Abstract
Purpose
Magnetic resonance image guided radiation therapy (MR-IGRT) has been used at our institution since 2014. We report on more than 2 years of clinical experience in treating patients with the world's first MR-IGRT system.
Methods and materials
A clinical service was opened for MR-IGRT in January 2014 with an MR-IGRT system consisting of a split 0.35T magnetic resonance scanner that straddles a ring gantry with 3 multileaf collimator-equipped 60Co heads. The service was expanded to include online adaptive radiation therapy (ART) MR-IGRT and cine gating after 6 and 9 months, respectively. Patients selected for MR-IGRT were enrolled in a prospective registry between January 2014 and June 2016. Patients were treated with a variety of radiation therapy techniques including intensity modulated radiation therapy and stereotactic body radiation therapy (SBRT). When applicable, online ART was performed and gating on sagittal 2-dimensional cine MR was used. The charts of patients treated with MR-IGRT were reviewed to report on the clinical and treatment characteristics of the initial patients who were treated with this novel technique.
Results
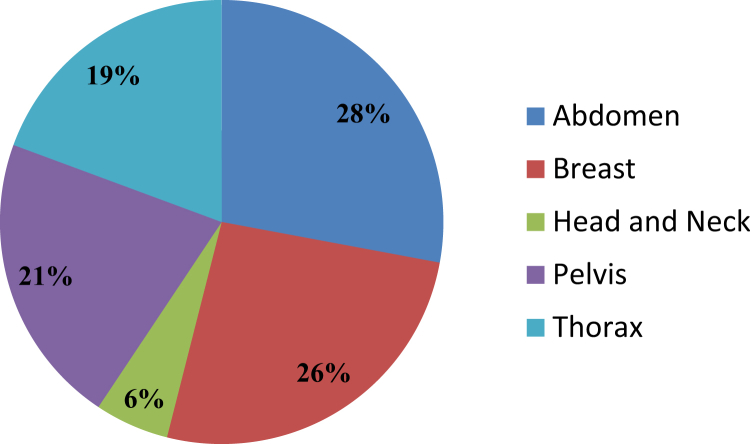
A total of 316 patients have been treated with the MR-IGRT system, which has been integrated into a high-volume clinic. The cases were most commonly selected for improved soft tissue visualization, ART, and cine gating. Seventy-six patients were treated with 3-dimensional conformal radiation therapy, 146 patients with intensity modulated radiation therapy, and 94 patients with SBRT. The most commonly treated disease sites were the abdomen (28%), breast (26%), pelvis (22%), thorax (19%), and head and neck (5%). Sixty-seven patients were treated with online ART over a total of 244 adapted fractions. Cine treatment gating was used for a total of 81 patients.
Conclusions
MR-IGRT has been successfully implemented in a high-volume radiation clinic and provides unique advantages in the treatment of a variety of malignancies. Additional clinical trials are in development to formally evaluate MR-IGRT in the treatment of multiple disease sites with techniques such as SBRT and ART.
Summary.
Magnetic resonance (MR) image guided radiation therapy (IGRT) has been successfully implemented in a high-volume radiation clinic and provides unique advantages in the treatment of patients with a variety of malignancies. We describe our initial clinical experience and integration of this new technology within a high-volume clinical practice, highlight the technical capabilities of the system, and describe the selection of patients who benefited most from MR-IGRT.
Introduction
Magnetic resonance (MR) image guided radiation therapy (IGRT) represents a treatment modality that offers potential solutions to the well-recognized challenges of radiation delivery. Compared with computed tomography (CT)-based strategies, MR imaging (MRI) for treatment guidance offers superior soft tissue definition that is potentially advantageous in numerous disease sites.1 From a patient-safety perspective, daily image guidance with MR also avoids undesirable radiation exposure inherent to the use of CT imaging guidance such as cone beam CT (CBCT). Moreover, cine MRI can be safely employed throughout a patient's entire treatment fraction and course to monitor and manage intrafraction motion. MR-IGRT enables daily imaging of sufficient quality to permit daily plan adjustments in response to interfraction changes in anatomy.2 This approach is valid even in disease sites that are typically poorly visualized with conventional x-ray imaging, such as soft tissues within the abdomen and pelvis.1 This daily plan adjustment, termed online adaptive radiation therapy (ART), has been found in dosimetric studies to potentially improve the therapeutic ratio of radiation therapy (RT) by enhanced sparing of organs-at-risk (OARs) and safe-dose escalation in disease sites where high-dose therapy has been limited.3, 4 Thus, MR-IGRT has the potential to improve the accuracy, precision, and safety of RT delivery.
Historically, MR-IGRT has been unavailable due to the challenges of protecting a radiation delivery device from the influence of a magnetic field and maintaining imaging quality in the presence of a treatment device. At our institution, the world's first commercially available device for MR-IGRT was clinically developed and implemented in routine clinical practice (MRIdian System; ViewRay Inc., Oakwood Village, OH). In the more than 2 years after the first patient treatment in January 2014, more than 300 patients have been treated in disease sites such as head and neck, breast, thorax, abdomen, and pelvis. In this study, we review our institutional experience with patients who were treated on the world's first MR-IGRT system. More specifically, we aim to describe our initial clinical experience and the integration of this new technology within a high-volume clinical practice, highlight the technical capabilities of the system, and describe the selection of patients who benefited most from MR-IGRT.
Methods and materials
Setting and patients
The radiation oncology department at our institution includes 21 attending radiation oncologists who service our main site and 5 additional satellite locations. The main facility includes 7 linear accelerators with a dedicated MR simulator, a Gamma Knife Perfexion (Elekta, Stockholm, Sweden), a single-gantry proton therapy system (Mevion, Littleton, MA), a cobalt 60-based MR-IGRT system (ViewRay Inc., Oakwood Village, OH), and a full brachytherapy suite. A linear accelerator–based MR-IGRT system is currently under construction. Our satellite facilities have an additional 7 linear accelerators. In 2015, approximately 3,400 patients were treated with external beam radiation therapy at our facility.5
All patients included in this study were enrolled in an institutional review board–approved prospective registry, and informed written consent for treatment was obtained. Patients were divided into groups on the basis of the anatomical site of the malignancy and the treatment technique (stereotactic body radiation therapy [SBRT], adaptive, gating). Additionally, the clinical rationale with regard to the selection of MR-IGRT compared with conventional linac-based treatment was evaluated (ie, improved soft tissue imaging, cine gating on the basis of daily anatomy, and online/offline adaptation) when available.
Magnetic resonance image guided radiation therapy system
A complete description of the system and treatment workflow has been previously described.6, 7 Briefly, the MR-IGRT system comprises open, split-solenoid low-field magnetic resonance imaging (MRI) and a 60Co radiation delivery device. The MRI has a field strength of 0.35T, and the RT delivery system includes a robotic 3-headed 60Co device with a nominal dose rate of 550 cGy/min from three 27.3 × 27.3 cm2 fields to the 105 cm isocenter.6
Simulation and planning
All patients underwent CT simulation, as well as MR simulation when applicable, per our institutional protocol and based on the site being treated. The CT planning simulation (Philips Brilliance Small Bore, Philips Medical System, Andover, MA) was planned to the emulate patient set-up that would occur during MR-IGRT. The MR simulation process included a high-resolution, volumetric MRI, followed by planar cine MR in the sagittal plane to evaluate target motion characteristics if necessary for planning purposes. The entire simulation procedure is approximately 1.5 hours in duration.
The MR-IGRT system has a dedicated treatment planning system (TPS). Although the TPS has image registration and contouring capabilities, following our standard clinic workflow CT and MR simulation images were transferred to a third-party software for contouring and then transferred to the MR-IGRT TPS for plan creation. The TPS is capable of conformal and intensity modulated radiation therapy (IMRT) planning and uses a fast Monte Carlo dose calculation algorithm.
On treatment days, patients were screened for MRI contraindication. Once in the treatment room, they were positioned and aligned to marks that were placed during CT simulation. Either an end-exhale breath hold or a free-breathing, volumetric MRI image then was acquired. When applicable, a clinical gross target volume (GTV) or GTV delineation was confirmed or manually recontoured at the MR-IGRT console to obtain a 2-dimensional MRI (cine) view of the target for gating on the treatment machine during MR simulation.
Online adaptive radiation therapy
Our institutional workflow has been described in a previous study for patients who were treated with online ART.2 Briefly, all patients underwent simulation with pretreatment planning as described previously. At the time of the first treatment fraction, all patients underwent a 17- or 25-second volumetric setup MRI at exhale breath hold with an in-plane resolution of 1.67 mm and a 3.0 mm slice thickness. The patient was localized to the target for maximal target overlap, and the electron density map and planning contours were transferred to the daily MR using deformable or rigid registration. The treating physician reviewed the daily MRI and the transferred contours and manually modified contours as necessary while the patient remained on the table. The initial plan was recalculated on the daily anatomy. Dose volume histograms were reviewed to evaluate dose to daily anatomy using an isotoxicity approach. If, by application of the initial plan to the anatomy of the day, OAR dose constraints were exceeded or target coverage could be improved, online plan adaptation was performed via reoptimization with original beam angles and optimization objectives. All adapted plans were subject to online quality assurance and verified by an independent Monte Carlo dose computation algorithm prior to treatment delivery while the patient remained on the table. The reoptimized plan was then delivered to the daily anatomy.
Treatment gating
Using the real-time planar cine MR images during treatment delivery, one sagittal plane image is acquired at 4 frames per second. A gating target is chosen (either tumor or OAR), and a gating boundary is selected at the discretion of the treating physician and defined on the volumetric MRI. Prior to the delivery of each fraction, a sagittal plane was chosen as the reference for gating. The system then deformed the selected gating target from this reference frame to each subsequent image acquired during treatment delivery and compared the resulting contour to the predefined gating boundary. If the target contour moved beyond the defined boundary during RT, the beam automatically turned off. The percentage of target outside of the defined boundary before the beam turns off can be specified by the physician to allow for minor errors in the deformed contour caused by noise in the MR images and uncertainties in deformable registration.
Statistical analysis
The data analysis was performed with Excel 2013 (Microsoft Corporation; Redmond, WA).
Results
Patient/treatment characteristics and selection for magnetic resonance image guided radiation therapy
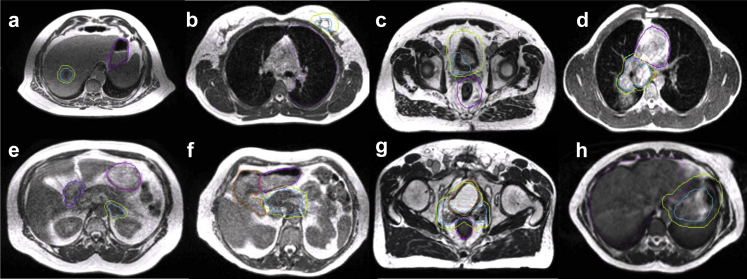
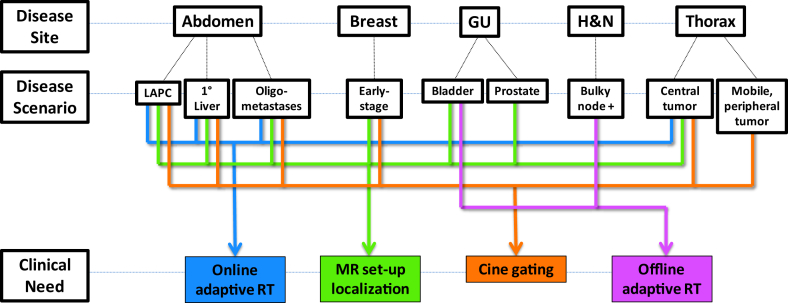
Between January 2014 and June 2016, a total of 316 patients were treated with the MR-IGRT system. Disease sites were divided anatomically as follows: abdomen, 88 patients (28%); breast, 82 patients (26%); head and neck, 17 patients (6%); pelvis, 68 patients (21%); and thorax, 61 patients (19%; Fig 1). Representative MR-IGRT plans for various disease sites are shown in Figure 2. Patients were selected for treatment with the MR-IGRT system as opposed to conventional linear accelerator–based treatment for a variety of indications, including improved soft tissue imaging (168 patients [53%]), cine gating (81 patients [26%]), online ART (67 patients [21%]), or, most commonly, a combination of these features. Figure 3 depicts the clinical workflow and decision process for a variety of disease sites and scenarios.
Figure 1.
Anatomical site of treatment with magnetic resonance image guided radiation therapy.
Figure 2.
Example anatomy for commonly treated disease sites, including gross tumor volumes and planning target volumes in blue and lime and relevant organs-at-risk in purple and orange. Sites include (a) intrahepatic cholangiocarcinoma, (b) early stage breast cancer, (c) bladder cancer, (d) central thorax malignancy, (e) adrenal metastases, (f) locally advanced pancreatic cancer, (g) postoperative prostate cancer, and (h) gastric mucosa-associated lymphoid tissue lymphoma.
Figure 3.
Workflow of common disease sites and clinical scenarios in which key features of magnetic resonance image guided radiation therapy are indicated. GU, genitourinary; H&N, head and neck; LAPC, locally advanced pancreatic cancer; liver, primary liver cancer.
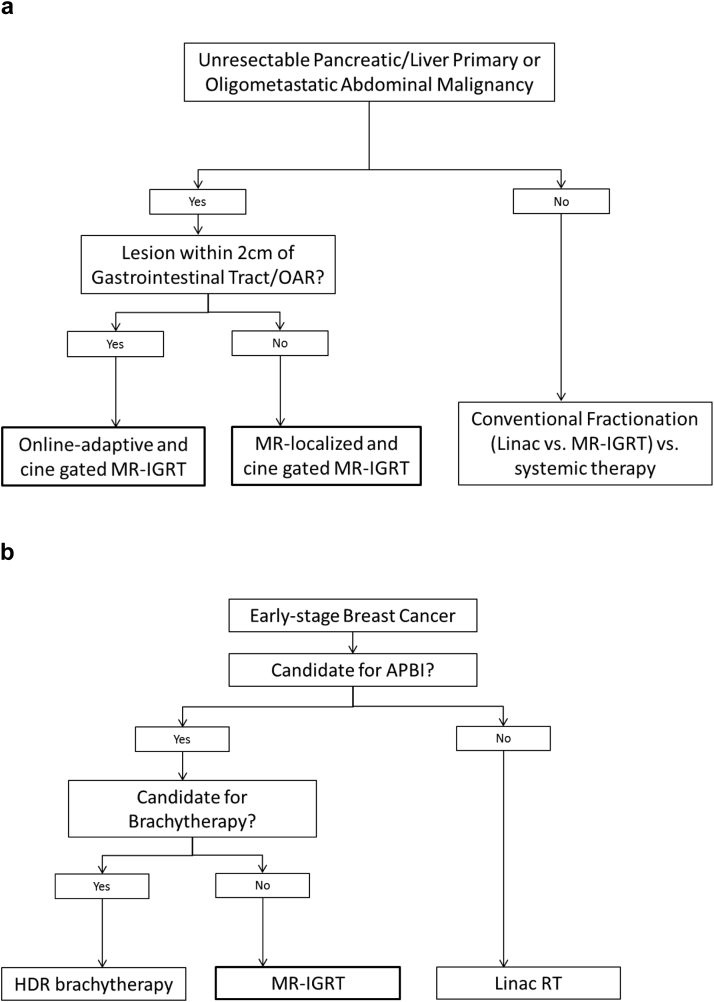
Representative clinical decision flowcharts for the treatment of oligometastatic disease and early stage breast cancer are shown in Figure 4. Seventy-six patients (24%) were treated with 3-dimensional conformal RT, 146 (46%) with IMRT, and 94 (30%) with SBRT. A total of 69 patients were treated in a prospective clinical trial. Nine patients were considered initially for MR-IGRT but were unable to tolerate the simulation due to claustrophobia and/or pain in the treatment position. Approximately 8 patients per day were treated on the machine (range, 0-15) with significant variation on the basis of the complexity of the therapy delivered. During these initial 2.5 years, the machine was offline for 39 business days (6%) for scheduled source change, software upgrades, and maintenance.
Figure 4.
Clinical decision flowchart for using magnetic resonance image guided radiation therapy for (a) unresectable pancreatic/liver primary or oligometastatic abdominal malignancy and (b) early stage breast cancer.
Online adaptive radiation therapy
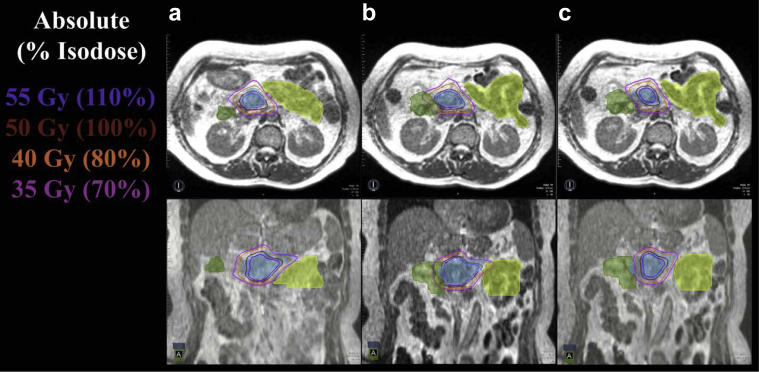
Online ART was implemented in September 2014. Sixty-seven patients (25.7% since the technology became available) have been treated with online ART. The adapted treatment course length ranged from 5 fractions to 28 fractions (median, 5 fractions). A total of 371 fractions were evaluated for daily online ART, and 244 of these of fractions (66%) were delivered with an adapted plan. Among the 67 patients who were treated, 26 were treated in a prospective trial for online-adaptive SBRT (Trial NCT02264886) and 12 in a dose escalation trial for inoperable pancreatic cancer (Trial NCT02283372). Within the subset of trial patients who were treated with 5-fraction SBRT, the incidence of plan adaptation was 84% (81 of 97 fractions). Overall, the subsites treated with online ART included 18 liver sites (27%), 24 pancreas sites (36%), 5 adrenal sites (7%), 7 central thorax sites (10%), 8 abdominal lymph node sites (12%), and 5 “other” cases (7%). An online ART plan for pancreas SBRT is given in Figure 5.
Figure 5.
(a) Magnetic resonance–based, adaptive plan for fraction 1 met all organ-at-risk constraints based on daily set-up anatomy from fraction 1. (b) Application of the fraction 1 plan to the fraction 2 magnetic resonance image of a patient with a pancreatic tumor (blue color wash) resulted in a violation of the hard duodenal (green color wash) and small bowel (lime color wash) constraints. (c) Daily adaptive planning for fraction 2 achieved resolution of the organ-at-risk constraint violation to the duodenum and small bowel while preserving target volume coverage.
Cine treatment gating
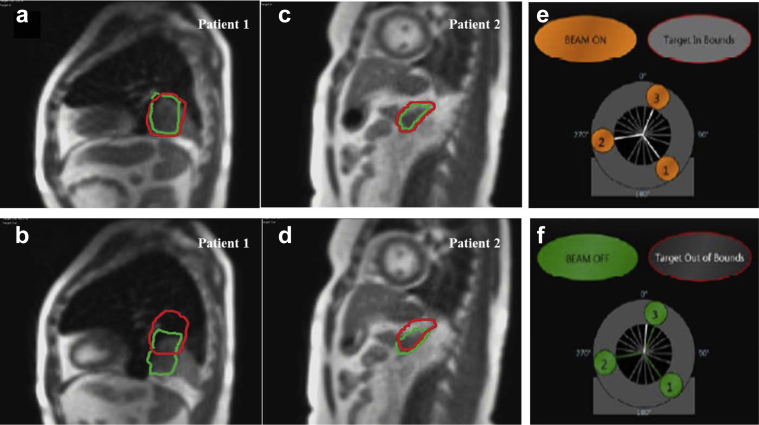
Sagittal cine treatment gating was first implemented in February 2015. To date, 81 patients (37% since the technology became available) have undergone treatment with cine gating. The most common clinical settings were abdominal (42 patients [52%]) and thoracic (20 patients [25%]) disease. All SBRT cases used cine gating, typically using the tumor plus a median 0.3 cm (range, 0.3-0.5 cm) margin for the gating structure. Figure 6 shows representative examples of cine gating for SBRT patients. The gating margin around the tumor in both examples was 0.3 cm, and the average treatment time was 28 minutes (range, 14-36 minutes) with a beam-on time of 93%. When >10% of the tumor volume was tracked outside of the gating boundary, the beam was automatically turned off with a latency of 246 ms to 527 ms.
Figure 6.
Magnetic resonance real-time cine image with (a, c) the target within the specified gating margin and the beam turned on and (b, d) >10% of the target located outside of the specified gating margin and the beam turned off. Patient 1 had oligometastatic rectal cancer and underwent stereotactic body radiation therapy to a solitary thorax lesion. Patient 2 had a history of stage IIIA non-small cell lung cancer with solitary adrenal metastasis and underwent stereotactic body radiation therapy.
Discussion
The world's first commercially available MR-IGRT system has been successfully used at our institution since 2014. The system features capabilities including onboard low-field MRI, gating based on real-time sagittal cine MR, and a built-in TPS that is capable of both conventional and online ART. This study sought to describe our initial clinical experience with integrating MR-IGRT in a high-volume RT center, characterize the disease sites that were treated with MR-IGRT, and highlight the technical capabilities of the system.
Over the past 3 years, approximately 3,500 patients per year were treated with external beam RT at our facility, including approximately 290 patients treated with proton RT and 316 patients treated with MR-IGRT between January 2014 and June 2016. Since implementing both proton RT and MR-IGRT, the overall volume of patients treated at our facility has steadily increased, thus allowing us to select a highly specific subgroup of patients who would likely benefit from MR-IGRT without a decrease in other treatment modalities in our department. Additionally, the incorporation of the MR-IGRT service into a high-volume clinic has allowed integration of current staff, simulation, and dosimetry without changes to other workflows. The group of initial patients and cases for MR-IGRT was selected based on the improved soft tissue visualization for setup, reduction of treatment margins, and/or increased ability to control for any observed changes during treatment.
The onboard low-field MRI offers improved visualization for selected RT targets and other critical structures compared with other onboard imaging modalities, including CBCT.1 The geometric accuracy and soft tissue definition of MRI can provide accurate and reproducible daily localization, leading to potential reduction in treatment margins, and can eliminate the need for fiducial markers. For example, >95% of female patients at our institution who are candidates for accelerated partial breast irradiation but unable to undergo high-dose rate brachytherapy (eg, procedural contraindications) are treated with MR-IGRT using treatment margins that are decreased compared with accelerated partial breast irradiation on a conventional linac (Fig 4b).8
We previously reported that the mean lumpectomy cavity displacement during treatment on the cine MR in the anterior-posterior and superior-inferior directions was 0.6 ± 0.4 mm and 0.6 ± 0.33 mm, respectively.8 Therefore, for these patients, we defined our clinical GTV as the surgical cavity plus a 1-cm margin (excluding chest wall and pectoral muscles and 5 mm from skin), and no additional margin was added for the PTV. This is a median treatment volume reduction of approximately 52% compared with using a 1-cm PTV margin, which may result in improved cosmesis.8 Intra- and interfraction tumor and OAR motion and potential margin reductions are also currently under investigation in the treatment of gastric mucosa-associated lymphoid tissue lymphoma and potentially bladder cancer. We also no longer require fiducial markers to be placed prior to treatment of adrenal metastases and prostate cancer with the MR-IGRT system given the excellent daily imaging used for patient set-up. With a median follow-up of more than 1.5 years, the local control of patients who were treated for oligometastatic adrenal tumors is 90%. Additionally, unlike other onboard imaging techniques such as CBCT, the MRI system does not deliver any additional radiation dose to the patient. Song et al. measured the average dose of 2 widely used onboard CBCT systems and found that the average dose ranged from 0.1 to 3.5 cGy and 1.1 to 8.3 cGy, which would not be included in the dose calculated by the TPS and can degrade the delivered plan quality by blurring the intended isodose lines.9
In addition to the improved setup accuracy and reduction in unplanned radiation exposure with daily MR setup imaging, online ART also improves the therapeutic precision of RT. Previously, the standard method of addressing interfraction target positioning variation was to use image guidance for daily patient repositioning on the basis of rigid anatomical registration using the planning image and the image acquired just before treatment, with subsequent delivery of the original plan. To a limited extent, MR-IGRT alone may manage large interfractional anatomic shifts and setup errors, but it cannot sufficiently account for known organ deformation, rotation, and independent motion between organs, such as within the abdomen.10 Instead, online ART that uses high-quality 0.35 T volumetric MR images acquired at the start of each fraction with the MR-IGRT system can fully account for interfraction volumetric changes in both target volumes and OARs using reoptimization of treatment plans based on the observed daily anatomy.
The projected benefits of online ART are many and include margin reduction and an increase in the therapeutic window of RT via reduced OAR dose and the possibility for PTV dose escalation.11, 12, 13 A dosimetric pilot study that was performed with the current MR-IGRT system demonstrated that simulated online ART MR-IGRT SBRT would eliminate 100% of unintended OAR constraint violations that occurred in 61% of unadapted fractions and permit simultaneous dose escalation.4 ART does require a time investment from the physician and daily physics support for online quality assurance of the adaptive plan prior to delivery. However, as we have previously reported, this is feasible within an acceptable clinical time frame (median, 26 minutes) for recontouring, reoptimization, and quality assurance.2 Efforts are also underway to train advanced therapy personnel to assist in the contouring tasks that account for the majority of the time required for adaptation. Presently, we are evaluating the clinical feasibility of online adaptive MR-guided SBRT for oligometastatic and unresectable primary malignancies of the abdomen and central thorax (Trial NCT02264886) and for dose escalation for inoperable pancreatic cancer with full dose concurrent chemotherapy (Trial NCT02283372). These trials use a fraction-by-fraction isotoxicity approach with planning prioritization of hard constraints for OARs to minimize toxicity and maximize the safe deliverable dose to the target. We have established that there were no instances of grade 3 or greater toxicities in our patients who were treated in a phase 1 study of online adaptive SBRT for abdominal and central thoracic malignancies despite the use of prescriptions for biological equivalent doses >100.
Our current MR-IGRT system also permits sagittal planar cine MR gating. The management of intrafraction motion during RT is a long-standing concern, particularly within the thorax and abdomen where respiratory motion and intrafraction physiologic motions challenge technology. Prior attempts to track target and OAR motion, such as the use of 4-dimensional CT planning, internal target volume construction, and fiducial markers are insufficient. Within the abdomen, internal target volume instability has been shown to range from 46% to 127% for tumors as small as 1 cm in diameter.14 A study by Ge et al. revealed that 4-dimensional CT inadequately represents the daily motion of abdominal tumors, with a discrepancy noted between planning CT and daily fluoroscopic video imaging in 90% of cases.15 Similarly, for patients who receive thoracic SBRT, in which treatment precision is paramount, daily intrafraction motion was found to exceed the mean target position by >2 mm for 41% of patients and by >5mm for 7% of patients.16 Even with fiducial use and daily adjustment of gating windows, studies have shown decreased gating accuracy over the course of a single treatment session for nearly half of the delivered fractions in the abdomen.17 In the absence of real-time tumor imaging, all surrogates for motion require some additional planning margin to account for these uncertainties, which that reduce therapeutic precision. However, with cine MR gating in real time, the GTV itself is the gating target, enabling potential reduction of PTV expansions and eliminating the inherent uncertainties of motion surrogates. Although there is some overall system latency in cine imaging processing, our institution has developed a policy of a 3 mm gating structure that is expanded from the GTV, which is smaller than the typical PTV expansion of 5 mm, to minimize the concern of organ motion beyond the gating target during that processing lag window. For patients with early stage non-small cell lung cancer, we selected treatment with MR-IGRT and cine gating for tumors that remain excessively mobile (>1 cm) after maximal abdominal compression (eg, tumors near the diaphragm). We have found that MR gating for such mobile tumors is also more time efficient compared with other gating systems, and the tumor can be directly visualized throughout treatment.
Although MR-IGRT has made significant strides in addressing the historic limitations of RT, this novel technology has limitations of its own. MR-IGRT, particularly adaptive treatment, requires additional clinical time for implementation, training, and treatment delivery. An ongoing phase 1 trial addresses the feasibility of online adaptive SBRT, and the timing data collected will be used for process improvement. Additionally, the use of a low-field MR-imaging unit could be considered a limitation of the current device. However, in our experience, the current, clinically available low-field MR is sufficient for daily imaging localization, cine gating, and online-adaptive planning. Diagnostic-quality imaging may have specialized applications in MR-IGRT, but its primary applications comprise initial staging/diagnosis and simulation; it is not essential for daily use. Other MR-IGRT units under development offer higher magnet strength and improved imaging resolution, but their clinical implementation has been impeded by challenges such as geometric distortion, dose distribution uncertainty, and undesirable patient heating.18, 19 We acknowledge that both the imaging and radiation delivery components of MR-IGRT devices will continue to improve with advancing technology, but we maintain that the current clinically implemented technology is sufficient for broad therapeutic use. Another limitation is the use of 60Co sources. Although the plan quality between 60Co and linac plans has been shown to be comparable, 60Co still has limitations such as less tissue penetration, greater low dose spread, and longer treatment times with source decay.7, 20 These limitations may be addressed by future technologies that use MR-linac systems. In our department, the cobalt radiation delivery system will be replaced with a 6MV linac while leaving the current MRI potion of the system in the treatment vault for use with the linear accelerator.
In addition to the limitations inherent to the MR-IGRT system, many other challenges were faced during the implementation of this technology. The machine was quickly integrated into clinical use and simultaneously available to all clinical services (eg, breast, thorax, gastrointestinal, genitourinary). Therefore, each service had to identify its unique challenges and/or specific patients who would benefit the most from the MR-IGRT technology. As new applications became available (ie, online adaptation, cine gating), each service also needed to determine when, how often, and for whom to use these features. For example, the thorax and gastrointestinal services have had specific challenges in determining when and how often to adapt hypofractionated treatment courses. Daily adaptations for extended treatment courses (ie, >10 fractions) is likely excessive and burdensome on the MR-IGRT workflow, and trials are in development to determine the ideal time point to adapt. Specific margin sizes and gating windows also remain challenges. These are questions that are still being answered today, and a collaboration between multiple institutions that have this technology has been extremely beneficial and informative.
MR-IGRT has been successfully implemented and provides unique advantages in the treatment of a variety of malignancies. Multiple clinical trials are in development to formally evaluate MR-IGRT in the treatment of various disease sites using techniques such as SBRT and adaptive RT. An in silico trial of MR-IGRT with mid-treatment adaptive planning for hypofractionated stereotactic RT in centrally located thoracic tumors is ongoing. Additionally, an analysis of online adaptive SBRT for patients with prostate cancer who use a hydrogel prostate-rectal spacer is currently underway. Other areas of interest include autosegmentation, dose accumulation, motion management, and the financial implications of MR-IGRT. Lastly, a multi-institutional registry is under development, which will allow for sharing of clinical outcomes and treatment techniques.
Footnotes
Conflicts of interest: Benjamin Fischer-Valuck, Lauren Henke, Olga Green, Rojano Kashani, Jeffrey Bradley, Cliff Robinson, Maria Thomas, Imran Zoberi, Jiayi Huang, Jeff Olsen, Parag Parikh, Sasa Mutic, and Jeff Michalski have received either a grant, honorarium, speaker's bureau, or travel expenses reimbursement from ViewRay Inc. outside the scope of this report.
References
- 1.Noel C.E., Parikh P.J., Spencer C.R. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015;54:1474–1482. doi: 10.3109/0284186X.2015.1062541. [DOI] [PubMed] [Google Scholar]
- 2.Acharya S., Fischer-Valuck B.W., Kashani R. Online magnetic resonance image guided adaptive radiation therapy: First clinical applications. Int J Radiat Oncol Biol Phys. 2016;94:394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Liu F., Yorke E.D., Belderbos J.S. Using generalized equivalent uniform dose atlases to combine and analyze prospective dosimetric and radiation pneumonitis data from 2 non-small cell lung cancer dose escalation protocols. Int J Radiat Oncol Biol Phys. 2013;85:182–189. doi: 10.1016/j.ijrobp.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henke L., Kashani R., Yang D. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: Characterization of potential advantages. Int J Radiat Oncol Biol Phys. 2016;96:1078–1086. doi: 10.1016/j.ijrobp.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contreras J., Zhao T., Perkins S. The world's first single-room proton therapy facility: Two-year experience. Pract Radiat Oncol. 2017;7:e71–e76. doi: 10.1016/j.prro.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Mutic S., Dempsey J.F. The ViewRay system: Magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Wooten H.O., Green O., Yang M. Quality of intensity modulated radiation therapy treatment plans using a (6)(0)Co magnetic resonance image guidance radiation therapy system. Int J Radiat Oncol Biol Phys. 2015;92:771–778. doi: 10.1016/j.ijrobp.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 8.Acharya S., Fischer-Valuck B.W., Mazur T.R. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: Evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys. 2016;96:785–792. doi: 10.1016/j.ijrobp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Song W.Y., Kamath S., Ozawa S. A dose comparison study between XVI and OBI CBCT systems. Med Phys. 2008;35:480–486. doi: 10.1118/1.2825619. [DOI] [PubMed] [Google Scholar]
- 10.Kishan A.U., Cao M., Mikaeilian A.G. Dosimetric feasibility of magnetic resonance imaging-guided tri-cobalt 60 preoperative intensity modulated radiation therapy for soft tissue sarcomas of the extremity. Pract Radiat Oncol. 2015;5:350–356. doi: 10.1016/j.prro.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Lui F.E., Yu B., Baron D.M., Lei C., Zapol W.M., Kluger R. Hemodynamic responses to a hemoglobin bis-tetramer and its polyethylene glycol conjugate. Transfusion. 2012;52:974–982. doi: 10.1111/j.1537-2995.2011.03421.x. [DOI] [PubMed] [Google Scholar]
- 12.Spoelstra F.O., Pantarotto J.R., van Sornsen de Koste J.R., Slotman B.J., Senan S. Role of adaptive radiotherapy during concomitant chemoradiotherapy for lung cancer: Analysis of data from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2009;75:1092–1097. doi: 10.1016/j.ijrobp.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz D.L., Garden A.S., Thomas J. Adaptive radiotherapy for head-and-neck cancer: Initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:986–993. doi: 10.1016/j.ijrobp.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James S.S., Mishra P., Hacker F., Berbeco R.I., Lewis J.H. Quantifying ITV instabilities arising from 4DCT: A simulation study using patient data. Phys Med Biol. 2012;57:L1–L7. doi: 10.1088/0031-9155/57/5/L1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge J., Santanam L., Noel C., Parikh P.J. Planning 4-dimensional computed tomography (4DCT) cannot adequately represent daily intrafractional motion of abdominal tumors. Int J Radiat Oncol Biol Phys. 2013;85:999–1005. doi: 10.1016/j.ijrobp.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Shah C., Grills I.S., Kestin L.L. Intrafraction variation of mean tumor position during image-guided hypofractionated stereotactic body radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:1636–1641. doi: 10.1016/j.ijrobp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Ge J., Santanam L., Yang D., Parikh P.J. Accuracy and consistency of respiratory gating in abdominal cancer patients. Int J Radiat Oncol Biol Phys. 2013;85:854–861. doi: 10.1016/j.ijrobp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Weygand J., Fuller C.D., Ibbott G.S. Spatial precision in magnetic resonance imaging-guided radiation therapy: The role of geometric distortion. Int J Radiat Oncol Biol Phys. 2016;95:1304–1316. doi: 10.1016/j.ijrobp.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 19.Raaijmakers A.J., Raaymakers B.W., Lagendijk J.J. Magnetic-field-induced dose effects in MR-guided radiotherapy systems: dependence on the magnetic field strength. Phys Med Biol. 2008;53:909–923. doi: 10.1088/0031-9155/53/4/006. [DOI] [PubMed] [Google Scholar]
- 20.Wooten H.O., Rodriguez V., Green O. Benchmark IMRT evaluation of a Co-60 MRI-guided radiation therapy system. Radiother Oncol. 2015;114:402–405. doi: 10.1016/j.radonc.2015.01.015. [DOI] [PubMed] [Google Scholar]