Abstract
Visceral fat accumulation as observed in Crohn's disease and obesity is linked to chronic gut inflammation, suggesting that accumulation of gut adipocytes can trigger local inflammatory signaling. However, direct interactions between intestinal epithelial cells (IECs) and adipocytes have not been investigated, in part because IEC physiology is difficult to replicate in culture. In this study, we originally prepared intact, polarized, and cytokine responsive IEC monolayers from primary or induced pluripotent stem cell-derived intestinal organoids by simple and repeatable methods. When these physiological IECs were co-cultured with differentiated adipocytes in Transwell, pro-inflammatory genes were induced in both cell types, suggesting reciprocal inflammatory activation in the absence of immunocompetent cells. These inflammatory responses were blocked by nuclear factor-κB or signal transducer and activator of transcription 3 inhibition and by anti-tumor necrosis factor- or anti-interleukin-6-neutralizing antibodies. Our results highlight the utility of these monolayers for investigating IEC biology. Furthermore, this system recapitulates the intestinal epithelium–mesenteric fat signals that potentially trigger or worsen inflammatory disorders such as Crohn's disease and obesity-related enterocolitis.
Abbreviations: ANOVA, analysis of variance; BSA, bovine serum albumin; CD, Crohn's disease; ChgA, Chromogranin A; CM, conditioned medium; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; DSS, dextran sodium sulfate; ECM, extracellular matrix; EGF, epidermal growth factor; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; FGF, fibroblast growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IBD, inflammatory bowel disease; IBMX, 3-isobutyl-1-methylxanthine; IEC, intestinal epithelial cell; IFN, interferon; IL, interleukin; iPSC, induced-pluripotent stem cell; MMP, matrix metalloproteinase; Muc2, mucin 2; NF-κB, nuclear factor-κB; PBS, phosphate-buffered saline; pIgR, polymeric immunoglobulin receptor; PPAR, peroxisome proliferator-activated receptor; RT-PCR, reverse transcription polymerase chain reaction; RPMI, Roswell Park Memorial Institute; R-spo1, R-spondin1; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; STAT3, signal transducer and activator of transcription 3; TER, transepithelial electrical resistance; TNF, tumor necrosis factor; UC, ulcerative colitis; WAT, white adipose tissue
Keywords: Crohn's disease, Intestinal epithelial cells, Induced-pluripotent stem cells, Organoids, Co-culture, Adipocytes
Highlights
-
•
Intact intestinal epithelial monolayers are developed from primary or induced pluripotent stem cell-derived organoids.
-
•
Intestinal epithelial cells and adipocytes mutually enhance inflammatory responses in the absence of immune cells.
-
•
The inflammatory responses are mediated by nuclear factor-κB and signal transducer and activator of transcription 3.
One of the inflammatory bowel diseases, Crohn's disease (CD), frequently accompanies mesenteric fat accumulation and inflammation; however, the biological significance has not been elucidated so far. Here, we co-cultured intact intestinal epithelial cells developed from mouse and human intestinal organoids with mature adipocytes, and found that inflammation responses were reciprocally induced. The responses were blocked by small molecule compounds and neutralizing antibodies. Our results indicate mesenteric fat abnormalities may lead to the initiation and/or progression of CD, and provide the possibility that disconnecting the inflammatory signaling would be a promising approach to treat CD.
1. Introduction
Crohn's disease (CD) is a recurrent inflammatory bowel disease (IBD) characterized by abdominal pain, diarrhea, weight loss, and fever. Around 40% of patients suffer from chronic inflammation in both small and large intestines, while 30% exhibit symptoms limited to the small intestine (Annese et al., 2012). Development of IBD is usually accompanied by dysregulation of intestinal epithelial cells (IECs), which function in nutrient absorption and as physical and immunological barriers to pathogen invasion (Peterson and Artis, 2014). It has been recognized for decades that the onset of CD is associated with accumulation of mesenteric white adipose tissue (WAT) (Desreumaux et al., 1999, Drouet et al., 2012, Goncalves et al., 2015, Peyrin-Biroulet et al., 2007), with CD patients exhibiting four times more adipocytes per unit area than healthy controls (Fink et al., 2012). The net effects of mesenteric fat abnormalities on CD patients, whether beneficial or harmful, have been debated. Some suggest that the balance of cell types in mesenteric fat abnormalities could exert an immunoprotective effect including M2 macrophage increase, while others stress the pathological effects based on clinical observations (Coffey et al., 2016). Emerging evidence suggests that mesentery including, the mesenteric fat tissues, could be a therapeutic target for CD by both operative and pharmacological means. In many cases, patchy or linear mucosal ulcerations are observed just beneath WAT, which implies local pathogenic signaling between mesenteric fat and intestinal epithelium (Peyrin-Biroulet et al., 2007). In addition, several pro-inflammatory cytokines, including tumor necrosis factor (TNF) and Interleukin (IL)-6, are produced in excess by WAT in CD patients (Goncalves et al., 2015). Similarly, obesity is linked to IBD pathogenesis (Boutros and Maron, 2011, Ding et al., 2010, Ding and Lund, 2011, Goncalves et al., 2015, Zhang et al., 2012), partly due to mesenteric WAT hypertrophy. It was reported that around 38% of IBD patients are overweight (Steed et al., 2009). High-fat diet and/or associated obesity can also cause mild bowel inflammation in experimental animal models (Ding et al., 2010, Gulhane et al., 2016). Furthermore, chemically or genetically induced enterocolitis is exacerbated by diet-induced obesity (Paik et al., 2013, Teixeira et al., 2011).
Adipocyte development and fat storage may be regulated by local tissue signaling. Transcriptional cascades for adipocyte differentiation, initiated by peroxisome proliferator-activated receptor (PPAR)γ, have been investigated in vitro using 3T3-L1 cells or mouse embryonic fibroblasts (MEFs) (Rosen and MacDougald, 2006). Differentiated adipocytes can release free fatty acids (FFAs) in response to lipolytic stimuli such as fasting that are utilized by peripheral tissues (Fruhbeck et al., 2014). However, hypertrophied adipocytes tend to release more FFAs in the steady state, which act as lipotoxicity and can lead to insulin resistance and inflammation in many other tissues (de Luca and Olefsky, 2008). Adipocytes also secrete various cytokines, such as leptin, adiponectin, and IL-6 (Peyrin-Biroulet et al., 2007, Rosen and Spiegelman, 2006). For instance, the secretion of some pro-inflammatory adipokines including TNF and resistin is augmented in obesity and is directly brought about by β-cell dysfunction or apoptosis, resulting in the progression of type II diabetes (Dunmore and Brown, 2013). Based on these findings, it would be possible that changes in the local number and activity of adipocytes induce the inflammation of IECs in CD and obese patients as IECs are prone to external stimuli and stress (Hosomi et al., 2015, Zeissig et al., 2004), but no direct evidence for this currently exists.
Cell lines are widely used as models of the intestinal epithelial monolayer, including Caco-2 and HT-29 cells (Rousset, 1986). However, these lines are derived from cancer cells and so exhibit chromosome aneuploidy and multiple mutations (Ghadimi et al., 2000). For more physiological assays, some recent studies have attempted to establish IEC cultures (Moon et al., 2013, VanDussen et al., 2015, Wang et al., 2015), but several technical issues remain, including recapitulation of in vivo physiology, operational simplicity, culture stability over time, and assay throughput.
Gut epithelial organoid culture is an emerging technique for investigating the molecular biology of IECs (Sato et al., 2009, Sato et al., 2011, Yui et al., 2012). Organoids derived from mouse small intestine contain enterocytes, Paneth cells, goblet cells, and enteroendocrine cells, and so may better reflect enteric characteristics in vivo. However, it is difficult to evaluate the specific functions of IECs, such as their response to bacterial infection and interactions with other types of cells, because organoid culture is conducted in a solidified three-dimensional (3D) environment and the villus-like luminal domains exist at the organoid core (Sato et al., 2009). Therefore, to improve our understanding of intestinal epithelial biology, it is critical to establish culture methods for more accessible IEC monolayers. On the other hand, it has recently been reported that intestinal organoids can be generated from human induced-pluripotent stem cells (iPSCs) (McCracken et al., 2011). These cells have several major advantages for organoid development; there are fewer ethical issues associated with iPSC technologies compared to embryonic stem (ES) cell studies, and iPSCs can be isolated from patients as disease models for basic research as well as for regenerative medicine.
In this study, we present evidence that intestinal inflammation induced by chronic dextran sodium sulfate (DSS) administration can cause mesenteric fat accumulation and inflammation in mice. To examine potential interactions between IECs and adipocytes, we developed a simple, original method for preparing physiological IECs from physically-fractured organoids. Co-culture of these IECs with mature adipocytes revealed direct inflammatory crosstalk between these cell types in the absence of immune cells, which strongly suggests that adipose tissues themselves can be a promising therapeutic target for chronic inflammatory diseases.
2. Materials and Methods
2.1. Materials
The recombinant proteins and neutralizing antibodies for mouse TNF and IL-6 were obtained from R&D Systems. Defined fetal bovine serum (FBS) was purchased from HyClone, vitronectin was from Thermo Fisher Scientific, and insulin, dexamethazone and a protease inhibitor cocktail were from Sigma. 3-Isobutyl-1-methylxanthine (IBMX) and pioglitazone were obtained from Wako. CAPE and Galiellalactone were from Tocris and Cayman Chemical, respectively. Antibodies for villin1 (ab3304), mucin 2 (Muc2) (ab11197 for human, ab76774 for mouse), and chromogranin A (ChgA) (ab15160) were purchased from Abcam. Anti-E-cadherin antibody (3195) was from Cell Signaling, anti-lysozyme antibody (A0099) was from Dako, and anti-perilipin1 antibody (GP29) was from Progen. Secondary antibodies for immunostaining were from Jackson ImmunoResearch.
2.2. Mice and Tissues
C57BL/6J mice were purchased from CLEA Japan (Tokyo, Japan). Mesenteric fat tissues from whole intestinal mesentery were isolated from male mice fed three cycles of 2.25% DSS in the drinking water for 5 days, followed by 2-week consumption of water. Mesenteric fat as well as other white adipose tissues were confirmed to float in phosphate-buffered saline (PBS) and were carefully separated from other associated tissues, including mesenteric lymph nodes and blood vessels. The tissues were rapidly immersed in RNA later (QIAGEN), and stored at 4 °C until use. Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacture's instruction. All mice were housed in specific pathogen-free conditions in the experimental animal facility of the Institute of Medical Science, The University of Tokyo. The Institutional Animal Care and Research Advisory Committee at The University of Tokyo approved all animal procedures.
2.3. Cell Culture
Primary MEFs were obtained from embryos at day 13 post-coitum, and adipocyte differentiation experiments were carried out at passage two, as described previously (Kim et al., 2007). HEK293T cells, L cells, Caco-2 cells, and MEFs were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. For differentiation, Caco-2 cells were monolayer-cultured in Transwells (Corning 3413) for over 10 days after they reached confluence. Human primary visceral pre-adipocytes were purchased from Lonza. Cell growth and adipocyte differentiation followed the supplier's protocols. The human iPSC line TkDN4-M (Takayama et al., 2010) was supplied by The University of Tokyo and was maintained as colonies in feeder-free conditions on plates coated with vitronectin (Gibco) with Essential 8 medium (Gibco). The cells were passaged every 3–4 days before reaching confluence. All cultures were incubated at 37 °C in 95% humidity with 5% CO2.
2.4. Preparation of Conditioned Medium
L cells stably expressing human R-spondin1 and human Noggin together with (WRN CM) or without mouse Wnt3a (RN CM) were established by lentiviral infection. The plasmids for lentiviral production (pCAG-HIVgp, pCMV-VSV-G-RSV-Rev, and pCSII-EF-MCS-IRES2-Venus) were kindly provided by Dr. Hiroyuki Miyoshi (BioResource Center, RIKEN, Japan). Each conditioned medium was prepared from the culture supernatants of the cells seeded at 1.4 × 106 cells/35-mm dish for 72 h.
2.5. Preparation and Culture of Mouse Intestinal Organoids
Small intestinal crypts were isolated from 8 to 12-week-old mice and the organoids were cultured as described previously (Sato et al., 2009). The crypts were embedded in 50 μL Matrigel (BD Biosciences) and placed in the center of each well (24-well plates). Following polymerization in a 37 °C, 5% CO2 incubator for 15 min, 500 μL mouse organoid culture medium (Supplementary Table 1) was added to each well. 25% RN CM can be replaced with 500 ng/mL mouse R-spondin1 and 100 ng/mL mouse Noggin. Epidermal growth factor (EGF) was added every second day and the entire medium was changed every 4 days. Organoid passage was carried out as described below.
2.6. Differentiation of Human Induced-Pluripotent Stem Cells Into Intestinal Organoids
Organoids were differentiated from human iPSCs following a previously described protocol (McCracken et al., 2011), with small modifications in differentiation medium. Briefly, 80%–90% confluent iPSCs were differentiated into definitive endoderm through treatment with DE1 medium (Supplementary Table 1) for 24 h, followed by DE2 medium (Supplementary Table 1) for 24 h, and then DE3 medium (Supplementary Table 1) for a further 24 h. For mid- and hindgut differentiation, definitive endoderm cells were cultured in mid- and hindgut differentiation medium (Supplementary Table 1) for up to 4 days. Free-floating spheroids were collected during the course of the culture, and transferred into three-dimensional cultures in Matrigel refed with intestine growth medium (Supplementary Table 1), which was replaced every 2–3 days for 14 days. Organoids were collected after several passages, following the protocol described below.
2.7. Organoid Passage
The organoids that had been cultured in Matrigel were washed with PBS and treated with cell recovery solution (BD Biosciences) for 30 min on ice. The recovered organoids were then collected by centrifugation at 440g for 5 min. Following removal of the supernatant, the organoids were suspended with 700 μL basal medium (Supplementary Table 1). The cell suspension was mildly passed through a 26G needle 10 times without bubbling and then centrifuged at 440g for 5 min. The organoids were resuspended in Matrigel with 20% organoid growth medium on ice and the suspensions were aliquoted into the wells of a 24-well plate, leaving the border of each well untouched, and solidified in a 37 °C, 5% CO2 incubator for 15 min. Following this, 500 μL mouse organoid culture medium or human organoid culture medium (Supplementary Table 1) was added to each well. The average passaging ratio was 1:2 for mouse organoids and 1:4 for human organoids. For mouse organoids, EGF was added every second day and passage was performed every 4 days, whereas for human organoids, the entire medium was changed every 3 days and passage was performed every 6 days.
2.8. Monolayer Culture of Organoid-derived Cells
After being recovered from Matrigel using cell recovery solution, the organoids were broken by passing a needle (26G for mouse organoids or 29G for human organoids) 10 times through the basal medium. Following collection by centrifugation at 440 g for 5 min, they were resuspended with each organoid culture medium and then seeded in type I collagen (Nitta Gelatin)-coated 24-well plates or Transwells. For mouse cell culture, 300 ng/mL recombinant mWnt3a was also added to the medium. On average, organoids from one well were plated into the upper compartment of two (mouse) or five (human) Transwells (100 μL per well), and an additional 600 μL culture medium was added to each lower compartment. The medium was replaced every 2–3 days. For the responsive assays, cells were pretreated with pre-stimulation medium (Supplementary Table 1) for 1 day to eliminate the growth factors. The medium was then replaced with pre-stimulation medium containing the respective cytokines for 2 days. For the co-culture assays, Transwells with IECs were transferred to 24-well plates with adipocytes, and co-cultured for 2 days with pre-stimulation medium.
2.9. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was extracted from the cultured cells and mesenteric fat tissues using the RNeasy Mini Kit (QIAGEN) or TRIzol reagent (Invitrogen), respectively. Reverse transcription was then performed using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems), and mRNA levels were quantified by fluorescence real-time PCR on a StepOnePlus (Applied Biosystems) using TaqMan Gene Expression Assays (Applied Biosystems) or PrimeTime qPCR Assays (Integrated DNA Technologies). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA (for IECs) or 18s rRNA (for adipocytes) levels were used as an internal control to normalize the mRNA levels of each gene.
2.10. Enzyme-linked Immunosorbent Assay (ELISA)
Adiponectin protein levels were determined using a commercial ELISA kit (R&D Systems). The assay was performed according to the manufacturer's instructions. The values were normalized to the corresponding culture supernatant before being subjected to co-culture with IECs.
2.11. Histology
Colonic tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin solution, followed by observation under a bright-field microscope using BZ-II Image Analysis Application (BZ-9000, Keyence). For staining with anti-perilipin1 antibody, sections blocked with 5% normal donkey serum (Jackson ImmunoResearch) for 60 min were incubated with anti-perilipin1 (1:100) antibody at 4 °C overnight. The slides were rinsed three times with 0.05% Tween-20 in PBS (PBS-T), and incubated with Cy3-conjugated donkey anti-guinea pig IgG (1:100) for 3 h at 4 °C. They were then washed three times in PBS-T and counterstained with DAPI (1:1000, Cell Biolabs) for 10 min at room temperature. Fluorescence signals were visualized using a Leica TCS SP2 confocal laser-scanning microscope.
2.12. Immunofluorescence Staining
2.12.1. Frozen Sections
The Transwell membrane was washed with PBS, fixed with 10% formalin for 10 min at room temperature, washed three times in 70% ethanol, and then cut out using a surgical blade. The membrane was embedded in Tissue-Tek Optimal Cutting Temperature (OCT) compound (Sakura Finetechnical) and frozen with liquid nitrogen. Following blocking with 5% normal donkey serum for 60 min, 8-μm cryostat sections were incubated with mouse anti-villin1 (1:50) and rabbit anti-E-cadherin (1:200) antibodies at 4 °C overnight. The slides were rinsed three times with PBS-T, and incubated with Cy3-conjugated donkey anti-mouse IgG (1:100) and Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:100) for 3 h at 4 °C. They were then washed three times in PBS-T and counterstained with DAPI (1:1000) for 10 min at room temperature. Fluorescence signals were visualized using a Leica TCS SP2 confocal laser-scanning microscope.
2.12.2. Whole-mounts
The monolayer IECs with Transwell membrane were fixed and permeabilized using the Cytofix/Cytoperm Kit (BD Biosciences). The specimens were then incubated with primary antibodies (rabbit anti-lysozyme (1:400), mouse anti-Muc2 (1:250), or rabbit anti-ChgA (1:100) antibodies) at 4 °C overnight. After being washed three times with Perm/Wash Buffer, the specimens were further incubated with Cy3-conjugated donkey anti-mouse IgG (1:100), Cy3-conjugated donkey anti-rabbit IgG (1:100), or Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:100) for 3 h at 4 °C, followed by counterstaining with DAPI for 10 min at room temperature. Fluorescence signals were visualized using a Leica TCS SP2 confocal laser-scanning microscope.
2.13. Oil Red O Staining
After fixing the cells with 4% paraformaldehyde in PBS for 1 h at room temperature, they were stained with Oil Red O solution (0.5% Oil red O in 2-propanol: water = 3:2 [v/v]) for 1 h at room temperature.
2.14. Statistical Analysis
All results are presented as mean ± standard error of the mean (SEM). Data from in vitro studies are representative from at least two independent experiments performed in triplicate. Data were analyzed using Student's t-test for two groups, and a one-way analysis of variance (ANOVA) for more than three groups followed by the Bonferroni procedure. Differences were considered significant at P < 0.05. These experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
3. Results
3.1. Mesenteric Fat Accumulation and Inflammation by DSS-induced Chronic Intestinal Inflammation
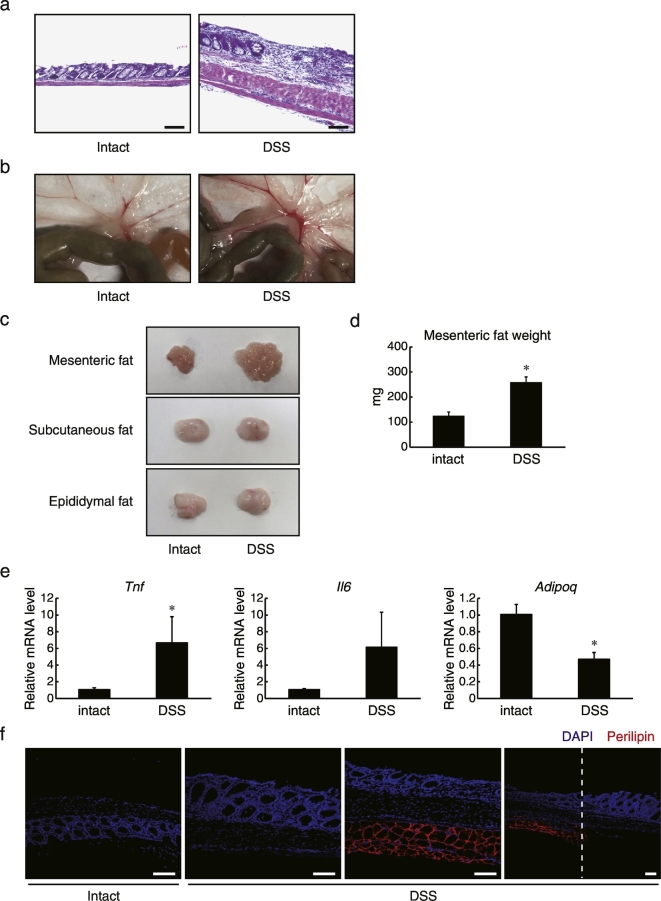
As mesenteric fat is enlarged and inflamed in CD patients (Peyrin-Biroulet et al., 2007), we investigated whether such a phenotype is also observed in an experimental animal model. We induced chronic inflammation in C57BL/6 mice by administering three cycles of 2.25% DSS via the drinking water, each cycle lasting 5 days at 2-week intervals. Irregular thickening of the muscle layer, damaged intestinal epithelia, and infiltration of inflammatory cells indicated that DSS-induced chronic inflammation proceeded successfully (Fig. 1A). At the same time, we observed increased mesenteric WAT accumulation as well as tissue vascularization in the weeks following DSS administration (Fig. 1B). It was confirmed that carefully isolated mesenteric fat tissues, but not subcutaneous and epididymal fat tissues, were enlarged by DSS administration (Fig. 1C). The weight of isolated mesenteric fat tissues increased by about twofold compared with that from the control group (Fig. 1D). Furthermore, gene expression analysis revealed that mRNA levels of the pro-inflammatory adipokines TNF and IL-6 were increased in mesenteric WAT from the DSS-induced colitis group, whereas anti-inflammatory adiponectin expression levels were decreased (Fig. 1E). A similar pathological phenotype was also reported in a 2,4,6-trinitrobenzenesulfonic acid-induced mouse colitis model (Karagiannides et al., 2006). Interestingly, severe epithelium damage was frequently observed in the area adjacent to the mesenteric fat tissues (Fig. 1F), which was identified by immunostaining against perilipin1, a protein localized to adipocyte lipid droplets (Takahashi et al., 2016). Taken together, these results indicate that local, chronic epithelial inflammation accompanies surrounding mesenteric fat accumulation and enhances the expression of inflammatory mediators by the tissue in mice, consistent with the histopathology of CD.
Fig. 1.
Induction of inflammatory responses in mesenteric fat tissues by chronic DSS treatment. C57BL/6 mice were fed 3 cycles of 2.25% DSS in their drinking water (5 days per cycle at 2 week intervals). Mice were then sacrificed from day 65 to 75. (a) Representative sections of colon from control and DSS-administered mice stained with hematoxylin and eosin. Scale bars, 100 μm. (b) Representative images of mesenteric fat beneath intestinal tissues from both treatment groups. (c) Representative images showing isolated mesenteric fat tissues, subcutaneous fat tissues, and epididymal fat tissues from both treatment groups. (d) Weight of mesenteric fat tissues from each group of mice (n = 10). Data are presented as mean ± SEM, *P < 0.05 (Student's t-test). (e) The relative mRNA levels of the indicated genes in mesenteric fat tissues were determined by quantitative RT-PCR and normalized to 18 s rRNA levels (n = 8). Data are presented as mean ± SEM, *P < 0.05 (Student's t-test). (f) Colon tissues from control and DSS-administered C57BL/6J mice were then isolated, fixed with 4% paraformaldehyde, and embedded in paraffin. 5 μm paraffin sections were immunostained with anti-perilipin1 antibody (red), followed by counterstaining with DAPI (blue). White dotted lines indicate borders between the presence and absence of mesenteric fat tissues. Scale bar, 100 μm.
3.2. Establishment of Monolayer IECs From Mouse and Human Intestinal Organoids
Sato and colleagues established a culture method for mouse primary intestinal organoids (Sato et al., 2009). Subsequently, development of intestinal organoids from human iPSCs as well as human tissues was reported (Sato et al., 2011, Spence et al., 2011). However, these organoids are not suitable for co-culture experiments because they require 3D culture, such as in solidified Matrigel. Therefore, we initially developed original IECs from mouse small intestinal organoids in two-dimensional (2D) culture on Transwell prior to co-culture with adipocytes to examine IEC–adipocyte interactions.
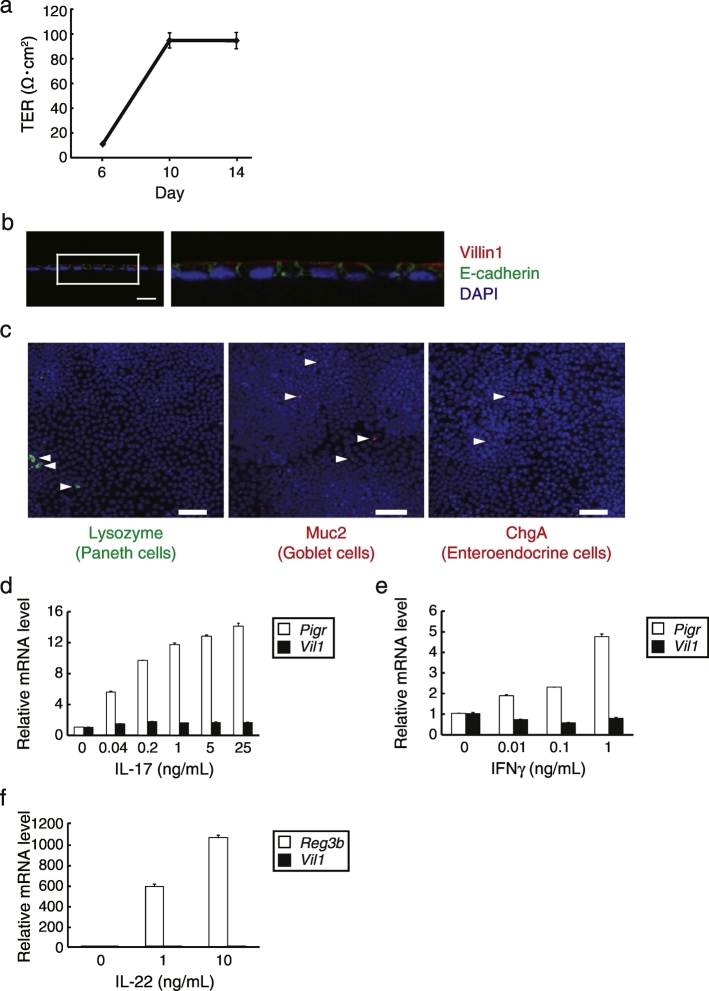
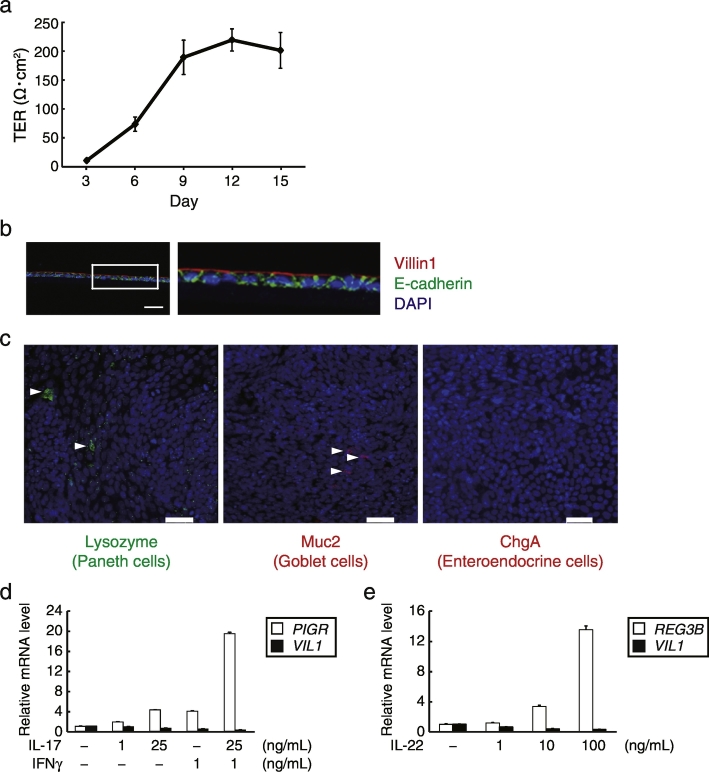
After disrupting mouse organoids by a 26G needle, the cells were seeded on a collagen I-coated Transwell. The transepithelial electrical resistance (TER) of the cells increased after 10 days of culture (Fig. 2A), suggesting the construction of tight junctions, a characteristic structure between epithelial cells (Grasset et al., 1984). Immunofluorescence staining of the frozen sections revealed that the cells formed monolayers with polarity, as evidenced by co-staining of nuclei (4′,6-diamidino-2-phenylindole [DAPI]), the apical brush border protein villin1, and the adhesion junction protein E-cadherin, indicating the distinctive features of IECs (Fig. 2B). Whole-mount staining showed that Paneth, goblet, and enteroendocrine cells were sparsely present in the monolayers (Fig. 2C). To determine the functional responses of monolayer cells in Transwell, we examined the effect of the cytokine IL-17 on the expression of polymeric immunoglobulin receptor (pIgR), which is involved in the formation and transport of IgA to the intestinal lumen (Cao et al., 2012). The mRNA levels of pIgR increased with stimulation by IL-17 in a dose-dependent manner, whereas villin1 mRNA levels were hardly affected (Fig. 2D). Interferon (IFN)γ also dose-dependently increased pIgR mRNA levels (Fig. 2E). We also treated mouse monolayer IECs with IL-22, which is another epithelial cell stimulation cytokine that is known to induce Reg3 family antimicrobial protein expression in IECs (Zheng et al., 2008), and showed that IL-22 dramatically increased the mRNA levels of Reg3β (Fig. 2F). Based on these results, we conclude that we successfully developed monolayer mouse IECs that are responsive to physiological stimuli. We also attempted to develop monolayer IECs from human iPSC (TkDN4-M)-derived organoids using our system. Notably, the establishment of human IECs required a finer 29G needle to eliminate remaining larger organoids, which can cause multilayer formation (Supplementary Fig. 1). Consequently, the proliferative cells succeeded to form monolayers which are polarized and responsive to physiological stimuli as well as mouse IECs (Figs. 3A–E).
Fig. 2.
Generation and characterization of mouse monolayer IECs from primary small intestinal organoids. (a) Mouse organoid-derived cells were broken by a 26G needle and seeded in type I collagen-coated Transwells. After they were cultured with mouse organoid culture medium supplemented with 300 ng/mL recombinant mWnt3a, TER values were measured on the indicated days in quadruplicate. Data are presented as mean ± SEM of one experiment representative of three independent experiments. (b) Immunohistochemistry analysis of the monolayer harvested with the Transwell membrane at 12 days of culture. Sections were stained with anti-E-cadherin (green) and anti-villin1 (red) antibodies, and counterstained with DAPI (blue). The right image is a magnification of the boxed region. Data are representative of three independent experiments. Scale bar, 20 μm. (c) Whole-mount images of the cells visualized by confocal laser microscopy after fixing and staining with antibodies toward the indicated molecules. Arrowheads indicate each positive cell. Data are representative of two independent experiments. Scale bars, 50 μm. (d–f) Mouse monolayer IECs that had been cultured for 13 days in Transwells and then replaced by pre-stimulation medium to starve cytokines for 1 day, followed by the stimulation with various concentrations of the indicated cytokines for 2 days. The relative mRNA level was determined by quantitative RT-PCR and normalized to GAPDH mRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM of one experiment representative of at least two independent experiments.
Fig. 3.
Generation and characterization of monolayer IECs from human iPSC-derived intestinal organoids. (a) TER values of the human organoid-derived cells that were broken by a 29G needle, seeded in collagen-coated Transwells with human organoid culture medium, were measured on the indicated days. The assay was performed in triplicate. Data are presented as mean ± SEM of one representative of three independent experiments. (b) Immunohistochemistry analysis of frozen sections of human organoid-derived cells described in (a) stained with anti-E-cadherin (green) and anti-villin1 (red) antibodies, and counterstained with DAPI (blue). The right image is a magnification of the boxed area. Data are representative of three independent experiments. A scale bar, 20 μm. (c) Whole-mount images of the human organoid-derived cells described in (a) stained with DAPI (blue), together with each antibody toward the indicated molecules. Arrowheads indicate each positive cell. Scale bars, 50 μm. (d–f) Human organoid-derived cells were cultured with human organoid culture medium for 6–8 days and then exposed to pre-stimulation medium for 1 day, followed by medium containing the indicated concentrations of recombinant cytokines for 2 days. Relative pIgR, Reg3β, and villin1 mRNA levels were determined by quantitative RT-PCR and normalized to GAPDH mRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM of one experiment representative of at least two independent experiments.
3.3. Induction of Inflammatory Responses in IECs Co-cultured With Differentiated Adipocytes
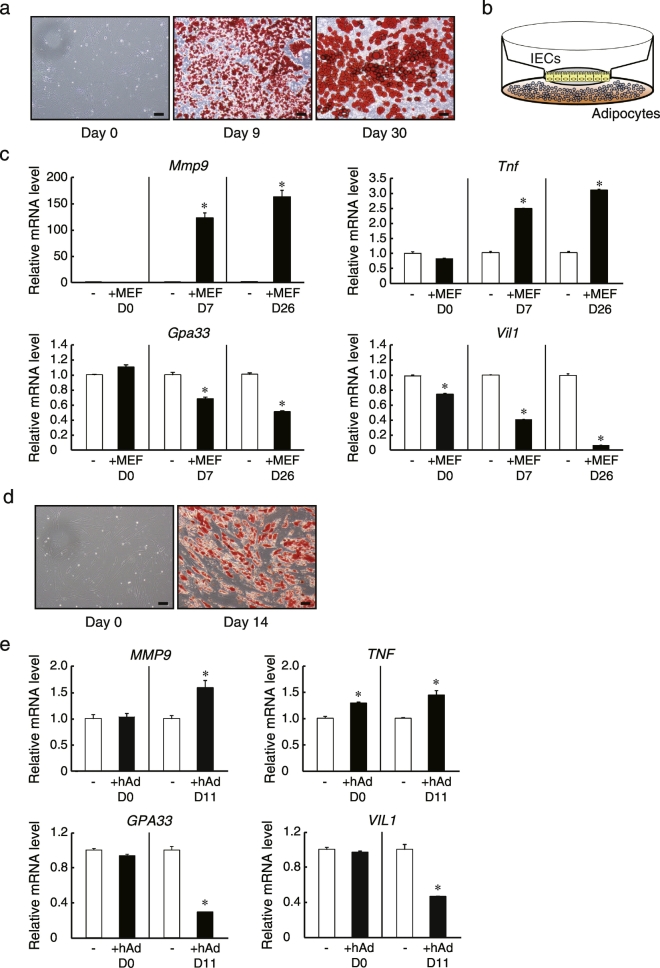
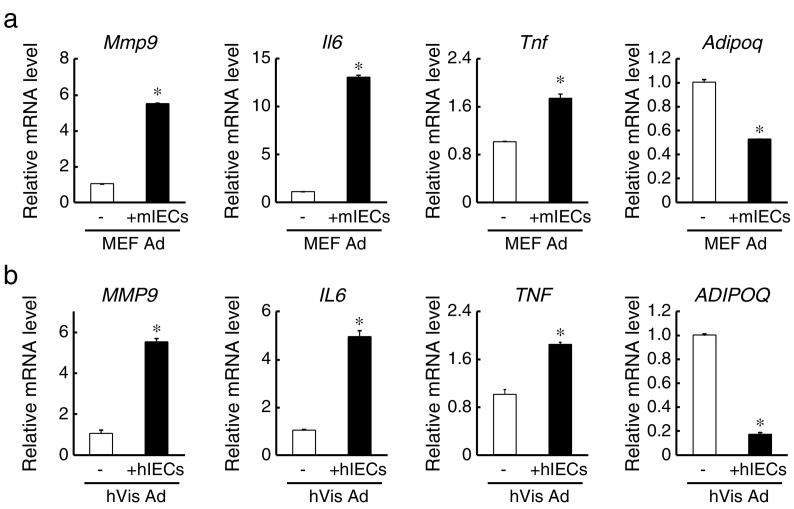
We next investigated the direct interaction between IECs and adipocytes using our Transwell system. Mouse embryonic fibroblasts (MEFs) were differentiated into adipocytes to co-culture with mouse IECs derived from primary small intestinal organoids. Oil red O staining showed that intracellular neutral lipids appeared efficiently in response to differentiation stimuli and that the lipid droplets became hypertrophic after additional days in culture (Fig. 4A). Subsequently, monolayer mouse IECs were co-cultured for 2 days in Transwell inserts above differentiated adipocytes attached to the outer wells so that cells could interact only via soluble factors (Fig. 4B). We then explored the expression of matrix metalloproteinase (MMP)-9 in the monolayer IECs following co-culture with adipocytes, as MMP-9 is induced in epithelial cells in response to inflammatory signals both in vivo and in vitro (Castaneda et al., 2005, Gan et al., 2001). We found that MMP-9 mRNA levels in mouse IECs were markedly increased by co-culture with MEF-derived mature adipocytes, and increased to a greater degree by co-culture with hypertrophied adipocytes, whereas they were relatively unchanged by co-culture with undifferentiated MEFs (Fig. 4C). These results indicate that the effects were mediated primarily by factors secreted from differentiated adipocytes, and that this signaling was stronger from adipocytes in a hypertrophied state. The expression of another inflammatory molecule, TNF was also increased in IECs by co-culture with differentiated adipocytes. In contrast, the expression levels of Villin1 and glycoprotein 33 (GPA33), both of which are known as intestinal epithelial markers, were reduced in IECs by co-culture with differentiated adipocytes and decreased to a greater extent by adipocytes possessing enlarged lipid droplets (Fig. 4C). Considering that decreased expression levels of Villin1 and GPA33 are observed in enterocytes from CD patients (Kersting et al., 2004, Williams et al., 2015) and these genes are shown to play anti-inflammatory roles through knockout studies (Wang et al., 2008, Williams et al., 2015), the results also indicate the occurrence of inflammation in IECs. Although it was unclear whether the decreased expression of these enterocyte markers was a cause or a result of inflammatory responses, similar inflammatory reactions were also observed when human IECs from iPSC-derived organoids were co-cultured with human primary adipocytes (Fig. 4D and E).
Fig. 4.
Inflammatory induction in monolayer IECs by co-culturing with differentiated adipocytes. (a) Images of differentiated mouse embryonic fibroblasts (MEFs; days 0, 9, and 30) stained with Oil Red O. (b) Schematic of co-culture system for IECs and adipocytes. (c) Monolayer IECs derived from mouse small intestinal organoids were cultured for 13 days in Transwells, and then co-cultured in pre-stimulation medium for 2 days with MEFs (days 0, 7, and 26). IECs were then harvested, and the relative mRNA levels were determined by quantitative RT-PCR and normalized to GAPDH mRNA levels. (d) Images of differentiated human primary visceral adipocytes (days 0 and 14) stained with Oil Red O. (e) Monolayer IECs derived from human iPSC-derived organoids were cultured for 13 days in Transwells, and then co-cultured in pre-stimulation medium for 2 days with human primary adipocytes (days 0 and 11). IECs were then harvested, and the relative mRNA levels were determined by quantitative RT-PCR and normalized to GAPDH mRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM, *P < 0.05 (Student's t-test). All data are representative of at least two independent experiments.
3.4. Induction of Inflammatory Responses in Mature Adipocytes Co-cultured With IECs
It has also been reported that MMP-9 expression is induced in adipocytes during fat mass development or adipocyte differentiation and plays a pivotal role in angiogenesis (Bouloumie et al., 2001). Consistent with this notion, we found that the expression of MMP-9 was upregulated in adipocytes co-cultured with mouse IECs (Fig. 5A). Furthermore, expression levels of the pro-inflammatory cytokines IL-6 and TNF were also increased in adipocytes by co-culture, whereas the expression of anti-inflammatory adiponectin was decreased, suggesting that pro-inflammatory signals were activated in adipocytes as well as IECs. Adiponectin protein levels were also decreased by co-culture with IECs (Supplementary Fig. 2). The similar inflammatory change in gene expression was also observed in human primary adipocytes following co-culture with human IECs (Fig. 5B). These results suggest that inflamed IECs can inflict inflammatory responses on adipocytes. Therefore, IECs and mature adipocytes may engage in reciprocal inflammatory signaling.
Fig. 5.
Inflammatory induction in adipocytes by co-culturing with monolayer IECs. (a) After co-cultured in pre-stimulation medium for 2 days with mouse IECs, MEF adipocytes (Day 9; MEF Ad) were harvested, and the relative mRNA levels were determined by quantitative RT-PCR and normalized to 18 s rRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM, *P < 0.05 (Student's t-test). (b) After co-cultured in pre-stimulation medium for 2 days with human IECs, human primary visceral adipocytes (Day 13; hVis Ad) were harvested, and the relative mRNA levels were determined by quantitative RT-PCR and normalized to 18 s rRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM, *P < 0.05 (Student's t-test). All data are representative of two independent experiments.
3.5. Initiation of intestinal Epithelial Inflammation by Adipocytes Through NF-κB and STAT3 Pathways
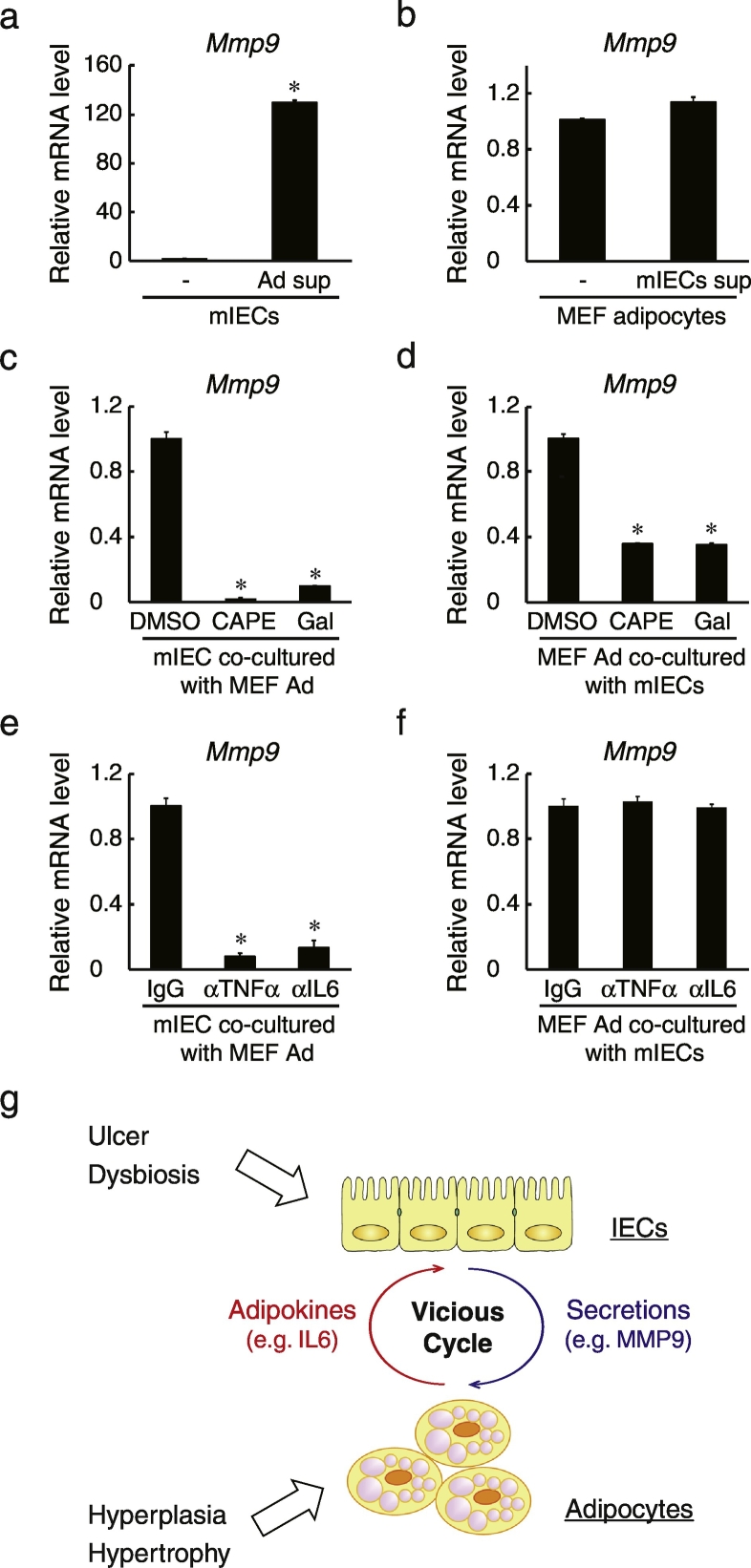
We next explored the molecular mechanisms of this putative inflammatory signaling between IECs and adipocytes by measuring mRNA levels of MMP-9 in mouse IECs and MEF-derived adipocytes cultured in conditioned media (culture supernatant) from the other cell type. MMP-9 expression was induced in IECs cultured in adipocyte conditioned media (Fig. 6A), whereas IEC conditioned media failed to increase the expression of MMP-9 in adipocytes (Fig. 6B). These results indicated that secretions from adipocytes triggered inflammatory reactions. It has been reported that adipocyte secretions such as FFAs and IL-6 can activate nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) (Hodge et al., 2005, Suganami et al., 2007). Therefore, we examined the effects of the NF-κB inhibitor caffeic acid phenethyl ester (CAPE) and the STAT3 inhibitor galiellalactone on the inflammatory responses in IEC–adipocyte co-culture. Expression of MMP-9 was significantly reduced by these inhibitors in both IECs (Fig. 6C) and adipocytes (Fig. 6D), indicating that downstream inflammatory signaling is mediated by these molecules in both cells. In contrast, when neutralizing antibodies against TNF and IL-6 were included in co-culture, MMP-9 expression was diminished in IECs (Fig. 6E) but not in adipocytes (Fig. 6F), suggesting that the inflammatory condition including the induction of MMP-9 is mediated by TNF and IL-6 in IECs but not in adipocytes.
Fig. 6.
Involvement of NF-κB and STAT3 signaling pathways in IEC–adipocyte inflammatory interactions. (a) Mouse IECs were cultured for 2 days in medium from MEF-derived adipocytes (Ad sup) or normal medium (–). Relative MMP-9 mRNA levels were determined by quantitative RT-PCR normalized to GAPDH mRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM, *P < 0.05 (Student's t-test). “Ad” denotes adipocytes. (b) MEF adipocytes (Day 11) were cultured for 2 days in medium from mouse IECs (mIEC sup) or normal medium (–). Relative MMP-9 mRNA levels were determined by quantitative RT-PCR normalized to 18 s rRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM. (c, d) Mouse IECs (c) and MEF adipocytes (d) were co-cultured with or without 10 μM CAPE (NF-κB inhibitor) or 10 μM Gal (STAT3 inhibitor) for 2 days. Each culture was then harvested and relative MMP-9 mRNA levels were determined by quantitative RT-PCR normalized to GAPDH mRNA (c) or 18 s rRNA (d) levels. The assay was performed in triplicate. Data are presented as mean ± SEM, *P < 0.05 (one-way ANOVA). (e, f) Mouse IECs (e) and MEF adipocytes (f) were co-cultured with 1 μg/mL IgG, anti-mouse TNF, or anti-IL-6 antibodies for 2 days. Each culture was then harvested and relative MMP-9 mRNA levels were determined by quantitative RT-PCR normalized to GAPDH mRNA (e) or 18 s rRNA (f) levels. The assay was performed in triplicate. Data are presented as mean ± SEM, *P < 0.05 (one-way ANOVA). (g) Schematic of the putative paracrine loop between IECs and adipocytes inducing a vicious inflammatory cycle. *P < 0.05. All data are representative of two independent experiments.
Taken together, we conclude that inflammatory responses by IECs and adipocytes may be triggered by a feed-forward loop of reciprocal cytokine signaling, even in the absence of immunocompetent cells (Fig. 6G).
4. Discussion
It has been widely known for decades that mesenteric mesenchymal abnormalities are the hallmarks of CD (Coffey et al., 2016). Although whether mesenteric fat augmentation (i.e., fat wrapping) is pathologic or not is debatable, it has been suggested that mesenteric fat might contribute to the progression of CD, especially from the evidence that mucosal ulceration accompanied by intestinal epithelium damage is confined under the surface of mesenteric fat tissues. Here, we demonstrated that chemically-induced chronic intestinal inflammation can induce hyperplasia and inflammation of mesenteric WATs, as found in human CD patients (Fig. 1). In addition, we established monolayer IECs from intestinal organoids (Fig. 2, Fig. 3) and revealed that IECs and mature adipocytes can induce mutual inflammatory responses through NF-κB and STAT3 signaling pathways (Fig. 4, Fig. 5, Fig. 6).
For the development of IECs, we simply seeded intestinal organoids disrupted by needles onto thin-layered type I collagen gel. We believe that the use of type I collagen is more physiological because this is a predominant ECM in the small intestine (Graham et al., 1988) and IECs are supported by type I collagen-producing intestinal mesenchymal cells in vivo (Speca et al., 2012). This method of IEC culture is convenient and reproducible as it requires neither feeder cells nor any specialized equipment. The resultant monolayer cells contained Paneth and goblet cells, which we also believe makes these cultures a more physiological model of the IEC layer in vitro than monolayers of established cell lines, such as Caco-2 and HT-29 cells. Moreover, TER values in our IECs were lower than in Caco-2 cell monolayers (Figs. 2C, 3D, and Supplementary Fig. 3), consistent with a previous study indicating that TER values are lower in small intestinal segments than in Caco-2 cells (Artursson et al., 1993). Notably, the monolayer IECs can self-propagate, which enhances the feasibility and versatility of our approach for a number of biological assays, as sufficient epithelial cell numbers can be obtained even if initial organoid numbers are limited. Taken together, we propose that our IEC culture system is a more robust physiological model for investigation of enteric cell–cell signaling and IBD pathogenesis.
Using our polarized IECs, we examined the potential interaction with differentiated adipocytes. We found that mature adipocytes in Transwell co-culture system elicited inflammatory responses in IECs (Fig. 4C and E), indicating that adipocyte-derived secretions affect the physiology and/or pathology of IECs. Undifferentiated adipocytes had a lesser inflammatory effect on IECs, indicating that adipocytes, but not preadipocytes, cause the induction of inflammatory genes, which is consistent with CD pathogenesis accompanied by mesenteric fat tissue augmentation and intestinal epithelium inflammation. In other words, the accumulation of mesenteric WAT in obese and CD patients could result in higher local cytokine concentrations, triggering inflammation in surrounding IECs. This notion is also supported by the evidence that expression levels of the pro-inflammatory cytokines TNF and IL-6, which are known to promote intestinal epithelium damage, are upregulated in mesenteric WAT from CD and obese patients (Bertin et al., 2010, Goncalves et al., 2015) as well as in that from DSS-administered mice (Fig. 1) and differentiated adipocytes co-cultured with IECs (Fig. 5). It should be noted that blockage of TNF as well as IL-6 signaling can be beneficial in both murine colitis models and human CD patients (Kaser et al., 2010).
MMP-9 is known as one of the inflammation markers in epithelial cells and knockout of this gene is reported to attenuate intestinal inflammation (Castaneda et al., 2005, Gan et al., 2001). We found that MMP-9 expression was increased in adipocytes as well as in IECs by co-culture with each other (Fig. 4, Fig. 5), and it is plausible that the induction of MMP-9 may contribute indirectly to intestinal inflammation by modulating the ECM (Parks et al., 2004). Although MMP-9 expression is induced during adipogenesis (Bouloumie et al., 2001), its induction in adipocytes by co-culture with IECs was more likely a pro-inflammatory response rather than part of the differentiation process because the expression of the anti-inflammatory factor adiponectin, which is also induced during adipogenesis (Takahashi et al., 2008), was not upregulated (Fig. 5). Moreover, considering that inflammatory reactions in adipocytes were also induced by inflamed epithelial cells, the activation of a mutual inflammatory program could be mediated by a positive feedback loop of cytokine signaling. This implies that selective disruption of this signaling loop would be effective for the treatment or prevention of CD. PPARγ agonists, therapeutic drugs for type II diabetes that target adipocytes (Chiarelli and Di Marzio, 2008), are possible candidates, and they are shown to be effective in some animal models of IBD (Annese et al., 2012).
Results suggested that secretions from mature adipocytes rather than IECs were the primary initiators of inflammation (Fig. 6A and B), consistent with evidence that mesenteric fat hyperplasia is frequently observed at the onset of CD (Desreumaux et al., 1999, Peyrin-Biroulet et al., 2007). On the other hand, it remains unclear why mesenteric WAT accumulates at or before the pathogenesis of CD, although some hypotheses have been proposed, such as changes in intestinal flora (Peyrin-Biroulet et al., 2007). In addition, from the results obtained by our co-culture experiments, we postulate that unknown molecules secreted from inflamed IECs also have such a biological impact on adipocyte properties. Researchers have reported that bone marrow-derived fibrocytes, whose quantity is increased in the mesentery in CD, can differentiate into adipocytes (Coffey et al., 2016); therefore, it may be possible that inflamed IECs can secrete factors which can stimulate fibrocytes to undergo adipogenesis. Hence, our co-culture systems would also be useful for identifying candidate factors secreted exclusively from IECs. Mesenteric fat increases are rarely observed in ulcerative colitis (UC) patients (Drouet et al., 2012), so revealing biopathological distinctions between CD and UC may also help elucidate the contributions of IEC- and adipocyte-derived signals to CD pathogenesis.
There is compelling evidence that high-fat diet-induced obesity contributes to the pathogenesis of bowel inflammation (Ding et al., 2010, Zhang et al., 2012), and we found that adipocytes with larger lipid droplets induced stronger inflammatory responses in IECs (Fig. 4C). Indeed, it has been reported that hypertrophied adipocytes differentiated from 3T3-L1 cells exhibit a more inflamed state with higher pro-inflammatory gene expression levels (Suganami et al., 2005). We also confirmed that TNF expression was induced, whereas adiponectin expression was diminished in MEF adipocytes with larger lipid droplets (Supplementary Fig. 4). Therefore, the extent of inflammatory progression in adipocytes can have a huge impact on the extent of inflammatory responses in IECs, and our co-culture system may be useful in investigating their relationships. Considering that macrophage infiltration is observed in mesenteric WAT from CD and obese patients (Bertin et al., 2010, Drouet et al., 2012), it would also be of interest to examine inflammatory signaling events and other signaling interactions among IECs, adipocytes, and macrophages in co-culture.
In summary, we developed an original method to generate organoid-derived murine and human monolayer IECs possessing many of the intrinsic characteristics of intestinal epithelium. Specifically, human IECs generated from iPSC-derived organoids would have multiple advantages for basic research on intestinal function, pathogenesis, drug screening, mucosal vaccine development, regenerative therapy, and personalized medicine. We observed mutually enhanced inflammatory responses when these IECs were co-cultured with adipocytes, suggesting a possible paracrine loop for the development of inflammation without the influence of immune cells. In aggregate, these results suggest that increased mesenteric fat tissues, which are frequently found in CD and obese patients as well as animal models of these conditions, may directly induce or exacerbate intestinal ulcerations. Our in vitro studies assessing isolated local tissue interactions may yield innovative concepts regarding gut homeostasis, disease pathogenesis, and treatment strategies.
Funding Sources
The project is supported by a joint grant from Japan Tobacco Inc.; grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in Aid for Young Scientists B [25860353 to S.S.], and for Scientific Research S [23229004 to H.K.]); from Agency for Medical Research and Development (AMED) (Practical Research Project for Allergic Diseases and Immunology [17ek0410032h0002 to H.K.]). All funds were used for data collection and for preparation of the manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions
Y. T., S.S., and Y. K. performed the experiments. Y. T., S. S., T. Y., M. H., and H. K. conceived the theme. C. L. and M. O. managed and provided human iPS cells. Y. T., S. S., and H. K. designed and directed the study, and wrote the paper. All authors approved the final version of the manuscript.
Acknowledgments
Acknowledgement
We thank Dr. Atsushi Miyawaki (Brain Science Institute, RIKEN, Japan) for providing us Venus containing plasmids, Dr. Haruna Takagi, Dr. Seiichi Matsumura, and Dr. Ayae Nishiyama for technical supports, and Prof. Ryuichiro Sato and Dr. Tatsuhiko Azegami for helpful discussions.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.07.027.
Contributor Information
Yu Takahashi, Email: yu.takahashi@jt.com.
Shintaro Sato, Email: shinta@biken.osaka-u.ac.jp.
Hiroshi Kiyono, Email: kiyono@ims.u-tokyo.ac.jp.
Appendix A. Supplementary data
Supplementary material
References
- Annese V., Rogai F., Settesoldi A., Bagnoli S. PPARgamma in inflammatory bowel disease. PPAR Res. 2012;2012:620839. doi: 10.1155/2012/620839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artursson P., Ungell A.L., Lofroth J.E. Selective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Pharm. Res. 1993;10:1123–1129. doi: 10.1023/a:1018903931777. [DOI] [PubMed] [Google Scholar]
- Bertin B., Desreumaux P., Dubuquoy L. Obesity, visceral fat and Crohn's disease. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:574–580. doi: 10.1097/MCO.0b013e32833cf0f4. [DOI] [PubMed] [Google Scholar]
- Bouloumie A., Sengenes C., Portolan G., Galitzky J., Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50:2080–2086. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- Boutros M., Maron D. Inflammatory bowel disease in the obese patient. Clin. Colon Rectal Surg. 2011;24:244–252. doi: 10.1055/s-0031-1295687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao A.T., Yao S., Gong B., Elson C.O., Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda F.E., Walia B., Vijay-Kumar M., Patel N.R., Roser S., Kolachala V.L., Rojas M., Wang L., Oprea G., Garg P., Gewirtz A.T., Roman J., Merlin D., Sitaraman S.V. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Chiarelli F., Di Marzio D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: current evidence and future perspectives. Vasc. Health Risk Manag. 2008;4:297–304. doi: 10.2147/vhrm.s993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J.C., O'Leary D.P., Kiernan M.G., Faul P. The mesentery in Crohn's disease: friend or foe? Curr. Opin. Gastroenterol. 2016;32:267–273. doi: 10.1097/MOG.0000000000000280. [DOI] [PubMed] [Google Scholar]
- Desreumaux P., Ernst O., Geboes K., Gambiez L., Berrebi D., Muller-Alouf H., Hafraoui S., Emilie D., Ectors N., Peuchmaur M., Cortot A., Capron M., Auwerx J., Colombel J.F. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- Ding S., Lund P.K. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:328–333. doi: 10.1097/MCO.0b013e3283478727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Chi M.M., Scull B.P., Rigby R., Schwerbrock N.M., Magness S., Jobin C., Lund P.K. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet M., Dubuquoy L., Desreumaux P., Bertin B. Visceral fat and gut inflammation. Nutrition. 2012;28:113–117. doi: 10.1016/j.nut.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Dunmore S.J., Brown J.E. The role of adipokines in beta-cell failure of type 2 diabetes. J. Endocrinol. 2013;216:T37–45. doi: 10.1530/JOE-12-0278. [DOI] [PubMed] [Google Scholar]
- Fink C., Karagiannides I., Bakirtzi K., Pothoulakis C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2012;18:1550–1557. doi: 10.1002/ibd.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeck G., Mendez-Gimenez L., Fernandez-Formoso J.A., Fernandez S., Rodriguez A. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 2014;27:63–93. doi: 10.1017/S095442241400002X. [DOI] [PubMed] [Google Scholar]
- Gan X., Wong B., Wright S.D., Cai T.Q. Production of matrix metalloproteinase-9 in CaCO-2 cells in response to inflammatory stimuli. J. Interf. Cytokine Res. 2001;21:93–98. doi: 10.1089/107999001750069953. [DOI] [PubMed] [Google Scholar]
- Ghadimi B.M., Sackett D.L., Difilippantonio M.J., Schrock E., Neumann T., Jauho A., Auer G., Ried T. Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosom. Cancer. 2000;27:183–190. [PMC free article] [PubMed] [Google Scholar]
- Goncalves P., Magro F., Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm. Bowel Dis. 2015;21:453–467. doi: 10.1097/MIB.0000000000000209. [DOI] [PubMed] [Google Scholar]
- Graham M.F., Diegelmann R.F., Elson C.O., Lindblad W.J., Gotschalk N., Gay S., Gay R. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257–265. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- Grasset E., Pinto M., Dussaulx E., Zweibaum A., Desjeux J.F. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am. J. Phys. 1984;247:C260–267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- Gulhane M., Murray L., Lourie R., Tong H., Sheng Y.H., Wang R., Kang A., Schreiber V., Wong K.Y., Magor G., Denman S., Begun J., Florin T.H., Perkins A., Cuiv P.O., McGuckin M.A., Hasnain S.Z. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci Rep. 2016;6:28990. doi: 10.1038/srep28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge D.R., Hurt E.M., Farrar W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Hosomi S., Kaser A., Blumberg R.S. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr. Opin. Gastroenterol. 2015;31:81–88. doi: 10.1097/MOG.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannides I., Kokkotou E., Tansky M., Tchkonia T., Giorgadze N., O'Brien M., Leeman S.E., Kirkland J.L., Pothoulakis C. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5207–5212. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersting S., Bruewer M., Schuermann G., Klotz A., Utech M., Hansmerten M., Krieglstein C.F., Senninger N., Schulzke J.-D., Naim H.Y., Zimmer K.-P. Antigen transport and cytoskeletal characteristics of a distinct enterocyte population in inflammatory bowel diseases. Am. J. Pathol. 2004;165:425–437. doi: 10.1016/S0002-9440(10)63308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.A., Kim J.H., Wang Y., Sul H.S. Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol. Cell. Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca C., Olefsky J.M. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken K.W., Howell J.C., Wells J.M., Spence J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C., VanDussen K.L., Miyoshi H., Stappenbeck T.S. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J., Fierce Y., Treuting P.M., Brabb T., Maggio-Price L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a −/− male mice. J. Nutr. 2013;143:1240–1247. doi: 10.3945/jn.113.174615. [DOI] [PubMed] [Google Scholar]
- Parks W.C., Wilson C.L., Lopez-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Chamaillard M., Gonzalez F., Beclin E., Decourcelle C., Antunes L., Gay J., Neut C., Colombel J.F., Desreumaux P. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E.D., MacDougald O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., Spiegelman B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986;68:1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Speca S., Giusti I., Rieder F., Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M., Shroyer N.F., Wells J.M. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed H., Walsh S., Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes. Facts. 2009;2:370–372. doi: 10.1159/000262276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T., Nishida J., Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- Suganami T., Tanimoto-Koyama K., Nishida J., Itoh M., Yuan X., Mizuarai S., Kotani H., Yamaoka S., Miyake K., Aoe S., Kamei Y., Ogawa Y. Role of the toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Ohoka N., Hayashi H., Sato R. TRB3 suppresses adipocyte differentiation by negatively regulating PPARgamma transcriptional activity. J. Lipid Res. 2008;49:880–892. doi: 10.1194/jlr.M700545-JLR200. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Shinoda A., Kamada H., Shimizu M., Inoue J., Sato R. Perilipin2 plays a positive role in adipocytes during lipolysis by escaping proteasomal degradation. Sci Rep. 2016;6:20975. doi: 10.1038/srep20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama N., Nishimura S., Nakamura S., Shimizu T., Ohnishi R., Endo H., Yamaguchi T., Otsu M., Nishimura K., Nakanishi M., Sawaguchi A., Nagai R., Takahashi K., Yamanaka S., Nakauchi H., Eto K. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J. Exp. Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L.G., Leonel A.J., Aguilar E.C., Batista N.V., Alves A.C., Coimbra C.C., Ferreira A.V., de Faria A.M., Cara D.C., Alvarez Leite J.I. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011;10:204. doi: 10.1186/1476-511X-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen K.L., Marinshaw J.M., Shaikh N., Miyoshi H., Moon C., Tarr P.I., Ciorba M.A., Stappenbeck T.S. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Srinivasan K., Siddiqui M.R., George S.P., Tomar A., Khurana S. A novel role for villin in intestinal epithelial cell survival and homeostasis. J. Biol. Chem. 2008;283:9454–9464. doi: 10.1074/jbc.M707962200. [DOI] [PubMed] [Google Scholar]
- Wang X., Yamamoto Y., Wilson L.H., Zhang T., Howitt B.E., Farrow M.A., Kern F., Ning G., Hong Y., Khor C.C., Chevalier B., Bertrand D., Wu L., Nagarajan N., Sylvester F.A., Hyams J.S., Devers T., Bronson R., Lacy D.B., Ho K.Y., Crum C.P., McKeon F., Xian W. Cloning and variation of ground state intestinal stem cells. Nature. 2015;522:173–178. doi: 10.1038/nature14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.B., Tebbutt N.C., Buchert M., Putoczki T.L., Doggett K., Bao S., Johnstone C.N., Masson F., Hollande F., Burgess A.W., Scott A.M., Ernst M., Heath J.K. Glycoprotein A33 deficiency: a new mouse model of impaired intestinal epithelial barrier function and inflammatory disease. Dis. Model. Mech. 2015;8:805–815. doi: 10.1242/dmm.019935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X., Ichinose S., Nagaishi T., Okamoto R., Tsuchiya K., Clevers H., Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5 + stem cell. Nat. Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- Zeissig S., Bojarski C., Buergel N., Mankertz J., Zeitz M., Fromm M., Schulzke J.D. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.R., Lam Y.Y., Ha C.W.Y., Campbell C.R., Mitchell A.J., Dinudom A., Oscarsson J., Cook D.I., Hunt N.H., Caterson I.D., Holmes A.J., Storlien L.H. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material