Abstract
Purpose
Guidelines for locally advanced non-small cell lung cancer (LA-NSCLC) recommend definitive chemoradiation therapy (CRT) for cN2-N3 disease, reserving surgery for patients with minimal nodal involvement at presentation. The current literature suggests that surgery after CRT for stage III NSCLC can improve freedom-from-recurrence (FFR) but has not consistently demonstrated an improvement in overall survival, perhaps partly due to the low (45-50.4 Gy) preoperative doses delivered that result in low rates of mediastinal nodal clearance. We therefore analyzed factors associated with trimodality therapy receipt and determined outcomes in patients with LA-NSCLC who were treated with definitive doses (≥60 Gy) of neoadjuvant CRT prior to surgery.
Methods and materials
We retrospectively analyzed 355 consecutive patients with LA-NSCLC who were treated with curative intent between January 2000 and December 2013. The Kaplan-Meier method was used to estimate the overall survival and FFR of patients who were initially planned to receive trimodality treatment but never underwent surgery (unplanned bimodality) compared with those who were never considered to be surgical candidates (planned bimodality) and those who underwent surgical resection after CRT (trimodality). Cox proportional hazards regression with forward selection was used for multivariate analyses, and the Fisher exact test was used to test contingency tables.
Results
Patients who received trimodality therapy had a longer median survival than those with unplanned or planned bimodality therapy at 59.9, 20.1, and 17.3 months, respectively (P < .001). The survival benefit with surgery persisted in patients with stage IIIB (P < .001) and N3 (P = .010) nodal disease when mediastinal nodal clearance was achieved. FFR was also improved with surgical resection (P = .001). Race (P < .001), stage (P < .001), performance status (P < .001), age (P < .001), and diagnosis of chronic obstructive pulmonary disease (P = .009) were significant indicators that influenced both the decision to initially choose trimodality therapy at consultation and to actually perform surgical resection.
Conclusions
Trimodality treatment significantly improves survival and FFR in patients with LA-NSCLC when definitive doses of radiation with neoadjuvant chemotherapy are employed. We identified important demographic features that predict the use of surgical intervention in patients with stage III NSCLC.
Introduction
Non-small cell lung cancer (NSCLC) is the most common cause of cancer deaths, and approximately 25% of patients will present with locally advanced disease.1 Five-year overall survival (OS) in patients with stage III NSCLC ranges from 19% to 36%,2 and the prognosis for this heterogeneous patient population often correlates with the extent of mediastinal nodal involvement at the time of diagnosis.3, 4, 5, 6, 7, 8, 9, 10 Accordingly, treatment options for locally advanced NSCLC can vary based on nodal disease burden. The National Comprehensive Cancer Network guidelines recommend definitive chemoradiation therapy (CRT; bimodality) for the majority of patients with stage III NSCLC, reserving trimodality treatment (ie, addition of surgery after concurrent CRT) for those with minimal N2 disease.
However, the use of trimodality therapy for locally advanced NSCLC (LA-NSCLC) remains controversial. In the Intergroup 0139 phase 3 trial, although the 5-year progression-free survival was improved in patients who received surgery after concurrent CRT compared with those who underwent bimodality therapy alone, this benefit did not translate to a survival advantage. This result was most likely influenced by the high postoperative mortality rate seen in patients who underwent pneumonectomies.10 Nevertheless, in an unplanned subset analysis, a subset of patients with limited nodal (N2) disease who received a lobectomy after neoadjuvant CRT were noted to have improved OS.10 Recently published population-based studies11 and single-institutional experiences also suggest superior survival with surgical resection after concurrent CRT, especially in the setting of mediastinal nodal clearance (MNC).12, 13
MNC after CRT is a known clinical factor that predicts outcomes after trimodality therapy. In most institutions, the preoperative radiation therapy dose has been limited to 45 to 50.4 Gy because in initial experiences, higher doses (>50-50.4 Gy) were associated with excessive postoperative complications.14, 15, 16 With improved radiation delivery and surgical techniques, select high-volume centers have considered planning full-dose radiation therapy (60 Gy) with concurrent chemotherapy as an approach to enhance MNC and potentially allow patients who are unable to undergo surgery to receive full, uninterrupted doses of definitive treatment.17, 18, 19 This approach was also evaluated in the prospective Radiation Therapy Oncology Group (RTOG) 02-29 study, which reported excellent early survival and further established the safety and feasibility of high-dose induction CRT before surgical resection.9
In the absence of randomized clinical trial evidence, continued evaluation of this approach of definitive preoperative radiation therapy with concurrent chemotherapy is necessary. We therefore performed a large retrospective analysis of patients with stage III NSCLC treated with curative intent at our institution to determine the survival and failure patterns of patients undergoing trimodality therapy compared with patients undergoing unplanned bimodality therapy (ie, surgery recommended pre-CRT but ultimately not performed) or planned bimodality therapy (ie, surgery never considered) in the setting of definitive doses (≥60 Gy) of neoadjuvant CRT. Although we recognize that not all patients with stage III NSCLC are candidates for trimodality, our hypothesis was that these carefully selected patients with stage III NSCLC undergoing trimodality therapy after high-dose CRT will have improved oncological outcomes, and by delivering definitive radiation therapy doses, those who are unable to undergo surgery will have outcomes comparable to those with planned bimodality therapy.
Finally, because general operative fitness criteria, including cardiopulmonary function prior to thoracic surgery, are well defined in the literature, we further aimed to explore additional pretreatment demographic and patient/disease-related clinical factors that may influence the consideration of trimodality therapy at initial consultation and post-CRT.
Methods and materials
Patient selection
The study population for this retrospective analysis included all patients with stage III NSCLC (American Joint Committee on Cancer staging criteria, 7th edition) treated with curative intent at our institution between January 2000 and December 2013, which allowed for 3 years or more of follow-up. Patients who could not complete the full course of curative treatment were still included in this study for intent-to-treat analyses. Patients who were treated with upfront surgical resection prior to receiving radiation or chemotherapy were excluded from this analysis.
Pretreatment evaluation
All patients included in this study were evaluated by a multidisciplinary team, including thoracic surgeons, medical oncologists, and radiation oncologists, at initial consultation. Patients underwent standard work-up, including systemic imaging (positron emission tomography [PET], PET/computed tomography [CT], CT, and/or bone scan), brain imaging (magnetic resonance imaging or, when contraindicated, CT), and cardiopulmonary function tests. All patients' baseline mediastinal disease was documented by cervical mediastinoscopy, endobronchial ultrasound, or PET (Table 1). Prior to surgery, patients underwent restaging scans and mediastinal nodal sampling to assess for disease progression/clearance.
Table 1.
Baseline patient, disease, and treatment characteristics for all patients (N = 355)
| Characteristic | Patients, n (%) |
|---|---|
| Age | |
| Median/Range (years) | 60/30-86 |
| Sex | |
| Male | 203 (57.2) |
| Female | 152 (42.8) |
| Race | |
| Black | 150 (42.3) |
| Non-Black | 205 (57.7) |
| ECOG Performance Status | |
| 0 | 165 (46.5) |
| ≥1 | 186 (52.4) |
| Unknown | 4 (1.1) |
| Marital Status | |
| Married | 185 (52.1) |
| Single | 170 (47.9) |
| Smoking | |
| Median/Range (pack-years) | 40/0-212 |
| Charlson Comorbidity Score | |
| ≤6 | 189 (53.2) |
| >6 | 165 (46.5) |
| Unknown | 1 (0.3) |
| Histology | |
| Adenocarcinoma | 117 (33) |
| Squamous Cell | 104 (29.3) |
| NSCLC (NOS) | 111 (31.3) |
| Other | 23 (6.5) |
| T stagea | |
| TX | 18 (5.1) |
| ≤T2 | 161 (45.4) |
| ≥T3 | 174 (49) |
| N stage | |
| NX | 3 (0.8) |
| ≤N1 | 56 (15.8) |
| N2 | 218 (61.4) |
| N3 | 76 (21.4) |
| Stage | |
| IIIA | 200 (56.3) |
| IIIB | 155 (43.7) |
| Mediastinal Staging | |
| PET/CT alone | 99 (27.9) |
| Mediastinoscopy alone | 15 (4.2) |
| EBUS alone | 31 (8.7) |
| PET+Mediastinoscopy | 100 (28.2) |
| EBUS+Mediastinoscopy | 82 (23.2) |
| Other | 28 (7.9) |
| Trimodality vs Bimodality | |
| Trimodality | 88 (24.8) |
| Unplanned bimodalityb | 89 (25.1) |
| Planned Bimodality | 178 (50.1) |
| Type of Surgery | |
| Lobectomy | 76 (86.4) |
| Pneumonectomy | 12 (13.6) |
| Type of Chemoradiation | |
| Concurrent | 327 (92.1) |
| Sequential | 28 (7.9) |
| Prescribed Radiation Dosec | |
| Median/Range (Gy) | 64.8/45-81.6 |
| Radiation Dose Deliveredc | |
| >60 Gy | 293 (83) |
| Radiation Techniqued | |
| 3-dimensional conformal | 235 (66.2) |
| IMRT | 84 (23.7) |
| Adjuvant Chemotherapye | |
| Yes | 212 (69.7) |
CT, computed tomography; EBUS, endobronchial ultrasound; ECOG, Eastern Cooperative Oncology Group; IMRT, intensity modulated radiation therapy; NSCLC (NOS), non-small cell lung cancer (not otherwise specified); PET, positron emission tomography.
T and N stage are not known for 2 patients.
Unplanned bimodality comprised patients who were initially planned for surgery after definitive chemoradiation but ultimately did not receive any surgical treatment.
Data are not available for 11 patients.
Data are not available for 36 patients.
Data are not available for 51 patients.
Treatment
Patients in this analysis typically were planned to receive high-dose CRT (concurrent or sequential). Including patients who were unable to complete the planned course of CRT, the median radiation therapy dose prescribed was 64.8 Gy (range, 45-81.6 Gy) given in 1.8 or 2 Gy per fraction. Radiation was delivered with either 3-dimensional conformal radiation therapy or intensity modulated radiation treatment (IMRT), the latter being more common after 2009. Radiation therapy was given to a target consisting of the primary tumor and involved nodes, with an additional margin for microscopic disease patient set up error. Respiratory motion was assessed in all cases, either with fluoroscopy or four4-dimensional CT simulation scans. Four-dimensional assessments were used starting in 2009 (16% of the patient cohort received 4-dimensional CT simulation scans). Elective nodal irradiation was routinely done on all patients prior to 2009 and involved-field nodal treatment was exclusively adopted thereafter. All patients received platinum-based chemotherapy, with the most common chemotherapeutic regimen being weekly carboplatin/paclitaxel (carboplatin, area under the curve of 2; paclitaxel, 50 mg/m2) for concurrent CRT. Among patients receiving initial carboplatin/paclitaxel, consolidation with 2 cycles of carboplatin/paclitaxel (area under the curve of 5-6/200-225 mg/m2) was given 4 to 6 weeks after completion of the CRT or surgical resection, as tolerated.
Statistical analysis
For this study, long-term follow-up data were retrospectively analyzed with institutional review board approval. Three patient cohorts were analyzed: trimodality, unplanned bimodality, and planned bimodality. The differences in patient demographic, disease, and treatment characteristics between the cohorts was examined using Fisher's exact and χ2 tests. Recurrences were identified as progressive abnormal findings from surveillance imaging (CT, PET/CT, or brain magnetic resonance imaging) with biopsy performed when feasible before initiation of salvage treatment. The date of failure was recorded as the date of the abnormal surveillance scan. Locoregional failures were classified as recurrences in the ipsilateral lung and/or nodal regions (hilum, mediastinum, or supraclavicular fossa). Distant failures were recorded as recurrences that were consistent with the M-classification as stated in the American Joint Committee on Cancer staging criteria, 7th edition. Pathology reports from the surgical resection were used to assess the treatment response from the neoadjuvant CRT in the lymph nodes. Patients were considered to have a complete pathologic response in the lymph nodes if no viable tumor was identified (MNC).
The Kaplan-Meier product limit method was used to estimate OS and freedom-from-recurrence (FFR) rates. Log-rank test statistics were used to assess the levels of statistical significance between selected prognostic factors. Logistic regression analysis with forward selection tested the predictive value of each demographic variable in influencing the recommendation for surgery, and Cox regression analysis assessed their impact on timed outcomes. Variables that were tested in multivariate analysis included age, sex, race, marital status, T stage, N stage, overall stage (IIIA vs IIIB), Eastern Cooperative Oncology Group (ECOG) performance status (at diagnosis), smoking pack-years, and median household income. SPSS software Version 21.0 (IBM Corporation, Armonk, NY) was used for the statistical analysis.
Results
Treatment cohorts
A total of 355 consecutive patients were included in this retrospective analysis. Patient demographic, disease, and treatment characteristics for the entire group are summarized in Table 1. Select demographic, disease, and treatment characteristics are compared among the 3 cohorts in Table 2. When reviewing the pretreatment demographic factors, significant differences in age among the 3 cohorts can be observed, with younger patients being more likely to undergo trimodality therapy (P < .001). Race (P < .001), performance status (P < .001), and marital status (P < .001) were also statistically different; patients who were of black race, those who had a poor performance status, and single individuals were more likely to receive bimodality treatment.
Table 2.
Select baseline patient, disease and treatment characteristics between cohortsa
| Characteristic | Trimodality | Unplanned Bimodality | Planned Bimodality | P-value |
|---|---|---|---|---|
| Age | ||||
| Median/range | 56/38-79 | 58/39-86 | 64/30-83 | < .001 |
| <60 | 52 (59.1) | 47 (54) | 68 (38.2) | |
| ≥60 | 36 (40.9) | 40 (46) | 110 (61.8) | |
| Sex | ||||
| Male | 50 (56.8) | 46 (51.7) | 107 (52.7) | .422 |
| Female | 38 (43.2) | 43 (48.3) | 71 (46.7) | |
| Race | ||||
| Black | 23 (26.1) | 33 (37.1) | 94 (52.8) | < .001 |
| Non-Black | 65 (73.9) | 56 (62.9) | 84 (47.2) | |
| ECOG Performance Status | ||||
| 0 | 63 (72.4) | 47 (53.4) | 55 (33.3) | < .001 |
| ≥1 | 24 (27.6) | 41 (46.6) | 121 (65.1) | |
| Smoking (pack-years) | ||||
| Median/range | 34/0-180 | 38/0-150 | 45/0-212 | .044 |
| COPD diagnosis | ||||
| Yes | 13 (14.9) | 22 (25.3) | 65 (36.9) | .001 |
| Charlson Comorbidity Status | ||||
| <6 | 60 (68.2) | 59 (55.7) | 80 (44.9) | .001 |
| ≥6 | 28 (31.8) | 39 (44.3) | 98 (55.1) | |
| Marital Status | ||||
| Married | 59 (67) | 53 (59.6) | 73 (41) | < .001 |
| Non-married | 29 (33) | 36 (40.4) | 105 (59) | |
| Median Household Income | ||||
| ≥43,723 | 56 (65.1) | 44 (51.8) | 72 (40.4) | .001 |
| Overall Stage | ||||
| IIIA | 66 (75) | 54 (60.7) | 80 (44.9) | < .001 |
| IIIB | 22 (25) | 35 (39.3) | 98 (55.1) | |
| T-stage | ||||
| TX | 0 (0) | 4 (4.5) | 14 (7.9) | .044 |
| ≤T2 | 48 (54.5) | 39 (44.3) | 74 (41.8) | |
| ≥T3 | 40 (45.5) | 45 (51.1) | 89 (50.3) | |
| N-stage | ||||
| NX | 1 (1.1) | 1 (1.1) | 1 (0.6) | .039 |
| ≤N1 | 14 (15.9) | 11 (12.5) | 31 (17.5) | |
| N2 | 64 (72.7) | 58 (65.9) | 96 (54.2) | |
| N3 | 9 (10.2) | 18 (20.5) | 49 (27.7) | |
| N2-stage | ||||
| Single station | 36 (56.3) | 25 (43.1) | 35 (36.5) | .128 |
| Multi-nodal | 22 (34.4) | 27 (46.6) | 53 (55.2) | |
| Unknown | 6 (9.4) | 6 (10.3) | 8 (8.3) | |
| Radiation Dose Delivered (Gy) | ||||
| Median/range | 61.2/39.6-69.6 | 66/10-70.2 | 63/19.8-81.6 | .108 |
| ≥60 Gy | 79 (93) | 73 (85.8) | 141 (81) | .040 |
| Adjuvant Chemotherapy | ||||
| Yes | 65 (80.2) | 54 (70.1) | 93 (63.7) | .034 |
COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group.
The number of patients in each cohort (%).
One hundred seventy-seven patients were initially considered for trimodality therapy at the time of consultation, but only 88 patients actually underwent surgery. These patients make up the trimodality cohort (86.4% of surgeries were lobectomies; Table 1). The other 89 patients who were considered for surgery after a multidisciplinary evaluation before initiation of definitive CRT but who did not receive trimodality treatment form the unplanned bimodality cohort. Major reasons for patients not undergoing surgery included detection of metastatic disease at restaging (20 patients; 22.5%), persistent mediastinal disease (16 patients; 18%), and continued unresectability after CRT (16 patients; 18%). Recognizing that neoadjuvant therapy is not typically used as a means to improve resectability, in our clinic, we pursue an open approach whereby patients with very early resectability or operability concerns are given the benefit of the doubt and treated with the intention to reassess after neoadjuvant therapy. Other reasons for patients not proceeding to surgery included exacerbation of comorbid illnesses after CRT (11 patients; 12.4%), declining surgery (6 patients; 6.7%), treatment-related toxicities (7 patient; 7.9%), requiring a pneumonectomy but not being medically fit to receive one (3 patients; 3.4%), and other reasons (10 patients; 11.1%). Finally, 178 patients were recommended upfront for bimodality therapy only (planned bimodality cohort).
Survival analysis
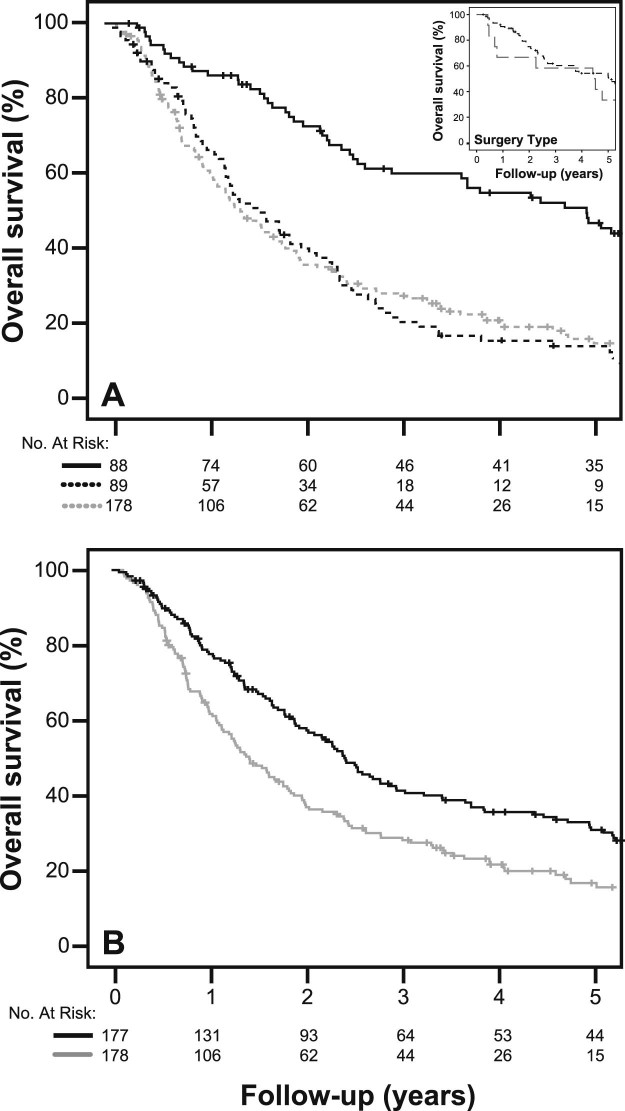
With a median follow-up of 15 months for all patients and 88 months for surviving patients (range, 1-184 months), the estimated median OS rates in the 3 cohorts (trimodality, unplanned bimodality, and planned bimodality) were 59.9, 20.1, and 17.3 months, respectively (trimodality vs planned bimodality: hazard ratio [HR], 0.688; 95% confidence interval [CI], 0.595-0.796; P < .001; Fig 1a). In the intention-to-treat analysis, median survival for the surgery initially planned group (unplanned bimodality + trimodality) was 29.2 months compared with 17.2 months for the planned bimodality (HR, 0.643; 95% CI, 0.505-0.819; P < .001; Fig. 1b). There was no difference in survival time between the unplanned and planned bimodality cohorts. The 30- and 90-day surgical mortality rate for the trimodality cohort was 4.5% and 6.8%, respectively. There was no significant difference in survival among patients who required pneumonectomies (n = 12) versus lobectomies (Fig 1a; inset; P = .513). Four patients died from postoperative complications within 30 days; 3 were pneumonectomies. Two patients developed acute respiratory distress syndrome, one experienced cardiac arrest, and one had an acute massive hemoptysis episode after developing a bronchopleural fistula.
Figure 1.
(A) Overall survival in the 3 patient cohorts: Trimodality (dark solid line), unplanned bimodality (dark dashed line), and planned bimodality (light dashed line). Hazard ratio (HR) comparing the trimodality and unplanned bimodality cohorts: HR, 0.688; 95% CI, 0.595-0.796; P < .001. Inset shows overall survival as a function of surgery type (lobectomy: dark dashed line; pneumonectomy: light dashed line) in patients who underwent trimodality treatment: P = .513; (B) Intention-to-treat overall survival curves comparing all trimodality patients (unplanned bimodality+trimodality; dark solid line) and planned bimodality cohort (light solid line). HR, 0.643; 95% CI, 0.505-0.819; P < .001.
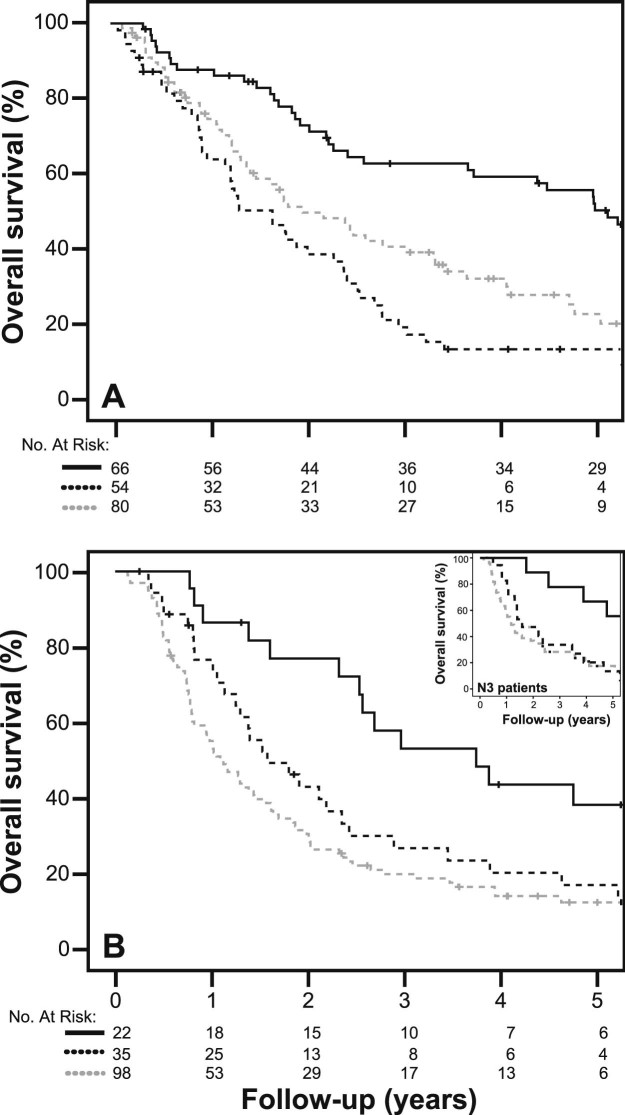
Patients who were pathologic N0 at the time of surgery (MNC) had a survival advantage compared with patients who still had residual nodal disease (P < .001; Table 3). The survival benefit with trimodality therapy was present even when stratified by clinical stage (IIIA vs IIIB; Figs 2a and 2b, including the inset) and pathological nodal grouping (N0 vs N1-N3; Table 3). Specifically, patients with IIIB disease who underwent surgery (n = 22) had a median survival of 45.1 months, compared with 19 and 13 months in the unplanned and planned bimodality cohorts, respectively (P = .002). Furthermore, in a small subset of clinical N3 patients (n = 9; 79% of N3 patients had contralateral hilar disease) who underwent trimodality therapy, the estimated median survival was 57.2 months in comparison with 14 and 19 months, respectively, with planned bimodality (n = 49) and unplanned bimodality (n = 18; P = .033) (HR, 0.625; 95% CI, 0.428-0.913; P = .010 for trimodality vs planned bimodality patients).
Table 3.
Median survival outcomes of patients with stage III NSCLC on the basis of treatment modality and nodal response after neoadjuvant CRTa
| Our Analysis | Intergroup 0139 | RTOG 0229 | RTOG 0617 | |
|---|---|---|---|---|
| Trimodality | ||||
| All patients | 59.9 | 23.6 | - | - |
| Lobectomies only | 61.0 | 33.6 | - | - |
| pN0 | 60.2 | 34.4 | MST not reachedc | - |
| pN1-N3 | 35.7 | 26.4 | 32.7 | - |
| No Surgery | 20.1 | 7.9 | 7.5 | - |
| Planned Bimodality | ||||
| All patients | 17.3 | 22.2 | - | - |
| Lobectomies only | - | 21.7b | - | - |
| 60 Gy | - | - | - | 28.7 |
| 74 Gy | - | - | - | 20.3 |
| P-values | ||||
| < .001e | .002,d < .001e |
.0002 | .004 |
CRT, chemoradiation therapy; MST, median survival time; NSCLC, non-small cell lung cancer; RTOG, radiation therapy oncology group.
Estimated median survival in months.
Unplanned exploratory matched analysis for this group.
Median survival time (MST) not reached.
When comparing lobectomy patients to bimodality.
When comparing pN0 to pN1-N3 and no surgery.
Figure 2.
Five-year overall survival in the 3 patient cohorts (trimodality [solid line], unplanned bimodality [dark dashed line], and planned bimodality [light dashed line]; P < .001), stratified by (A) stage IIIA and (B) stage IIIB. Inset shows overall survival in patients with clinical N3 disease (P = .014).
Freedom-from-recurrence and patterns of failure
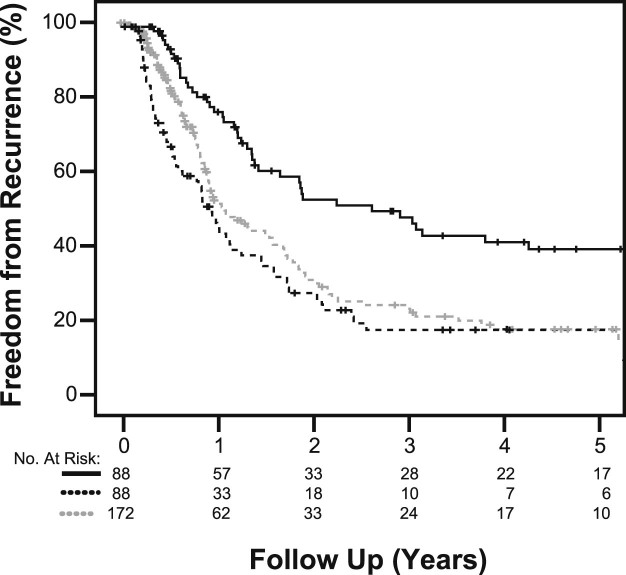
Five-year FFR rates and median time to failure were improved with surgery and were 38.3% (32.1 months), 17.9% (11.9 months), and 17.8% (13.5 months) for trimodality, unplanned bimodality, and planned bimodality cohorts, respectively (HR, 0.766; 95% CI: 0.651-0.900; P = .001; Fig 3).
Figure 3.
Five-year freedom from recurrence in the 3 patient cohorts (planned trimodality [solid line], unplanned bimodality [dark dashed line], planned bimodality [light dashed line]; P < .001).
Clinical indicators for treatment choice
Overall stage (odds ratio [OR], 0.225; 95% CI, 0.127-0.400; P < .001, favoring stage IIIA), ECOG performance status (OR, 0.299; 95% CI, 0.174 0.513; P < .001, favoring ECOG 0), race (OR, 0.320; 95% CI, 0.178 0.577; P < .001, favoring patients of non-black race), having chronic obstructive pulmonary disease (COPD; OR, 0.412; 95% CI, 0.225-0.754; P = .004, favoring patients with no COPD), age (OR, 0.949; 95% CI, 0.924-0.974; P < .001, favoring younger age), marital status (OR, 2.150; 95% CI, 1.249-3.702; P = .006, favoring being married), and T stage (OR, 1.902; 95% CI, 1.182-3.062; P = .008, favoring T0-T2 vs T3-T4 primaries) were all significant predictors of patients being initially considered for trimodality treatment at the time of first consultation.
Among patients who were initially considered for surgery, the clinical factors that predicted for actually proceeding to trimodality treatment after definitive doses of CRT were race (OR, 0.279; 95% CI, 0.146-0.531; P < .001, favoring non-black patients), overall stage (OR, 0.284; 95% CI, 0.153-0.527; P < .001, favoring stage IIIA), ECOG performance status (OR, 0.287; 95% CI, 0.158-0.521; P < .001, favoring ECOG 0), age (OR, 0.945; 95% CI, 0.919-0.972; P < .001, favoring younger patients), and COPD diagnosis (OR, 0.379; 95% CI, 0.183-0.785; P = .009, favoring patients with no COPD).
ECOG performance status (OR, 0.452; 95% CI, 0.236-0.864; P = .016, favoring ECOG greater than 0) was the only statistically significant factor that predicted for a change in treatment (ie, from trimodality therapy to unplanned bimodality status).
Discussion
In this largest report of a single institutional analysis of trimodality therapy after high-dose CRT, we demonstrate a significant OS and FFR advantage with trimodality treatment when compared with patients who receive bimodality therapy in a carefully selected patient population (Figure 1, Figure 3; Table 3). In contrast to the median survival of 28.7 months reported in the recent randomized phase 3 cooperative group trial of standard-dose bimodality therapy alone for stage III NSCLC,20 we have demonstrated a doubling of the median survival with the addition of surgical resection after the same high-dose CRT, when feasible (Table 3).
Our outcomes are also considerably superior to those seen with low-dose (45 Gy) CRT prior to surgery in the Intergroup 0139 randomized trial, which did not show an OS advantage with trimodality therapy. This was most likely due to the high postoperative mortality rate observed among patients who underwent pneumonectomy (26%).10 In contrast, in our institutional experience, definitive doses of CRT (≥60 Gy) were delivered to patients with LA-NSCLC with trimodality therapy performed when feasible with minimal surgical mortality. Even in the RTOG 02-29 trial, postoperative mortality was only 3% after definitive doses (61.2 Gy) of CRT.9
The critical factors leading to reduced complications were improved surgical techniques (including consideration of vascularized muscle flaps at the stump19), monitoring postoperative hydration, and limiting barotrauma. Advancements in radiation techniques also may have played a role in the improved outcomes seen after definitive doses of neoadjuvant CRT. Indeed, in the secondary analysis done on RTOG 0617, despite patients who received IMRT being more likely to have stage IIIB disease and larger target volume to lung ratios compared with 3-dimensional conformal radiation therapy, IMRT was still significantly associated with lower doses to the lungs and heart, resulting in a decrease in treatment-related toxicities after CRT.21 Reductions in postoperative toxicities while maximizing the benefits of local control with trimodality treatment can translate into improved OS, as seen in several modern retrospective studies that used low-dose CRT and supported by a population analysis.11, 12, 13
Careful selection of patients for trimodality therapy is essential to maximize outcomes. One critical factor in the selection process for trimodality therapy identified in Intergroup 0139 and RTOG 0229 was MNC.9, 10 A large institutional analysis from Korea also demonstrated that the initial bulk or extent of mediastinal nodal disease did not influence prognosis for patients planned for trimodality therapy as long as pathological nodal clearance was achieved.20 Our data further corroborate this trend with a survival benefit that was predominantly seen in patients who achieved MNC (Table 3). Another benefit of definitive doses of neoadjuvant CRT for patients who are candidates for surgical resection is a greater likelihood of achieving MNC, as seen in RTOG 02-29 when comparing MNC rates to those from the Intergroup 0139 trial (63% vs 47%), which potentially augments the local benefits of surgical resection in this patient population.19, 22, 23
Furthermore, delivering high-dose CRT to all patients with stage III disease maximizes the opportunity to undergo surgical intervention irrespective of potential confounding factors. With widespread availability of modern technologies such as IMRT at smaller community centers, there is potential for extension of this work to a broader population.
Despite the improved OS benefit seen with the addition of surgical resection after CRT, we recognize the selection bias inherent to such an analysis that could lead to similar results. For example, we were unable to determine if OS was improved solely due to MNC (and these patients were thus chosen to complete trimodality treatment) or if the addition of surgical resection was an added benefit that resulted in improved clinical outcomes. We also observed that patients in our surgical cohort had a statistically significant lower burden of disease (ie, smaller primaries and fewer N3-patients; Table 2). It is plausible that patients with a favorable tumor biology and smaller volume of disease may do well despite locally aggressive treatment. However, the considerably improved outcomes seen in our trimodality cohort when compared with the RTOG 0617 study results and the challenges seen with radiation dose escalation beyond 60 Gy to enhance local therapy in that same study justify an exploration of surgical resection as an alternate approach in select patients.
In our analysis, we did not explore the objective measurements of operability, including pulmonary function test results and echocardiograms, because they are well described in the literature. In our series, planned bimodality patients had higher comorbidities, hence their exclusion from consideration for trimodality therapy. Furthermore, nearly one-quarter of our unplanned bimodality group could not proceed with surgery because of a decline in medical fitness after concurrent chemoradiation.
However, the purpose of our analysis was to explore these selection biases, which are inherent to the functioning of a multidisciplinary clinic. Clinical factors such as cardiopulumonary function, fitness for anesthesia to undergo thoracotomy, and tolerance to CRT often determine whether a patient is a candidate for surgical resection and are well described in the literature.19, 22, 23, 24, 25 Yet, there are no published data that describe the influence of baseline demographic factors in the selection process for trimodality therapy. We therefore elected to review our experience to identify these demographic factors that might influence consideration for surgery both at the time of initial evaluation and again post-CRT.
We found that race, overall stage, performance status, age, and COPD diagnosis were predictive factors for patients to be initially considered for trimodality treatment and to actually undergo surgical resection. In our cohort, patients of black race were less likely to undergo surgery, a trend described in other studies.11, 26 On subgroup analysis, the survival benefit with trimodality therapy persisted in both black and non-black patients (data not shown), which leaves in question whether race truly affects the decision for surgery. On closer inspection, black patients were less likely to be married (71% and 29%, respectively; P < .001) and to have an ECOG performance status score of 0 (67% and 32%, respectively; P < .001), either or both of which could have influenced recommendations at initial consultation and surgical decision after definitive CRT.
Previous data from our institution support the observation that married patients with LA-NSCLC have superior outcomes when compared with their single counterparts.27 Marital status is often a surrogate for improved social support with better adherence to recommended treatment modalities; thus, married patients may have been more likely to be considered for surgical intervention. Age was also a significant predictor for surgical intervention, with younger patients more likely to be considered for trimodality therapy. Select older patients, however, were able to successfully complete trimodality treatment (30% of trimodality patients were >65 years old) in our cohort. Given the significant clinical benefit of surgical resection after definitive doses of CRT, age alone should not deter providers from offering trimodality treatment. The strong survival benefit with trimodality treatment seen in our analysis suggests that an open-ended methodology may be justified for patients at the time of initial consultation. Patients who are older or those with a higher stage should still be considered for trimodality treatment, especially if they are able to achieve MNC post-CRT and continue to retain a healthy performance status.
Conclusion
In our retrospective analysis, survival benefits and improvement in tumor recurrence rates were seen in patients who underwent surgery after high-dose CRT. This survival benefit was seen irrespective of stage and nodal group, although MNC played a decisive role in the ultimate outcomes. Among demographic and clinical variables, race, overall stage, performance status, age, and COPD diagnosis were significant factors that were associated with the initial choice of trimodality treatment at the time of consultation inLA-NSCLC and for actual surgical intervention. The strong surival benefit seen suggests pursuing a flexible approach to allow for maximal consideration of trimodality therapy in the LA-NSCLC patient population, independent of stage, age, race, or marital status and guided more by performance status after neoadjuvant CRT and MNC.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The open access fee for this article was paid for by a 2017 grant from the Radiation Oncology Institute (ROI).
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P., Chansky K., Crowley J. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Varlotto J.M., Yao A.N., DeCamp M.M. Nodal stage of surgically resected non-small cell lung cancer and its effect on recurrence patterns and overall survival. Int J Radiat Oncol Biol Phys. 2015;91:765–773. doi: 10.1016/j.ijrobp.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe Y., Hayashi Y., Shimizu J., Oda M., Iwa T. Mediastinal nodal involvement and the prognosis of non-small cell lung cancer. Chest. 1991;100:422–428. doi: 10.1378/chest.100.2.422. [DOI] [PubMed] [Google Scholar]
- 5.Sakao Y., Miyamoto H., Yamazaki A. Prognostic significance of metastasis to the highest mediastinal lymph node in nonsmall cell lung cancer. Ann Thorac Surg. 2006;81:292–297. doi: 10.1016/j.athoracsur.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 6.De Waele M., Hendriks J., Lauwers P. Nodal status at repeat mediastinoscopy determines survival in non-small cell lung cancer with mediastinal nodal involvement, treated by induction therapy. Eur J Cardiothorac Surg. 2006;29:240–243. doi: 10.1016/j.ejcts.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Crvenkova S., Pesevska M. Important prognostic factors for the long-term survival in non-small cell lung cancer patients treated with combination of chemotherapy and conformal radiotherapy. J BUON. 2015;20:775–781. [PubMed] [Google Scholar]
- 8.Sayar A., Turna A., Kilicgun A., Solak O., Urer N., Gurses A. Prognostic significance of surgical-pathologic multiple-station N1 disease in non-small cell carcinoma of the lung. Eur J Cardiothorac Surg. 2004;25:434–438. doi: 10.1016/j.ejcts.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Suntharalingam M., Paulus R., Edelman M.J. Radiation therapy oncology group protocol 02-29: A phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 2012;84:456–463. doi: 10.1016/j.ijrobp.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 10.Albain K.S., Swann R.S., Rusch V.W. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speicher P.J., Englum B.R., Ganapathi A.M., Onaitis M.W., D'Amico T.A., Berry M.F. Outcomes after treatment of 17 378 patients with locally advanced (T3N0-2) non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;47:636–641. doi: 10.1093/ejcts/ezu270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziel E., Hermann G.B., Sen N. Survival benefit of surgery after chemoradiotherapy for stage III (N0–2) non-small-cell lung cancer is dependent on pathologic nodal response. J Thorac Oncol. 2009;10:1475–1480. doi: 10.1097/JTO.0000000000000639. [DOI] [PubMed] [Google Scholar]
- 13.Lee H., Ahn Y.C., Pyo H. Pretreatment clinical mediastinal nodal bulk and extent do not influence survival in N2-positive stage IIIA non-small cell lung cancer patients treated with trimodality therapy. Ann Surg Oncol. 2014;21:2083–2090. doi: 10.1245/s10434-014-3540-x. [DOI] [PubMed] [Google Scholar]
- 14.Albain K.S., Rusch V.W., Crowley J.J. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 15.Farray D., Mirkovic N., Albain K.S. Multimodality therapy for stage III non-small-cell lung cancer. J Clin Oncol. 2005;23:3257–3269. doi: 10.1200/JCO.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt W.E., Albain K.S., Pass H. Induction treatment before surgery for non-small cell lung cancer. Lung Cancer. 2003;42:S9–S14. doi: 10.1016/s0169-5002(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 17.Edelman M.J., Suntharalingam M., Burrows W. Phase I/II trial of hyperfractionated radiation and chemotherapy followed by surgery in stage III lung cancer. Ann Thorac Surg. 2008;86:903–910. doi: 10.1016/j.athoracsur.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Edelman M.J., Gandara D.R., Roach M., 3rd, Benfield J.R. Multimodality therapy in stage III non-small cell lung cancer. Ann Thorac Surg. 1996;61:1564–1572. doi: 10.1016/0003-4975(96)00044-6. [DOI] [PubMed] [Google Scholar]
- 19.Sonett J.R., Krasna M.J., Suntharalingam M. Safe pulmonary resection after chemotherapy and high-dose thoracic radiation. Ann Thorac Surg. 1999;68:316–320. doi: 10.1016/s0003-4975(99)00593-7. [DOI] [PubMed] [Google Scholar]
- 20.Bradley J.D., Moughan J., Graham M.V. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: Phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys. 2010;77:367–372. doi: 10.1016/j.ijrobp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun S.G., Hu C., Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2016;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R., Ferguson M.K. Video-assisted versus open lobectomy in patients with compromised lung function: A literature review and meta-analysis. PLoS ONE. 2015;10:e0124512. doi: 10.1371/journal.pone.0124512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J.S., Fischel R., Brenner M., Gelb A., Invernizzi F., Wagner W. Pulmonary function tests in preoperative pulmonary evaluation. Respir Med. 2004;98:598–605. doi: 10.1016/j.rmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Kummer F. The assessment of cardiopulmonary function in thoracic surgery. Thorac Cardiovasc Surg. 1983;31:329–330. doi: 10.1055/s-2007-1022011. [DOI] [PubMed] [Google Scholar]
- 25.Semik M., Schmid C., Trösch F., Broermann P., Scheld H.H. Lung cancer surgery–preoperative risk assessment and patient selection. Lung Cancer. 2001;33:S9–S15. doi: 10.1016/s0169-5002(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 26.American Lung Association Too many cases, too many deaths: Lung cancer in African Americans. 2017. http://www.lung.org/assets/documents/research/ala-lung-cancer-in-african.pdf Available at:
- 27.Feliciano J.L., Bentzen S.M., Lam V.K. Marital status is strongly prognostic and associated with more favorable nutritional status in locally advanced non-small cell lung cancer. J Thor Oncol. 2015;10:S451–S452. [Google Scholar]