individual physician preference or consensus of the multidisciplinary group. The survival follow-up was carried out through our institution's tumor registry, electronic medical records, and/or the Social Security Death Index. OS was calculated from the date of diagnosis to the date of death or censoring at the last follow-up. LRR was defined as any recurrence at the initial primary disease site or in the regional lymph nodes. Nodal regional recurrence could be either in-field or out-field; however, recurrences outside of the potential areas of coverage by radiation therapy (celiac nodes for proximal disease or supraclavicular nodes for distal disease) were scored as distant metastases. Locoregional recurrence-free survival (LRFS) was defined as the duration between the radiation therapy end date and the LRR date or death and the last follow-up date (if no LRR). Disease-free survival (DFS) was defined as the duration between the radiation therapy end date and LRR or distant metastasis date or death and last follow-up date (if no LRR or distant recurrence).
Radiation-related toxicity was scored in accordance with the Common Terminology Criteria for Adverse Events, Version 4.0. Toxicity data were extracted and graded according to physician-reported toxicities recorded at each of the clinical visits during chemoradiation therapy and at follow-up visits after treatment. Because radiation-related toxicities could initially present during treatment, the time to toxicity was calculated from the radiation start date (pneumonitis was evaluated up to 1 year after completion of CCRT, and other toxicities were calculated up to 1 month after completion of CCRT). For perioperative complications, we recorded pulmonary (pneumonia, acute respiratory distress syndrome, pleural effusion, or respiratory insufficiency), gastrointestinal (anastomatic leak, ileus, fistula, bowel obstruction, or bowel necrosis), cardiac (atrial fibrillation, other arrhythmias, myocardial infarction, congestive heart failure), and wound healing complications up to 30 days postoperatively. Moreover, the median length of hospital stay, readmission to the hospital within 60 days, and death within 30 days of surgery were also recorded.
Statistical analysis
Pearson's χ2 (Fisher's exact) test was used to assess measures of association in frequency tables. The equality of group medians was assessed with nonparametric tests for equality. OS, LRFS, distant metastases–free survival (DMFS), and DFS were calculated with Kaplan-Meier estimators. Statistical analysis was performed by using Stata/MP 14.0 for Windows (StataCorp LLC, College Station, TX). A P-value of .05 or less was considered statistically significant. Statistical tests were based on a two-sided significance level.
Results
Patient, tumor, and treatment characteristics
Table 1 summarizes the clinical characteristics of the 587 patients included in this analysis. Briefly, the median age was 62 years (range, 20-89 years). Most patients were male (82.5%), and most had adenocarcinoma (78.4%). Most tumors were located in the distal portion of the esophagus (84.3%), and the median tumor length was 5 cm (1-19 cm). At baseline staging, 2.0% of patients had T1, 10.7 % had T2, 79.6% had T3, and 4.9% had T4 tumors. Most patients (60.5%) had N-positive disease, and 4.8% of patients had celiac nodal involvement. All patients received CCRT, with 36.3% receiving induction chemotherapy. The median radiation dose was 50.4 Gy delivered in daily fractions of 1.8 to 2.0 Gy. Most patients had no residual disease (78.9%) in the first post-CCRT EGD biopsy. Just under half of the patients (47.4%) underwent surgery after CCRT. The most common reason for not undergoing surgery was observation (48.6%) due to patient or physician choice on the basis of a good clinical response.
Table 1.
Demographic, tumor, and treatment characteristics of the 587 patients with esophageal or esophagogastric-junction cancer
| Characteristic | N = 587 | % |
|---|---|---|
| Age (y) | ||
| Median | 62 | – |
| Range | 20-89 | – |
| Sex | ||
| Male | 484 | 82.5 |
| Female | 103 | 17.5 |
| Race/ethnicity | ||
| Caucasian | 504 | 85.9 |
| Other | 83 | 14.1 |
| Tumor histology | ||
| Adenocarcinoma | 460 | 78.4 |
| Squamous-cell carcinoma | 108 | 18.4 |
| Other | 19 | 3.2 |
| Tumor length(cm) | ||
| Median | 5 | – |
| Range | 1-19 | – |
| Tumor location | ||
| Proximal | 48 | 8.2 |
| Mid | 44 | 7.5 |
| Distal/Gastro-Esophageal Junction/Cardiac | 495 | 84.3 |
| EUS T stagea | ||
| T1 | 11 | 1.9 |
| T2 | 63 | 10.7 |
| T3 | 467 | 79.6 |
| T4 | 29 | 4.9 |
| Unknown | 17 | 2.9 |
| EUS N stagea | ||
| N0 | 205 | 34.9 |
| N1 | 355 | 60.4 |
| Unknown | 27 | 4.4 |
| FNA resultsa | ||
| Negative | 52 | 9.1 |
| Positive | 115 | 20.2 |
| Not done | 403 | 70.7 |
| Clinical M stageb | ||
| M0 | 531 | 90.4 |
| M1a | 28 | 4.8 |
| Unknown | 28 | 4.8 |
| Clinical Stageb | ||
| I | 12 | 2.0 |
| II | 199 | 33.9 |
| III | 323 | 55.0 |
| IVa | 27 | 4.6 |
| Unknown | 26 | 4.4 |
| Karnofsky Performance Status | ||
| 80-100 | 531 | 90.5 |
| 50-70 | 56 | 9.5 |
| Chemotherapy modality | ||
| Induction chemotherapy | 213 | 36.3 |
| Concurrent chemotherapy alone | 374 | 63.7 |
| PTV (Gy) | ||
| Median | 50.4 | – |
| Range | 3.6-66 | – |
| <50.4 Gy | 72 | 12.3 |
| 50.4 Gy | 467 | 79.6 |
| >50.4 Gy | 48 | 8.1 |
| First post-radiation therapy EGD biopsy response | ||
| No residual | 463 | 78.8 |
| Residual | 74 | 12.6 |
| Not done | 25 | 4.3 |
| Unknown | 25 | 4.3 |
| Reason for no surgery after CCRT (N = 309) | ||
| Unresectable | 37 | 12.0 |
| Medically inoperable | 58 | 18.8 |
| Observe | 135 | 43.7 |
| Metastasis | 70 | 22.7 |
| Death before surgery | 6 | 1.9 |
| Unknown | 3 | 0.9 |
| Surgery | 278 | 47.4 |
CRT, concurrent chemoradiation therapy; EGD, esophagogastroduodenoscopy; EUS, endoscopic ultrasound; FNA, fine-needle aspiration; IMRT, intensity modulated radiation therapy; PET/CT, positron emission tomography/computed tomography; PTV, planning target volume.
Only 570 patients received EUS.
Clinical stage was assessed by means of endoscopic ultrasonography or CT or PET/CT and was classified according to the sixth editions of the American Joint Committee on Cancer TNM system.
Treatment outcomes
As of July 2015, the median follow-up times were 31.3 months (range, 2.9-130.7 months) for all patients and 61.8 months (range, 7.7-130.7 months) for survivors.
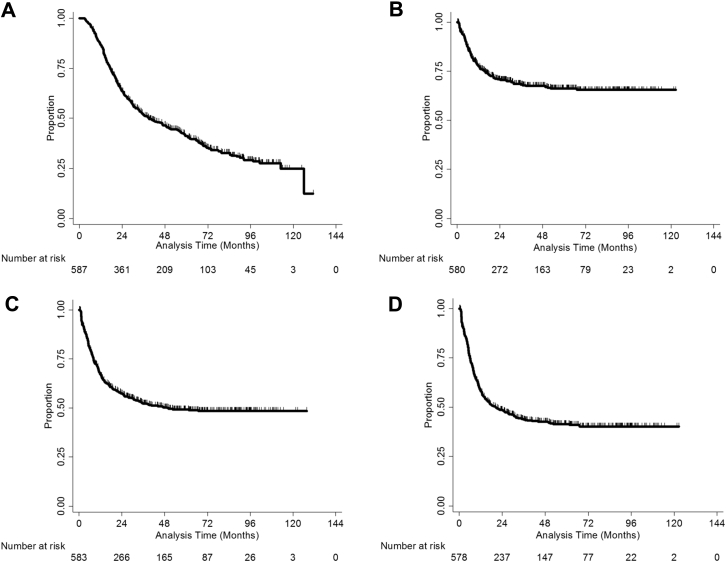
For all patients, the median OS was 38.9 months, and the 1-, 3-, and 5-year OS rates were 86.7%, 51.8%, and 41.2%, respectively (Fig 1A). The 1-, 3-, and 5-year-LRFS rates were 77.6%, 68.2%, and 66.1%, respectively (Fig 1B), and the 1-, 3-. and 5-year DMFS rates were 67.5%, 52.3%, and 49.3%, respectively (Fig 1C). Finally, the 1-, 3-, and 5-year DFS rates were 58.6%, 43.7%, and 41.4%, respectively (Fig 1D).
Figure 1.
Clinical outcomes for the study cohort from the end of radiation therapy to the date of the event in terms of overall survival (A), locoregional recurrence-free survival (B), distant metastatic-free survival (C), and disease-free survival (D).
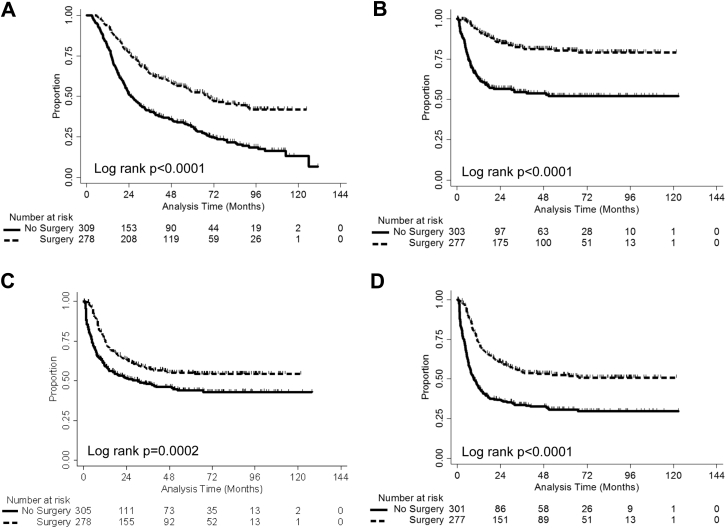
For 309 patients who only received chemotherapy and IMRT without upfront surgical intervention, the estimated median OS was 25.6 months, and the 1-, 3-, and 5-year OS rates were 80.2%, 40.8%, and 31.2%, respectively (Fig 2A). The estimated median LRFS was not reached, but the 1-, 3-, and 5-year LRFS rates were 62.8%, 54.6%, and 52.0%, respectively (Fig 2B). The estimated median DMFS was 30.3 months, and the 1-, 3-, and 5-year DMFS were 59.5%, 47.8%, and 44.0%, respectively (Fig 2C). The estimated median DFS was 9.2 months, and the 1-, 3-, and 5-year DFS rates were 44.5%, 33.6%, and 30.7%, respectively (Fig 2C).
Figure 2.
Clinical outcomes for the study group stratified by whether patients had surgery or not. Outcomes were counted from the last day of radiation therapy to the event in terms of overall survival (A), locoregional recurrence-free survival (B), distant metastatic-free survival (C), and disease-free survival (D).
Of the 278 patients who received surgery after IMRT, 265 patients (95.3%) achieved an R0 resection. According to the tumor regression grade (TRG) criteria, 73 of 278 patients (26.3%) achieved a pathologic complete response (pCR; TRG 1), 82 patients (29.5%) achieved TRG 2, 86 patients (30.9%) achieved TRG 3, 32 patients (11.5%) achieved TRG 4, and 5 were unknown.4 A median of 20 lymph nodes (interquartile range, 14-26) was resected in 278 patients. The estimated median OS was 69.1 months, and the 1-, 3-, and 5-year OS rates were 93.9%, 64.1%, and 52.4%, respectively (Fig 2A). The estimated median LRFS was not reached, but the 1-, 3-, and 5-year LRFS rates were 92.7%, 82.0%, and 80.4%, respectively (Fig 2B). The estimated DMFS was not reached, but the 1-, 3-, and 5-year DMFS rates were 75.9%, 57.3%, and 55.0%, respectively (Fig 2C). The estimated median DFS was 35.1 months, and the 1-, 3-, and 5-year DFS rates were 73.2%, 54.2%, and 52.5%, respectively (Fig 2D).
Treatment-related toxicities and perioperative complications
Sixteen of 587 patients (2.7%) developed grade ≥3 hematologic toxicity, and one developed grade 4 hematologic toxicity. Eight patients (1.4%) experienced grade ≥3 radiation-induced pneumonitis, including 4 grade 5 events (0.7%). Seventy-two patients (12.6%) developed grade ≥3 esophagitis, including 2 patients (0.3%) with grade 4 toxicity. Forty-six patients (12.3%) developed grade ≥3 dysphagia, with 1 patient (0.2%) having developed grade 4 dysphagia. All other nonhematologic grade 3 events occurred in less than 10% of patients. The types of adverse events that occurred during treatment are summarized in Table 2.
Table 2.
Toxicities experienced for 587 patients with esophageal or esophagogastric-junction cancer during concurrent chemoradiation therapy
| Toxicity | Patients, n (N = 587) | % |
|---|---|---|
| Weight loss | ||
| Grade 0 | 324 | 55.2 |
| Grade 1 | 196 | 33.4 |
| Grade 2 | 64 | 10.9 |
| Grade 3 | 3 | 0.5 |
| Fatigue | ||
| Grade 0 | 208 | 35.4 |
| Grade 1 | 168 | 28.6 |
| Grade 2 | 185 | 31.5 |
| Grade 3 | 26 | 4.4 |
| Dermatitis | ||
| Grade 0 | 324 | 55.2 |
| Grade 1 | 215 | 36.6 |
| Grade 2 | 39 | 6.6 |
| Grade 3 | 9 | 1.5 |
| Dysphagia | ||
| Grade 0 | 163 | 27.8 |
| Grade 1 | 146 | 24.9 |
| Grade 2 | 232 | 33.5 |
| Grade 3 | 45 | 7.7 |
| Grade 4 | 1 | 0.1 |
| Esophagitis | ||
| Grade 0 | 130 | 22.1 |
| Grade 1 | 98 | 16.7 |
| Grade 2 | 285 | 48.6 |
| Grade 3 | 72 | 12.3 |
| Grade 4 | 2 | 0.3 |
| Pneumonitis | ||
| Grade 0 | 530 | 90.3 |
| Grade 1 | 38 | 6.5 |
| Grade 2 | 11 | 1.9 |
| Grade 3 | 4 | 0.7 |
| Grade 5 | 4 | 0.7 |
| Nausea | ||
| Grade 0 | 272 | 46.3 |
| Grade 1 | 103 | 17.5 |
| Grade 2 | 176 | 30.0 |
| Grade 3 | 36 | 6.1 |
| Anorexia | ||
| Grade 0 | 388 | 66.1 |
| Grade 1 | 7482 | 14.0 |
| Grade 2 | 108 | 18.4 |
| Grade 3 | 9 | 1.5 |
| Hematologic | ||
| Grade 0 | 526 | 89.6 |
| Grade 1 | 24 | 4.1 |
| Grade 2 | 20 | 3.4 |
| Grade 3 | 16 | 2.7 |
| Grade 4 | 1 | 0.2 |
The most common categories of perioperative complications after esophagectomy were pulmonary (20.5%) and gastrointestinal (18.3%). Pneumonia was the most common pulmonary complication and accounted for 9.7% of patients. The most common gastrointestinal complication was anastomotic leakage (11.2%). Thirty-five patients (12.6%) developed atrial fibrillation, 40 patients (14.4%) had a readmission within 60 days of surgery, and 4 patients (1.4%) died within 30 days of surgery (Table 3).
Table 3.
Complications experienced for 278 patients with esophageal or esophagogastric-junction cancer after surgery
| Complications | Patients, n (N = 278) | % |
|---|---|---|
| Pulmonary | ||
| Pneumonia | 27 | 9.7 |
| Acute respiratory distress syndrome | 5 | 1.8 |
| Pleural effusion | 18 | 6.5 |
| Respiratory insufficiency | 3 | 1.1 |
| Pneumothorax | 2 | 0.7 |
| Pulmonary embolism | 2 | 0.7 |
| Gastrointestinal | ||
| Anastomotic leakage | 31 | 11.2 |
| Tracheoesophageal fistula | 1 | 0.4 |
| Anastomotic stricture | 8 | 2.9 |
| Intra-abdominal infected hematoma | 1 | 0.4 |
| Ileus | 6 | 2.2 |
| Small bowel and colonic perforation | 1 | 0.4 |
| Peritonitis | 1 | 0.4 |
| Omental infarction | 1 | 0.4 |
| Gastric outlet obstruction | 1 | 0.4 |
| Cardiac | ||
| Atrial fibrillation | 35 | 12.6 |
| Atrial tachyarrhythmia | 1 | 0.4 |
| Readmission within 60 d | 40 | 14.4 |
| Death within 30 d | 4 | 1.4 |
Predictors of clinical outcomes and toxicities
Univariate and multivariate analyses of all-cause mortality for surgical and nonsurgical patients are shown in Supplemental Tables 1 and 2. For surgical patients, only histologic grade 3 was significantly associated with worse survival, but for nonsurgical patients, tumor location (gastro-esophageal junction vs upper/middle), nodal staging (N+ vs N0), and higher baseline PET (maximum standardized uptake values) all portended a poorer prognosis.
Next, we assessed if the effect of concurrent chemotherapy type used could influence the toxicity burden on patients. The χ2 analysis found that grade 3-4 esophagitis varied significantly among the different chemotherapy regimen, with nearly a doubling of grade 3-4 esophagitis for taxane/5-FU-based (17%) versus platinum/5-FU-based (8.7%) chemotherapy (Supplementary Table 3). This remained significant on multivariate analysis, among other factors (Supplementary Table 4).
Discussion
The current standard treatment strategy for localized EC is either chemoradiation therapy followed by surgery (trimodality therapy [TMT]) or definitive chemoradiation therapy (bimodality therapy).3, 14 Although radiation therapy plays an important role in the management of EC, it can cause significant toxicities that are largely determined by the radiation quality and the techniques used. Advanced techniques, such as IMRT, can significantly reduce cumulative doses within adjacent normal organs, such as the heart and lungs, compared with traditional techniques such as 3-dimensional CRT. The clinical benefits of the dose sparing of IMRT are only beginning to emerge.7, 8, 15 Freilich et al reported that IMRT-based CRT resulted in comparable survival but significantly decreased grade ≥3 toxicity compared with 3-dimensional CRT (overall response, 0.51; P = .050).
In this report, we present the results of a large cohort of patients who were treated with chemotherapy and IMRT and provide their OS, LRFS, DFS, CCRT toxicity, and surgery complication outcomes. We found excellent survival outcomes in this cohort, with low treatment-related morbidity and mortality rates. In particular, patients who underwent surgical resection after chemoradiation had a median OS of 69.1 months and a 5-year OS of 52.4%. Although it is tempting to conclude that the excellent outcomes of the TMT group were due to surgery, this is not a valid comparison because there is substantial selection bias toward better outcomes in the TMT group as patients who develop metastatic disease, poor health, or unresectable disease were excluded from undergoing surgery. On the basis of recent studies, patients who are potentially resectable and undergo observation after excellent clinical response can have good outcomes because patients who recur with local-only disease are often salvageable without compromising survival.5, 16, 17, 18, 19 The need for surgery after CCRT is an ongoing debate because 2 randomized trials demonstrated no survival benefit with the addition of surgery.20, 21
We benchmarked the survival outcomes of the TMT patients to the patients treated with TMT in the CROSS trial. A detailed comparison of the clinical and pathologic characteristics of our study with CROSS trial can be found in Supplementary Table 5. Although there were many differences between these 2 groups (eg, radiation dose, chemotherapy used, radiation delivery [3-dimensional vs IMRT]), we felt that the comparison was still valid because there is no radiation dose relationship in pathologic response rate or treatment outcomes with the type of concurrent chemotherapy used. Nearly a third (36.3%) of patients in our study also received induction chemotherapy, which was not administered to the patients in the CROSS trial. However, we know that this is not necessarily a predictor of good outcomes because it is our common practice to add induction chemotherapy for patients with multiple or bulky nodal disease because of the risk of micrometastatic disease.
A recent prospective randomized trial demonstrated no advantage with the addition of induction chemotherapy if given in an unselected setting.22 In comparison with the CROSS trial, our study cohort also had significantly more male patients with worse performance status and more adenocarcinomas with slightly longer tumors. Most importantly, there were no differences in the TNM stage of these patients. Despite these differences, the median OS in our cohort versus the CROSS trial was 69.1 months versus 49.4 months, and the 5-year OS rates were 52.4% versus 47%. Therefore, the OS of our study cohort is at least as good as, if not better than, that of the patients in the CROSS trial.
There could be some explanation for this difference. Better survival results could be associated with ongoing improvements in surgical techniques, patient selection, and staging methods over the years, although the time period of the patients in our study falls within the timing of the CROSS study. This also was not due to differences in the 30-day postoperative mortality because no differences were observed (1.4% in our cohort and 2% in the CROSS study). Although our R0 resection rate was a little higher (95.3%) than that in the CROSS study (92%), our pCR rate (26.1%) was less than that observed in the CROSS study (29%) and in other studies.4, 23, 24 The difference in pCR rate could be due to the proportion of adenocarcinoma versus squamous cell carcinoma in the various study cohorts because the majority of the patients in our study (89.6%) had adenocarcinoma, compared with 75% in the CROSS study. The pCR rate seems substantially different between adenocarcinomas (23%) and squamous cell carcinoma (49%) in the CROSS trial, which is what we observed as well (7 of 16 squamous cell carcinoma [43.8%] vs 62 of 267 adenocarcinomas [23.2%]).
Another potential explanation is the radiation modality used. In the CROSS study, all patients received concurrent 3-dimensional CRT with chemotherapy. Lin et al compared the long-term outcomes with 3-dimensional CRT and IMRT and reported that OS, locoregional control, and cardiac death were significantly better after IMRT than after 3-dimensional CRT.8 This appears to be in part due to the increased cardiac mortality seen in the patients who received 3-dimensional CRT, which was seen at both the single institutional and population levels.8, 15 This can be due to the ability of IMRT to reduce the radiation dose to the heart.6, 25, 26, 27 For all patients, the estimated median OS was 38.9 months, and the 3- and 5-year OS rates were 51.8% and 41.2%, respectively, which was similar to the previously reported IMRT results from a smaller cohort of patients (N = 263).8 For the 309 patients from the current study who received chemotherapy and IMRT without surgery, the estimated median OS was 25.2 months and the 3- and 5-year OS rates were 40.8% and 35.9%, respectively. These numbers also appear higher than those reported in the literature on patients treated with definitive chemoradiation therapy, but this could be the result of stage migration due to improvements in staging and diagnosis of patients.20, 21
Another retrospective study also reviewed the outcomes of patients treated with IMRT and 3D-CRT and observed no significant difference on the basis of radiation technique with respect to median survival (32 vs 29 months, P = .74), but there was a significant decrease in grade ≥3 toxicity in the IMRT group (24.6% vs 37.2%, P = .04). This suggests that IMRT-based chemoradiation for esophageal cancer did not affect survival but did reduce toxicity.7 A small prospective randomized trial by Lin et al showed no statistical differences in terms of 3-year survival (66.7% vs 63.3%, P > .05), grade ≥3 esophagitis (10.0% vs 6.7%, P > .05), and grade ≥3 pneumonitis between IMRT and 3-dimensional CRT, although lung V20 (21.2% vs 24.9%, P < .05) and lung V30 (15.3% vs 17.5%, P < .05) were significantly lower with IMRT.28 Supplementary Table 6 summarizes the results from these studies.
Our study is limited by its retrospective nature, and the outcomes are reflective of our single institutional practice. There is also some heterogeneity in the chemotherapy that was used, and a substantial percentage of patients received induction chemotherapy, which may contribute to increased toxicities without adding a benefit to patient outcomes. However, in this large series of patients treated with IMRT and chemotherapy, we had excellent survival outcomes with low rates of grade ≥3 toxicities, both during chemoradiation and postoperatively. We believe that by minimizing the higher radiation dose exposure to the heart and lungs with IMRT, the late toxic effects of radiation could also be minimized in patients who are cured of EC. In patients who are healthy enough to undergo CCRT and surgery, the 5-year survival outcomes can exceed 50%.
Conclusions
We report excellent clinical outcomes in the largest series of patients with EC treated with IMRT and chemotherapy, with or without surgery. Because of the mid and distal esophageal location from which most of the esophageal adenocarcinomas arise, the uniform and unnecessary exposure of the heart and lungs from entrance and exit doses of radiation can contribute to significant morbidity and possibly mortality of potentially curative therapies. Using advanced radiation delivery technologies such as IMRT, a substantial reduction in the unnecessary exposure of critical organs is possible, and better clinical outcomes can be expected. Whether additional benefits could be afforded with particle beam, such as proton beam therapy, is currently being studied in a prospective randomized trial (NCT01512589).
Footnotes
Sources of support: Funding was provided in part by The University of Texas, MD Anderson Cancer Center and by the National Cancer Institute (Cancer Center Support Grant CA016672).
Conflicts of interest: S.H.L. has received research funding from Elekta, STCube Pharmaceuticals, Peregrine Pharmaceuticals, Hitachi Chemical, and Roche/Genentech; has served as a consultant for AstraZeneca; and received honoraria from US Oncology and ProCure. All other authors have no conflicts of interest to declare.
Supplementary material for this article (http://dx.doi.org/10.1016/j.adro.2017.04.002) can be found at www.advancesradonc.org.
Supplementary data
References
- 1.American Cancer Society . 2nd edition. American Cancer Society; Atlanta, GA: 2011. Global Cancer Facts & Figures. [Google Scholar]
- 2.American Cancer Society . American Cancer Society; Atlanta, GA: 2015. Cancer Facts & Figures 2015. [Google Scholar]
- 3.Ajani J.A., Barthel J.S., Bentrem D.J. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P., Hulshof M.C., van Lanschot J.J. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Sudo K., Xiao L., Wadhwa R. Importance of surveillance and success of salvage strategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol. 2014;32:3400–3405. doi: 10.1200/JCO.2014.56.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu V.W., Sham J.S., Kwong D.L. Inverse planning in three-dimensional conformal and intensity-modulated radiotherapy of mid-thoracic oesophageal cancer. Br J Radiol. 2004;77:568–572. doi: 10.1259/bjr/19972578. [DOI] [PubMed] [Google Scholar]
- 7.Freilich J., Hoffe S.E., Almhanna K. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus. 2015;28:352–357. doi: 10.1111/dote.12203. [DOI] [PubMed] [Google Scholar]
- 8.Lin S.H., Wang L., Myles B. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene F.L., Page D.L., Fleming I.D., Fritz A.G., Balch C.M., Haller D.G., Morrow M., editors. AJCC Cancer Staging Manual. 6th ed. Springer-Verlag; New York, NY: 2002. [Google Scholar]
- 10.Ajani J.A., Correa A.M., Hofstetter W.L. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23:2638–2642. doi: 10.1093/annonc/mds210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajani J.A., Correa A.M., Walsh G.L. Trimodality therapy without a platinum compound for localized carcinoma of the esophagus and gastroesophageal junction. Cancer. 2010;116:1656–1663. doi: 10.1002/cncr.24935. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network . National Comprehensive Cancer Network; Washington, PA: 2013. NCCN guidelines, Version 2. 2013. [Google Scholar]
- 13.Grosshans D., Boehling N.S., Palmer M. Improving cardiac dosimetry: Alternative beam arrangements for intensity modulated radiation therapy planning in patients with carcinoma of the distal esophagus. Pract Radiat Oncol. 2012;2:41–45. doi: 10.1016/j.prro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Ajani J.A., Mansfield P.F., Janjan N. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774–2780. doi: 10.1200/JCO.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Lin S.H., Zhang N., Godby J. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer. 2016;12:917–928. doi: 10.1002/cncr.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennequin C., Quero L., Baruch-Hennequin V., Maylin C. Do locally advanced esophageal cancer still need surgery? Cancer Radiother. 2008;12:831–836. doi: 10.1016/j.canrad.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hofstetter W.L. Salvage esophagectomy. J Thorac Dis. 2014;6:S341–S349. doi: 10.3978/j.issn.2072-1439.2014.03.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markar S., Gronnier C., Duhamel A. Salvage surgery after chemoradiotherapy in the management of esophageal cancer: Is it a viable therapeutic option? J Clin Oncol. 2015;33:3866–3873. doi: 10.1200/JCO.2014.59.9092. [DOI] [PubMed] [Google Scholar]
- 19.Piessen G., Messager M., Mirabel X. Is there a role for surgery for patients with a complete clinical response after chemoradiation for esophageal cancer? An intention-to-treat case-control study. Ann Surg. 2013;258:793–799. doi: 10.1097/SLA.0000000000000228. discussion 799-800. [DOI] [PubMed] [Google Scholar]
- 20.Bedenne L., Michel P., Bouche O. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 21.Stahl M., Stuschke M., Lehmann N. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 22.Ajani J.A., Xiao L., Roth J.A. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2013;24:2844–2849. doi: 10.1093/annonc/mdt339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gannett D.E., Wolf R.F., Takahashi G.W. Neoadjuvant chemoradiotherapy for esophageal cancer using weekly paclitaxel and carboplatin plus infusional 5-fluorouracil. Gastrointest Cancer Res. 2007;1:132–138. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.L., Park S.I., Kim S.B. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15:947–954. doi: 10.1093/annonc/mdh219. [DOI] [PubMed] [Google Scholar]
- 25.Chandra A., Guerrero T.M., Liu H.H. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol. 2005;77:247–253. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Fenkell L., Kaminsky I., Breen S. Dosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagus. Radiother Oncol. 2008;89:287–291. doi: 10.1016/j.radonc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Wu V.W., Kwong D.L., Sham J.S. Target dose conformity in 3-dimensional conformal radiotherapy and intensity modulated radiotherapy. Radiother Oncol. 2004;71:201–206. doi: 10.1016/j.radonc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Lin X.D., Shi X.Y., Zhou T.C., Zhang W.J. Intensity-modulated or 3-D conformal radiotherapy combined with chemotherapy with docetaxel and cisplatin for locally advanced esophageal carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:1264–1267. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.