Abstract
Purpose
Stereotactic radiation therapy (SRT) enables focused, short course, high dose per fraction radiation delivery to brain tumors that are less ideal for single fraction treatment because of size, shape, or close proximity to sensitive structures. We sought to identify optimal SRT treatment regimens for maximizing local control while minimizing morbidity.
Methods and materials
We performed a retrospective review of patients treated with SRT for solid brain metastases using variable dose schedules between 2001 and 2011 at 3 academic hospitals. Endpoints included (1) local control, (2) acute toxicity (Common Toxicity Criteria for Adverse Events v3.0), and (3) symptomatic radionecrosis. Kaplan-Meier and a competing risks methodology were used to estimate the actuarial rate of local failure and assess the association of clinical and treatment covariates with time to local failure.
Results
A total of 156 patients was identified. Common tumor histologies included breast (21%), non-small cell lung (32%), melanoma (22%), small cell lung (9%), and renal cell carcinoma (6%). The majority of lesions were supratentorial (57%). Median target volume was 3.99 mL (range, 0.04-58.42). Median total SRT dose was 25 Gy (range, 12-36), median fractional dose was 5 Gy (range, 2.5-11), and median number of fractions was 5 (range, 2-10). Cumulative incidence of local progression at 3, 6, 12, 18, and 24 months was 11%, 22%, 29%, 34%, and 36%. Total prescription dose was the only factor significantly associated with time to local progression on univariate (P = .02) and multivariable analysis (P = .01, adjusted hazards ratio, 0.87). Five patients experienced seizures within 10 days of SRT and 5 patients developed radionecrosis. All patients with documented radionecrosis received prior radiation to the index lesion.
Conclusions
Our series of SRT for brain metastases found total prescription dose to be the only factor associated with local control. Both acute and long-term toxicity events from SRT were modest.
Summary.
Stereotactic radiation therapy uses precisely focused radiation, often delivered in short courses, to brain metastases unsuited for single fraction treatment, but the optimal dose and fractionation schema remain unclear. We performed a multi-institutional retrospective review of patients treated with various stereotactic radiation therapy regimens. Total prescription dose was the only factor significantly associated with local control. Dose per fraction was not found to be associated with tumor control.
Introduction
Approximately 20% to 40% of cancer patients will develop brain metastases, resulting in more than 200,000 new cases in the United States each year.1, 2 Whereas whole brain radiation therapy (WBRT) has been the standard treatment for patients with multiple brain metastases for decades,3, 4, 5, 6, 7 increasing survival of these patients has led to greater concern for the neurotoxic effects of radiation8, 9, 10 and a shift from WBRT to increase use of stereotactic radiosurgery (SRS).11, 12 SRS has proven to be effective and well-tolerated,11, 12 but may not be an appropriate treatment for lesions that are large, irregularly shaped, or geographically proximal to radiation sensitive normal tissues.11, 12, 13, 14
Stereotactic radiation therapy (SRT) addresses these concerns by using smaller fractional doses of radiation while using the precise localization techniques of SRS to deliver highly conformal radiation. Local control rates of 40% to 90% are achieved with few serious toxicities.15, 16, 17, 18, 19, 20 To date, the ideal dose and fractionation scheme for SRT is unknown and published regimens vary widely across institutions.15, 16, 17, 18, 19, 20 We performed a pooled analysis of patients treated with SRT for solid brain metastases at 3 academic cancer centers to determine the SRT characteristics most predictive of local tumor control.
Methods and materials
Patients and clinical data
All patients aged 18 years or older who received SRT to a solid brain metastasis at Brigham and Women's Hospital, Massachusetts General Hospital, or Beth Israel Deaconess Medical Center from January 1, 2001, to December 31, 2011, were included. Patients were identified by a review of radiation therapy records at each institution. A common institutional review board approved the study protocol.
Data collected for this study included patient, tumor, imaging, and treatment characteristics.
For the purposes of this study, only 1 index lesion was identified and followed per patient, such that a 1:1 ratio of patient and index brain metastasis existed in the database. The rationale for this approach was to minimize potential confounding factors that a per-person analysis could introduce including variations in the number and treatment of the patients' other brain metastases. If a patient had multiple brain metastases treated with SRT, the earliest recorded treatment was selected for inclusion in the analysis. In the few cases in which more than 1 brain metastasis received SRT at the same time, the index lesion was selected at random. Only intact solid tumor metastases were included. Prior surgery to any nonindex brain lesion and prior SRS or WBRT, inclusive of the index lesion, were permissible.
Radiation treatment
Radiation planning and immobilization technique varied by institution; however, in all cases patients were immobilized using either a thermoplastic mask or a rigid frame. Computed tomography–based planning with fusion to contrast enhanced T1-weighted magnetic resonance imaging (MRI) scan was used to define the gross tumor volume. When MRI was contraindicated, a contrast enhanced computed tomography scan was used. A margin of 1 to 3 mm was added to the gross tumor volume to generate the planning target volume. The dose and fractionation scheme were chosen at the discretion of the treating radiation oncologist; additional details regarding dose and fractionation are detailed in the Results section. However, to best reflect the short course nature of therapy, we included any treatments delivered in larger than conventional radiation doses of 1.8 to 2 Gy per fraction and included any number of treatments up to a total of 10.
Patient outcomes and statistical analysis
The primary endpoints in this study were local control and toxicity. Local progression was defined by the modified MacDonald Criteria as an interval change greater than 25% of the largest axial diameter of the index lesion on contrast enhancing T1-weighted MRI without spontaneous regression or clinical progression resulting in initiation of salvage radiation or surgery.21 Local control was then defined as any lesion that did not meet criteria for local progression. Patients were censored if the lesion did not meet criteria for local progression at last imaging.
Both a Kaplan-Meier (KM) and a competing risks methodology were used to estimate local control. For the purposes of the competing risks analysis, 2 events were defined to compete with local progression: (1) death without local progression and (2) WBRT or surgery related to distant brain metastases. Additionally, the relationship between clinical and demographic covariates and time to local progression was estimated using both a Cox proportional hazards model and a Fine and Gray competing risks regression method. Variables included in the multivariable model were selected based on clinical significance.
A nonmixture cure fraction model was also used to estimate the probability of lesion control based on dose response. For the purposes of this analysis, photon doses were analyzed in Gy and proton doses were adjusted using a relative biological effectiveness weighted of 1.1 expressed in Gy.
Descriptive statistics were used to report toxicity. Toxicity was defined as symptoms referable to the target site following treatment that were not related to disease progression. Potential adverse effects were graded using the Common Terminology Criteria for Adverse Events, version 3.0. Long-term toxicity was restricted to symptomatic radionecrosis, defined as a treated lesion with radiographic correlate for potential radiation injury and neurologic symptoms that ultimately necessitated either: more than 6 months of corticosteroid use from the time of treatment without evidence of tumor progression to the treated lesion on serial MRI or surgical excision with pathology revealing no viable cancer cells. Analyses were performed using Stata (StataCorp 2013, Stata Statistical Software: release 13; College Station, TX).
Results
Patient and treatment characteristics
Patient clinical and treatment characteristics are described in Table 1. A total of 156 eligible patients were identified, of which 100 were female. The median age at receipt of radiation was 60 years (range, 20-92) and the median Karnofsky performance status at the time of radiation was 80 (range, 50-100). Tumor histologies included breast 21%, non-small cell lung 32%, melanoma 22%, small cell 9%, and renal cell carcinoma 6%.
Table 1.
Baseline patient clinical and treatment characteristics
| Baseline characteristics | N | % |
|---|---|---|
| Recruitment site | ||
| Brigham and Women's Hospital | 76/156 | 48.7 |
| Massachusetts General Hospital | 51/156 | 32.7 |
| Beth Israel Deaconess Medical Center | 29/156 | 18.6 |
| Patient age at treatment; median (range), y | 60 (20-92) | |
| Gender, female | 100/156 | 64.1 |
| Karnofsky Performance Status, median (range) | 80 (50-100) | |
| Primary cancer type | ||
| Breast | 33/156 | 21.2 |
| Non-small cell lung carcinoma | 50/156 | 32.1 |
| Melanoma | 35/156 | 22.4 |
| Small cell lung carcinoma | 14/156 | 9.0 |
| Renal cell carcinoma | 9/156 | 5.8 |
| Other | 15/156 | 9.6 |
| Prior radiation to index lesion | ||
| Any | 98/156 | 62.8 |
| WBRT only | 88/156 | 56.4 |
| SRS only | 2/156 | 1.3 |
| WBRT + SRS, focal RT or repeat WBRT | 8/156 | 5.1 |
| Planning target volume; median (range), mL | 3.99 (0.04-58.42) | |
| Index lesion size (maximum axial dimension) | ||
| <2.0 cm | 70/156 | 44.8 |
| 2.0-2.9 cm | 57/156 | 36.5 |
| 3.0-3.9 cm | 21/156 | 13.5 |
| ≥4.0 cm | 6/156 | 3.8 |
| Index lesion location | ||
| Frontal lobe | 47/156 | 30.1 |
| Temporal lobe | 11/156 | 7.1 |
| Parietal lobe | 20/156 | 12.8 |
| Occipital lobe | 9/156 | 5.8 |
| Cerebellum | 37/156 | 23.7 |
| Brainstem | 14/156 | 9.0 |
| Thalamus/basal ganglia | 12/156 | 7.7 |
| Other | 6/156 | 3.8 |
| Total prescription dose; median (range), Gy | 25 (12-36) | |
| Fraction size, median | ||
| ≤3 Gy | 12/156 | 7.7 |
| 4-6 Gy | 104/156 | 66.7 |
| 7-9 Gy | 29/156 | 18.6 |
| ≥10 Gy | 10/156 | 6.4 |
| Number of fractions, median (range) | 5 (2-10) | |
| Number of other brain metastases at treatment | ||
| 0 | 49/156 | 31.4 |
| 1-2 | 54/156 | 34.6 |
| 3-4 | 21/156 | 13.5 |
| ≥5 | 32/156 | 20.5 |
RT, radiation therapy; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
In general, SRT was used for lesions that were larger than 3 cm, irregularly shaped, or located in proximity to dose-limiting normal structures such as the brainstem or optic chiasm. Most lesions measured less than 2 cm (45%), 37% were between 2 and 2.9 cm, and 17% were ≥3 cm (range, 0.4-6.4). Median target volume was 3.99 mL (range, 0.04-58.42). Median total SRT dose was 25 Gy (range, 12-36), median fractional dose was 5 Gy (range, 2.5-11), and median number of fractions was 5 (range, 2-10). A total of 22 different dose and fractionation schedules were represented in our patient cohort. The most common dose schedules were 25 Gy in 5 fractions (49%), 24 Gy in 3 fractions (14%), 20 Gy in 5 fractions (8%), and 20 Gy in 2 fractions (5%). The majority of patients, 132/156 (85%), underwent photon SRT with either a linac-based (n = 103) or robotic stereotactic radiation therapy (n = 29) technique, whereas the remaining 24 patients received proton radiation.
Of the 156 patients, 98 (62.8%) had received cranial radiation therapy before SRT, either with WBRT (56.4%; n = 88; median dose, 35 Gy), SRS (1.3%; n = 2, median dose; 14 Gy), or a combination of WBRT and either SRS or fractionated, nonstereotactic, focal radiation therapy (5.1%; n = 8; median WBRT dose, 35 Gy). The median cumulative dose to the index lesion before SRT was 35 Gy (range, 8-60) and the median time from prior radiation therapy to the initiation of SRT was 26 weeks (range, 3-554). Although it is unclear how many physicians planned the SRT treatment as a boost, only 4/98 (4%) patients who received prior WBRT initiated SRT within 30 days of completion of WBRT. All received different SRT regimens and there were no gross difference among these patients regarding the likelihood of tumor progression. Approximately 90% (140/156) of patients received daily treatments 5 days per week, whereas the remainder received fractions spaced greater than 1 day apart (range, 2-21 days apart; median, 2 days apart).
Local progression
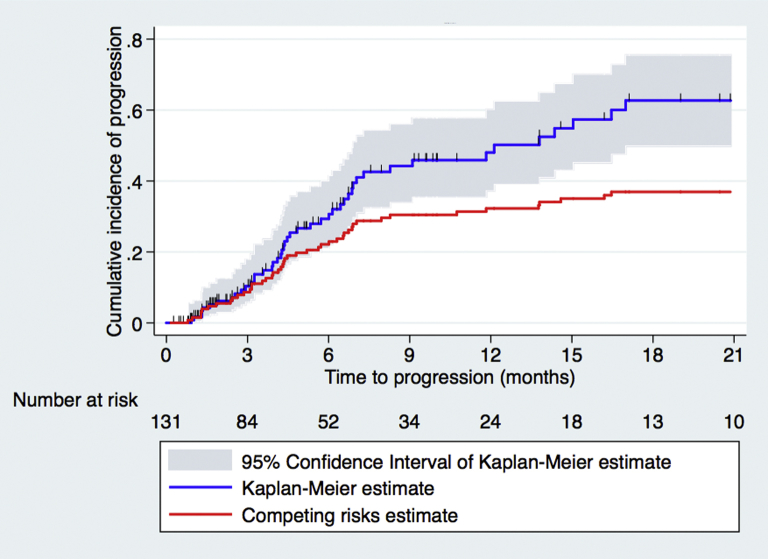
Of 156 patients treated with SRT, 131 were included in the analysis for local progression; the remainder lacked adequate follow-up imaging. With a median survival of 7.5 months (95% confidence interval [CI], 6.1-9.4 months) and a median imaging follow-up of 4.5 months, the cumulative incidence of local progression at 3, 6, 12, 18, and 24 months per Kaplan-Meier and competing risks estimates was 13%, 29%, 44%, 60%, and 64% and 11%, 22%, 29%, 34%, and 36%, respectively (Fig 1). In the univariable competing risks analysis, total prescribed dose was the only variable significantly associated with the risk of local progression (Table 2).
Figure 1.
Kaplan-Meier and competing risks estimates of local progression.
Table 2.
Univariate competing-risks analysis for local progression (N = 131 lesions)
| HR | 95% CI | P value | |
|---|---|---|---|
| Age at SRT, y | 0.99 | 0.96-1.02 | .4 |
| KPS at initiation of SRT | 1.008 | 0.98-1.04 | .5 |
| Primary cancer type (breast vs…) | |||
| Non-small cell lung carcinoma | 0.66 | 0.32-1.3 | .2 |
| Melanoma | 0.50 | 0.21-1.2 | .1 |
| All other | 0.71 | 0.28-1.8 | .5 |
| Radioresistant histology (melanoma + renal cell carcinoma vs other) | 0.98 | 0.48-2.0 | .9 |
| Prior WBRT to index lesion | 1.1 | 0.58-2.1 | .8 |
| Lesion size (product of cross-sectional axial tumor dimensions, cm) | 0.73 | 0.49-1.1 | .1 |
| Planning target volume, cm3 | 0.97 | 0.92-1.02 | .2 |
| Tumor location (brainstem vs…) | |||
| Cerebellum | 1.2 | 0.41-3.8 | .7 |
| Frontal lobe | 0.91 | 0.28-2.9 | .9 |
| Temporal lobe | 0.58 | 0.12-2.8 | .5 |
| Parietal lobe | 0.94 | 0.25-3.5 | .9 |
| Occipital lobe | 1.002 | 0.25-3.9 | .9 |
| Deep structures (thalamus/basal ganglia) | 0.84 | 0.18-3.8 | .8 |
| Supratentorial vs infratentorial/deep structures | 1.08 | 0.59-1.97 | .8 |
| Total prescription dose, Gy | 0.87 | 0.79-0.97 | .02 |
| Fraction size, Gy | 1.005 | 0.85-1.2 | .9 |
| Number of fractions | 0.94 | 0.77-1.1 | .5 |
| Biologically equivalent dose (alpha/beta = 2 Gy) | 0.98 | 0.94-1.02 | .3 |
| Biologically equivalent dose (alpha/beta = 30 Gy) | 0.87 | 0.79-0.96 | .008 |
| Number of other brain metastases | 0.99 | 0.85-1.2 | .9 |
| Time from diagnosis to first brain metastasis, y | 0.96 | 0.89-1.03 | .3 |
| Protons | 1.2 | 0.54-2.5 | .7 |
CI, confidence interval; HR, hazard ratio; KPS, Karnofsky Performance Scale; SRT, stereotactic radiation therapy. Other abbreviation as in Table 1.
Of note, biologically effective dose (BED) was not a significant predictor of local control. An alpha/beta ratio inclusive of the spectrum from 1 to 100 Gy for each tumor type was included in the analysis and, as the alpha/beta ratio rose, its association with local control increased, suggesting independence between tumor response and fractionation. For example, assuming an alpha/beta ratio of 2 Gy, the hazard ratio for local progression was 0.98 (95% CI, 0.94-1.02; P = .3), whereas assuming an alpha/beta ratio of 30 Gy yielded a hazard ratio of 0.87 (95% CI, 0.79-0.96; P = .008) (Table 2).
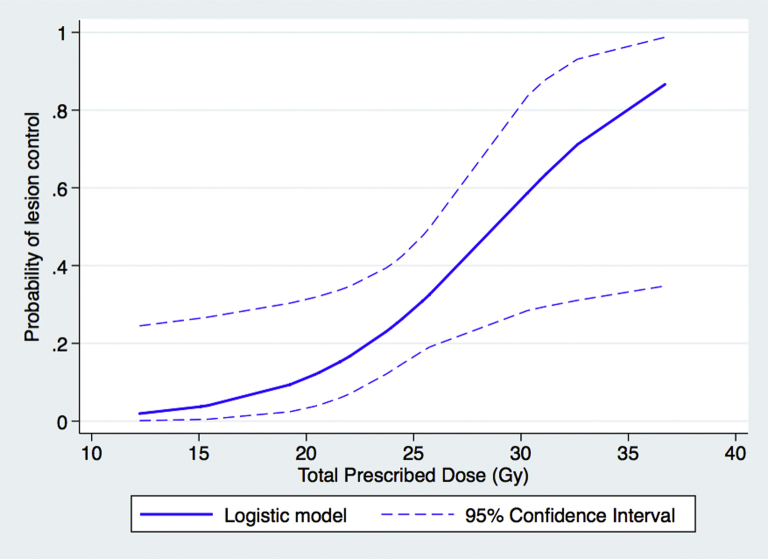
On multivariable analysis, which included total prescription dose, planning target volume, tumor location, and tumor histology (defined as radioresistant ie, melanoma and renal cell carcinoma vs other), total prescription dose remained the only factor significantly associated with time to local progression (P = .01; adjusted hazard ratio, 0.87) (Table 3). Additionally, a sigmoidal relationship between dose and the probability of lesion control was derived from actuarial analysis using a nonmixture cure fraction model with logistic model of dose-response (Fig 2).
Table 3.
Multivariable competing-risks regression model for local progression
| HR | 95% CI | P value | |
|---|---|---|---|
| Total prescription dose (Gy) | 0.87 | 0.79-0.97 | .01 |
| Lesion size (product of cross-sectional axial tumor dimensions, cm) | 0.94 | 0.83-1.06 | .3 |
| Tumor location Supratentorial vs infratentorial/deep structures |
1.1 | 0.61-2.2 | .7 |
| Radioresistant histology Melanoma + renal cell carcinoma vs other |
1.1 | 0.51-2.3 | .2 |
Abbreviations as in Table 2.
Figure 2.
Probability of tumor control by total prescription dose.
Acute toxicity
Of 156 patients, 23.7% (37/156) experienced at least 1 adverse symptom potentially associated with SRT. The most common symptoms were fatigue and headache, with 18 cases of grade 1-2 fatigue and 13 reports of grade 1-3 headache. Additionally, 6 patients experienced grade 1-3 nausea and 3 patients demonstrated grade 1 cognitive impairment, manifesting as temporary confusion immediately following treatment.
There were 5 patients with a documented seizure occurring during or within 10 days of completion of SRT. Two patients experienced generalized seizures, 1 while on levetiracetam prophylaxis and the remaining 3 experiencing focal seizures. All were successfully managed with the initiation of antiepileptic medication, and the single patient experiencing seizure while on prophylaxis was successfully managed with phenytoin. There was no common SRT dose or fractionation regimen among these patients.
Radionecrosis
At a median of 6 months following completion of SRT (range, 3-9 months), 5 patients had developed symptoms concerning for radionecrosis and ultimately met study criteria for necrosis by surgical resection (n = 1) or greater than 6 months of systemic steroid use (n = 4). There was no consistent SRT dose or fractionation regimen among these patients, but all of the patients with documented radionecrosis received prior cranial irradiation, either in the form of WBRT (n = 2), SRS to the index lesion (n = 1), or both WBRT and nonstereotactic, conventionally fractionated, focal radiation to the index lesion (n = 2). These prior courses of radiation occurred at a median of 238 days (range, 56-910) days before SRT.
Discussion
In our multi-institutional cohort of patients treated with varying SRT schedules for brain metastases based on physician preference, total prescription dose was the sole factor significantly associated with time to local failure. Additionally, when the probability of local failure was evaluated as a function of total prescription dose, a sigmoidal relationship was observed with the greatest increase in local control observed at the 25 Gy threshold. BED, dose per fraction, tumor size, and tumor histology were not associated with local failure, nor were any other clinical or demographic characteristics. This finding stands in contrast to some prior single-institution experiences, often of smaller patient populations treated with uniform dose schedules that have demonstrated different local control rates based on both tumor size and tumor histology. For example, Aoyama and colleagues found a significantly improved 1-year local control rate for tumor volumes less than or equal to 3 mL (96% vs 59%), and data from Kwon et al suggest a decrease in local control for tumors >3 cm in maximum tumor dimension.15, 16 More recent studies, however, suggest that larger tumor sizes, as reported by Navarria et al and Kim et al, may not result in a decrement in local control.17, 18 Additionally, Minniti and colleagues reported a significant association between melanoma histology and a decrease in local control.19
It is unclear why our results are in contrast to some other experiences. The linear-quadratic model does not accurately estimate biological effectiveness at larger fraction sizes and may be a factor for this difference.22, 23, 24 Alternative models for BED, including the multitarget model and universal survival curve model, have been developed in response to the observation that cell survival in vitro exhibits a linear rather than a continuously curving relationship with dose, particularly at high doses per fraction. This suggests that BED in SRT may depend on the total dose delivered rather than the cumulative number of fractions.25, 26, 27, 28 These models require clinical validation and resolution of conflicting mathematical models and of cell colony assay and in vivo studies.28, 29
Our results demonstrate comparable local failure rates to both prior reports of SRT and to small institutional series of single-fraction SRS administered to eloquent areas of the brain.16, 17, 18, 19, 20, 30, 31 Using the KM method, the estimated 1-year local failure rate in our cohort was 36%, whereas the 1-year competing risks estimate of local failure was 21%. The discrepancy between rates generated with the KM method and those estimates with a competing risks methodology are the result of our patient population in whom both death without local failure and intervention from distant metastases are high. In regard to toxicity, results were also in keeping with previously published reports. Acute toxicities were largely restricted to grade 1-2 headache, nausea, and fatigue. Five patients in our cohort developed symptomatic radionecrosis after SRT, all whom had received prior radiation to the index lesion; this compares favorably to prior studies reporting radionecrosis rates of approximately 3% to 9%.16, 17, 18, 19, 20
There are several limitations to this study. First, this study is retrospective and cannot account for nuanced differences in the utilization of SRT across the 3 participating institutions. Both the decision to use SRT and the decision to use a particular radiation scheme was at the discretion of the treating physician. Furthermore, our results only apply to the specific dose and fractionations used in our practice and do not provide insight regarding comparisons of each permutation of fraction size for the same total dose, (eg, 30 Gy delivered in 5 6-Gy fractions vs 3 10-Gy fractions).
Additionally, details regarding treatment planning and delivery, including institutional differences in contouring, planning software, or patient setup, could not be fully compared. Finally, although most patients in this study received daily treatments, a minority of patients did receive fractions separated by more than 1 day. These treatments tended to be among patients receiving larger fraction sizes with probable intent to reduce normal tissue injury; however, it may impact local control and reduce the ability to detect differences between treatment schemes because of repopulation and reoxygenation effects.
Nevertheless, this study demonstrates that SRT, delivered with varying dose and fractionation schemes can be a safe and effective treatment for brain metastases while simultaneously demonstrating that total prescription dose and not BED or dose per fraction is a primary predictor of local failure. This study suggests that total doses exceeding 25 Gy should be used to ensure adequate tumor control rates; however, prospective and randomized comparisons of dose and fractionation accounting for BED are needed to better elucidate potential differences in local control.
Footnotes
Conflicts of interest: None.
References
- 1.Gavrilovic I.T., Posner J.B. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 2.Mehta M., Vogelbaum M.A., Chang S. Neoplasms of the central nervous system. In: DeVita V.T. Jr., Lawrence T.S., Rosenberg S.A., editors. Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. pp. 1700–1749. [Google Scholar]
- 3.Borgelt B., Gelber R., Kramer S. The palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 4.Patchell R.A., Tibbs P.A., Regine W.F. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 5.Kocher M., Soffietti R., Abacioglu U. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyama H., Shirato H., Tago M. Stereotactic radiosurgery plus whole-brain radiation therapy versus stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Bentzen S.M., Renschler M. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 8.Crossen J.R., Garwood D., Glatstein E. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 9.Pui C., Cheng C., Leung W. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 10.Sun A., Kyounghwa B., Gore E.M. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw E., Scott C., Souhami L. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: Initial report of radiation therapy oncology group protocol (90-05) Int J Radiat Oncol Biol Phys. 1996;34:647–654. doi: 10.1016/0360-3016(95)02106-x. [DOI] [PubMed] [Google Scholar]
- 12.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 13.Mayo C., Yorke E., Merchant T.E. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S36–S41. doi: 10.1016/j.ijrobp.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leber K.A., Bergloff J., Langmann G. Radiation sensitivity of visual and oculomotor pathways. Stereotact Funct Neurosurg. 1995;64(1 Suppl):233–238. doi: 10.1159/000098784. [DOI] [PubMed] [Google Scholar]
- 15.Aoyama H., Shirato H., Onimaru R. Hypofractionated stereotactic radiotherapy alone without whole brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003;56:793–800. doi: 10.1016/s0360-3016(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 16.Kwon A.K., Dibiase S.J., Wang B. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer. 2009;115:890–898. doi: 10.1002/cncr.24082. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.W., Park H.R., Lee J.M. Fractionated stereotactic gamma knife radiosurgery for large brain metastases: A retrospective, single center study. PLoS One. 2016;11:e0163304. doi: 10.1371/journal.pone.0163304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarria P., Pessina F., Cozzi L. Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol. 2016;11:76. doi: 10.1186/s13014-016-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minniti G., D'Angelillo R.M., Scaringi C. Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol. 2014;117:295–301. doi: 10.1007/s11060-014-1388-3. [DOI] [PubMed] [Google Scholar]
- 20.Ernst-Stecken A., Ganslandt O., Lambrecht U. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: Results and toxicity. Radiother Oncol. 2006;81:18–24. doi: 10.1016/j.radonc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald D.R., Cascino T.L., Schold S.C., Jr. Response criteria for phase II studies of supratentorial malignant glioma. J Cli Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 22.Otsuka S., Shibamoto Y., Iwata H. Compatibility of the linear-quadratic formalism and biologically effective dose concept to high-dose-per-fraction irradiation in a murine tumor. Int J Radiat Oncol Biol Phys. 2011;81:1538–1543. doi: 10.1016/j.ijrobp.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Miyakawa A., Shibamoto Y., Otsuka S. Applicability of the linear-quadratic model to single and fractionated radiotherapy schedules: An experimental study. J Radiat Res. 2013;55:451–454. doi: 10.1093/jrr/rrt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheu T., Molkentine J., Transtrum M.K. Use of the LQ model with large fraction sizes results in underestimation of isoeffect doses. Radiother Oncol. 2013;109:21–25. doi: 10.1016/j.radonc.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Wennberg B., Lax I. The impact of fractionation in SBRT: Analysis with the linear quadratic model and the universal survival curve model. Acta Oncol. 2013;52:902–909. doi: 10.3109/0284186X.2012.728292. [DOI] [PubMed] [Google Scholar]
- 26.Park C., Papiez L., Zhang S. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 27.Andisheh B., Edgren M., Belkić D. A comparative analysis of radiobiological models for cell surviving fractions at high doses. Technol Cancer Res Treat. 2013;12:183–192. doi: 10.7785/tcrt.2012.500306. [DOI] [PubMed] [Google Scholar]
- 28.Franken N.A., Oei A.L., Kok H.P. Cell survival and radiosensitization: Modulation of the linear and quadratic parameters of the LQ model (review) Int J Oncol. 2013;42:1501–1515. doi: 10.3892/ijo.2013.1857. [DOI] [PubMed] [Google Scholar]
- 29.Brown J.M., Carlson D.J., Brenner D.J. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–262. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kased N., Huang K., Nakamura J.L. Gamma knife radiosurgery for brainstem metastases: The UCSF experience. J Neurooncol. 2008;86:195–205. doi: 10.1007/s11060-007-9458-4. [DOI] [PubMed] [Google Scholar]
- 31.Kelly P.J., Lin Y.B., Yu A.Y. Linear accelerator-based stereotactic radiosurgeryfor brainstem metastases: The Dana-Farber/Brigham and Women’s Cancer Center experience. J Neurooncol. 2011;104:553–557. doi: 10.1007/s11060-010-0514-0. [DOI] [PubMed] [Google Scholar]