Abstract
Purpose
Whole brain radiation therapy (WBRT) remains the standard of care for patients with multiple brain metastases, but more than half of treated patients will develop intracranial progression. Because there is no clear consensus on the optimal therapeutic approach, a prognostic index would be helpful to guide treatment options at progression. We explored whether the recursive partitioning analysis (RPA) score prior to repeat WBRT is predictive of survival.
Methods and materials
This multi-institutional pooled analysis included patients with 2 or more brain metastases from any solid primary tumor that was treated with 2 courses of WBRT. Information on demographics, disease characteristics, and intervals between courses was collected. RPA class was abstracted or retrospectively assigned, and descriptive statistics calculated. Median survival (MS) was determined using the Kaplan-Meier method and compared using log rank tests. Univariate and multivariate analyses were performed via Cox regression analysis.
Results
For 205 patients, the median age was 55 years (range, 25-83 years), 68% were female, 40.5% had non-small cell lung cancer, and 31.2% had small cell lung cancer. Prior to the second WBRT, 4.9% of patients were RPA class 1, 36.6% were RPA2, and 58.5% were RPA3, with an MS of 7.5 months (95% confidence interval [CI], 4.7-10.3), 5.2 months (95% CI, 3.7-6.7 months), and 2.9 months (95% CI, 2.2-2.9 months), respectively (P = .001). On univariate and multivariate analyses, a Karnofsky Performance Status of <80, extracranial metastases, interval between courses <9 months, small cell lung cancer histology, and uncontrolled primary significantly correlated with shorter MS. By assigning a score of 1 to each of these factors, a new prognostic index was created, the reirradiation (ReRT) score. Survival on the basis of ReRT score grouping ranged from 2.2 to 7.2 months and demonstrated significant differences in MS.
Conclusions
In the largest reported cohort to receive repeat WBRT, application of the RPA score was not predictive of MS. The new ReRT score is a simple tool based on readily available clinical information.
Summary.
More than half of patients who are treated for multiple brain metastases with whole brain radiation therapy (WBRT) will develop intracranial progression. This multi-institutional pooled analysis included 205 patients from 9 centers who received 2 courses of whole brain radiation therapy, both for multiple metastases. In this cohort, the application of the recursive partitioning analysis score was not predictive of median survival, leading to the construction of a new index, the reirradiation (ReRT) score.
Introduction
Brain metastases (BM) occur in approximately one-quarter of patients with cancer.1 They are more common in patients with lung and breast cancers and melanoma.2, 3, 4 BM are associated with significant symptoms, including headache, weakness, cognitive disturbance, and seizures. If untreated, they result in a median survival (MS) of 1 to 2 months.5, 6 The choice of treatment approach depends on patient factors (eg, age, performance status), tumor factors (eg, size, number of metastases, primary histology, and status of extracranial disease), and the availability of different therapeutic modalities.7, 8, 9
Treatment options for BM have expanded in recent years from surgery and conventional external beam whole brain radiation therapy (WBRT) to include high-dose conformal treatment to 1 or more lesions, or a resection cavity, via stereotactic radiosurgery (SRS) or stereotactic fractionated radiation therapy.9, 10, 11 Although the addition of an SRS boost after WBRT for patients with a single metastasis improved survival, local control, performance status, and steroid dependence in a pivotal phase 3 randomized trial for patients with 2-3 metastases, there was no survival advantage or impact on the rate of neurological death.12 SRS alone for patients with 1 to 3 BM is an appealing choice, with recent randomized evidence that demonstrates no difference in MS between SRS or SRS and WBRT, in light of the detrimental neurocognitive effects at 3 months for patients who receive both modalities.13 Although SRS is being actively explored, there are technical challenges for implementation in patients with multiple metastases, and it is considered investigational for those with >4 lesions.14
Approximately 50% of patients with BM present with multiple metastases.1, 15, 16 For this group, WBRT remains the standard of care, with a reported MS after treatment ranging from 2.3 to 7.1 months.10, 16 Symptomatic improvement after WBRT occurs in 60% to 90% of cases7, 15 with 1-year local control rates of approximately 70%.8, 12, 17 However, intracranial progression after WBRT is common and has been reported as the cause of death in one-third to one-half of patients.2, 3, 4 Radiologically uncontrolled BM are associated with symptom recurrence or progression, along with cognitive decline, with an average decrease of 6.3 points on the mini-mental status examination.5, 6 Options for salvage at the time of progression after WBRT are limited, especially for those who progress in the form of multiple metastases. Improvements in neurologic signs or symptoms have been reported in up to 80% of patients after repeat WBRT.7, 8, 9
Predicting which patients may benefit from reirradiation (ReRT) versus best supportive care (BSC) would guide treatment recommendations and discussions on the goals of care. Both the Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) and the graded prognostic assessment (GPA) are validated tools that can be used to predict survival at the time of initial BM presentation. The RPA is based on age, status of extracranial disease, and performance status,10 whereas GPA includes primary histology.18, 19 However, the use of these tools at the time of ReRT for in-brain recurrence or progression have been reported sparingly.20, 21 In this study, we aimed to evaluate the factors that are associated with survival after a second course of WBRT for multiple BM, determine the utility of RPA in this setting, and design a specific index to predict survival after ReRT.
Methods and materials
Study design
A literature search (MEDLINE, to October 2013) was undertaken to identify all reported studies of repeat WBRT for patients with BM (Appendix). We identified 11 peer-reviewed publications that met the search criteria. Two groups agreed to provide individual patient data. Of Canadian centers with dedicated palliative radiation oncology clinics and routine prospective data collection strategies, data from 6 additional centers that treated patients between 2000 and 2010 were added to our own.
The eligibility criteria included patients >18 years old with any solid primary tumor who had received 2 courses of WBRT, both for the indication of multiple BM, defined as >2 lesions. Those undergoing prophylactic cranial radiation, SRS (boost or salvage), or partial cranial radiation therapy were excluded. Biopsy or resection (subtotal or gross total) of a dominant lesion was not an exclusion criterion.
Patient characteristics (age, sex, Karnofsky Performance Status [KPS]), radiation therapy dose and fractionation, and disease characteristics (primary histology, status of extracranial metastases, control of primary) were abstracted where available or determined retrospectively when possible. KPS was assigned retrospectively for 77.3% of patients at the time of first WBRT and prior to ReRT for 80.4%. KPS was converted from the Eastern Cooperative Oncology Group performance status for 33.3% of patients at the time of first WBRT and for 29.4% prior to ReRT. For the remainder, KPS was assigned retrospectively by 1 of 2 reviewers on the basis of documented oncologic history and physical examination. RTOG neurologic function score was determined retrospectively. An RTOG neurologic function score of 1 indicates absent/minimal neurologic findings, 2 indicates neurologic impairment that does not require nursing care, 3 reflects impairment that requires nursing care, and 4 indicates inability to communicate/comatose state.15 The interval between courses was calculated from the date of the first fraction of the first course to the first fraction of the second course. RPA class as described by Gaspar et al10 was assigned where possible for both the first and second course of WBRT. GPA as described by Sperduto et al18, 19 could not be calculated for this cohort due missing data (eg, for primary breast and lung histologies) and due to the inclusion of primary histologies for which GPA does not exist presently (eg, carcinoma of unknown primary, ovary).
Institutional review board approval for data collection and sharing was obtained in accordance with the requirements of each center.
Statistical analysis
Summary statistics were calculated, including medians and ranges for continuous variables and frequencies and proportions for categorical variables. MS was analyzed from the first day of each course of WBRT to the date of death using the Kaplan Meier method with 95% confidence intervals. Log rank tests compared survival curves by RPA class and primary histology. Cox proportional hazard analysis described factors associated with survival. Factors that were significant at the P < .10 level on univariate analysis (UVA) were incorporated into multivariate analysis, with hazard ratios and 95% confidence intervals reported. The final multivariate model included factors that were significant at the P < .05 level, which were then used to construct a prognostic index (the ReRT score). A score of 1 was assigned to each variable for ease of clinical use. MS was determined for each ReRT score group (scores of 0-2, 3, or 4-5) using the Kaplan-Meier method and compared using the log rank test. UVA was also performed using a logistic binary regression analysis to explore factors associated with very short survival (≤30 days). A two-sided P-value of <.05 was considered significant. All statistical analyses were conducted using IBM SPSS Statistics Version 15 (IBM Corp., Armonk, NY).
Results
Demographics and radiation therapy
Of 205 patients from 9 centers, the majority were female (68.3%) with non-small cell lung cancer (NSCLC) as the main histology (40.5%; Table 1). The median interval between courses was 9.1 months (range, 0.5-68.3 months). The median KPSs were 70 and 60, respectively. At the time of first WBRT, the RPA class was 1 for 14 patients, 2 for 105 patients, 3 for 73 patients, and not assignable for 13 patients. The most frequently used dose and fractionation schedule was 20 Gy in 5 fractions for both courses, with a total dose that ranged from 12 to 48 Gy for the first WBRT and 4 to 30.6 Gy for the second. Twenty-nine patients (14.1%) underwent surgical intervention (biopsy, subtotal or gross total resection) in the interval prior to ReRT.
Table 1.
Demographics of patients (n = 205) at the time of reirradiation
| Demographics | N (%) |
|---|---|
| Sex | |
| Female | 140 (68.3) |
| Male | 65 (31.7) |
| Age, median (range), y | 55 (25-83) |
| Primary histology | |
| NSCLC | 83 (40.5) |
| Breast | 64 (31.2) |
| SCLC | 33 (16.1) |
| Othera | 25 (12.2) |
| RPA | |
| Class 1 | 10 (4.9) |
| Class 2 | 75 (36.6) |
| Class 3 | 120 (58.5) |
| Extracranial metastases | |
| Yes | 25 (12.2) |
| No | 137 (66.8) |
| Unknown | 43 (21.0) |
| KPS | |
| ≥80 | 40 (19.5) |
| <80 | 164 (80.0) |
| Unknown | 1 (0.5) |
| Interval between RT courses | |
| ≥9 mo | 107 (52.2) |
| <9 mo | 98 (47.8) |
| Primary | |
| Controlled | 65 (31.7) |
| Uncontrolled | 131 (63.9) |
| Unknown | 9 (4.4) |
| RTOG neurological function status | |
| 1 | 132 (64.4) |
| 2 | 53 (25.8) |
| Unknown | 20 (9.8) |
KPS, Karnofsky Performance Status; NSCLC, non-small cell lung cancer; RPA, recursive partitioning analysis; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group; SCLC, small cell lung cancer.
Includes head and neck, gynecologic, melanoma, lymphoma, and colorectal.
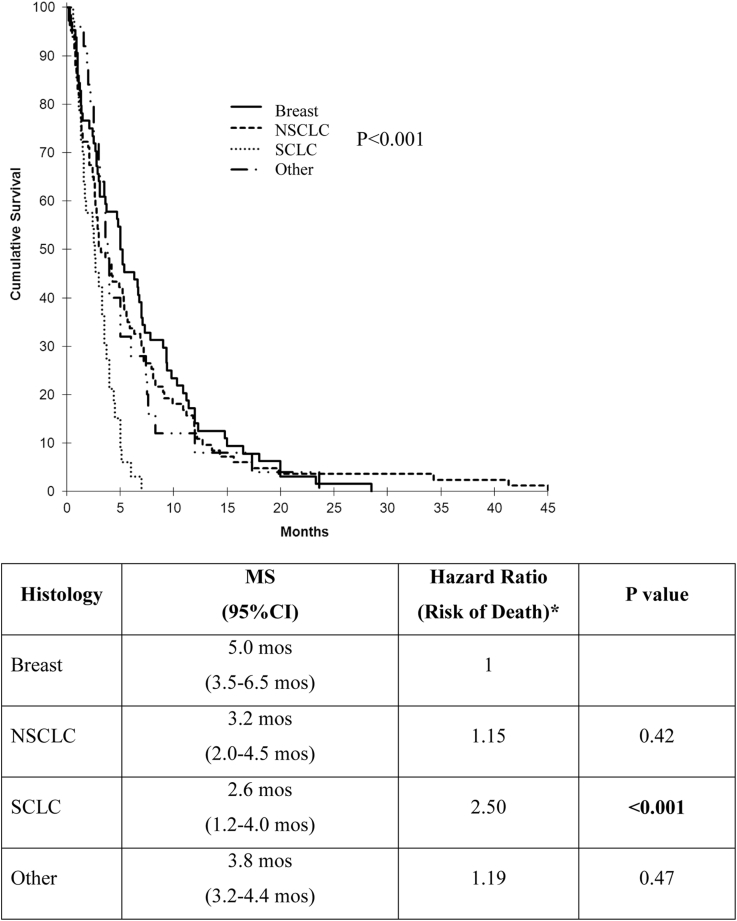
The MS was 14.5 months (range, 3.0-85.0 months) measured from the first WBRT and 3.6 months (range, 0.2-45.0 months) starting from ReRT. There were significant differences depending on primary histology (Fig 1). Patients with small cell lung cancer (SCLC) had a risk of death after repeat WBRT that was 2.5 times higher than those with breast cancer (MS: 2.6 vs 5.0 months; P < .001).
Figure 1.
Median survival by primary histology (*Ref = breast primary). MS, median survival; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Application of the recursive partitioning analysis score at the time of reirradiation
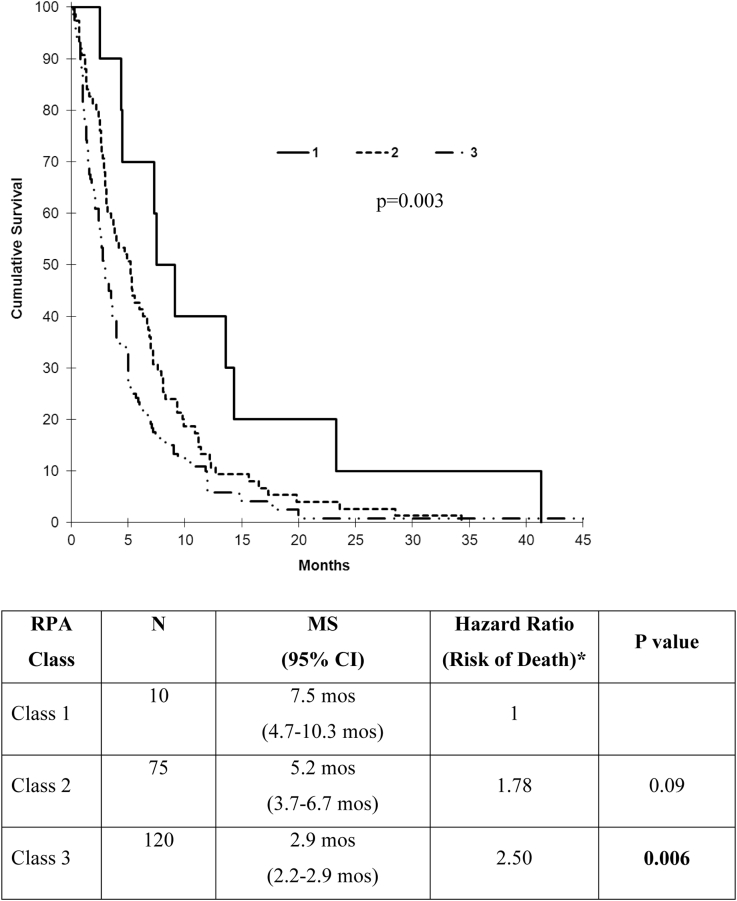
At the time of repeat WBRT, more than half of patients were RPA class 3 (Table 1). The MS by RPA class that was calculated from the first day of ReRT demonstrated a significant difference when comparing class 1 and class 3 (7.5 vs 2.9 months; P = .003; Fig 2). On multivariate analysis, the presence of extracranial metastases, KPS <80 (the median value of the cohort), <9-month interval between courses (the median value), uncontrolled primary, and SCLC histology were independently associated with lower survival (Table 2). Neither age nor RTOG score were predictive.
Figure 2.
Survival by recursive partitioning analysis (RPA) class after repeat WBRT. (*Ref = RPA class 1). CI, confidence interval; MS, median survival.
Table 2.
Univariate and multivariate analysis for predictors of survival at the time of ReRT
| Factor | UVA |
MVA |
||
|---|---|---|---|---|
| HR | P value | HR | P value | |
| Presence of extracranial metastases | 1.89 | <0.001 | 2.27 | 0.002 |
| KPS <80 | 1.72 | <0.001 | 1.85 | 0.005 |
| Interval between courses <9 mo | 1.59 | <0.001 | 1.53 | 0.02 |
| Primary uncontrolled | 1.63 | <0.001 | 1.52 | 0.03 |
| Primary histologya | ||||
| Breast | 1.00 | |||
| NSCLC | 1.15 | 0.42 | 1.42 | 0.11 |
| SCLC | 2.50 | <0.001 | 2.69 | <0.001 |
| Other | 1.19 | 0.47 | 1.21 | 0.55 |
| RTOG neuro function score >2 | 1.38 | 0.05 | 1.16 | 0.51 |
| Age ≥65 y | 1.11 | 0.44 | ||
HR, hazard ratio; KPS, Karnofsky Performance Status; MVA, multivariate analysis; neuro, neurological; NSCLC, non-small cell lung cancer; RTOG, Radiation Therapy Oncology Group; SCLC, small cell lung cancer; UVA, univariate analysis; WBRT, whole brain radiation therapy.
Ref=Breast primary.
Reirradiation score
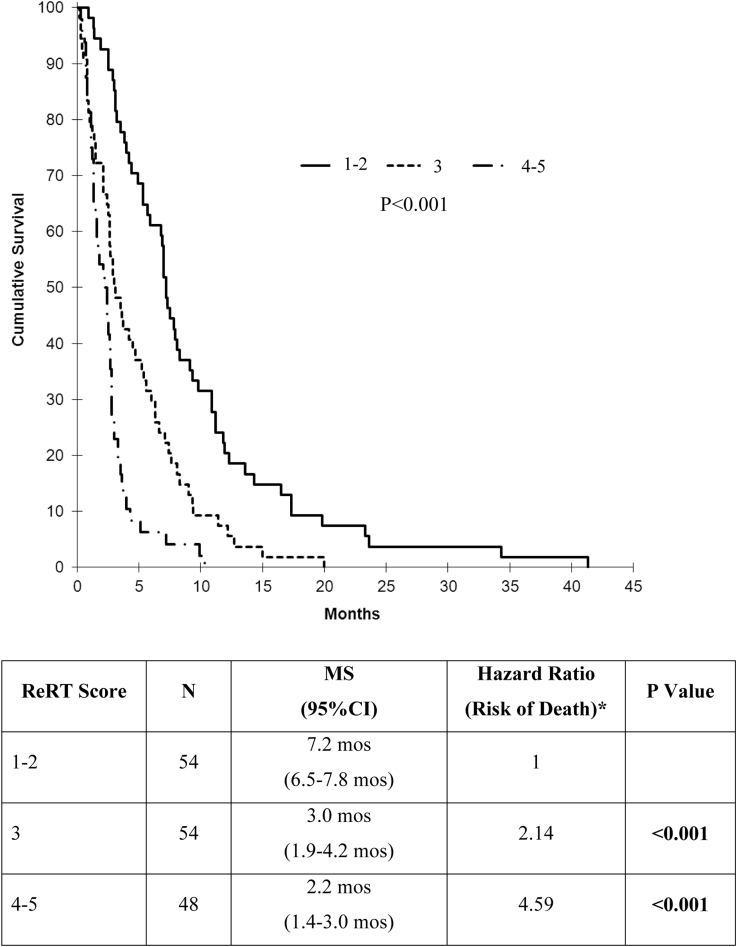
The aforementioned 5 factors were combined to generate a new prognostic scoring system, the ReRT score. For 156 of 205 patients for whom all data were available, 1 point was assigned for each of the following poor prognostic factors: SCLC; presence of extracranial metastases; KPS <80, interval between radiation therapy courses <9 months; and uncontrolled primary. The sum ranged from 0 to 5; however, no patients in this study scored 0. Of 156 patients, 54 (34.6%) scored 1-2, 54 (34.6%) scored 3, and 48 (30.8%) scored 4-5. Survival based on ReRT score as measured from the first day of ReRT demonstrated a significant difference between ReRT score groups. (P < .001; Fig 3). When comparing classification by ReRT versus RPA score, 45 patients (28.8%) were categorized into a group with a higher expected survival, 34 (21.8%) had a lower expected survival, and the remainder had a similar survival regardless of the index used.
Figure 3.
Survival after repeat whole brain radiation therapy by reirradiation (ReRT) score group (*Ref = ReRT score 1-2).
Very short survival
Twenty-eight patients (13.7%) survived 30 days or fewer (as measured from the first day of ReRT). None of the factors listed in Table 2 predicted very short survival (all P > .11 on UVA). For 20 of these 28 patients, the ReRT score could be calculated: 2.6% scored 2, 35.7% scored 3, and 32.1% scored 4-5.
Discussion
In this pooled analysis examining the applicability of RPA classifications to predict survival at the time of ReRT, there was a significant difference in MS between classes 1 and 3 (7.5 vs 2.9 months, respectively), but class 2 patients could not be distinguished from class 3 patients. The American College of Radiology advocates consideration of “patient characteristics and preferences, previous treatments employed, and potential risks and toxicities of treatment” to determine the optimal therapeutic approach in this clinical setting.16 An adaptation of the RPA scoring system may thus be helpful to guide treatment selection in patients with intracranial progression after WBRT, especially as the incidence of BM continues to increase.16
The results of the Quality of Life after Treatment for Brain Metastases (QUARTZ) trial, which randomized patients with NSCLC who were not eligible for SRS or surgery into cohorts of WBRT or BSC, revealed no difference between treatment arms.22 However, the patients accrued to that particular clinical study were expected to have a poor prognosis at baseline and are therefore not likely generalizable to the cohort in this study.23
Early reports of outcomes after repeat WBRT described survivals on the order of 8 weeks.7, 15 Subsequent studies suggested an MS of 3 to 5 months, with some favorable symptomatic responses and minimal complications.8, 17, 24 Recent reviews have indicated that ReRT may be beneficial in carefully selected patients with longer predicted survival.25, 26, 27, 28, 29 The general principles remain that the goals of care, tolerance to prior course of radiation therapy, tumor biology, actionable mutations, and eligibility for clinical trial must also be integrated into the decision-making process when considering ReRT.
Similar to other studies (Table 3), we found the following to be associated with shorter survival: extracranial metastases,17 KPS,26, 27, 28, 29 interval between courses,26, 27 uncontrolled primary,8, 28 and SCLC histology.28 An RTOG neurologic function status score of >2 was found to be significant by other groups24, 25 but not in the present study, which could be due to the retrospective nature by which it was assigned. Interestingly, lung histology (both NSCLC alone and combined SCLC and NSCLC) correlated with higher MS elsewhere.29
Table 3.
Summary of repeat WBRT studies
| Reference | N | ≥2 Metastases | Median Dose, First WBRT (Range) | Median Dose, Second WBRT (Range) | MS (95% CI) | Predictors of Poor Prognosis on UVA | Predictors of Poor Prognosis on MVA |
|---|---|---|---|---|---|---|---|
| Aktan et al27 | 34 | 100% | 30 Gy (25-30 Gy) |
25 Gy (20-30 Gy) |
5.3 mo (4.1-6.6 mo) |
NR |
|
| Scharp et al28 | 134 | NR | 30 Gy (30-40 Gy) |
20 Gy (2-30 Gy) |
2.8 mo (0-28 mo) |
|
|
| Ozgen et al26 | 28 | 86% | 30 Gy (20-30 Gy) |
25 Gy (20-30 Gy) |
3 mo (1.8-4.1 mo) |
NR |
|
| Son et al8 | 17 | 100% | 35 Gy (28–40 Gy) |
21.6 Gy (14–30 Gy) |
5.2 mo (1.3-8.7 mo) |
|
NR |
| Akiba et al29 | 31 | NR | 30 Gy (26-42 Gy) |
30 Gy (3-40 Gy) |
4.0 mo (NR) |
|
|
| Karam et al20a | 37 | NR | NR | NR (15-20 Gy) |
6.9 mo (NR) |
|
NR |
| Sadikov et al25 | 72 | 86% | 20 Gy (20-30 Gy) |
NR | 4.1 mo (NR) |
|
NR |
| Abdel-Wahab et al30b | 15 | NR | 30 Gy (30-55 Gy) |
30 Gy (30-35 Gy) |
3.2 mo (NR) |
NR | NR |
| Wong et al17 | 86 | 55% | 30 Gy (1.5-50.6 Gy) |
20 Gy (8-30.6 Gy) |
4.0 mo (0.25-72 mo) |
|
|
| Cooper et al24 | 52 | 45% | NR | NR | 16.3 wk (NR) |
|
NR |
| Hazuka et al7 | 44 | NR | 30 Gy (30-36 Gy) |
25 Gy (6-36 Gy) |
8 wk (NR) |
NR | NR |
| Kurup et al31 | 56 | NR | NR (18-30 Gy) |
20 Gy (20 Gy) |
3.5 mo (0.25-16 mo) |
NR | NR |
| Shehata et al32 | 35 | NR | NR | NR | NR | NR | NR |
CI, confidence interval; CNS, central nervous system; KPS, Karnofsky Performance Status; MS, median survival; MVA, multivariate analysis; NR, not reported; ReRT, reirradiation; RPA, recursive partitioning analysis; RT, radiation therapy, RTOG, Radiation Therapy Oncology Group; SCLC, small cell lung cancer; UVA, univariate analysis; WBRT, whole brain radiation therapy.
Included breast histology only.
Included patients who received partial WBRT.
In an attempt to more precisely estimate survival at the time of repeat WBRT, we developed the ReRT score. Patients with an ReRT score of 1 to 2 are likely to have as long a survival time as those with RPA class 1 at initial diagnosis of BM and should be considered for ReRT. Patients with an ReRT score of 4 to 5 have an estimated survival of approximately 2 months, and BSC with hospice enrollment is likely to be more appropriate. For patients with an ReRT score of 3, the expected potential benefits versus risks of ReRT in relation to BSC should be discussed.
Comparing the ReRT score with the RPA, the presence of extracranial metastases and uncontrolled primary remained strongly predictive for both indices. KPS emerged with a modified cut-point (≥80) separating patients. Age was not a significant predictor for MS after ReRT; however, the interval to treatment was significant and may be a surrogate for the underlying biology of the disease and/or responsiveness to treatment.
WBRT within 30 days of death has recently been discussed in the setting of quality care indicators.33 Patients with very short predicted survival are better suited to BSC than to radiation therapy, from which they are not likely to live long enough to benefit, especially in the case of protracted fractionation. However, we failed to find significant factors that were associated with very short survival.
Although this is the largest patient cohort reported, conclusions remain limited by retrospective calculation of the GPA, the proportion of patients for whom KPS was retrospectively assigned, and heterogeneous primary histologies. Prior to adoption into routine practice, prospective validation is required. Recently, the impact of biomarkers on prognostication for patients with BM from primary breast and NSCLC has led to modifications of the GPA.34, 35 This information is not available within the current cohort, which may also be a limitation; however, in practice, these data may either be unavailable or rendered irrelevant once patients have progressed through all available lines of systemic therapy. Additionally, we were unable to abstract quality of life, steroid dependence, setting of care, and KPS after completion of repeat WBRT. Specific symptoms have been reported previously as correlating with prognosis17, 24, 25, 27 but could not be examined in this cohort. Although we attempted to collect information on toxicity, this was rarely available. Finally, this review did not include patients who received SRS. As more centers use SRS for salvage in patients with >4 metastases, future work should focus on the potential clinical utility of the ReRT score for this population.
Conclusions
In the largest reported cohort to receive repeat WBRT, application of the RPA score was not independently predictive of MS for all patients. The new ReRT score, based on histology, KPS, stability of the primary, interval between courses, and absence of extracranial metastases, is a simple tool that is based on readily available clinical information and has been shown to be predictive of survival after repeat WBRT in this pooled population. However, internal and external prospective validation is required to confirm robustness prior to routine clinical implementation.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: None.
Supplementary material for this article (http://dx.doi.org/10.1016/j.adro.2017.05.010) can be found at www.advancesradonc.org.
Supplementary data
References
- 1.Posner J.B., Chernik N.L. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- 2.Lagerwaard F.J., Levendag P.C., Nowak P.J., Eijkenboom W.M., Hanssens P.E., Schmitz P.I. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 3.Posner J.B. Brain metastases: 1995. A brief review. J Neurooncol. 1996;27:287–293. doi: 10.1007/BF00165486. [DOI] [PubMed] [Google Scholar]
- 4.Graham P.H., Bucci J., Browne L. Randomized comparison of whole brain radiotherapy, 20 Gy in four daily fractions versus 40 Gy in 20 twice-daily fractions, for brain metastases. Int J Radiat Oncol Biol Phys. 2010;77:648–654. doi: 10.1016/j.ijrobp.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Regine W.F., Scott C., Murray K., Curran W. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: An analysis from Radiation Therapy Oncology Group Study 91-04. Int J Radiat Oncol Biol Phys. 2001;51:711–717. doi: 10.1016/s0360-3016(01)01676-5. [DOI] [PubMed] [Google Scholar]
- 6.Markesbery W.R., Brooks W.H., Gupta G.D., Young A.B. Treatment for patients with cerebral metastases. Arch Neurol. 1978;35:754–756. doi: 10.1001/archneur.1978.00500350058012. [DOI] [PubMed] [Google Scholar]
- 7.Hazuka M.B., Kinzie J.J. Brain metastases: Results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15:433–437. doi: 10.1016/s0360-3016(98)90026-8. [DOI] [PubMed] [Google Scholar]
- 8.Son C.H., Jimenez R., Niemierko A., Loeffler J.S., Oh K.S., Shih H.A. Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:e167–e172. doi: 10.1016/j.ijrobp.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Tsao M.N., Rades D., Wirth A. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspar L., Scott C., Rotman M. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 11.Videtic G.M., Gore E.M., Bradley J.D. Multiple brain metastases. American College of Radiology. ACR Appropriateness Criteria®. Am Coll Radiol. 2014:9. [PubMed] [Google Scholar]
- 12.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 13.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto M., Serizawa T., Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 15.Borgelt B., Gelber R., Kramer S. The palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 16.Robbins J.R., Elson A., Buatti J.M. ACR Appropriateness Criteria® follow-up and retreatment of brain metastases. Am Coll Radiol. 2014:11. [Google Scholar]
- 17.Wong W.W., Schild S.E., Sawyer T.E., Shaw E.G. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996;34:585–590. doi: 10.1016/0360-3016(95)02156-6. [DOI] [PubMed] [Google Scholar]
- 18.Sperduto P.W., Berkey B., Gaspar L.E., Mehta M., Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 19.Sperduto P.W., Kased N., Roberge D. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karam I., Nichol A., Woods R., Tyldesley S. Population-based outcomes after whole brain radiotherapy and re-irradiation in patients with metastatic breast cancer in the trastuzumab era. Radiat Oncol. 2011;6:181. doi: 10.1186/1748-717X-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernhardt D., Bozorgmehr F., Adeberg S. Outcome in patients with small cell lung cancer re-irradiated for brain metastases after prior prophylactic cranial irradiation. Lung Cancer. 2016;101:76–81. doi: 10.1016/j.lungcan.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Mulvenna P., Nankivell M., Barton R. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaidar-Person O., Anders C.K., Zagar T.M. Whole brain radiotherapy for brain metastases: Is the debate over? JAMA. 2016;316:393–395. doi: 10.1001/jama.2016.8692. [DOI] [PubMed] [Google Scholar]
- 24.Cooper J.S., Steinfeld A.D., Lerch I.A. Cerebral metastases: Value of reirradiation in selected patients. Radiology. 1990;174:883–885. doi: 10.1148/radiology.174.3.2305074. [DOI] [PubMed] [Google Scholar]
- 25.Sadikov E., Bezjak A., Yi Q.L. Value of whole brain re-irradiation for brain metastases–single centre experience. Clin Oncol (R Coll Radiol) 2007;19:532–538. doi: 10.1016/j.clon.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Ozgen Z., Atasoy B.M., Kefeli A.U., Seker A., Dane F., Abacioglu U. The benefit of whole brain reirradiation in patients with multiple brain metastases. Radiat Oncol. 2013;8:186. doi: 10.1186/1748-717X-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aktan M., Koc M., Kanyilmaz G., Tezcan Y. Outcomes of reirradiation in the treatment of patients with multiple brain metastases of solid tumors: a retrospective analysis. Ann Transl Med. 2015;3:325. doi: 10.3978/j.issn.2305-5839.2015.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharp M., Hauswald H., Bischof M., Debus J., Combs S.E. Re-irradiation in the treatment of patients with cerebral metastases of solid tumors: Retrospective analysis. Radiat Oncol. 2014;9:4. doi: 10.1186/1748-717X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akiba T., Kunieda E., Kogawa A., Komatsu T., Tamai Y., Ohizumi Y. Re-irradiation for metastatic brain tumors with whole-brain radiotherapy. Jpn J Clin Oncol. 2012;42:264–269. doi: 10.1093/jjco/hys007. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Wahab M.M., Wolfson A.H., Raub W. The role of hyperfractionated re-irradiation in metastatic brain disease: A single institutional trial. Am J Clin Oncol. 1997;20:158–160. doi: 10.1097/00000421-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Kurup P., Reddy S., Hendrickson F.R. Results of re-irradiation for cerebral metastases. Cancer. 1980;46:2587–2589. doi: 10.1002/1097-0142(19801215)46:12<2587::aid-cncr2820461209>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Shehata W.M., Hendrickson F.R., Hindo W.A. Rapid fractionation technique and re-treatment of cerebral metastases by irradiation. Cancer. 1974;34:257–261. doi: 10.1002/1097-0142(197408)34:2<257::aid-cncr2820340206>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Jones J.A., Lutz S.T., Chow E., Johnstone P.A. Palliative radiotherapy at the end of life: A critical review. CA Cancer J Clin. 2014;64:296–310. doi: 10.3322/caac.21242. [DOI] [PubMed] [Google Scholar]
- 34.Sperduto P.W., Kased N., Roberge D. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112:467–472. doi: 10.1007/s11060-013-1083-9. [DOI] [PubMed] [Google Scholar]
- 35.Sperduto P.W., Yang T.J., Beal K. The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys. 2016;96:406–413. doi: 10.1016/j.ijrobp.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.