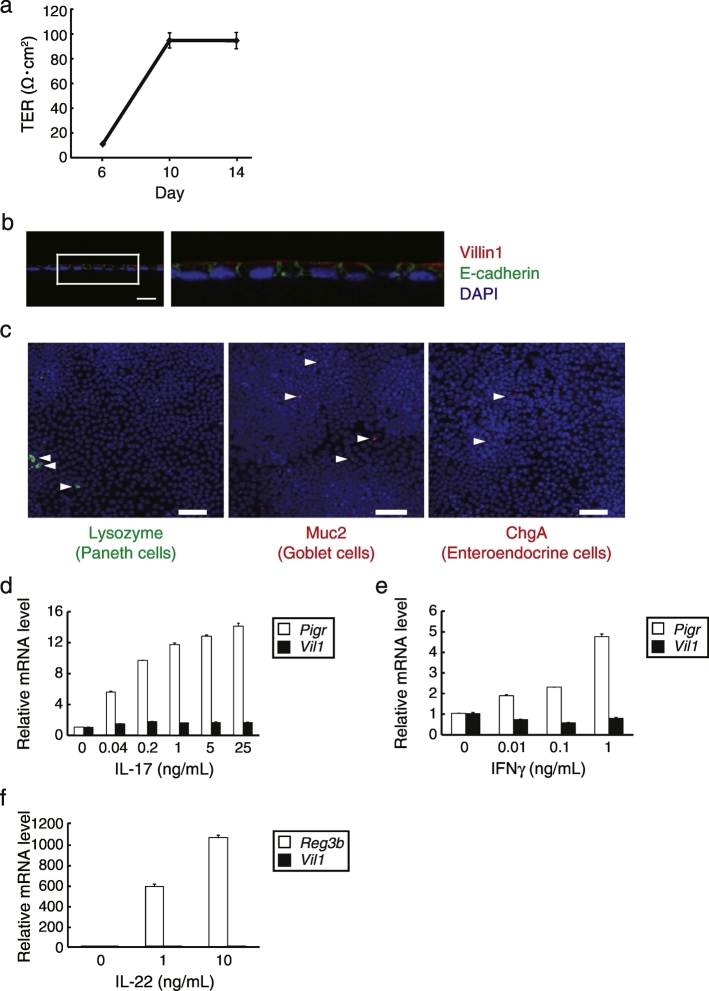
Fig. 2.
Generation and characterization of mouse monolayer IECs from primary small intestinal organoids. (a) Mouse organoid-derived cells were broken by a 26G needle and seeded in type I collagen-coated Transwells. After they were cultured with mouse organoid culture medium supplemented with 300 ng/mL recombinant mWnt3a, TER values were measured on the indicated days in quadruplicate. Data are presented as mean ± SEM of one experiment representative of three independent experiments. (b) Immunohistochemistry analysis of the monolayer harvested with the Transwell membrane at 12 days of culture. Sections were stained with anti-E-cadherin (green) and anti-villin1 (red) antibodies, and counterstained with DAPI (blue). The right image is a magnification of the boxed region. Data are representative of three independent experiments. Scale bar, 20 μm. (c) Whole-mount images of the cells visualized by confocal laser microscopy after fixing and staining with antibodies toward the indicated molecules. Arrowheads indicate each positive cell. Data are representative of two independent experiments. Scale bars, 50 μm. (d–f) Mouse monolayer IECs that had been cultured for 13 days in Transwells and then replaced by pre-stimulation medium to starve cytokines for 1 day, followed by the stimulation with various concentrations of the indicated cytokines for 2 days. The relative mRNA level was determined by quantitative RT-PCR and normalized to GAPDH mRNA levels. The assay was performed in triplicate. Data are presented as mean ± SEM of one experiment representative of at least two independent experiments.