Abstract
Objectives
More than one-third of hospitalized patients have hyperglycemia. Despite evidence that improving glycemic control leads to better outcomes, achieving recognized targets remains a challenge. The objective of this study was to evaluate the implementation of a computerized insulin order set and titration algorithm on rates of hypoglycemia and overall inpatient glycemic control.
Methods
A prospective observational study evaluating the impact of a glycemic order set and titration algorithm in an academic medical center in non-critical care medical and surgical inpatients. The initial intervention was hospital-wide implementation of a comprehensive insulin order set. The secondary intervention was initiation of an insulin titration algorithm in two pilot medicine inpatient units. Point of care testing blood glucose reports were analyzed. These reports included rates of hypoglycemia (BG < 70 mg/dL) and hyperglycemia (BG >200 mg/dL in phase 1, BG > 180 mg/dL in phase 2).
Results
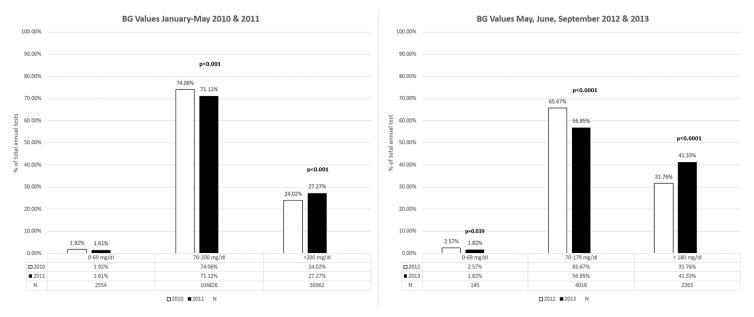
In the first phase of the study, implementation of the insulin order set was associated with decreased rates of hypoglycemia (1.92% vs 1.61%; p <0.001) and increased rates of hyperglycemia (24.02% vs 27.27%; p <0.001) from 2010 to 2011. In the second phase, addition of a titration algorithm was associated with decreased rates of hypoglycemia (2.57% vs 1.82%; p = 0.039) and increased rates of hyperglycemia (31.76% vs 41.33%; p<0.001) from 2012 to 2013.
Conclusions
A comprehensive computerized insulin order set and titration algorithm significantly decreased rates of hypoglycemia. This significant reduction in hypoglycemia was associated with increased rates of hyperglycemia. Hardwiring the algorithm into the electronic medical record may foster adoption.
Keywords: Hypoglycemia, Hyperglycemia, Inpatient Glycemic Control, Computerized Order Sets
1. Introduction
Achieving optimal glycemic control in the inpatient setting continues to be a tremendous challenge. It is estimated that 38% of hospitalized patients have hyperglycemia. Of this number, 26% have pre-existing diabetes with the remaining 12% having either newly diagnosed diabetes or stress hyperglycemia.1 Although hyperglycemia is not the primary diagnosis in most cases, it has adverse effects on health outcomes.2 There are numerous detrimental consequences to the immune, cardiovascular and central nervous systems leading to poor outcomes.3 Patients with hyperglycemia have an increased infection rate secondary to decreased leukocyte mobilization and phagocytic activity.4 Hyperglycemia is an important marker of poor clinical outcome and mortality not only in critically ill patients but also in those admitted to general medicine and surgery units.1 Hypoglycemia is also associated with a higher risk of mortality in hospitalized patients. 5 In addition, poor glycemic control leads to higher admission rates as well as an increased need for support at home or in a facility following discharge. 6 The benefits of treating both hypo- and hyperglycemia result in decreased rates of morbidity and mortality as well as in cost savings such as decreased length of stay and rate of readmissions.7
Despite the evidence that improved inpatient glycemic control yields better outcomes, achieving recognized targets remain elusive. Insulin is the preferred agent in the inpatient setting due to its rapid onset of action and flexibility in adjusting doses as clinical status changes.3 Hospitalized patients have rapidly changing insulin requirements due to varying nutritional intake as well as fluctuations in the severity of their underlying illness.8 The 2013 American Society of Health-System Pharmacists (ASHP) Research and Education Foundation recommendations designated insulin as a high-alert medication across all inpatient settings.9 In annual surveys conducted by The Institute for Safe Medication Practices (ISMP), insulin continues to rank as one of the top high-risk medications in hospitals.10 The appropriate initiation and titration of insulin therapy is an ongoing challenge that contributes to clinical inertia, or the failure to advance therapy when needed.11 Common root causes of clinical inertia when dosing insulin include lack of comfort level when prescribing, fear of inducing hypoglycemia, various training levels amongst prescribers as well as having to balance competing patient and institutional priorities.12
The primary aim of this study is to evaluate the safety and efficacy of a comprehensive insulin order set in the inpatient computer-based provider order-entry system (CPOE) to facilitate insulin dosing and its impact on glycemic control at NewYork-Presbyterian Hospital/Weill Cornell Medicine (NYPH/WC). Previous studies have suggested that structured insulin order sets promote insulin safety and efficacy.13 We posited that a standardized protocol with initial starting doses of both basal and bolus insulin would enhance the quality of care by improving overall glycemic control and reducing both insulin medication errors and rates of hypoglycemia.
2. Methods
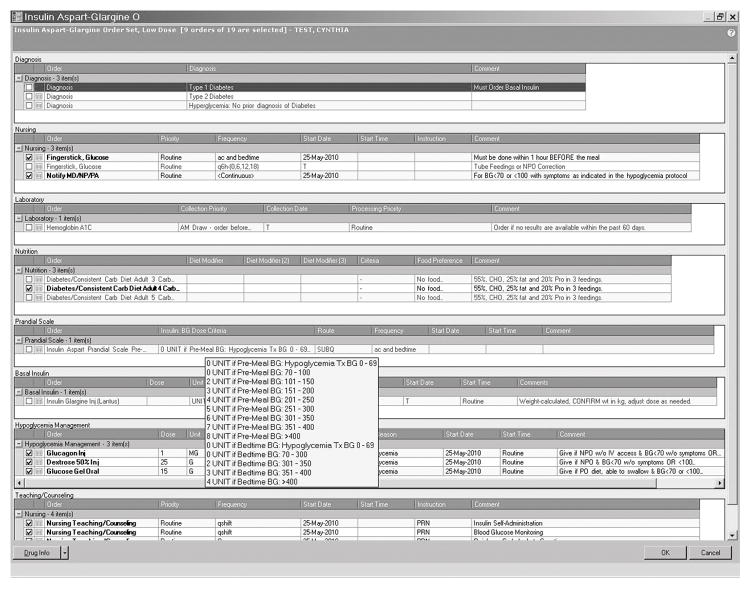
The setting was an academic teaching hospital with an average of 60,000 inpatients per year and an overall inpatient mortality rate of 6.9%. The type 1 diabetes subgroup had an average A1c of 9.3% ± 2.7% and the type 2 diabetes subgroup an A1c of 7.7% ± 2.0%. Additional relevant demographics can be found in Table 1. In the first phase of this study, five comprehensive subcutaneous insulin order sets were launched in November 2010. These order sets were available hospital wide in all adult non-critical care areas, except for labor and delivery. The launch of the order sets was hospital-wide therefore all patients receiving subcutaneous insulin were using these order sets. There were no patients in the non-critical care medical and surgical floors that did not have their insulin ordered through these order sets. These order sets provided initial starting doses based on levels of insulin sensitivity for adult inpatients with hyperglycemia. The following five order sets were available: NPO, very low, low, medium and high dose. (See Figure 1- Screenshot of Aspart/Glargine Low Dose Order Set & Table 2 – Initial insulin doses for order sets). Clinicians received minimal training with a handout that included screenshots of the order sets and how to place orders. There was wide adoption of the order sets because of its simple and comprehensive nature, with pre-selected orders for diet, blood glucose (BG) monitoring, correction and mealtime insulin, treatment of hypoglycemia and diabetes education. Safety and efficacy of the insulin order sets was determined based on point of care BG testing (POCT) records stored in a database in the POCT laboratory. Reports were generated on a daily, monthly and annual basis and included rates of hypoglycemia (BG <70 mg/dL) and hyperglycemia (BG >200 mg/dL), as well as frequencies of BGs within the recommended range at that time of 70–200 mg/dl. These reports were based on aggregated data with no identifiable individual patient information included. BG POCT reports were analyzed on a monthly basis, comparing 2010 BG data to 2011 BG data.
Table 1.
Demographics of the Inpatient Population in 2013
| Number of patients admitted in 2013 | n=61,289 |
|---|---|
| Gender | |
| Male | 29,526 (48.2%) |
| Female | 31,672 (51.7%) |
| Unknown | 91 (0.2%) |
| Age (years) | |
| ≤18 | 8,841 (14.4%) |
| 19–24 | 1,398 (2.2%) |
| 25–34 | 4,068 (6.6%) |
| 35–44 | 6,842 (11.2%) |
| 45–54 | 5,172 (8.4%) |
| 55–64 | 6,291 (10.3%) |
| 65–74 | 8,248 (13.5%) |
| 75–84 | 8,129 (13.3%) |
| ≥85 | 12,300 (20.1%) |
| Race | |
| White | 21,349 (34.8%) |
| African-American | 5,072 (8.3%) |
| Asian | 2,796 (4.6%) |
| American Indian or Alaska Native | 132 (0.2%) |
| Native Hawaiian or Pacific Islander | 60 (0.1%) |
| Unknown | 31,777 (51.8%) |
| Insurance | |
| Medicaid | 2,988 (4.9%) |
| Medicare | 19,754 (32.2%) |
| Commercial | 38,091 (62.1%) |
| Uninsured | 449 (0.7%) |
| Length of Stay (days) | |
| Total Inpatient Population | 9.2 ± 18.5 |
| Type 1 Diabetes (n= 301; 0.01%) | 17.9 ± 43.3 |
| Type 2 Diabetes (n=3179; 5.19%) | 10.8 ± 15.1 |
| BMI (kg/m2) | |
| ≤18.5 | 9,759 (15.9%) |
| 18.6–25 | 17,320 (28.3%) |
| 25.1–30 | 14,091 (23.0%) |
| 30.1–40 | 8,967 (14.6%) |
| >40.1 | 1,920 (3.1%) |
| Not Recorded | 9.232 (15.1%) |
Figure 1.
Screenshot of Aspart/Glargine Low Dose Order Set
Table 2.
Initial insulin doses for order sets
| INSULIN ASPART GLARGINE SUBCUTANEOUS ORDER SETS | |||||
|---|---|---|---|---|---|
| NPO | Poor PO Intake | Low | Medium | High | |
| Indication | Use for short term NPO; If Type 1 Diabetes, must order basal insulin | Use for poor oral intake < 50% of tray | Use for elderly Type 1 Diabetes Lean body type Renal insufficiency; pancreatectomy |
Use for average body size | Use for obesity; taking steroids |
| Glargine | None | 0.1 unit/kg | 0.15 unit/kg | 0.2 unit/kg | 0.3 unit/kg |
| BG frequency | Q 6hours (00,6,12,18) | AC (with meals and bedtime) | AC (with meals and bedtime) | AC (with meals and bedtime) | AC (with meals and bedtime) |
| BG | Initial Aspart Doses | ||||
| 70–100 | 0 units | 0 units | 0 units | 0 units | 2units |
| 101–150 | 0 units | 0 units | 0 units | 4 units | 6 units |
| 151–200 | 1 units | 2 units | 3 units | 5 units | 8 units |
| 201–250 | 2 units | 3 units | 4 units | 6 units | 10 units |
| 251–300 | 3 units | 4 units | 5 units | 7 units | 12 units |
| 301–350 | 4 units | 5 units | 6 units | 8 units | 14 units |
| 351–400 | 5 units | 6 units | 7 units | 9 units | 16 units |
| >400 | 6 units | 7 units | 8 units | 10 units | 18 units |
| Bedtime BG | Initial Aspart Doses | ||||
| 250–300 | 0 units | 0 units | 0 units | 4 units | |
| 301–350 | 2 units | 2 units | 4 units | 6 units | |
| 351–400 | 3 units | 3 units | 5 units | 8 units | |
| >400 | 4 units | 4 units | 6 units | 10 units | |
Upon implementation of the order sets, the researchers discovered that there was reluctance on the part of prescribers to titrate the initial basal and the bolus insulin doses since the titrations were not auto-calculated. In response to this need, a second phase of the study was initiated and piloted in two general medicine units. It involved the development of a simple evidence-based titration algorithm based on BG target ranges to facilitate safe, timely and effective titration of both basal and bolus subcutaneous insulin. The algorithm was based on several inpatient studies that used dosing algorithms successfully.14,15,16 It is important to note that in 2012 we adopted the newly released Endocrine Society management of hyperglycemia guideline to maintain BG <180 mg/dL in the non-critical care setting.16 The new titration algorithm was designed to guide prescribers to adjust insulin based on patterns of glucose control over the previous 24-hour period. In May 2013, the inpatient diabetes nurse practitioner (JJS) piloted the algorithm with groups of medicine house staff responsible for ordering and titrating insulin. The education consisted of 15-minute group sessions offered twice a month on the medicine unit. To reinforce the teaching, a pocket card was distributed containing the key elements of the algorithm (Fig. 2 - Algorithm Version 1.0). In June 2013, house staff feedback indicated a lack of comfort calculating and entering the dose adjustments into the CPOE. This was due to an unforeseen hesitation when deciding which direction to round a fraction to a whole number and the cumbersome hand entry process into the EMR. To address this concern, the intervention was modified by creating a new version of the algorithm that pre-calculated the doses for all the glucose ranges in the high dose order set by adding 10% when BG was between 180–250 mg/dL and 20% when BG was greater than 250 mg/dL. (See Fig 2- Algorithm Version 2.0). In September 2013, the inpatient diabetes nurse practitioner (JJS) re-educated the house staff in the new version of the algorithm and distributed a revised pocket card. After the education was completed, we analyzed three months of BG POCT data from the pilot medicine unit and compared it to the same three calendar months from the previous year.
Figure 2.
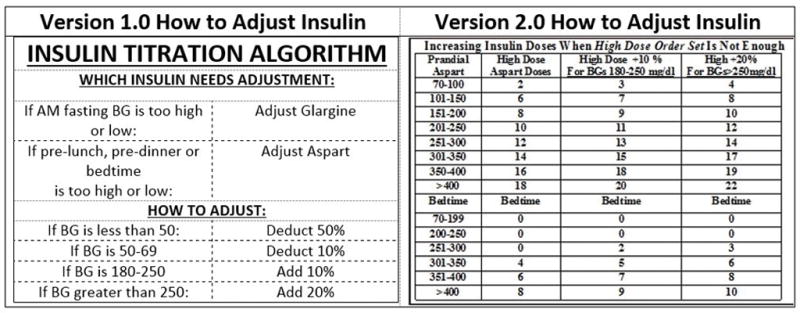
Algorithm Versions 1.0 and 2.0
Rates of hypoglycemia, BG in-target range, and hyperglycemia were calculated by year. The chi square test was used to compare the rates within each BG category by year. All statistical tests were two-sided and p < 0.05 was considered statistically significant. Analyses were performed in SAS version 9.1 (SAS Institute, Inc., Cary, North Carolina).
The study was approved by the Weill Cornell Medicine Institutional Review Board.
3. Results
In the first phase of the study, implementation of the insulin order sets was associated with a significant decrease in the rates of hypoglycemia from 2010 to 2011 (1.92% and 1.61%, respectively; p <0.001). At the same time, BG levels in target range decreased (74.06% vs 71.12%, respectively; p<0.001) and rates of hyperglycemia increased (24.02% vs 27.27%, respectively; p<0.001). In the second phase, with the addition of a titration algorithm to address hyperglycemia, rates of glycemia were re-evaluated. There was a significant decrease in hypoglycemia from 2012 to 2013 (2.57% vs 1.82% respectively; p = 0.039). In addition, there was a significant decrease in blood glucose levels in the target range from 2012 to 2013 (65.67% vs 56.85%; p<0.0001). Rates of hyperglycemia increased (31.76% vs 41.33%; p<0.001). (See Fig. 3)
Figure 3.
Rates of Glycemia Following Both Phases of Study
4. Discussion
Our study showed that a comprehensive computerized insulin order set and titration algorithm was safe and resulted in decreased hypoglycemia with the unforeseen consequence of increased hyperglycemia. In response to the increase in hyperglycemia, the second phase of our study introduced an insulin titration algorithm to help with prescriber intensification of insulin doses based on glucose patterns. This titration algorithm did not appear to work optimally for the following reasons: Despite the distribution of a pocket card of the algorithm as an additional resource at the time of the educational intervention, adoption was slow. Careful examination of algorithm usage revealed resident reluctance to manually calculate doses and hand enter them into the computerized provider order entry (CPOE). To address this significant barrier, a new pocket card was created that pre-calculated the doses by adding 10% or 20% depending on the BG range. Although this strategy was intended to improve clinical practice behavior, the rate of adoption remained disappointing. In subsequent discussions with medical residents asked to use the algorithm, many complained about hand entering the new doses and requested that auto-calculation be incorporated into the CPOE. One important future direction would include hardwiring the algorithm into our EMR with assistance from our information technology colleagues.
A strength of our study was the large BG POCT dataset captured from an 800 bed academic medical center. Another strength was the widespread adoption of the order set since it replaced the previous cumbersome method of separately entering insulin and other related orders such as BG monitoring and hypoglycemia treatment. The primary limitation of the study is that the effect of the intervention was restricted to changes in glycemic rates instead of patient outcomes. The clinical significance of the decrease in rates of hypoglycemia was not evaluated. The low p-values due to large numbers may contribute to the statistical significance observed in the rates of hypoglycemia. However, the rates of hypoglycemia are, fortunately, very uncommon in this hospital’s setting so that a small difference in rates may be of clinical importance. Another limitation of working with a large BG POCT dataset is that specific information regarding the type of diabetes (ie. type 1, type 2 or new hyperglycemia) or degree of control (A1c ) was not available. It was also not possible to perform an assessment of comorbidities using an Elixhauser or Charlson comorbidity score. Another limitation was that the implementation of the insulin titration algorithm was piloted on two general medicine units each with an average census of 35 patients and may not be generalizable to other inpatient units. The rates of compliance and adherence to the titration algorithm were not reported other than what was gathered through feedback at the time of the teaching sessions. This limits the conclusions that can be drawn about the efficacy of the algorithm.
Our study reinforces the ongoing difficulty in achieving recommended glycemic targets in the inpatient setting. Rogers’s Diffusion of Innovations, a theoretical framework that helps address barriers to following clinical practice recommendations and offers possible solutions, can guide the development and testing of a comprehensive electronic insulin order set for prescribers in the inpatient setting when glycemic targets are not reached.17 A “Call to Action” consensus conference hosted by the American Association of Clinical Endocrinologists and the American Diabetes Association identified institutional insulin management protocols and standardized insulin order sets as essential interventions to address key barriers to inpatient glycemic control.18 An ASHP expert consensus panel concurred with these recommendations as well as the need to eliminate sliding scale insulin dosing and substitute it with protocol-driven evidence based insulin order sets.9 Since that time, many hospitals have built comprehensive insulin order sets with dosing recommendations to promote both safety and efficacy. Standardization of order sets along with staff education in glycemic control strategies are two ways that have been shown to mitigate common errors associated with insulin use.19
The fear that insulin use leads to an increased risk of hypoglycemia remains a strong contributor to clinical inertia. Interventions that increase prescriber comfort and knowledge when initiating and intensifying insulin therapy are important steps toward improving glycemic management in the hospital setting. A number of innovative strategies have been utilized to reduce clinical inertia and facilitate timely initiation and intensification of insulin therapy. These include the use of computer-based algorithms and information systems for decision support, automatic reminders of when to order tests and single “click” electronic ordering. 20 Donihi et al, found that insulin usage in the inpatient setting was associated with a number of medication errors along with adverse events such as hypoglycemia and hyperglycemia. The researchers instituted a standardized insulin order set along with pre-printed prescriber orders forms. One year after protocol implementation, prescribing errors were significantly reduced from 10.3 per 100 patient days to 1.2 per 100 patient days (p=0.03). Additionally, hyperglycemia episodes 1 year after implementation decreased from 55.9 to 16.3 per 100 patient days. There was no associated increase in hypoglycemia.21
5. Conclusion
The implementation of a comprehensive glycemic management order set is an important tool to improve patient safety and quality. Prior studies provided evidence that the implementation of standardized insulin protocols for the management of inpatient hyperglycemia can lead to decreased episodes of hyperglycemia as well as decreased prescribing errors. It is important to assess the effects of implementation of insulin order sets on outcomes. Our study showed significant decreases in rates of hypoglycemia but rates of hyperglycemia increased secondary to lack of appropriate intensification of insulin treatment. An important next step will be to explore hardwiring the algorithm into our EMR to facilitate adoption by enhancing ease of use and availability of decision support.
Acknowledgments
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Science of the National Institute of Health under Award Number UL1TR000457.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of inhospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi A, Deakins DA, Reynolds LR. Obstacles to optimal management of inpatient hyperglycemia in noncritically ill patients. Hosp Pract. 2012 Apr 1;40(2):36–43. doi: 10.3810/hp.2012.04.968. [DOI] [PubMed] [Google Scholar]
- 3.Mendez CE, Umpierrez GE. Pharmacotherapy for Hyperglycemia in Noncritically Ill Hospitalized Patients. Diabetes Spectrum. 2014 Aug 1;27(3):180–8. doi: 10.2337/diaspect.27.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler S, Btaiche I, Alaniz C. Relationship between hyperglycemia and infection in critally ill patients. Pharmacotherapy. 2005 Aug;25(7):963–76. doi: 10.1592/phco.2005.25.7.963. [DOI] [PubMed] [Google Scholar]
- 5.Kalfon P, Giraudeau B, Ichai, et al. Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensive Care Med. 2014 Feb;40(2):171–81. doi: 10.1007/s00134-013-3189-0. [DOI] [PubMed] [Google Scholar]
- 6.Society of Hospital Medicine Glycemic Control Task Force. The Glycemic Control Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. [Accessed 05/4/2016];Glycemic Control Quality Improvement Implementation Tool Kit. 2016 Available at http://www.hospitalmedicine.org.
- 7.Hermayer KL, Cawley P, Arnold P, et al. Impact of improvement efforts on glycemic control and hypoglycemia at a university medical center. Journal of Hosp Medicine. 2009 Jul 1;4(6):331–9. doi: 10.1002/jhm.449. [DOI] [PubMed] [Google Scholar]
- 8.Ryan DB, Swift CS. The mealtime challenge: nutrition and glycemic control in the hospital. Diabetes Spectrum. 2014 Aug 1;27(3):163–8. doi: 10.2337/diaspect.27.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobaugh DJ, Maynard G, Cooper L, et al. Enhancing insulin-use safety in hospitals: Practical recommendations from an ASHP Foundation Expert Consensus Panel. Am J Health-Syst Pharm. 2013;70:1404–13. doi: 10.2146/ajhp130169. [DOI] [PubMed] [Google Scholar]
- 10.Institute for Safe Medication Practices. [Accessed April 6, 2016];ISMP List of High-Alert Medications in Acute Care Setting. 2016 Available at https://www.ismp.org/tools/institutionalhighAlert.asp.
- 11.Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. 2007. Diabetes Care. 30:367–389. doi: 10.2337/dc06-1715. [DOI] [PubMed] [Google Scholar]
- 12.Mackey P, Boyle M, Walo P, Castro J, Cheng MR, Cook C. Care directed by a specialty-trained nurse practitioner or physician assistant can overcome clinical inertia in management of inpatient diabetes. Endocrine Practice. 2013 Sep 6; doi: 10.4158/EP13201.OR. [DOI] [PubMed] [Google Scholar]
- 13.Maynard G, Lee J, Phillips GW, Fink E, Renvall M. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: Effect of structured subcutaneous Insulin orders and insulin management algorithm. J Hosp Med. 2009;4:3–15. doi: 10.1002/jhm.391. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell ME, Umpierrez GE. Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrinol Metab Clin North Am. 2012;41:175–20. doi: 10.1016/j.ecl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30:2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 16.Umpierrez GE, Hellman R, Korytkowski, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2012;97:16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 17.Rogers EM. A prospective and retrospective look at the diffusion model. Journal of Health Communication. 2004;9(S1):13–19. doi: 10.1080/10810730490271449. [DOI] [PubMed] [Google Scholar]
- 18.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard G, Wesorick DH, O’Malley C, Inzucchi SE. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29–41. doi: 10.1002/jhm.354. [DOI] [PubMed] [Google Scholar]
- 20.Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers. Diabetes Care. 2005;28:337–342. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donihi AC, DiNardo MM, DeVita MA, Korytkowski MT. Use of a standardized protocol to decrease medication errors and adverse events related to sliding scale insulin. Qual Saf Health Care. 2006;15(2):89–91. doi: 10.1136/qshc.2005.014381. [DOI] [PMC free article] [PubMed] [Google Scholar]