Abstract
Purpose
Four-dimensional computed tomography (CT) images are typically used to quantify the necessary internal target volumes for thoracic and abdominal tumors. However, 4-dimensional CT is typically associated with excessive imaging dose to patients and the situation is exacerbated when using repeat 4-dimensional CT imaging on a weekly or daily basis throughout fractionated therapy. The aim of this work is to evaluate an iterative reconstruction (IR) algorithm that helps reduce the imaging dose to the patient while maintaining imaging quality as quantified by point spread function and contrast-to-noise ratios (CNRs).
Methods and materials
An IR algorithm, SAFIRE, was applied to CT data of a phantom and patients with varying CT doses and reconstruction kernels. Phantom data enable measurements of spatial resolution, contrast, and noise. The impact of SAFIRE on 4-dimensional CT was assessed with patient data acquired at 2 different dose levels during image guided radiation therapy with an in-room CT.
Results
Phantom data demonstrate that IR reduces noise approximately in proportion to the number of iterations indicated by the strength (SAFIRE 1 to SAFIRE 5). Spatial resolution and contrast are conserved independent of dose and reconstruction parameters. The CNR increases with an increase of imaging dose or an increase in the number of iterations. The use of IR on CT sets confirms the results that were derived from phantom scans. The IR significantly enhances single breathing phase CTs in 4-dimensional CT sets as assessed by CT number discrimination. Furthermore, the IR of the low dose 4-dimensional CT features a 45% increase in the CNR in comparison with the standard dose 4-dimensional CT.
Conclusions
The use of IR algorithms reduces noise while preserving spatial resolution and contrast, as evaluated from both phantom and patient CT data sets. For 4-dimensional CT, the IR can significantly improve image quality and reduce imaging dose without compromising image quality.
Summary.
Time-sorted 4-dimensional computed tomography (CT) scans are used in radiation therapy for motion management; however, these scans are associated with elevated dose to the patient. Iterative image reconstruction algorithms are known to reduce the imaging dose by 30% to 50% while maintaining the signal-to-noise ratio. This study aims to evaluate whether one such iterative reconstruction algorithm, SAFIRE, can reduce the imaging dose while maintaining the contrast-to-noise to generate clinically valid 4-dimensional CT data sets.
Introduction
The management of respiratory motion in radiation therapy (RT) starts with a 4-dimensional computed tomography (CT) during the initial simulation, followed by periodic checks with either additional 4-dimensional CT or 4-dimensional cone beam CT scans during RT delivery.1 A disadvantage of 4-dimensional CT is exposure of the patient to substantially elevated radiation doses. The radiation dose from 4-dimensional CT can be 2 to 4 times higher than the dose from a conventional CT (ie, 3-dimensional CT).2, 3 Although the imaging dose is relatively lower compared with the dose delivered during RT, repeated use of imaging during fractional RT can deter the use of appropriate imaging when clinically necessary.
Four-dimensional CT sets are CT scans that are time sorted with respect to the phases of the patient's breathing. Hence, the images that are associated with a specific breathing phase are reconstructed from a small fraction of the total 4-dimensional CT dose. Unfortunately, the quality of a single-phase reconstruction can be degraded by high levels of noise due to restriction of the imaging dose to a patient or machine limitations. The relationship between the noise in those images and the imaging dose is well understood. Increasing the imaging dose reduces the noise but unfortunately is hazardous to the patient as well. Several novel methods have been developed to minimize imaging dose while preserving image quality for CT.4, 5 One of those methods is the iterative reconstruction (IR) algorithm, which works in the raw data domain to reduce noise without diminishing spatial resolution and contrast.1
An example of IR methods is the sinogram-affirmed IR algorithm (SAFIRE; Siemens Medical Solutions, Malvern, PA).6 SAFIRE has been shown to improve the signal-to-noise ratio by reducing image noise and preserving contrast.7 A competing directive is the need to reduce the imaging dose. Numerous studies have demonstrated SAFIRE's ability to preserve image quality at reduced doses.7, 8, 9, 10, 11 Several studies have also quantified the method's success by means of phantom measurements and subjective polls of expert radiologists.10, 12, 13, 14 Studies of SAFIRE report imaging dose reductions of 50% without compromising image quality.8, 11 Furthermore, SAFIRE has been applied to diagnostic 4-dimensional CT for chronic obstructive pulmonary disease15 and coronary artery stenosis.16
To our knowledge, IR methods have not been applied to 4-dimensional CT for RT motion management. In this work, we explore the use of SAFIRE to maintain/enhance CT scan quality for 4-dimensional CT with reduced imaging doses. We aimed to quantify the noise reduction of SAFIRE on the basis of both phantom and patient data. Furthermore, we sought to investigate the image quality therein.
In the absence of a reference image, the most accepted method of grading quality is a subjective evaluation by human observers. Subjective image quality analysis has been performed on SAFIRE images, and the results are mixed.9 Naturally, different observers will arrive at different grades even for the same image. Furthermore, we posit that experienced observers (eg, radiologists, medical physicists, dosimetrists, and radiation oncologists) employed in these studies may demonstrate a bias toward the image reconstructions with which they are most familiar, and that bias may not reflect clinical efficacy. Thus, more objective methods are desirable. Typically, for the purpose of CT image quality assessment, phantoms with known characteristics are employed. A phantom provides well-defined landmarks that aid in the assessment of image quality metrics, including contrast, noise, and the modulation transfer function or point spread function. We present the performance of IR scans in terms of these metrics as measured from a phantom.
It is important to note that phantom-derived image quality measurements may not be sufficient for the evaluation of clinical performance. Obviously, the end goal is to enhance the image quality of CT scans of human subjects. Human subjects are vastly different from synthetic phantoms in a multitude of ways, and those differences limit the utility of measurements derived from phantom scans. Additionally, studies have shown that target volume delineation is observer dependent.17, 18, 19 The intraobserver variations likely confound attempts to quantify image quality improvements with observer studies. The noise reduction of IR should reduce these variations and thus meaningfully reduce uncertainty in radiation treatment planning. Similarly, enhancements in image quality have been shown to improve image guided RT (IGRT) delivery20 and deformable image registration.21
In this study, we sought quantitative assessments of the image quality of the patient scans in part due to the previously mentioned variation in subjective image quality analysis.9 Therefore, we supplemented the phantom-based evaluations with a comparative analysis of IR (SAFIRE) versus filtered back projection (FBP) reconstructions of representative patient IGRT scans. The 4-dimensional CT scans suffer from unusually high levels of noise due to the imaging dose constraints. Therefore, the noise reduction resulting from IR algorithms can dramatically enhance image quality for this modality or enable reduced imaging dose scans without compromising image quality.
Methods and materials
All CT data were acquired on a SOMATOM Definition AS CT Scanner (Siemens Healthcare, Malvern, PA). The scanner was installed in a linac room on a sliding gantry (ie, CT on rails). In addition to the previously mentioned SAFIRE strength parameter, the CT dose and reconstruction algorithm were systematically varied. All phantom scans were performed with a kernel size of 30 and a pitch factor of 0.6, unless stated otherwise.
The phantom used in the study was a CatPhan 500 (Phantom Laboratory Inc. Greenwich, NY), 15 cm in diameter. The CatPhan 500 is of a modular design, which enables the evaluation of multiple image quality metrics with a single scan. For our purposes, we were interested in spatial resolution and the contrast-to-noise ratio (CNR), which were analyzed from scans of the CTP528 and CTP515 modules, respectively. The CTP528 high resolution module has 21 line pairs per centimeter gauge and a tungsten bead point source, which was used to measure the point spread function (PSF). This metric of spatial resolution was measured by identifying the voxels that were associated with the bead, subtracting the background, normalizing the resulting curve, and fitting it to a Gaussian.
CTP515 is a low-contrast module with supra-slice and sub-slice contrast targets that vary in length and width. The CNR was measured from scans of the CTP515 module. The contrast was calculated by identifying the supra-slice 1.0% elements in the module, acquiring a circular region of interest (ROI) that was slightly smaller than the element; finding the average CT number in Hounsfield units of those voxels, and subtracting the average CT number of an identically sized adjacent ROI. Noise was calculated as the average of the standard deviations of the 2 ROIs. Mathematically, CNR is described by a simple formula
| (1) |
where is the average CT numbers in the 2 ROIs, and σ is the standard deviation of CT number in the same ROI.
As a supplement to the phantom measurements, we analyzed SAFIRE and FBP images that were reconstructed from representative patient scans obtained in our clinic using MATLAB routines that were written in-house. The patient 4-dimensional CT sets were acquired on the Siemens CT on rails at 120 kVp with a small pitch of 0.09 such that the CT couch would travel a scan range that was equal to the width of the detector array in a time that was longer than 1 full breathing cycle. The rotation time period was 0.5 seconds. To reduce the imaging dose and avoid overheating of the x-ray tube, the dose of a 4-dimensional scan (ie, CTDIvol of 29.2 mGy) is approximately twice that of a 3-dimensional CT scan, which results in rather noisy 4-dimensional CT phase-sorted images.
SAFIRE can be applied to the single-phase images to reduce the noise without increasing the image dose. The longest IRs (strength 5) require 16 seconds for a representative patient, or approximately 7 seconds longer than an FBP. The enhancement in image quality is quantified by the noise or CNR (when applicable), using the same ROI technique previously described. To further demonstrate the image quality enhancement as it pertains to target delineation, the noise reduction was illustrated by plotting the distribution of CT number in the tumor and surrounding tissue. The noisier the image, the broader this distribution will become, which degrades tumor and organ at risk (OAR) delineation. Conversely, a sharper image will feature narrower peaks, which enable a clearer distinction between target and OAR.
This study was approved by our institutional review board. To explore the feasibility of reducing imaging dose for 4-dimensional CT through the application of IR, two 4-dimensional CT data sets were acquired with different imaging dose levels as part of daily IGRT for a patient with liver cancer. The first data set was acquired at the clinical protocol with an imaging dose of 29.2 mGy CTDIvol using the same parameters as previously described. The second data set was acquired at a different treatment fraction with a reduced imaging dose of 17.50 mGy CTDIvol. This lower dose was chosen to match the one used in the 3-dimensional CT protocol.
Results
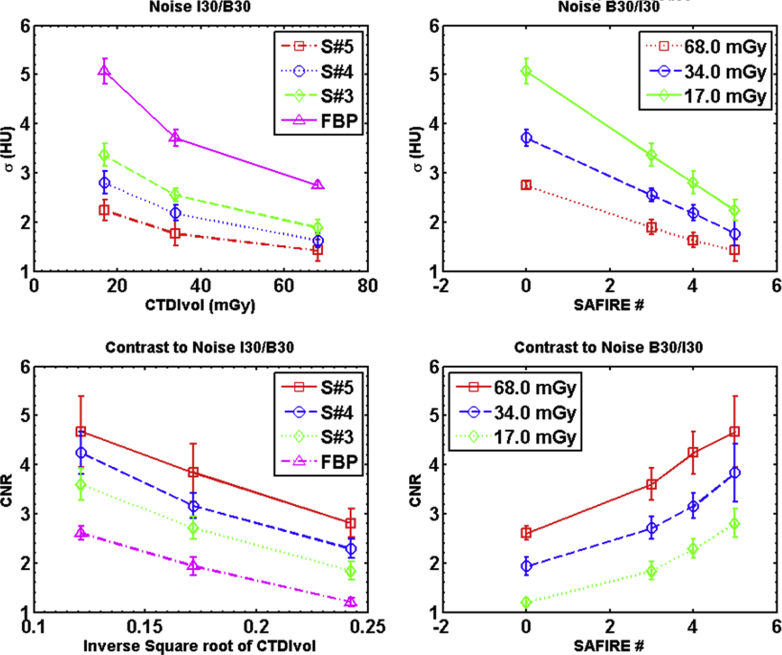
IRs of phantom scans demonstrated their clear advantages over FBP (Figs 1 and 2). All error bars in the figures were calculated by measuring the standard deviation of the plotted metric across multiple measurements. For the phantom measurements, 4 scans were performed.
Figure 1.
Noise and contrast-to-noise ratio (CNR) are plotted as a function to exposure and reconstruction type. The noise is measured from the substrate in the CTP515 low contrast module. The noise is quantified as the standard deviation of a selection of voxels in Hounsfield units. CNR is calculated using Equation 1. The contrasting regions of interest are in the 1% elements of CTP515 and the adjacent substrate.
Figure 2.
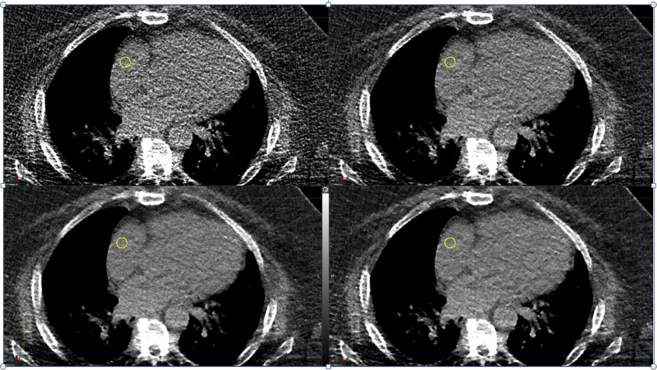
Detail of axial slices from 4 reconstructions of the same 4-dimensional computed tomography, time sorted to the 0% expiration phase. The kernel was 30f, CTDIvol was 29.2 mGy, and the tube voltage 120 kVp. The top left is the filtered back projection and the remaining 3 are the SAFIRE reconstructions from 3 to 5 in strength.
The difference between the reconstructions is most clearly illustrated by Fig 1A and B, which show the measured noise. The noise diminishes substantially as the dose is increased, in accordance with expectations. The noise is also reduced by the use of IR approximately in proportion to the strength of the iterative method, where the strength is a variable related to the number of iterations. Figure 1C and D display the CNR, the increase in which is entirely driven by the reduction in noise. For these figures, we plotted the dependence on the imaging dose as a function of the inverse square root of effective CTDIvol to highlight the expected relationship between noise reduction and dose.
Spatial resolution was not affected by reconstruction type or dose level. The average standard deviation of the PSF for SAFIRE reconstructions was 0.55 ± 0.05 pixels; for FBP reconstructions, it was 0.56 ± 0.06 pixels. For both types of reconstructions, the largest spread in PSF was at 17 mGy CTDIvol, the dose of a 3-dimensional CT. The standard deviation of the PSF for SAFIRE I30 strength 5 at 17 mGy CTDIvol was 0.57 pixels, and for the B30 reconstruction, the standard deviation of the PSF at that dose was 0.60 pixels.
Two key observations arise from the phantom data. First, the contrast and spatial resolution of IR images are equivalent to those of the FBP images. The PSF did not vary with reconstruction type or dose. The variation in contrast was well within the expected statistical fluctuations. Second, the noise was reduced by increasing the number of iterations in the SAFIRE reconstruction. The lowest noise images had a higher dose with more iterations, and these gains in image quality did not sacrifice image contrast or spatial resolution.
The first clinical example analyzed was a 4-dimensional CT set of the chest. The images were time sorted to give temporal information for respiration motion management in RT. As previously discussed, image quality of the time-sorted reconstructions suffers from a lack of photons, resulting in very noisy images. Figure 2 shows 3 SAFIRE reconstructions and the FBP of an axial slice through the heart and lungs at the 0% lung expiration phase (ie, the end of the exhale).
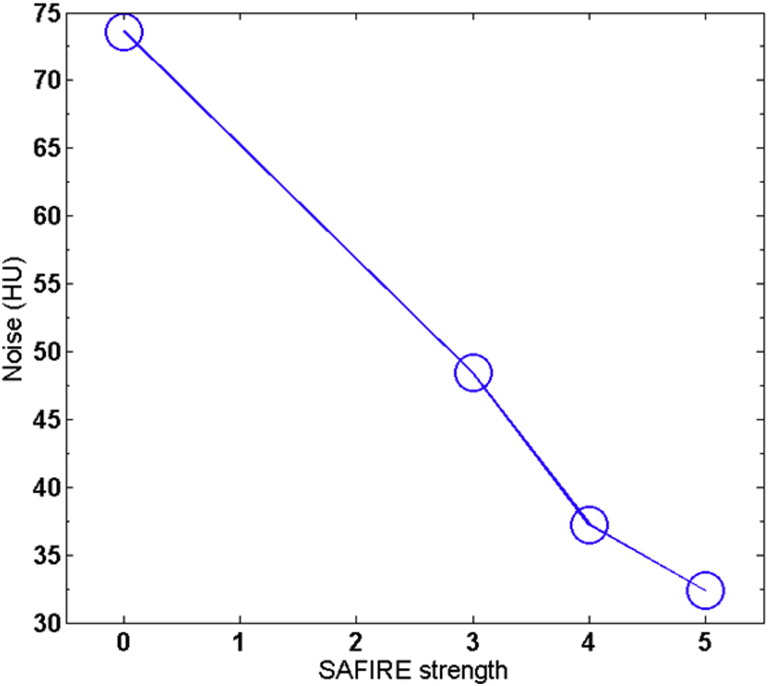
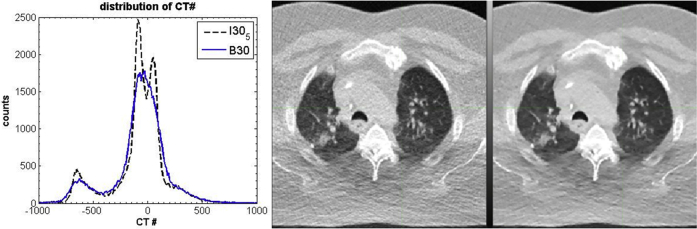
In analogy to phantom measurements, we chose a small ROI to demonstrate the noise reduction that was produced by the IR algorithm. In this case, we focused on a collection of voxels inside the right ventricle of the heart. The noise was calculated as the standard deviation of the CT number of the voxels in the ROI and plotted in Fig 3. Furthermore, histogram analysis was applied to demonstrate the potential clinical benefit. The right 2 panels of Fig 4 show the voxels used in the analysis and highlight a cross section of the target and the surrounding OAR. The noise reduction in the SAFIRE#5 image in comparison with the FBP image is immediately apparent and enables a clear distinction of the lung, soft tissues (including the tumor), and bone. The CT distribution of the axial scan detail from the right 2 panels of Fig 4 is shown in the left panel of the figure.
Figure 3.
The noise calculated from 4 regions of interest in 6 adjacent axial slices. The SAFIRE number of 0 indicates a filtered back projection. The scan parameters are the same as in Figure 2.
Figure 4.
The distribution of computed tomography (CT) numbers in detail from the two 4-dimensional CT axial slices on the right. The 2 reconstructions are from the same 4-dimensional CT data set, time sorted to the 0% expiration phase. The scan parameters are identical to those used to produce Figure 3. The image in the middle is the standard filtered back projection reconstruction (B30f), and the image on the right is the SAFIRE 5 reconstruction (I30f#5).
The largest peaks in the CT number distribution are at approximately −600 HU and 0 HU and are evident in both reconstructions. These peaks are easily identified as associated with the lung and soft tissue in Fig 4. (The detail from the axial slice chosen has insufficient bone to give a peak expected around 300 HU.) Significantly, the noise reduction in the IR makes it possible to identify a splitting in the soft tissue peak around 0 HU in the SAFIRE distribution. Fatty tissue has a slightly lower electron density than water and muscle has a slightly higher electron density than water, resulting in a splitting in the CT number between these 2 types of soft tissues. Hence, in the SAFIRE reconstruction, the types of tissues can be clearly distinguished whereas in the FBP reconstruction, the soft tissue makes a single broad peak where the differences are smoothed over by the noise. Thus, more complex features can be identified in the 4-dimensional CT, potentially enhancing the accuracy of treatment planning.
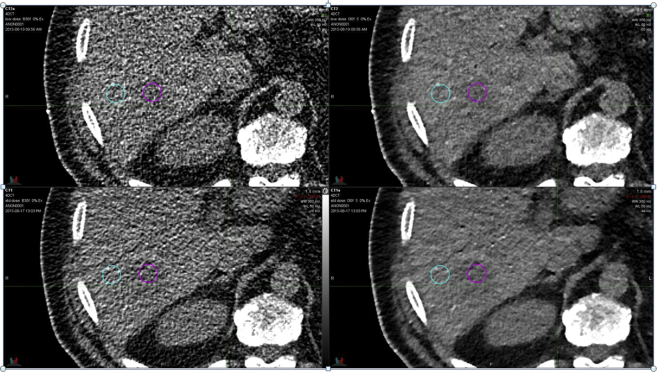
The second 4-dimensional CT data set was acquired during an IGRT on a patient with liver cancer (Fig 5). The first scan used the standard protocol imaging dose (29.2 mGy CTDIvol), and the second used slightly more than half of that dose (17.5 mGy CTDIvol). Both scans, axial images at the end of the expiration phase, were reconstructed with an FBP and the SAFIRE algorithm with a strength of 5. The 2 scans were rigidly registered, and ROIs were identified both inside and adjacent to the tumor (Fig 5).
Figure 5.
Detail from axial slices of the single-phase computed tomography at the end of expiration. The top row is a low-dose scan (17.5 mGy CTDIvol), and the bottom row is a standard-dose scan (29.2 mGy CTDIvol). The images on the left are reconstructed with the standard filtered back projection algorithm (B30f), and the images on the right are reconstructed with SAFIRE (I30f#5). The maroon ring is a region of interest (ROI) inside the gross tumor volume (GTV), and the teal ring is an ROI adjacent to the GTV.
As expected, the image quality of the FBP reconstructions suffered significantly from noise. In this case, a solid tumor can be clearly delineated when SAFIRE is applied. To demonstrate the image quality enhancement, the CNR can be calculated from Equation 1 for the tumor identified by the maroon ring in Fig 5. At a CTDIvol of 29.2 mGy (bottom row of Fig 5), the CNR is 0.25 and 0.54 for the FBP and SAFIRE strength 5, respectively. By reducing the imaging dose to the standard abdominal CTDIvol of 17.5 mGy (top row of Fig 5), the CNR drops to 0.20 and 0.40 for the FBP and SAFIRE strength 5, respectively. The low-dose SAFIRE reconstruction of I30 strength 5 features a CNR that is 60% higher than the standard dose FBP reconstruction of B30.
Discussion
Repeat 4-dimensional CT imaging either weekly or daily depending on clinical practice guidelines can help in assessing the changes in the internal target volume margins over the course of treatment. However, the cost of the additional imaging dose of 4-dimensional CT must be balanced by the benefit of better motion management for radiation therapy. In practice, that trade-off results in images that capture a single, albeit noisy, phase of patient breathing. IR that is designed to reduce the imaging dose without increasing noise is ideal for restoring the image quality so that single-phase CT in a 4-dimensional CT set can have a CNR that is comparable to a conventional volumetric CT image.
In this work, we show that it is possible to acquire 4-dimensional CT images at dose levels that are comparable with traditional 3-dimensional scans and with equivalent CNR. For the case studied, the CNR of the 17 mGy CTDIvol iteratively reconstructed 4-dimensional CT is superior to the contrast-to-noise ratio of the FBP reconstruction at 29 mGy CTDIvol. This protocol would enable clinicians to capture reasonable, quality CT images that measure intrafractional motion without exposing the patient to additional radiation doses.
Conclusions
IR methods such as SAFIRE enhance 4-dimensional CT image quality as measured from both phantom and patient CT scans. Primarily, substantial noise reduction is evident without compromising contrast or spatial resolution. As expected, CT noise decreases with an increasing dose. Furthermore, increasing the number of iterations (quantified by SAFIRE strength) also decreases noise. The enhancement of CT quality with IR is demonstrated with patient 4-dimensional CT scans and indicates that the substantially higher 4-dimensional CT dose can be reduced by the application IR without sacrificing image quality. Improved image quality and/or reduced imaging dose with IR are highly desirable in radiation therapy planning and delivery guidance.
Acknowledgments
The authors acknowledge Dr Nilesh Mistry of Siemens Medical Solutions, whose input was very helpful.
Footnotes
Sources of support: This study was supported in part by Siemens Medical.
References
- 1.Rietzel E., Pan T., Chen G.T. Four-dimensional computed tomography: Image formation and clinical protocol. Med. Phys. 2005;32:875–889. doi: 10.1118/1.1869852. [DOI] [PubMed] [Google Scholar]
- 2.Mori S., Ko S., Ishii T., Nishizawa K. Effective doses in four-dimensional computed tomography for lung radiotherapy planning. Med Dosim. 2009;34:87–90. doi: 10.1016/j.meddos.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard P., Callahn J., Cramb J., Budd R., Kron T. Audit of radiation dose delivered in time-resolved four-dimensional computed tomography in a radiotherapy department. J Med Imag Radiat Oncol. 2015;59:346–352. doi: 10.1111/1754-9485.12284. [DOI] [PubMed] [Google Scholar]
- 4.McCollough C.H., Primak A.N., Braun N., Kofler J., Yu L., Christner J. Strategies of reducing radiation dose in CT. Radiol Clin North Am. 2009;47:27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra M.K., Maher M.M., Toth T.L. Strategies for CT radiation dose optimization. Radiology. 2004;230:619–628. doi: 10.1148/radiol.2303021726. [DOI] [PubMed] [Google Scholar]
- 6.Grant K., Raupach R. SAFIRE: Sinogram affirmed iterative reconstruction. http://imaging.ubmmedica.com/all/editorial/diagnosticimaging/pdfs/SAFIRE.pdf Available at: Accessed August 6, 2013.
- 7.Becce F., Ben Salah Y., Verdun F.R. Computed tomography of the cervical spine: Comparison of image quality between a standard-dose and a low-dose protocol using filtered back-projection and iterative reconstruction. Skeletal Radiol. 2013;42:937–945. doi: 10.1007/s00256-013-1576-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang H., Tan B., Zhao B., Liang C., Xu Z. Raw-data-based iterative reconstruction versus filtered back projection: image quality of low-dose chest computed tomography examinations in 87 patients. Clin Imaging. 2013;37:1024–1032. doi: 10.1016/j.clinimag.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Winklehner A., Karlo C., Puippe G. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011;21:2521–2526. doi: 10.1007/s00330-011-2227-y. [DOI] [PubMed] [Google Scholar]
- 10.Nie P., Li H., Duan Y. Impact of sinogram affirmed iterative reconstruction (SAFIRE) algorithm on image quality with 70 kVp-tube-voltage dual-source CT angiography in children with congenital heart disease. PLoS One. 2014;9:e91123. doi: 10.1371/journal.pone.0091123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beister M., Kolditz D., Kalender W.A. Iterative reconstruction methods in x-ray CT. Phys Med. 2012;28:94–108. doi: 10.1016/j.ejmp.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Ghetti C., Palleri F., Serreli G., Ortenzia O., Ruffini L. Physical characterization of a new CT iterative reconstruction method operating in sinogram space. J Appl Clin Med Phys. 2013;14:4347. doi: 10.1120/jacmp.v14i4.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz B., Beeres M., Bodelle B. Performance of iterative image reconstruction in CT of the paranasal sinuses: A phantom study. AJNR Am J Neuroradiol. 2013;34:1072–1076. doi: 10.3174/ajnr.A3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalra M.K., Woisetschläger M., Dahlström N. Radiation dose reduction with sinogram affirmed iterative reconstruction technique for abdominal computed tomography. J Comput Assist Tomogr. 2012;36:339–346. doi: 10.1097/RCT.0b013e31825586c0. [DOI] [PubMed] [Google Scholar]
- 15.Wielputz M.O., Eberhardt R., Puderbach M., Weinheimer O., Kauczor H.U., Heussel C.P. Simultaneous assessment of airway instability and respiratory dynamics with low-dose 4D-CT in chronic obstructive pulmonary disease. Respiration. 2014;87:294–300. doi: 10.1159/000357448. [DOI] [PubMed] [Google Scholar]
- 16.Baxa J., Hromadka M., Sedivy J. Regadenoson-stress dynamic myocardial perfusion improves diagnostic performance of CT angiography in assessment of intermediate coronary artery stenosis in asymptomatic patients. Biomed Res Int. 2015;2015:105629. doi: 10.1155/2015/105629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leunens G., Menten J., Weltens C., Verstraete J., van der Schueren E. Quality assessment of medical decision making in radiation oncology: Variability in target volume delineation for brain tumours. Radiother Oncol. 1993;29:169–175. doi: 10.1016/0167-8140(93)90243-2. [DOI] [PubMed] [Google Scholar]
- 18.Weltens C., Menten J., Feron M. Interobserver variations in gross tumor volume delineation of brain tumors on computed tomography and impact of magnetic resonance imaging. Radiother Oncol. 2001;60:49–59. doi: 10.1016/s0167-8140(01)00371-1. [DOI] [PubMed] [Google Scholar]
- 19.Riegel A.C., Berson A.M., Destian S. Variability of gross tumor volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int J Radiat Oncol Biol Phys. 2006;65:726–732. doi: 10.1016/j.ijrobp.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Morrow N.V., Lawton C.A., Qi X.S., Li X.A. Impact of computed tomography image quality on image-guided radiation therapy based on soft tissue registration. Int J Radiat Oncol Biol Phys. 2012;82:e733–e738. doi: 10.1016/j.ijrobp.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 21.Hart V, Chen GP, Li XA. The effect of CT image quality on deformable image registration in radiotherapy. Presented at: Radiological Society of North America 2013 Scientific Assembly and Annual Meeting. December 1-6, 2013; Chicago, IL.