Abstract
Purpose
The hypothesis is that 2-dimensional kV orthogonal imaging with fiducial markers (kV-FM) and soft-tissue cone beam computed tomography (ST-CBCT) are equally reproducible for daily positional alignments for image guided (IG) intensity modulated radiation therapy (IMRT) for prostate cancer.
Methods and materials
Ten patients undergoing definitive treatment for prostate cancer with IG-IMRT were imaged daily with kV-FM and ST-CBCT. For each acquired kV and CBCT image, offline alignments to the digitally reconstructed radiograph or planning CT, respectively, were made twice by the same physician to assess intraobserver test-retest reproducibility. The 256 kV and 142 CBCT images were analyzed, and the test-retest analysis was performed again on a subset of images by another physician to verify the results.
Results
The results demonstrated that kV-FM had better intraobserver test-retest reproducibility in the anterior-posterior (AP; 95% confidence interval [CI] Pearson correlation coefficient [r], 0.987-0.991), left-right (LR; 95% CI r, 0.955-0.969), and superior-inferior (SI; 95% CI r, 0.971-0.980) directions for daily IG alignments compared with ST-CBCT (AP: 95% CI r, 0.804-0.877; LR: 95% CI r, 0.877-0.924; SI: 95% CI r, 0.791-0.869). Errors associated with intraobserver test-retest reproducibility were submillimeter with kV-FM (AP: 0.4 ± 0.7 mm; RL: 0.4 ± 1.0 mm; SI: 0.5 ± 0.7 mm) compared with ST-CBCT (AP: 2.1 ± 2.2 mm; LR: 1.3 ± 1.4 mm; SI: 1.2 ± 1.8 mm). The mean shift differences between kV-FM and ST-CBCT were 0.3 ± 3.8 mm for AP, −1.1 ± 8.5 mm for LR, and −2.0 ± 3.7 mm for SI. Dose-volume histograms were generated and showed that test-retest variability associated with ST-CBCT IG-alignments resulted in significantly increased dose to normal structures and a reduced planning target volume dose in many patients.
Conclusions
The kV-FM–based daily IG alignment for IMRT of prostate cancer is more precise than ST-CBCT, as assessed by a physician's ability to reproducibly align images. Given the magnitude of the error introduced by inconsistency in making ST-CBCT alignments, these data support a role for daily kV imaging of FM to enhance the precision of external beam dose delivery to the prostate.
Summary.
The intraobserver reproducibility of daily shifts made with 2-dimensional kV orthogonal imaging with fiducial markers (kV-FM) and soft-tissue cone beam computed tomography (ST-CBCT) was evaluated using a test-retest strategy to assess their reproducibility for daily image guided intensity modulated radiation therapy (IMRT) for prostate cancer. The kV-FM demonstrated better intraobserver test-retest reliability in all 3 dimensions compared with the ST-CBCT when daily images were aligned by a radiation oncologist experienced in treating prostate cancer with IG-IMRT.
Introduction
Prostate cancer is the most commonly diagnosed malignancy among men in the United States.1 Definitive external beam radiation therapy is a cornerstone of treatment for prostate cancer. The use of intensity modulated radiation therapy (IMRT) decreases the dose delivered to the rectum and bladder compared with 3-dimensional conformal radiation therapy (CRT), thereby allowing for dose escalation that results in improved biochemical disease-free survival, improved distant metastasis-free survival rates, and reduced toxicities.2, 3, 4, 5, 6, 7 The subsequent introduction of image guidance techniques has brought about a reduction in margins around the prostate from approximately 10 mm to ≤5 mm, with the goal of reducing toxicity.8, 9 Recent reports suggest that acute and chronic genitourinary and gastrointestinal toxicities associated with image guided (IG) IMRT are reduced compared with non-IG 3-dimensional CRT.10, 11 Numerous studies and a recent meta-analysis have demonstrated comparable or improved efficacy of IMRT compared with 3-dimensional CRT.12 Studies have confirmed that the addition of daily image guidance may further improve outcomes in IMRT.13, 14
Two on-board imaging modalities are widely in use for IG-IMRT: 2-dimensional kV orthogonal imaging with fiducial markers (kV-FM) and cone beam computed tomography aligned to soft tissue (ST-CBCT). Both of these imaging modalities offer a means of visualizing prostate motion relative to bony anatomy such that interfractional prostate motion may be accounted for during daily IG alignments.15, 16, 17 Substantial work has been done to evaluate which imaging modality provides better precision and accuracy for daily IG alignments in prostate cancer; however, no true consensus has been reached, and the literature presents a mixed preference among various centers.18, 19, 20
As others have noted, both imaging modalities have advantages and disadvantages.18 Most practitioners agree that kV-FM has the advantage of minimal imaging dose and reduced image acquisition and alignment time but carries a low risk of prostate infection associated with the procedure that is required to place the fiducial markers (FMs). On the other hand, ST-CBCT offers a better visualization of the bladder and rectum and avoids the risk of infection at the expense of higher imaging dose and longer acquisition and alignment time. Regardless of the IG system used, practitioners generally agree that significant errors in set-up and prostate localization exist and that improved target identification may contribute to a reduction in treatment margins and toxicities.21, 22
Research to determine the best method of image guidance is hampered by the fact that there is no method to demonstrate the true location of the prostate. Given that true accuracy is difficult to assess, we suggest that the best method of image guidance is the one that is most reproducible, but we acknowledge that precision does not imply accuracy. Test-retest is the most direct method of assessing reproducibility. Therefore, we had 2 independent observers with expertise in prostate cancer perform test-retest assessments on a large number of non-overlapping images. For the kV-FM and ST-CBCT comparisons, we eliminated interobserver variability by having a single physician observer perform the alignments of both the kV-FM and ST-CBCT images for each daily image set.
Previous studies have attempted to assess the agreement between kV-FM and ST-CBCT in daily alignments18, 23, 24 and the reproducibility of each of these methods.19, 25, 26, 27 However, these studies did not ensure that a pair of images (eg, a 2-dimensional kV image and its corresponding ST-CBCT image or a CBCT–CBCT image pair) was assessed by the same observer and thereby introduced a significant confounding factor. These studies also did not ensure that the same observer assessed all the images of a particular type or from a particular patient. In several studies, radiation therapists made the alignments, which further clouded the accuracy of the shifts, especially for ST-CBCT images with which therapists would not have much experience.23, 25, 26, 28 Therefore, any differences observed between imaging modalities may be due to the various observers who made these assessments.
Methods and materials
Human experimentation
The procedures followed were in accordance with the ethical standards of the institutional review board and the 1975 Declaration of Helsinki as revised in 2000. All patients were informed of the risks and benefits associated with participation and consented prior to participation in this study.
Patient selection
The 10 patients enrolled in the study had localized disease and were definitively treated with IG-IMRT. Patient characteristics are listed in Table 1.
Table 1.
Prostate disease characteristics in the study population
| TNM Stage | Gleason Score | Serum PSA [ng/mL] | Stage | NCCN Risk |
|---|---|---|---|---|
| T2bN0M0 | 6 | 7.28 | IIA | Intermediate |
| T2bN0M0 | 8 | 51.7 | IIB | High |
| T2aN0M0 | 4+3 | 7.47 | IIA | Intermediate |
| T3bN0M0 | 3+4 | 13.1 | III | Very High |
| T2bN0M0 | 4+3 | 9.22 | IIA | Intermediate |
| T2bN0M0 | 4+5 | 34.5 | IIB | High |
| T1cN0M0 | 3+4 | 3.59 | IIA | Intermediate |
| T1cN0M0 | 3+4 | 5.7 | IIA | Intermediate |
| T1cN0M0 | 3+4 | 64.9 | IIB | High |
| T1cN0M0 | 8 | 20.7 | IIB | High |
NCCN, National Comprehensive Cancer Network; PSA, prostate-specific antigen; TNM, tumor, node, metastases.
Fiducial markers, daily imaging, and alignments
Three to four FMs (PolyMark polymer markers, Civco Medical Solutions, Orange City, IA) were placed under ultrasound guidance with simultaneous axial and sagittal imaging, as previously described.29 The kV and CBCT images were acquired with the on-board imaging system for the TrueBeam Linear Accelerator (Varian Medical Systems, Palo Alto, CA) at the time of treatment. For clinical care, the kV 2-dimensional images were used for image guidance. The CBCT was used only for this research.
Test-retest analysis
For the purpose of this study, offline alignments were made by a single physician observer with expertise in prostate cancer (corresponding author, R.D.E.; MD1) for each kV- (n = 256) and CBCT- (n = 142) acquired image. Each of these alignments was made twice to determine the test-retest reproducibility in making daily alignments for each modality. To confirm the findings reported in the Results section, a second physician observer, a brachytherapy fellow (MD2), repeated the test-retest alignments with a non-overlapping set of kV- (n = 178) and CBCT- (n = 100) acquired images. Alignments did not include rotations. Fiducial markers were ignored for the purpose of ST-CBCT–based alignments. The ST-CBCT alignments did not begin with an automated registration. There was no defined protocol for aligning the prostate gland by ST-CBCT such that the entire prostate gland, with the recto-prostatic interface being an important landmark, was used. The 2 daily shifts that were generated offline were plotted as (x,y) coordinates to represent the test and retest values. Best-fit curves were generated with linear regression, and Pearson correlation coefficients (r) with 95% confidence intervals (CIs) were calculated for each data set. The magnitude of the daily intraobserver variability is the arithmetic difference of the test-retest shift values made by 1 observer. The mean and standard deviation (SD) of the absolute values of these differences are presented in the Results section.
Intraobserver comparison between kV+FM and ST-CBCT
The first offline alignment for each kV and CBCT image pair made by the same observer was used to calculate the intraobserver difference between shifts determined with the 2 imaging modalities. The magnitude of the intraobserver variability between kV-FM– and ST-CBCT–acquired daily images is the arithmetic difference between shifts made by the same physician observer. A total of 142 kV- and CBCT-acquired image pairs that were aligned by the corresponding author were included in this analysis.
Set-up error calculation
Data were analyzed to calculate the mean (or group systematic) error (M) of the interfractional daily shifts for the 10 patients as well as the SD or systematic error (Σ) and the root mean squared or random error (σ), as previously described by van Herk et al.,30 for both kV-FM and ST-CBCT. The mean error is the mean of means for the daily shifts from each of the 10 patients. The systematic error is the SD of the mean of means, and the random error is the root mean squared as calculated from the SDs of the individual patient means.
Analysis of needed margins for ST-CBCT alignment
Assuming that kV-FM is a truer method of assessing prostate location (see Results), an analysis was performed considering the difference of ST-CBCT from the kV-FM–determined shift to be a positioning error. Margins required if ST-CBCT were used were then calculated according to van Herk's formula (2.5 × Σ + 0.7 × σ), which ensures that 90% of the patients would receive 95% of the prescribed radiation therapy dose to the planning target volume (PTV).31
Intensity modulated radiation therapy treatment and planning
IMRT plans were developed on the Varian Eclipse Treatment Planning System, Version 11. Standard treatment plans incorporated 5 mm expansions from the clinical target volume (CTV; including the prostate and seminal vesicle) to the PTV in all dimensions. All analyses for this research were performed offline after treatment had been delivered. Alternative dose-volume histograms (DVHs) for research purposes were generated as follows. The first DVH was from the original treatment plan, and the next 3 DVHs were derived by applying the fluence of the original plan to new isocenters with posterior shifts of 0.5, 1, and 2 SD of the intraobserver variability for shifts made with ST-CBCT–based IG (ie, 1.1, 2.2, and 4.4 mm from the isocenter used for treatment). To generate the fifth DVH, a new IMRT plan was generated for the PTV with expanded margins that were determined by the variability in ST-CBCT–based IG as described in the Results section (ie, the standard 5 mm in all dimensions with an additional 5 mm anterior-posterior [AP] and left-right [LR] and 10 mm superior-inferior [SI]). Each of these simulations was run for the 10 patients included in the study, and DVH data were extracted for each contoured structure for each patient. The AP direction was chosen for this simulation because the majority of treatment toxicities were derived from radiation to the bladder and rectum.
Statistics
Standard statistical analyses were performed in GraphPad Prism v6.0h. Statistical significance for mean difference between test-retest alignments was assessed by the t test. Statistical significance for DVHs was assessed by a 2-way analysis of variance.
Results
Errors associated with daily interfractional shifts in the study population
IG alignments were made offline for both kV-FM– and ST-CBCT–based imaging, and the associated errors are presented Table 2. These findings are similar to what others have found when assessing the shifts determined by daily IG using a variety of methods.
Table 2.
Errors associated with daily interfractional shifts (mm)
| A-P | L-R | S-I | |
|---|---|---|---|
| kV-FM | |||
| Mean Error (M) | −2.0 | 0.3 | −3.3 |
| Systematic Error (Σ) | 4.8 | 2.6 | 2.9 |
| Random Error (σ) | 2.8 | 3.3 | 2.3 |
| ST-CBCT | |||
| Mean Error (M) | −3.2 | −0.4 | −1.8 |
| Systematic Error (Σ) | 5.2 | 3.3 | 3.3 |
| Random Error (σ) | 3.7 | 3.3 | 2.4 |
A-P, anterior-posterior; kV-FM, kV orthogonal imaging with fiducial markers; L-R, left-right; S-I, superior-inferior; ST-CBCT, soft-tissue cone beam computed tomography.
Intraobserver reproducibility for test-retest of interfractional shifts for daily image guided alignments
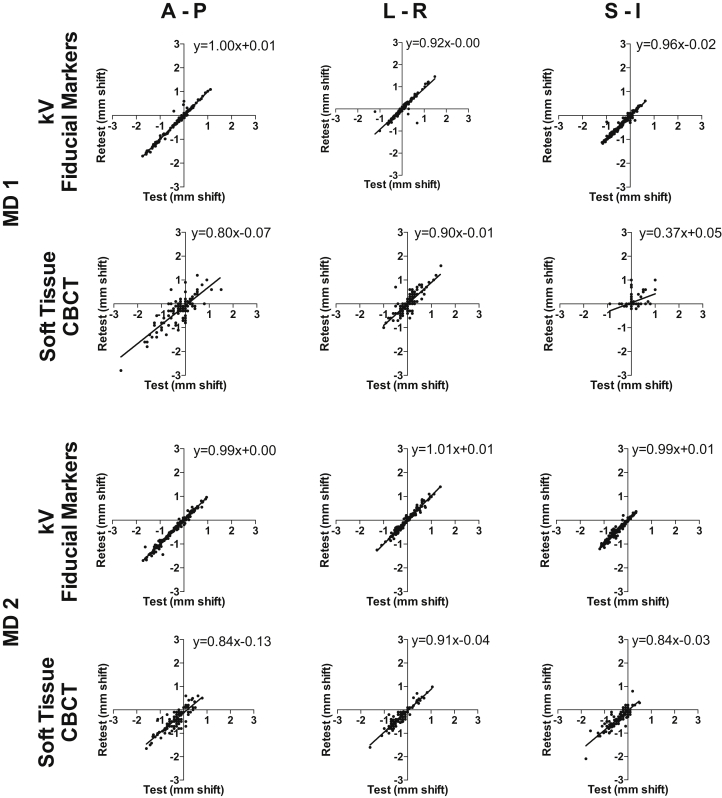
The magnitudes of the test-retest shifts in each dimension were plotted against one another to visualize their correlation (Fig 1). A higher correlation of the test-retest shifts in the kV-FM is observed in all 3 dimensions, and r with 95% CIs are presented in Table 3. Statistically higher correlations with non-overlapping 95% CIs were observed for the test-retest shifts in the kV-FM–mediated alignments compared with ST-CBCT.
Figure 1.
Intraobserver reproducibility for test-retest of interfractional shifts for daily image guided alignments. Offline alignments to the planning computed tomography were made twice each (test and retest) by 2 radiation oncologists (observers 1 and 2) for each set of daily images for kV orthogonal imaging with fiducial markers and soft-tissue cone beam computed tomography. The test and retest shift (in millimeters) for each image in each axis were plotted against one another so that correlations could be calculated (Table 3).
Table 3.
Correlation of intraobserver test-retest interfractional shifts, Pearson correlation coefficients with 95% confidence intervals
| A-P | L-R | S-I | |
|---|---|---|---|
| MD1 | |||
| kV-FM | .989 | .935 | .974 |
| .986-.991 | .918-.949 | .967-.980 | |
| ST-CBCT | .830 | .848 | .422 |
| .771-.875 | .794-.888 | .277-.548 | |
| MD2 | |||
| kV-FM | .988 | .986 | .977 |
| .984-.991 | .981-.989 | .970-.983 | |
| ST-CBCT | .863 | .930 | .871 |
| .803-.906 | .897-.952 | .814-.912 | |
| MD1 & MD2 | |||
| kV-FM | .989 | .963 | .976 |
| .987-991 | .955-.969 | .971-.980 | |
| ST-CBCT | .845 | .903 | .834 |
| .804-.877 | .877-.924 | .791-.869 |
A-P, anterior-posterior; kV-FM, kV orthogonal imaging with fiducial markers; L-R, left-right; S-I, superior-inferior; ST-CBCT, soft-tissue cone beam computed tomography.
Magnitude of intraobserver variability for shifts associated with daily alignments
To quantify the magnitude of the intraobserver variability in the test-retest daily alignments, the difference between the test and retest shift was calculated for each daily alignment for both kV-FM and ST-CBCT. The data with SDs are presented in Table 4, and the magnitude of the difference between the intraobserver test-retest shifts was significantly less in the kV-FM–based alignments as assessed by a 2-tailed, unpaired t test (P < .0001 in all dimensions).
Table 4.
Intraobserver variability for test-retest shifts. Mean difference of test-retest shifts±standard deviation (mm)
| kV-FM | ST-CBCT | t test P-value | |
|---|---|---|---|
| A-P | 0.4 ± 0.7 | 2.1 ± 2.2 | < .0001 |
| L-R | 0.4 ± 1.0 | 1.3 ± 1.4 | < .0001 |
| S-I | 0.5 ± 0.7 | 1.2 ± 1.8 | < .0001 |
A-P, anterior-posterior; kV-FM, kV orthogonal imaging with fiducial markers; L-R, left-right; S-I, superior-inferior; ST-CBCT, soft-tissue cone beam computed tomography.
Errors associated with ST-CBCT IG alignments may lead to decreased PTV coverage and increased normal tissue dosimetry
The differences in shifts for the daily alignments for ST-CBCT relative to kV-FM (ΔShiftkV-CBCT ± SD), the associated errors, and the margins needed to account for these errors are presented in Table 5. Only 73%, 70%, and 72% of shifts in the AP, LR, and SI dimensions, respectively, were within 3 mm of one another for each pair of images aligned by kV-FM and ST-CBCT. In addition, 85%, 85%, and 79% of shifts in the AP, LR, and SI dimensions, respectively, were within 5 mm.
Table 5.
Errors and margins associated with intraobserver shift differences between kV-FM and ST-CBCT (mm)
| A-P | L-R | S-I | |
|---|---|---|---|
| ΔShiftkV-CBCT ± SD | 0.3 ± 3.8 | −1.1 ± 8.5 | −2.0 ± 3.7 |
| Mean Error (M) | 0.5 | −0.3 | −2.3 |
| Systematic Error (Σ) | 1.0 | 1.3 | 3.2 |
| Random Error (σ) | 3.4 | 2.9 | 2.2 |
| Margins | 4.8 | 5.4 | 9.5 |
A-P, anterior-posterior; kV-FM, kV orthogonal imaging with fiducial markers; L-R, left-right; S-I, superior-inferior; SD, standard deviation; ST-CBCT, soft-tissue cone beam computed tomography.
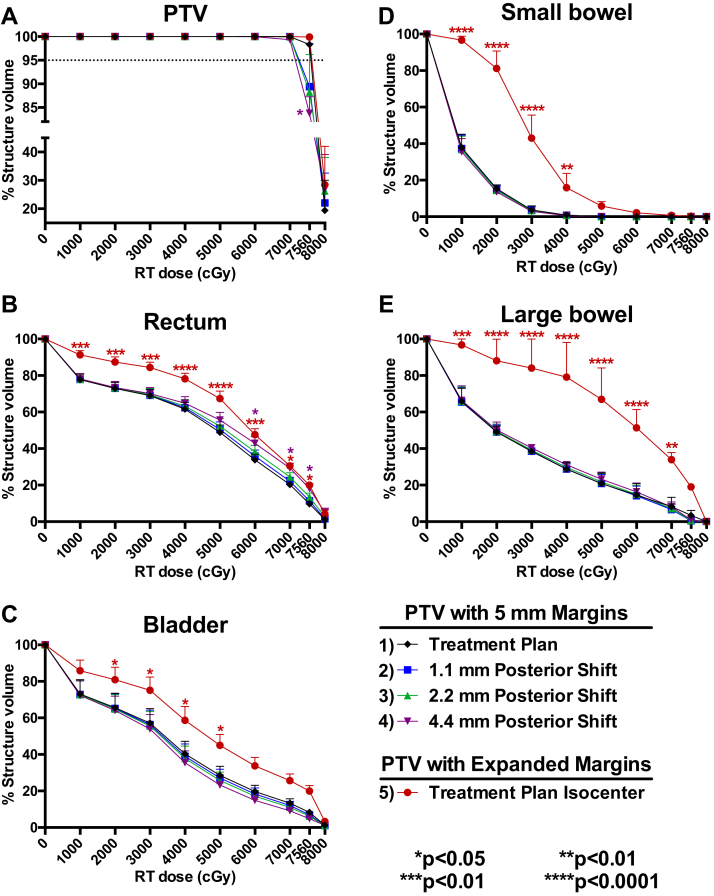
DVH data that were simulated to model the effects of variability across a wide range of errors (0.5, 1, and 2 SD) in ST-CBCT–mediated IG are presented in Figure 2 to illustrate how PTV coverage and healthy tissue exposure may be affected by the errors associated with intraobserver variability of IG alignments. With a 1.1 mm (0.5 SD) posterior shift, PTV coverage with the prescribed dose of 7560 cGy was reduced to 89.4%. A 2.2 mm (1 SD) posterior shift reduced PTV coverage to 88.3%, and a 4.4 mm (2 SD) posterior shift resulted in a significantly reduced PTV coverage of 83.4% (Fig 2A). With the 4.4 mm (2 SD) posterior shift, the volume of rectal tissue receiving a high dose of radiation (6000-7560 cGy) was also significantly increased (Fig 2B). The CTV coverage was not diminished by these shifts.
Figure 2.
Errors associated with image guided alignments may lead to decreased planning target volume (PTV) coverage or increased toxicity. Dose-volume histograms (DVHs) were generated from five different intensity modulated radiation therapy plans for each of the 10 patients in the study. The first DVH was from the original treatment plan and the next 3 DVHs were derived by applying the fluence of the original plan to new isocenters with posterior shifts of 0.5, 1, and 2 standard deviations of the intraobserver variability for shifts made with soft-tissue cone beam computed tomography–based image guidance (ie, 1.1, 2.2, and 4.4 mm from the isocenter used for treatment). To generate the fifth DVH, a new intensity modulated radiation therapy plan was generated for the PTV with expanded margins determined by the variability in soft-tissue cone beam computed tomography–based image guidance, as described in the Results section (ie, the standard 5 mm in all dimensions with an additional 5 mm anterior-posterior and left-right and 10 mm superior-inferior). Each of these simulations was run for the 10 patients included in the study, and DVH data were extracted for each contoured structure for each patient. Pooled patient DVH data were plotted for the (A) PTV including circumferential 5 mm margins (n = 10); (B) rectum (n = 10); (C) bladder (n = 10); (D) small bowel (n = 3); and (E) large bowel (n = 2). Two-way analysis of variance was used to test statistically significant differences in the percent structure volume receiving a given radiation therapy dose for plans 2 to 5 relative to plan 1. PTV with 5 mm margins at isocenter was used for treatment.
As shown in Table 5, significant additional margins are needed when using ST-CBCT to account for the positioning errors determined on the basis of the test-retest alignments with ST-CBCT. DVH data indicate that the use of these expanded margins may lead to a significant increase in dose delivered to all healthy tissues (Figs 2B-E).
Discussion
Here, we describe the reproducibility of these 2 imaging modalities for daily image alignment using a test-retest strategy, whereby the variability introduced by having variable observers assess such shifts is eliminated. Two radiation oncologists independently performed the test-retest analyses in this study. Previous studies have primarily focused on quantifying interobserver variability for a given imaging modality and have relied on multiple observers (often therapists) when comparing alignments between ST-CBCT– and kV-FM–based IG-alignments. We determined via the intraobserver test-retest alignments that kV-FM is more reproducible in the AP, LR, and SI directions than ST-CBCT.
Furthermore, by using kV- and CBCT-acquired image pairs that were aligned by the same physician observer, we demonstrated that the choice of imaging modality is relevant given the significant variability in daily alignments between the modalities, in agreement with previous reports.18 We observed variability between the imaging modalities that could affect daily prostate coverage when using narrow expansion margins to define PTVs. By relying on a single physician with expertise in prostate cancer to align the daily images, we removed interobserver variability. Controlling for interobserver variability, we found that kV-FM–based imaging was more reproducible than ST-CBCT for making daily shifts.
Without the ability to define the true location of the prostate, we suggest that the most reproducible imaging modality is most suitable for IG-IMRT. However, it must be acknowledged that it is possible to obtain highly precise alignments of FMs while achieving poor accuracy. Any systematic errors in the process of image acquisition and alignment could be reproducible, but inaccurate. However, given the quality of the equipment used (Varian TrueBeam linear accelerator) and the proper quality assurance performed on the equipment, we believe this to be unlikely.
Another possible source of disconnect between precision and accuracy arises because kV-FM imaging provides a surrogate for the prostate rather than visualizing the organ itself (ie, FMs can move within the prostate). Here, 3 to 4 FMs were implanted in each case and were easily aligned to one another throughout the course of treatment, which suggests that the FMs did not move relative to one another. With the observed stability of FMs, it is unlikely that all markers would shift in 3-dimensional space in the same direction simultaneously such that their orientation relative to one another remained the same but changed in relationship to the prostate. Therefore, a disconnect between precision and accuracy is also unlikely.
The magnitude of the errors associated with the test-retest alignments in kV-FM and ST-CBCT indicates an important role for FMs in maximizing the reliability of daily IG alignments. We characterized and quantified the effect of intraobserver variation with ST-CBCT versus kV-FM for daily set-up and then illustrated how these errors could potentially affect PTV coverage or healthy tissue dose. We modeled a range of errors, from 0.5 SD to 2 SD, to include both common and unlikely case scenarios. For illustrative purposes, we chose to model a posterior shift because rectal toxicities are relatively common. Although for many fractions, the dosimetric consequences of these misalignments would be small, the same can be said for treatment without image guidance. As such, awareness of the residual uncertainty associated with ST-CBCT IG is important. Physicians who want to ensure precise alignment for the entire treatment course may consider kV-FM IG.
Furthermore, the implementation of margins <5 mm may not be prudent when ST-CBCT is used because the associated uncertainties would likely affect the CTV coverage. This has implications for the design of novel treatment regimens that involve significant dose escalation or hypofractionation, in which the use of narrow treatment margins may be critical in maintaining acceptable toxicity profiles. However, ST-CBCT has certain advantages over kV-based imaging. Importantly, CBCT visualizes the rectum and bladder and may play a key role in the avoidance of these structures, which ensures that the degree of filling of these organs is similar to that of the simulation. Therefore, a combined imaging regimen including kV-FM–based alignment with ST-CBCT may be optimal for accurate and precise target alignment while minimizing doses to healthy tissue.
This study was robust in the number of test-retest alignments performed using both imaging modalities; however, because only 2 radiation oncologists performed the alignments, we cannot wholly exclude practitioner bias. FMs are also visible by CBCT, so they were ignored when making the ST-CBCT–based alignments. One might have expected that the presence of the markers would bias the study toward greater reproducibility in the ST-CBCT alignments, but this was not observed in this study, nor was this limitation considered a significant confounder in a previous study with the same limitation.18
Conclusions
In summary, kV-FM–based daily IG alignment for IMRT of prostate cancer is more precise than ST-CBCT as assessed by a physician's ability to reproducibly align daily images to the planning computed tomography. Given the magnitude of the error introduced by ST-CBCT alignment as demonstrated in the test-retest analyses, these data support a role for daily kV imaging of FMs to enhance the precision of external beam dose delivery to the prostate.
Footnotes
Conflicts of interest: None.
References
- 1.Howlader N., Noone A.M., Krapcho M. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. http://seer.cancer.gov/csr/1975_2013/ Available at: Accessed June 21, 2017.
- 2.Cahlon O., Zelefsky M.J., Shippy A. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky M.J., Levin E.J., Hunt M. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Michalski J.M., Yan Y., Watkins-Bruner D. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spratt D.E., Pei X., Yamada J., Kollmeier M.A., Cox B., Zelefsky M.J. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekelman J.E., Mitra N., Efstathiou J. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–e334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuban D.A., Tucker S.L., Dong L. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Crehange G., Mirjolet C., Gauthier M. Clinical impact of margin reduction on late toxicity and short-term biochemical control for patients treated with daily on-line image guided IMRT for prostate cancer. Radiother Oncol. 2012;103:244–246. doi: 10.1016/j.radonc.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Maund I.F., Benson R.J., Fairfoul J., Cook J., Huddart R., Poynter A. Image-guided radiotherapy of the prostate using daily CBCT: The feasibility and likely benefit of implementing a margin reduction. Br J Radiol. 2014;87:20140459. doi: 10.1259/bjr.20140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wortel R.C., Incrocci L., Pos F.J. Acute toxicity after image-guided intensity modulated radiation therapy compared to 3D conformal radiation therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2015;91:737–744. doi: 10.1016/j.ijrobp.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Wortel R.C., Incrocci L., Pos F.J. Late side effects after image guided intensity modulated radiation therapy compared to 3D-conformal radiation therapy for prostate cancer: Results from 2 prospective cohorts. Int J Radiat Oncol Biol Phys. 2016;95:680–689. doi: 10.1016/j.ijrobp.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Yu T., Zhang Q., Zheng T. The effectiveness of intensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: A meta-analysis of the literatures. PLoS One. 2016;11:e0154499. doi: 10.1371/journal.pone.0154499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelefsky M.J., Kollmeier M., Cox B. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 14.Wong W.W., Vora S.A., Schild S.E. Radiation dose escalation for localized prostate cancer: intensity-modulated radiotherapy versus permanent transperineal brachytherapy. Cancer. 2009;115:5596–5606. doi: 10.1002/cncr.24558. [DOI] [PubMed] [Google Scholar]
- 15.Mechalakos J.G., Mageras G.S., Zelefsky M.J. Time trends in organ position and volume in patients receiving prostate three-dimensional conformal radiotherapy. Radiother Oncol. 2002;62:261–265. doi: 10.1016/s0167-8140(01)00466-2. [DOI] [PubMed] [Google Scholar]
- 16.Schallenkamp J.M., Herman M.G., Kruse J.J., Pisansky T.M. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys. 2005;63:800–811. doi: 10.1016/j.ijrobp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Shirato H., Harada T., Harabayashi T. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:240–247. doi: 10.1016/s0360-3016(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 18.Barney B.M., Lee R.J., Handrahan D., Welsh K.T., Cook J.T., Sause W.T. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT) Int J Radiat Oncol Biol Phys. 2011;80:301–305. doi: 10.1016/j.ijrobp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Moseley D.J., White E.A., Wiltshire K.L. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys. 2007;67:942–953. doi: 10.1016/j.ijrobp.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen R., Kron T., Foroudi F. Comparison of CT on rails with electronic portal imaging for positioning of prostate cancer patients with implanted fiducial markers. Int J Radiat Oncol Biol Phys. 2009;74:906–912. doi: 10.1016/j.ijrobp.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 21.McPartlin A.J., Li X.A., Kershaw L.E. MRI-guided prostate adaptive radiotherapy - A systematic review. Radiother Oncol. 2016;119:371–380. doi: 10.1016/j.radonc.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Byrne T.E. A review of prostate motion with considerations for the treatment of prostate cancer. Med Dosim. 2005;30:155–161. doi: 10.1016/j.meddos.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Ye J.C., Qureshi M.M., Clancy P., Dise L.N., Willins J., Hirsch A.E. Daily patient setup error in prostate image guided radiation therapy with fiducial-based kilovoltage onboard imaging and conebeam computed tomography. Quant Imaging Med Surg. 2015;5:665–672. doi: 10.3978/j.issn.2223-4292.2015.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logadottir A., Korreman S., Petersen P.M. Comparison of the accuracy and precision of prostate localization with 2D-2D and 3D images. Radiother Oncol. 2011;98:175–180. doi: 10.1016/j.radonc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Snir J.A., Battista J.J., Bauman G., Yartsev S. Evaluation of inter-fraction prostate motion using kilovoltage cone beam computed tomography during radiotherapy. Clin Oncol (R Coll Radiol) 2011;23:625–631. doi: 10.1016/j.clon.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Deegan T., Owen R., Holt T. Assessment of cone beam CT registration for prostate radiation therapy: Fiducial marker and soft tissue methods. J Med Imaging Radiat Oncol. 2015;59:91–98. doi: 10.1111/1754-9485.12197. [DOI] [PubMed] [Google Scholar]
- 27.McNair H.A., Harris E.J., Hansen V.N. Magnitude of observer error using cone beam CT for prostate interfraction motion estimation: Effect of reducing scan length or increasing exposure. Br J Radiol. 2015;88:20150208. doi: 10.1259/bjr.20150208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bylund K.C., Bayouth J.E., Smith M.C., Hass A.C., Bhatia S.K., Buatti J.M. Analysis of interfraction prostate motion using megavoltage cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;72:949–956. doi: 10.1016/j.ijrobp.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Shinohara K., Roach M. Technique for implantation of fiducial markers in the prostate. Urology. 2008;71:196–200. doi: 10.1016/j.urology.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 30.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 31.van Herk M., Remeijer P., Rasch C., Lebesque J.V. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]