Abstract
Purpose
The population of patients aged 80 years or older who are diagnosed with diffuse large B-cell lymphoma (DLBCL) continues to increase, but an optimal treatment strategy has not been established. We sought to examine the influence of consolidative radiation therapy (RT) on outcome and toxicity among the very elderly diagnosed with stage I-IV DLBCL.
Methods and materials
We evaluated 131 patients treated at a single institution between 2002 and 2014 who were eligible for RT after successful treatment with chemotherapy.
Results
The median age was 83 years (range, 80-96). Advanced-stage disease was present in 61.8% of patients. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone was administered to 80% of patients (n = 108), and 23.7% of patients received consolidative RT. Among early-stage (ES) patients treated with 3 to 4 cycles of chemotherapy and RT (n = 12) versus 6 to 8 cycles of chemotherapy alone (n = 17), there were no statistically significant differences in 3-year disease-free, progression-free, or overall survival rates. The 3 year disease-free survival was 91.7% versus 88.2% among patients treated with combined modality therapy versus chemotherapy alone (P = .78). The 3-year overall survival was 82.5% versus 87.5% among patients treated with combined modality therapy compared with chemotherapy alone (P = .852). Anemia and neuropathy occurred more frequently among ES patients who received 6 to 8 cycles of chemotherapy alone. Among advanced-stage patients with bulky disease (n = 35), consolidative RT to sites of bulky disease may have improved local control (3-year local control, 100% vs 60.3%, P = .160).
Conclusions
Among patients aged 80 years or older who have with ES DLBCL, 3 to 4 cycles of chemotherapy followed by RT is at least equivalent in efficacy to chemotherapy alone and is associated with lower levels of toxicity, which suggests that it may be a better choice for therapy when trying to balance treatment efficacy and tolerability.
Summary.
In a cohort of 131 patients aged 80 years or older with diffuse large B-cell lymphoma and with chemosensitivity, outcomes rivaled those of younger patients. For limited stage disease, abbreviated chemotherapy followed by consolidative radiation therapy was equivalent in efficacy to chemotherapy alone (6-8 cycles) but was associated with lower rates of myelosuppression, neuropathy, and congestive heart failure. In advanced-stage patients, radiation therapy appeared to improve local control. Older patients should be considered for consolidative radiation therapy after immunochemotherapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin lymphoma, is a common neoplasm among the elderly with a median age of diagnosis approaching the seventh decade of life. Modern treatment with chemoimmunotherapy can be curative among 60% of patients over 65 years of age; however, this treatment can be complicated by preexisting comorbidities among the very elderly (ie, patients over 80 years of age). Given that the incidence of DLBCL has been growing, with the largest increases among patients over the age of 60 years, coupled with longer life expectancies that result in a growing population of individuals over the age of 80 years, therapeutic approaches that optimize treatment efficacy while minimizing toxicity are needed.1, 2
For all patients with DLBCL, data continue to emerge that suggest the benefit of consolidative radiation therapy (RT) in improving local control (LC), progression-free survival (PFS), and potentially overall survival (OS).3, 4 For the 25% to 30% of patients who present with early-stage (ES) disease, oncologists consider an abbreviated course of 3 to 4 cycles of chemotherapy followed by consolidative RT on the basis of randomized data from the pre-rituximab era. In the Southwest Oncology Group (SWOG) 8736 trial, 3 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) followed by involved field RT was compared with 8 cycles of CHOP alone.5 Five-year rates of PFS and OS were superior in the RT arm, and life threatening toxicity was more common among patients who received extended chemotherapy. However, with longer follow-up, there was no difference in OS at 10 years, presumably due to late relapses outside of the radiation field.6, 7
Given the vulnerability of the very elderly population to treatment-related toxicity, a course of abbreviated chemotherapy followed by RT is an attractive therapeutic option.8, 9 Indeed, in a Surveillance, Epidemiology and End Results (SEER) Medicare study that compared the outcome of 3 to 4 cycles of CHOP with RT versus 6 to 8 cycles of CHOP alone among 874 patients who were age 65 years or older and had ES disease, OS was similar in both treatment groups.10 Abbreviated chemotherapy followed by RT was associated with lower odds of neutropenia and a lower risk of second-line treatment.
Among patients with stage III/IV DLBCL with bulky disease treated with 6 to 8 cycles of rituximab plus CHOP (R-CHOP), RT leads to improvements in PFS, disease-free survival (DFS), and OS.3, 11, 12, 13 In patients over the age of 80 years who receive therapy for advanced-stage (AS) disease, there is potentially a benefit from RT with regard to LC, but whether this benefit translates into increases in EFS and OS is influenced by preexisting comorbidities and competing risks of death.
We previously evaluated the efficacy of various chemotherapy regimens among 207 patients age 80 years or older who were treated at our institution and found that patients who received anthracycline-based regimens such as R-CHOP and rituximab-etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH) had good outcomes that rivaled those of younger patients with DLCBL.14 In the current study, we examine the effect of consolidative RT on the outcome of patients aged 80 years or older with an indication for postimmunochemotherapy, RT, limited stage disease, and bulky AS DLBCL.
Methods and materials
After receiving institutional review board approval, we evaluated consecutive patients with DLBCL who were diagnosed at age 80 years or older between 2002 and 2014 at our institution. Biopsy review by a specialized hematopathologist confirmed the diagnosis. Baseline patient, laboratory, and treatment characteristics were obtained via review of electronic medical records.
Responses to frontline chemotherapy were based on the revised International Working Group Guidelines established by Lugano in 2011.15 We excluded patients with progressive disease during or at completion of the initial systemic therapy because these patients would not have been eligible for consolidative RT. Patients with significant missing information on follow-up were excluded.
DFS was defined from the date of diagnosis to disease relapse or death due to lymphoma, and PFS was defined from the date of diagnosis to disease relapse/progression or death of any cause. OS was defined from the date of diagnosis to death from any cause. LC was defined as the absence of disease relapse/progression within an initially presenting site of lymphoma, regardless of RT. The Kaplan-Meier method was used to estimate DFS, PFS, OS, and LC rates. Differences between survival and LC curves by RT were compared using the log rank test. Differences in the median number of chemotherapy cycles between cohorts was compared with the Mann-Whitney test. P-values that were less than or equal to .05 were considered significant. All statistical analyses were performed with SPSS Statistics 22 (IBM Corporation, New York, NY).
Adverse events were recorded from the initiation of chemotherapy to 12 months from the diagnosis date and graded on the basis of the Common Terminology Criteria for Adverse Events, Version 4. Toxicity grades 1 to 5 were recorded after each chemotherapy cycle and during and after radiation therapy for patients who received radiation. Differences in toxicity incidence were compared between groups using a two-sample t test to compare proportions with Fisher exact and Wilcoxon rank-sum tests.
Results
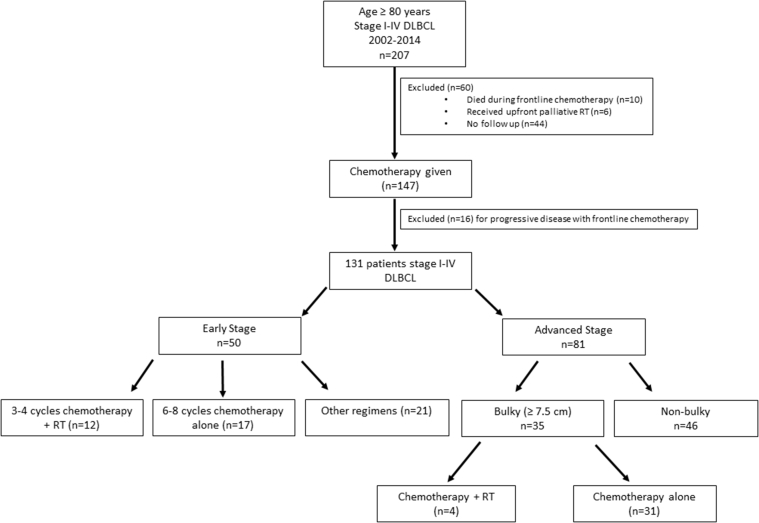
We identified 207 patients who were age 80 years or older and newly diagnosed with stage I-IV DLBCL. Details on the final patient cohort are depicted in Figure 1. Ten patients died during frontline chemotherapy and were excluded. Nine of these deaths were treatment related, and 1 was due to lymphoma. Sixteen patients were excluded for progressive disease during or at completion of systemic therapy. Six patients received upfront palliative RT. For 44 patients, follow-up data were unavailable or details with regard to receipt of RT were unknown. Of the 131 patients remaining who achieved a complete response (n = 118, 90.1%) or partial response (n = 13, 9.9 %) to systemic therapy, 29 had ES disease that was treated with 3 to 4 cycles of immunochemotherapy followed by consolidative RT (n = 12) or immunochemotherapy alone (n = 17). To evaluate the role of consolidative RT among AS patients, we evaluated 35 patients with bulky AS disease who were treated with (n = 4) or without (n = 31) consolidative RT.
Figure 1.
Patient cohort selection.
The clinical and treatment characteristics of the 131 patients are outlined in Table 1. The median age was 83 years (range, 80-96). AS disease was present in 61.8% of patients. Twenty-seven percent of patients had poor performance status (PS; defined as Eastern Cooperative Oncology Group score ≥2) at diagnosis. Bulky disease (defined as ≥7.5 cm) was evident among 24% and 43.2% of patients with ES and AS disease, respectively. More than 80% of patients were treated with R-CHOP chemotherapy (n = 108). Other regimens included rituximab, cyclophosphamide, etoposide, vincristine, and prednisone (n = 9); rituximab, cyclophosphamide, vincristine, and prednisone (n = 2); rituximab, cyclophosphamide, and prednisone, (n = 1); rituximab, cyclophosphamide, and prednisone (n = 1); rituximab and prednisone (n = 1); and R-EPOCH (n = 9). Positron emission tomography/computed tomography (PET/CT) scans were used to assess response in 84.0% of patients (n = 110).
Table 1.
Patient and treatment characteristics
| Characteristic | Entire cohort (n = 131) | Early Stage (n = 50) | Advanced Stage (n = 81) | Early Stage Final Cohorta (n = 29) | Advanced Stage Bulkyb (n = 35) |
|---|---|---|---|---|---|
| Age, y, median (range) | 83 (80-96) | 83 (80-96) | 82 (80-94) | 83 (80-96) | 83 (80-92) |
| Female | 63 (48.1%) | 20 (40.0%) | 43 (53.1%) | 12 (41.4%) | 16 (45.7%) |
| Stage | |||||
| I | 23 (17.6%) | 23 (46%) | -- | 17 (58.6%) | -- |
| II | 27 (20.6%) | 27 (52%) | -- | 12 (41.4%) | -- |
| III | 35 (26.7%) | -- | 35 (43.2%) | -- | 16 (45.7%) |
| IV | 46 (35.1%) | -- | 46 (56.8%) | -- | 19 (54.3%) |
| B symptoms | 27 (20.6%) | 6 (12.0%) | 21 (25.9%) | 3 (10.3%) | 12 (34.3%) |
| Bulky diseasea | 47 (35.9%) | 12 (24.0%) | 35 (43.2%) | 5 (17.2%) | 35 (100%) |
| Performance status score 2-4 | 35 (26.7%) | 12 (24.0%) | 23 (28.4%) | 5 (17.2%) | 10 (28.6%) |
| International Prognostic Index | |||||
| Low | 19 (14.5%) | 19 (38.0%) | 0 | 14 (48.3%) | 0 |
| Low-intermediate | 43 (32.8%) | 19 (38.0%) | 24 (29.6%) | 8 (27.6%) | 11 (31.4%) |
| Intermediate-high | 30 (22.9%) | 5 (10.0%) | 25 (30.9%) | 2 (6.9%) | 11 (31.4%) |
| High | 21 (13.0%) | 0 | 21 (25.9%) | 0 (0%) | 10 (28.6%) |
| Missing | 18 (13.7%) | 7 (14.0%) | 11 (13.6%) | 5 (17.2%) | 3 (8.6%) |
| LDH level > ULN | 53 (40.5%) | 18 (36.0%) | 35 (43.2%) | 8 (27.6%) | 17 (48.6%) |
| Extranodal disease ≥2 | 16 (12.2%) | 0 | 16 (19.8%) | 0 (0%) | 6 (17.1%) |
| Cycles of chemotherapy, median (range) | 6 (1-8) | 6 (1-8) | 6 (3-8) | 6 (3-8) | 6 (3-8) |
| 3-4 | 31 (23.7%) | 18 (36.0%) | 13 (16.0%) | 12 (41.4%) | 5 (14.3%) |
| 6-8 | 90 (68.7%) | 27 (54.0%) | 63 (77.9%) | 17 (58.6%) | 29 (82.9%) |
| Other | 10 (7.6%) | 5 (10%) | 5 (6.2%) | 0 (0%) | 1 (2.9%) |
| R-CHOP Chemotherapy | 108 (82.4%) | 43 (86%) | 65 (80.2%) | 24 (82.8%) | 26 (74.3%) |
| Response to Chemotherapy | |||||
| CR | 118 (90.1%) | 45 (90.0%) | 73 (90.1%) | 28 (96.6%) | 32 (91.4%) |
| PR | 13 (9.9%) | 5 (10%) | 8 (9.9%) | 1 (3.4%) | 3 (8.6%) |
| Consolidative radiation therapy | 31 (23.7%) | 23 (46%) | 8 (9.9%) | 12 (41.4%) | 4 (11.4%) |
CR, complete response; LDH, lactate dehydrogenase; PR, partial response; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; ULN, upper limit of normal.
Final early stage cohort comprises patients who were treated with abbreviated chemotherapy with radiation therapy or extended chemotherapy without radiation therapy.
Bulky disease is defined as ≥7.5 cm.
Among the entire cohort, 23.7% of patients received consolidative RT to a median dose of 36 Gy (range, 27-60 Gy). Almost half of ES patients received radiation therapy (n = 23, 46%) compared with 11.4% (n = 4) of patients with AS DLBCL and bulky disease (n = 35).
Outcome of entire patient cohort
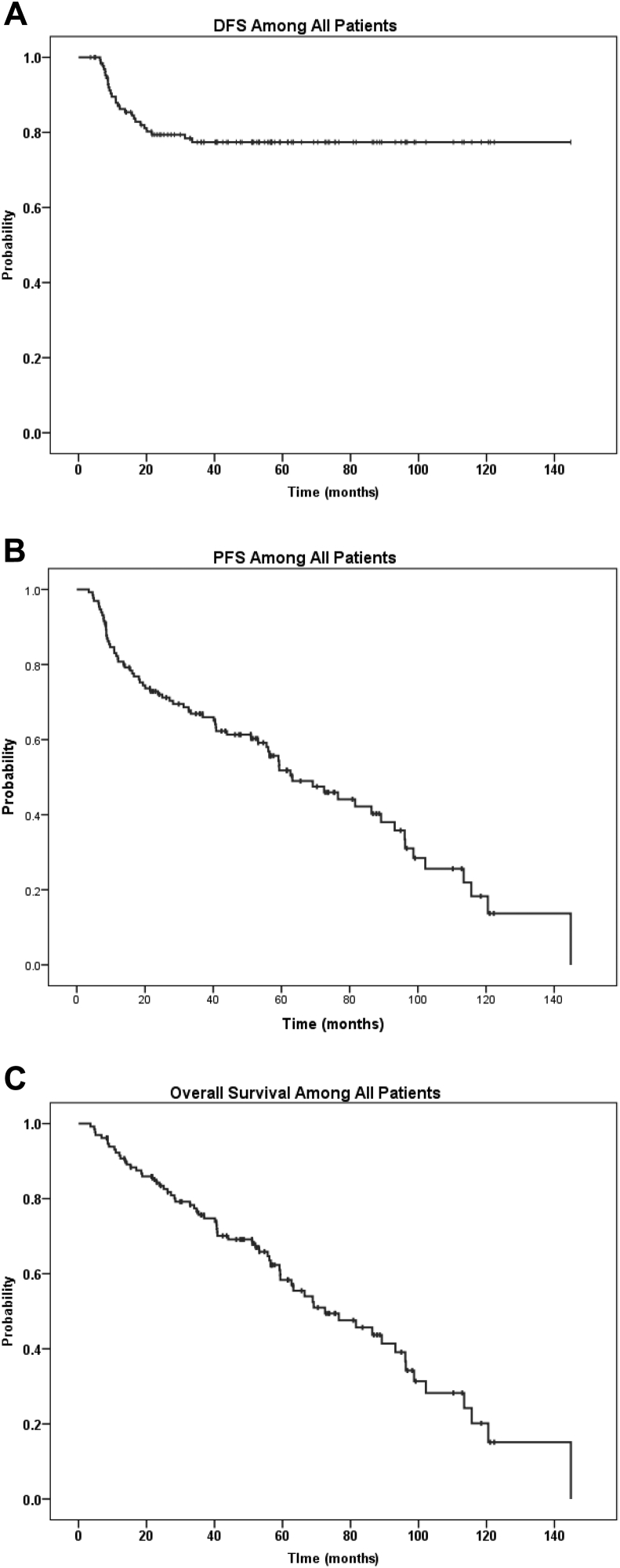
With a median follow-up of 51.3 months (range, 3.5-144.83), the 3-year DFS, PFS, and OS rates were 77.4%, 66.9%, and 75.7%, respectively (Fig 2). Median PFS and OS were 63.2 months (95% confidence interval [CI], 46.3-80.1) and 72.5 months (95% CI, 51.9-93.1), respectively. Twenty-seven patients experienced disease relapse (20.5%) at a median time of 10.9 months (range, 6.3-33.4 months), and 65 patients (49.6%) died. Causes of death were unrelated to lymphoma or therapy in 23 cases (35.4%), unknown in 20 patients (30.7%), due to lymphoma in 17 cases (26.2%), and treatment related in 5 patients (7.7%).
Figure 2.
Outcomes among 131 older elderly patients who were treated for stage I-IV diffuse large B-cell lymphoma. (A) Disease-free survival, (B) progression-free survival, and (C) overall survival.
ES patients
Among the 50 patients with ES DLBCL, 46% received consolidative RT (n = 23). Patients who did not receive RT were treated with increased cycles of chemotherapy (median = 6) compared with those who did receive RT (median = 4; P = .014). Among all ES patients, there was no significant difference in the 3-year rates of DFS, PFS, or OS among patients who did or did not receive consolidative RT (data not shown).
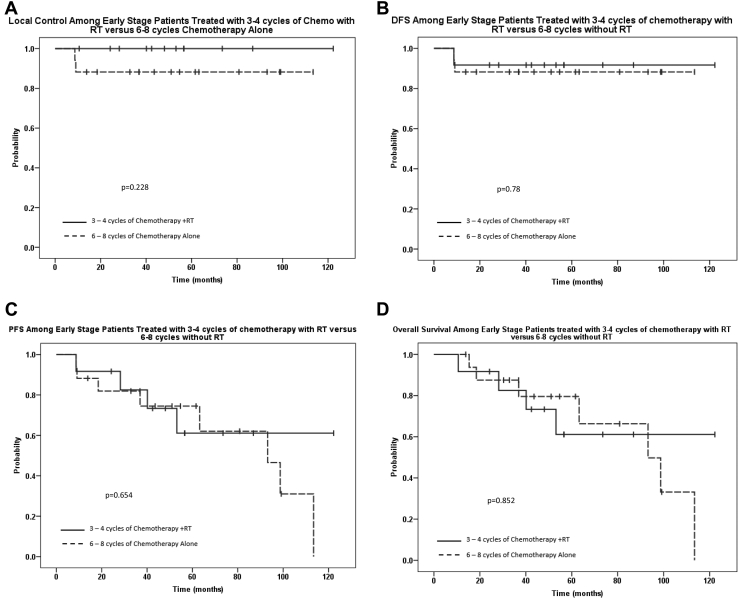
We sought to compare the outcome of ES patients treated with abbreviated systemic therapy (3-4 cycles) followed by consolidative RT (n = 12) versus those who received 6 to 8 cycles of systemic therapy alone (n = 17). The Kaplan-Meier curves for LC, DFS, PFS, and OS are shown in Figure 3. At a median follow up of 51 months (range, 10.6-122.3), the median OS for all 29 patients was 98.7 months (95% CI, 56.4-141). The 3-year LC was 100% for patients who received combined modality therapy (CMT) compared with 88.2% for patients treated with 6 to 8 cycles of systemic therapy alone (P = .228). There were no statistically significant differences in the 3-year DFS, PFS, or OS rates between these 2 cohorts. The 3-year DFS, PFS, and OS rates for patients who received CMT were 91.7%, 82.5%, and 82.5%, respectively, compared with 88.2% (P = .78), 81.9% (P = .654), and 87.5% (P = .852) among those treated with 6 to 8 cycles of chemotherapy without RT.
Figure 3.
Outcomes among patients with early stage disease who were treated with abbreviated chemotherapy (3-4 cycles) and radiation therapy versus chemotherapy alone (6-8 cycles). (A) local control, (B) disease-free survival, (C) progression-free survival, and (D) overall survival.
AS patients
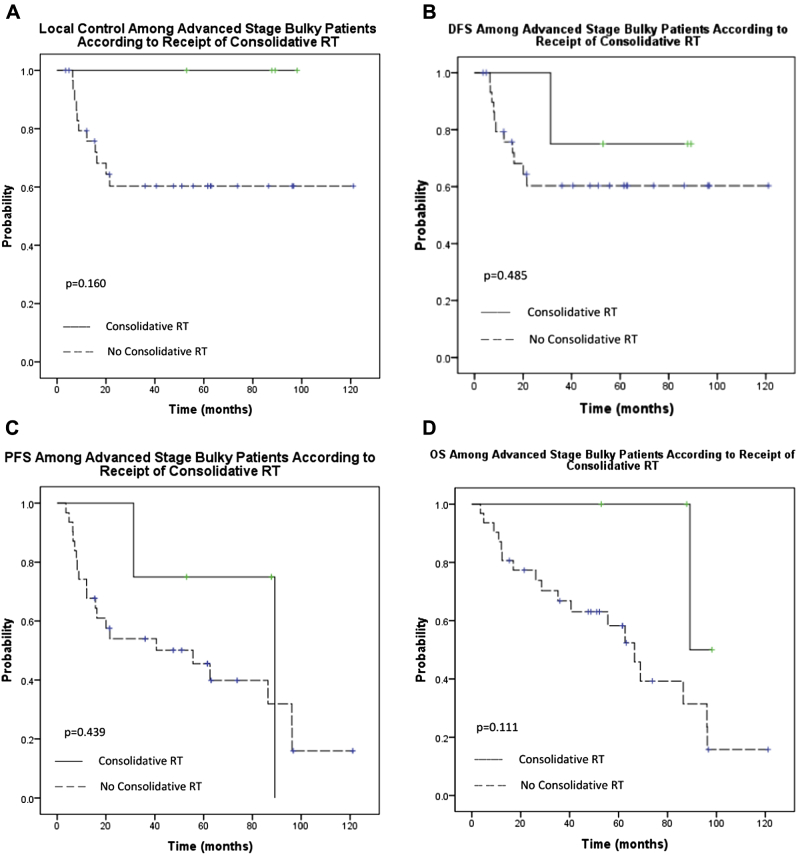
To ascertain the efficacy of RT among patients with AS lymphoma, we considered patients who would have been eligible for RT upon completion of systemic therapy (ie, those with bulky disease). We compared the outcome of patients treated with (n = 4) and without consolidative RT (n = 31 for AS DLBCL with bulky involvement [defined as ≥7.5 cm]). For the entire cohort of 35 patients, median OS was 68.9 months (95% CI, 40.3-97.4). At a median follow-up of 52.0 months (range, 3.5-121.1), the 3 year LC was 100% among patients that received RT compared with 60.3% for patients who did not (P = .160; Fig 4A). The 3-year DFS, PFS, and OS rates were 75%, 75%, and 100%, respectively, for patients who received consolidative RT and 60.3% (P = .485), 54.0% (P = .439), and 66.7% (P = .111) for those who did not (Fig 4B-D).
Figure 4.
Outcomes among patients with advanced-stage bulky disease according to receipt of radiation therapy. (A) Local control, (B) disease-free survival, (C) progression-free survival, and (D) overall survival.
Toxicity
The toxicity among ES patients treated with CMT compared with 6 to 8 cycles of chemotherapy alone is summarized in Table 2. Toxicity details were unavailable for 1 patient who received chemotherapy alone. Myelosuppression occurred more frequently among patients treated with extended chemotherapy. Thrombocytopenia occurred in 87.5% of patients treated with 6 to 8 cycles of therapy (n = 14) compared with 58.3% of patients treated with CMT (n = 7; P = .078). Anemia was observed in 93.8% of those who received chemotherapy alone (n = 15) versus 50.0% of patients treated with CMT (n = 6; P = .008). Neuropathy occurred more frequently in the chemotherapy alone cohort (50%; n = 8) than among patients who received RT after abbreviated chemotherapy (8.3%; n = 1; P = .0273). Congestive heart failure (CHF) occurred in 12.5% of patients who were treated with extended chemotherapy and none of the patients who received CMT (P = .2150). Half of patients who were treated with CMT were originally planned for extended chemotherapy but ultimately had treatment plan modifications due to poor tolerance of systemic therapy with initial chemotherapy cycles.
Table 2.
Toxicity among patients with early-stage disease
| Characteristic | 3-4 Cycles + Radiation Therapy (n = 12) | 6-8 Cycles Alone (n = 16) | P-value |
|---|---|---|---|
| Febrile neutropenia | 25% (3) | 37.5% (6) | .4896 |
| Neutropenia | 41.7% (5) | 68.8% (11) | .1634 |
| Thrombocytopenia | 58.3% (7) | 87.5% (14) | .0784 |
| Anemia | 50.0% (6) | 93.8% (15) | .0080 |
| Infection | 33.3% (4) | 50% (8) | .3849 |
| Cardiotoxicity | 16.7% (2) | 25% (4) | .6009 |
| Congestive heart failure | 0% (0) | 12.5% (2) | .2150 |
| Deep vein thrombosis | 8.3% (1) | 6.3% (1) | .8406 |
| Pulmonary toxicity | 0% (0) | 25% (4) | .0727 |
| Gastrointestinal toxicity | 25% (3) | 62.5% (10) | .0597 |
| Neuropathy | 8.3% (1) | 50% (8) | .0273 |
| Transfusion | 25.0% (3) | 43.8% (7) | .3138 |
| Mucositis | 41.7% (5) | 12.5% (2) | .0892 |
| Hospitalization | 50% (6) | 50% (8) | 1.0000 |
| Dose reduction or cycle delay | 66.7% (8) | 68.8% (11) | .9071 |
| Change in treatment plan | 50% (6) | 0% (0) | .0037 |
P values in bold indicate statistical significance (< or = 0.05).
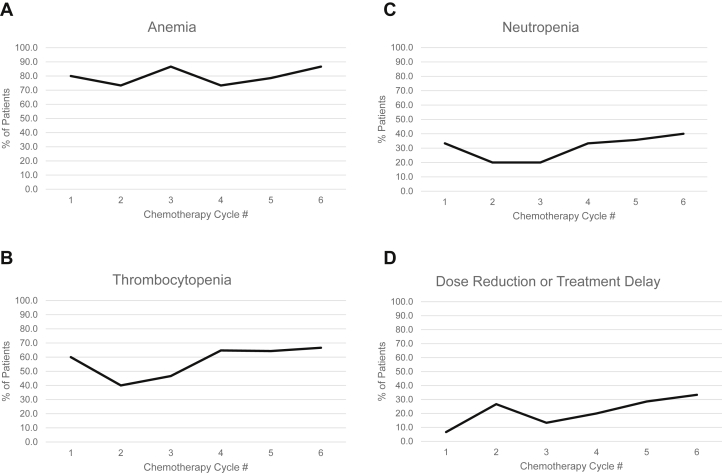
To examine the influence of increasing chemotherapy cycles on hematologic toxicity, we evaluated the incidence of grade 1 or higher anemia, thrombocytopenia, and neutropenia according to cycle of systemic therapy among ES patients who received 6 to 8 cycles of chemotherapy alone (Fig 5A-C). We also determined the frequency of chemotherapy dose reduction or delay across cycles in the same patients (Fig 5D). Myelosuppression tended to decrease after cycle 1, with a corresponding increase in chemotherapy dose reduction/delay. However, after cycle 3 the incidence of thrombocytopenia, neutropenia, and anemia increased despite further increases in chemotherapy dose delays and reductions.
Figure 5.
Incidence of Common Terminology Criteria for Adverse Events grade 1 or higher hematologic toxicity according to cycle of chemotherapy among patients with early-stage disease who were treated with 6 to 8 cycles of chemotherapy (n = 17). (A) Anemia, (B) thrombocytopenia, (C) neutropenia, and (D) chemotherapy dose reduction or delay.
Discussion
In this cohort of very elderly patients with newly diagnosed DLBCL, we examined the impact of consolidative RT on outcome among patients with an indication for RT: those with ES disease and patients with AS DLBCL and bulky disease. We observed that among patients with ES disease, DFS, PFS, and OS were not significantly different among patients who received CMT compared with 6 to 8 cycles of chemotherapy alone. However, toxicity was increased among patients who received longer courses of chemotherapy, with those who received 6 to 8 cycles experiencing higher rates of myelosuppression and neuropathy. For the patients with AS DLBCL and bulky disease, consolidative RT improved LC.
We previously demonstrated that among the entire cohort of 207 patients aged 80 years or older with newly diagnosed DLBCL, the leading cause of death was lymphoma in 50% and treatment-related complications in 14% of patients.14 Other studies have also demonstrated that even among a vulnerable population of patients over the age of 80 years who are often plagued by comorbidities that make treatment challenging, the leading cause of death is still lymphoma. This suggests that standard curative therapy should be offered to very elderly patients with DLBCL.16, 17 In our selected cohort of 131 patients with a good response to frontline chemotherapy, the leading source of mortality was unrelated to lymphoma or therapy in 35.4% of cases. Death due to lymphoma occurred in 26% and was treatment related in less than 10% of cases. This highlights the relative success of therapy in this selected population with chemosensitive disease and underscores the need to administer curative therapy while minimizing treatment-related toxicity.
We found no difference in survival outcomes at 3 years among ES patients aged 80 years or older who received 3 to 4 cycles of chemotherapy and RT versus extended immunochemotherapy alone. Our results are in agreement with a recent retrospective comparative analysis of 182 patients aged 70 years or older who had stage I-II DLBCL and were treated with R-CHOP chemotherapy with or without consolidative RT.18 In this large Italian study, the median number of chemotherapy cycles was 6 for the immunochemotherapy alone cohort and 4 for the combined modality group. The 5-year OS rates were similar in both arms (74% for immunochemotherapy; 72% for combined modality; P = .476).
The 5-year rates of PFS were also comparable in both groups. However, all grades of hematologic toxicity occurred more frequently in the immunochemotherapy arm (P = .034). Interestingly, in the SEER study by Odejide et al that compared the effectiveness of abbreviated chemotherapy and consolidative RT and 6 to 8 cycles of chemotherapy alone for patients aged 65 years or older, CMT was associated with a lower risk of second-line treatment (hazard ratio [HR], 0.71; P = .02).10 Similarly, in the initial publication of the SWOG 8736 trial, a 5-year PFS and OS benefit was demonstrated among patients with ES disease (5-year PFS: 77% vs 64%, P = .03; 5-year OS: 82% vs 72%; P = .02).5 However, with extended follow-up (median 17.7 years), there were continuous treatment failures in the CMT arm, resulting in similar PFS and OS for patients who received 3 cycles of CHOP followed by RT compared with those who received 8 cycles of CHOP.7 It is presumed that the late failures observed in the CMT arm are due to the inadequacy of abbreviated chemotherapy to control systemic disease.
However, in the very elderly patient population, post-therapy competing risks of death, coupled with a higher risk of severe toxicities from more extended immunochemotherapy, are competing risks for late systemic failures, which may instead have a negligible impact. Indeed, our cohort of ES patients treated with abbreviated chemotherapy and RT contained a significant proportion of patients who had their treatment plan changed due to poor tolerance of the initial cycles of chemotherapy. Fifty percent of patients who received CMT were planned to receive 6 to 8 cycles of chemotherapy prior to the initiation of treatment. Instead, these patients had chemotherapy suspended early and were referred for RT due to toxicity and poor tolerability of systemic treatment. We found no difference in survival among ES patients based on treatment approach, which suggests that with regard to survival outcomes and treatment efficacy in the very elderly population with ES DLBCL, abbreviated chemotherapy and RT is a valid therapeutic option.
Although treatment efficacy should be a prominent factor in guiding treatment decisions among very elderly patients with DLBCL, this must be weighed against treatment-related toxicity. We found that among ES patients, abbreviated chemotherapy and consolidative RT was associated with a more favorable side effect profile with regard to myelotoxicity and neuropathy. In patients over the age of 80 years, anemia, thrombocytopenia, and neutropenia caused a more significant burden than in younger patients.19
In both the SWOG 8736 trial and the SEER study, increased toxicity was significantly associated with a longer course of chemotherapy. In the SWOG trial, death as a result of CHF occurred in 7 patients in the extended chemotherapy arm compared with 1 patient in the combined modality arm.7 A SEER Medicare study found that, among DLBCL patients aged 65 years or older, there was a 47% increased risk of CHF after 6 or more cycles of R-CHOP.20 We saw a trend toward increased rates of CHF among older elderly ES patients treated with 6 to 8 cycles of anthracycline-based chemotherapy, with 12.5% of patients experiencing CHF compared with 0% in the CMT cohort (P = .215).
Myelosuppression is a significant source of toxicity in this patient population. In addition to causing patient morbidity, myelosuppression has the potential to adversely affect treatment efficacy by affecting dose intensity if it results in cycle delays or dose reductions in an effort to abrogate toxicity. We did observe a correlation between anemia, thrombocytopenia, and neutropenia and dose reduction. Decreases in chemotherapy doses were common after cycle 1 when a peak of myelosuppression was observed. As a result, decreased rates of cytopenia were observed after cycle 2. However, despite further treatment delays or additional decreases in chemotherapy dose, the incidence of cytopenia increased with later cycles of chemotherapy. Because dose reductions and delays have been shown to adversely affect treatment efficacy and outcome in this patient population, this should be a consideration when treatment decisions are made.21, 22 Our toxicity data suggest that in this patient population, abbreviated chemotherapy and consolidative RT may be superior to 6 to 8 cycles alone with regard to tolerability of therapy.
Very elderly AS patients pose a significant challenge to oncologists. Six cycles of chemotherapy represent standard therapy, but comorbid conditions, PS, and tolerance of intensive chemotherapy may be a challenge in older elderly patients. For patients with AS DLBCL, RT decreases local recurrence at bulky sites, which can translate into PFS and even OS benefits.3, 4, 11, 12, 13, 23
In the RICOVER-60 trial (rituximab with CHOP over age 60 years), the role of consolidative RT to sites of bulky (≥7.5 cm) or extralymphatic disease was investigated among patients aged 61 to 80 years.13 In the analysis according to treatment administered, RT improved the 3-year PFS (88% vs 62%; P < .001) and OS (90% vs 65%; P = .001). In our cohort of AS patients with disease ≥7.5 cm, there was a nonsignificant trend toward improved LC with consolidative RT at 3 years (100% vs 53.8%; P = .121). No statistically significant difference was observed with regard to DFS, PFS, or OS. The small number of patients in this subanalysis preclude any strong conclusions; however, LC of bulky sites is valuable in a population of frail patients who may have poor tolerance of salvage therapies for relapsed or refractory disease.
In the era of involved site radiation therapy and modern RT planning, acute toxicity is expected to be limited among patients with DLBCL who are treated with moderate doses of 30 Gy for consolidation after a complete response to chemotherapy.24 In our study, the median RT dose was 36 Gy, with one patient receiving RT to a dose as high as 60 Gy. This likely reflects concern with regard to residual disease in patients who are restaged with CT imaging and not PET/CT after chemotherapy. This highlights the importance of using proper staging assessments in this population of older patients in whom PET/CT will affect RT planning and dose, with implications for radiation-related toxicity. In our study, RT was generally well tolerated without significant side effects related to RT. There was a trend toward mucositis among ES patients treated with RT compared with chemotherapy alone (41.7% vs 12.5%; P = .0892).
Radiation oncologists should be cautious and have a high regard for limiting normal tissue exposure in these frail patients. With regard to long-term toxicities, secondary malignancies that occur a decade or more after RT are less of an issue among older patients with DLBCL.25, 26, 27 In a study of 128 patients with DLBCL treated between 1988 and 2003, no increased risk of second tumors was found among those over the age of 59 years.26 In the updated analysis of SWOG 8736, there were no significant differences in the cumulative incidence of secondary malignancies in either arm (2-sided P = .44).7 In the population of patients aged 80 years or older, secondary malignancy is not a valid concern and should not influence decisions with regard to RT.
Important limitations to this retrospective, single-center study warrant discussion. In addition to the inherent selection bias, we may be reporting outcomes for a favorable group of patients that do not reflect real-life experiences. We cannot conclude that similar favorable outcomes can be reproduced in the community setting. The retrospective collection of data on side effects may have underestimated the incidence of toxicity. In addition, we also have limited information on how reduced dose chemotherapy (mini-R-CHOP) would have compared with regard to tolerability because mini-R-CHOP is not commonly pursued in this population at our institution.14 Given that our results compare favorably with historical outcomes for younger patients with previously untreated DLBCL and that consolidative RT is highly specialized, these findings suggest that a referral to an academic center with experience in administering RT to patients with lymphoma should be considered.
In this single-institutional study of patients aged 80 years or older to evaluate the role of consolidative RT among patients with stage I-IV DLBCL, we demonstrated that survival outcomes were similar for ES patients who received abbreviated chemotherapy and consolidative RT compared with 6 to 8 cycles of chemotherapy alone. However, CMT was associated with decreased myelosuppression and neuropathy, which suggests that 3 to 4 cycles of systemic therapy and RT may be a superior choice for therapy when balancing treatment efficacy and tolerability. Furthermore, patients with AS DLBCL and bulky disease likely benefit from consolidative RT with regard to LC. We advise that even for this unique patient age group, curative intent should be the goal when considering management strategies, similar to how we approach their younger counterparts. We also strongly advise consideration of existing comorbidities, PS, and aggressive supportive care for this population. We conclude that abbreviated chemotherapy along with consolidative RT can achieve outcomes similar to those reported in younger patient populations with a tolerable safety profile.
Footnotes
Conflicts of interest: None.
References
- 1.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116:5103–5110. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- 2.Morrison V.A., Hamlin P., Soubeyran P. Approach to therapy of diffuse large B-cell lymphoma in the elderly: the International Society of Geriatric Oncology (SIOG) expert position commentary. Ann Oncol. 2015;26:1058–1068. doi: 10.1093/annonc/mdv018. [DOI] [PubMed] [Google Scholar]
- 3.Ng A.K., Dabaja B.S., Hoppe R.T., Illidge T., Yahalom J. Re-examining the role of radiation therapy for diffuse large B-cell lymphoma in the modern era. J Clin Oncol. 2016;34:1443–1447. doi: 10.1200/JCO.2015.64.9418. [DOI] [PubMed] [Google Scholar]
- 4.Pinnix C.C. Radiation therapy for diffuse large B-cell lymphoma: Indications, outcomes, and controversies. Int J Radiat Oncol Biol Phys. 2016;94:641–644. doi: 10.1016/j.ijrobp.2015.12.357. [DOI] [PubMed] [Google Scholar]
- 5.Miller T.P., Dahlberg S., Cassady J.R. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998;339:21–26. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 6.Miller T.P., LeBlanc M., Spier C. CHOP alone compared to CHOP plus radiotherapy for early stage aggressive non-Hodgkin's lymphomas: Update of the Southwest Oncology Group (SWOG) randomized trial. Blood. 2001;98:724a–725a. [Google Scholar]
- 7.Stephens D.M., Li H., LeBlanc M.L. Continued risk of relapse independent of treatment modality in limited-stage diffuse large B-cell lymphoma: Final and long-term analysis of Southwest Oncology Group study S8736. J Clin Oncol. 2016;34:2997–3004. doi: 10.1200/JCO.2015.65.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh R.R., Yahalom J. Older patients with early-stage diffuse large B-cell lymphoma: The role of consolidation radiotherapy after chemoimmunotherapy. Leuk Lymphoma. 2017;58:614–622. doi: 10.1080/10428194.2016.1205739. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy R.J., Jegadeesh N., Switchenko J. The role of radiotherapy for patients over age 60 with diffuse large B-cell lymphoma in the rituximab era. Leuk Lymphoma. 2016;57:1876–1882. doi: 10.3109/10428194.2015.1120866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odejide O.O., Cronin A.M., Davidoff A.J., LaCasce A.S., Abel G.A. Limited stage diffuse large B-cell lymphoma: comparative effectiveness of treatment strategies in a large cohort of elderly patients. Leuk Lymphoma. 2015;56:716–724. doi: 10.3109/10428194.2014.930853. [DOI] [PubMed] [Google Scholar]
- 11.Phan J., Mazloom A., Medeiros L.J. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170–4176. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- 12.Held G., Zeynalova S., Murawski N. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol. 2013;31:4115–4122. doi: 10.1200/JCO.2012.48.0467. [DOI] [PubMed] [Google Scholar]
- 13.Held G., Murawski N., Ziepert M. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol. 2014;32:1112–1118. doi: 10.1200/JCO.2013.51.4505. [DOI] [PubMed] [Google Scholar]
- 14.Chihara D., Westin J.R., Oki Y. Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer. 2016;122:3145–3151. doi: 10.1002/cncr.30173. [DOI] [PubMed] [Google Scholar]
- 15.Cheson B.D., Fisher R.I., Barrington S.F. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thieblemont C., Grossoeuvre A., Houot R. Non-Hodgkin's lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol. 2008;19:774–779. doi: 10.1093/annonc/mdm563. [DOI] [PubMed] [Google Scholar]
- 17.Trebouet A., Marchand T., Lemal R. Lymphoma occurring in patients over 90 years of age: characteristics, outcomes, and prognostic factors. A retrospective analysis of 234 cases from the LYSA. Ann Oncol. 2013;24:2612–2618. doi: 10.1093/annonc/mdt282. [DOI] [PubMed] [Google Scholar]
- 18.Ciammella P., Filippi A.R., Simontacchi G. Alternative options for elderly patients with limited stage diffuse large B-cell lymphoma: R-chemotherapy vs. R-chemotherapy plus radiotherapy. Leuk Lymphoma. 2016;57:2677–2680. doi: 10.3109/10428194.2016.1153088. [DOI] [PubMed] [Google Scholar]
- 19.Millar A., Ellis M., Mollee P. Deliverability and efficacy of R-CHOP chemotherapy in very elderly patients with diffuse large B-cell lymphoma: An Australian retrospective analysis. Intern Med J. 2015;45:1147–1153. doi: 10.1111/imj.12889. [DOI] [PubMed] [Google Scholar]
- 20.Hershman D.L., McBride R.B., Eisenberger A., Tsai W.Y., Grann V.R., Jacobson J.S. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:3159–3165. doi: 10.1200/JCO.2007.14.1242. [DOI] [PubMed] [Google Scholar]
- 21.Hirakawa T., Yamaguchi H., Yokose N., Gomi S., Inokuchi K., Dan K. Importance of maintaining the relative dose intensity of CHOP-like regimens combined with rituximab in patients with diffuse large B-cell lymphoma. Ann Hematol. 2010;89:897–904. doi: 10.1007/s00277-010-0956-7. [DOI] [PubMed] [Google Scholar]
- 22.Utsu Y., Takaishi K., Inagaki S. Influence of dose reduction of vincristine in R-CHOP on outcomes of diffuse large B cell lymphoma. Ann Hematol. 2016;95:41–47. doi: 10.1007/s00277-015-2514-9. [DOI] [PubMed] [Google Scholar]
- 23.Dorth J.A., Prosnitz L.R., Broadwater G. Impact of consolidation radiation therapy in stage III-IV diffuse large B-cell lymphoma with negative post-chemotherapy radiologic imaging. Int J Radiat Oncol Biol Phys. 2012;84:762–767. doi: 10.1016/j.ijrobp.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 24.Specht L., Yahalom J. The concept and evolution of involved site radiation therapy for lymphoma. Int J Clin Oncol. 2015;20:849–854. doi: 10.1007/s10147-015-0863-y. [DOI] [PubMed] [Google Scholar]
- 25.Mudie N.Y., Swerdlow A.J., Higgins C.D. Risk of second malignancy after non-Hodgkin's lymphoma: A British cohort study. J Clin Oncol. 2006;24:1568–1574. doi: 10.1200/JCO.2005.04.2200. [DOI] [PubMed] [Google Scholar]
- 26.Sacchi S., Marcheselli L., Bari A. Second malignancies after treatment of diffuse large B-cell non-Hodgkin's lymphoma: A GISL cohort study. Haematologica. 2008;93:1335–1342. doi: 10.3324/haematol.12918. [DOI] [PubMed] [Google Scholar]
- 27.Tward J.D., Wendland M.M., Shrieve D.C., Szabo A., Gaffney D.K. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:108–115. doi: 10.1002/cncr.21971. [DOI] [PubMed] [Google Scholar]