Abstract
Esophageal cancer is one of the leading causes of cancer-related death and is associated with high morbidity and mortality. It carries a poor prognosis as more than half of patients present with advanced and unresectable disease. One contributing factor is the increased risk of lymph node metastases at early stages of disease. As such, it is essential to detect squamous cell neoplasia (SCN) at an early stage. In order to risk stratify lesions, endoscopists must be able to perform image enhanced endoscopy including magnification and Lugol’s chromoendoscopy. The assessment of both the horizontal extent and depth of any lesion is also of utmost importance prior to treatment. Endoscopic mucosal resection and submucosal dissection remain the standard of care with literature supportive their respective use. Radiofrequency ablation and other endoscopic treatments are currently available although should not be considered first line at this time. Our objective is to review the current options for the endoscopic diagnosis and treatment of esophageal SCN.
Keywords: Esophageal squamous cell neoplasia, Image enhanced endoscopy, Esophageal squamous cell carcinoma, endoscopic detection, Chromoendoscopy, Endoscopic mucosal resection, Endoscopic submucosal dissection
Core tip: Esophageal squamous cell carcinoma is one of the leading causes of cancer death. Improving the detection of early stage lesions remains of utmost importance as these lesions can be cured with endoscopic therapy. Endoscopists have many advanced imaging modalities available to assist in risk stratifying lesions. Endoscopic mucosal resection and submucosal dissection remain the standard of care with literature supportive their respective use. Radiofrequency ablation and other endoscopic treatments are currently available although should not be considered first line at this time. As we await improved endoscopic technologies, endoscopists everywhere must remain vigilant in their endoscopic evaluation of the esophagus during each and every endoscopy performed.
INTRODUCTION
Esophageal cancer is one of the leading causes of cancer-related death and is associated with high morbidity and mortality[1,2]. There are two predominant histologic types of esophageal cancer; squamous cell carcinoma (SCC) and Barrett’s esophagus related adenocarcinoma. The incidence of SCC is higher along two geographic belts, one from North-central China through central Asia to Northern Iran, and the other from Eastern to Southern Africa. Despite the predominance of esophageal adenocarcinoma in Western countries, SCC remains the most common subtype worldwide. It carries a poor prognosis as more than half of patients present with advanced and unresectable disease[3]. One contributing factor to this phenomenon is the increased risk of lymph node metastases at early stages of disease. While the risk of lymph node metastases is almost zero for EP (intraepithelial) and LPM (lamina propria) lesions, the risk increases to 8%-15% for lesions invading into the muscularis mucosa (MM), 11%-53% for lesions invading SM1 (submucosal layer to 200 μm or less) and 30%-54% for SM2 and deeper lesions[4-7]. For patients with adenocarcinoma confined to the MM however, the rate of lymph node metastases ranges from 0%-4% and has been estimated at 15%-25% for those with submucosal invasion[8-10]. With data showing a higher risk of lymph node metastases in SCC, detecting squamous cell neoplasia (squamous high grade intraepithelial neoplasia, HGIN, and early SCC) at an early stage becomes extremely important with subsequent interventions that can improve patient outcomes.
The objective of this article is to review the current options for the endoscopic diagnosis and treatment of esophageal squamous cell neoplasia (SCN).
ESOPHAGEAL SCC
Esophageal SCC is most prevalent in the sixth and seventh decades; with a male to female ratio of 3:1[11]. Known risk factors include regular alcohol consumption, smoking, aldehyde dehydrogenase type 2 deficiency, low fruit and vegetable intake, selenium, zinc, and vitamin E deficiency, high exposure to areca nuts and polycystic aromatic hydrocarbons and poor oral hygiene. Caustic injuries, tylosis, achalasia, and human papillomavirus (HPV) infection are other known risk factors[3,12-15]. As well, the risk of developing synchronous and/or metachronous lesions is high in patients with a history of head and neck and esophageal SCC. All of the aforementioned risk factors must be considered when determining who may benefit from screening.
There is currently no globally accepted endoscopic screening program for esophageal SCC. This is in spite of an improved understanding of the risk factors. The data in support of screening is not as robust as for Barrett’s esophagus and the detection of Barrett’s associated dysplasia. Studies on endoscopic surveillance for high-risk patients with histories of head and neck cancer have shown that it is feasible and effective[16-18]. A recent study from China revealed that endoscopic screening and intervention significantly lowered mortality caused by esophageal SCC[2]. Wei et al[2] compared the incidence and mortality of esophageal SCC in two communities (endoscopic screening vs control). In the intervention group, detected lesions were treated according to their respective stages. Although there was an initial increased incidence of SCC in the intervention group, likely related to the effect of screening, the cumulative incidence (over the full ten year follow-up period) of esophageal SCC in the screened group became lower than in the control group (4.2% vs 5.9%, respectively, P < 0.01). A reduction in cumulative mortality was seen in the intervention group (3.35% vs 5.05% respectively, P < 0.001). This study supports the notion that screening (and subsequent intervention) can lead to a reduction in the incidence of and mortality from esophageal SCC.
ENDOSCOPIC DETECTION
Endoscopic screening/detection is performed by using a combination of conventional and high-definition white light imaging (WLI), Lugol’s chromoendoscopy (CE), and image-enhanced endoscopy (IEE). The detection of early lesions with WLI can be challenging since the mucosal abnormalities present are often difficult to observe. The features used to detect dysplasia using WLI include the disappearance of the mucosal vascular network, nodular surface, subtle white coating, and erythema. The endoscope should be maneuvered slowly and carefully to allow for a thorough assessment of the entire esophagus paying special attention to commonly missed areas on the right lateral wall and in the narrow cervical esophagus. Observation using moderate insufflation is recommended as excessive insufflation may make it more difficult to identify flat lesions. Lao-Sirieix et al[19] showed that the sensitivity and specificity of WLI for the detection of severe dysplasia or cancer was 62% and 79% respectively. One must also pay close attention to the oropharynx when inserting the gastroscope, especially in patients known to have esophageal squamous neoplasia given the high risk of synchronous lesions. A recent study showed that 8.6% of patients followed over the 2-year study period were found to have metachronous head and neck SCC even after treatment of their esophageal SCC[20]. Asking patients to perform a valsalva maneuver can be considered to facilitate clear visualization of the hypopharyngeal area.
Chromoendoscopy with Lugol’s iodine has become the standard of care for the detection of esophageal SCC and synchronous lesions as well as defining the extent of lesions. Lugol’s iodine adheres to the glycogen of normal squamous epithelium leading to staining. Neoplastic lesions lack glycogen and therefore remain unstained. A thorough examination with Lugol’s CE includes an assessment for the following: (1) pink color sign (PC sign): This finding can be seen following Lugol’s staining where unstained areas change to a pink color after 2-3 min. While not well understood, it is thought that this phenomenon is most likely related to an absence of the keratinous layer[21]. This finding can be used to distinguish HGIN and SCC from LGIN, inflammation, and epithelial atrophy and has been shown to have high diagnostic accuracy with a 91.9% sensitivity and 94.0% specificity[22] (Figure 1); (2) tatami sign: This is the pattern commonly seen after iodine staining and is named after “Tatami”, a type of mat used as flooring material in traditional Japanese rooms. This is seen as regular, fine circular folds of the unstained area. This occurs with lesions invading no deeper than the MM[23] (Figure 2); and (3) multiple iodine unstained areas: This is a frequent finding seen in patients with esophageal SCN and is also known as “leopard-skin appearance”. This finding implies a high risk of synchronous lesions and/or metachronous recurrence[24]. A recent study following patients for 2 years after treatment of esophageal SCC revealed that 24.7% of patients who had 10 or more Lugol’s unstained areas developed metachronous SCC[20].
Figure 1.
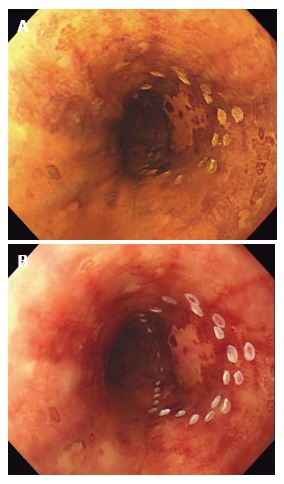
Chromoendoscopy with Lugol’s iodine. A: Unstained area seen within the marking after spraying diluted Lugol’s solution with spray catheter; B: After observing for several minutes, the unstained area turned into pink color, suggesting HGIN and squamous cell carcinoma.
Figure 2.
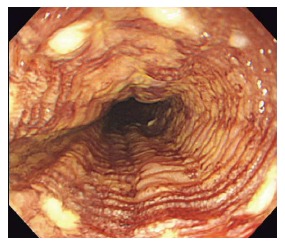
“Tatami sign” is commonly seen after iodine staining. It is characterized by regular, fine circular folds of the Lugol’s unstained area. This is typically seen when lesions are confined to the muscularis mucosal.
By picking up on the above findings with Lugol’s CE, lesions requiring endoscopic treatment can be identified. However, there are some disadvantages to using it. Firstly, staining with Lugol’s iodine is known to cause retrosternal chest discomfort and carries a risk of allergic reaction. Secondly, the aforementioned finding of unstained areas has been shown to have high sensitivity but low specificity for detecting HGIN and early SCC. Finally, re-epithelialization after mucosal damage, often caused by the chemical esophagitis from Lugol’s staining, may obscure delineation of lesions when performing subsequent endoscopic resection[25]. To overcome some of these concerns, less concentrated Lugol’s solution with concentrations of 0.5%-1% or less (vs 3% traditionally) are now used to reduce mucosal irritation. In addition, spraying 20 mL of 20% sodium thiosulfate solution (STS; 10% Detoxol, Banyu Pharmaceutical Co. Ltd., Tokyo, Japan) can be performed to neutralize the Lugol’s iodine solution[26]. Aspiration of the residual Lugol’s iodine from the stomach also minimizes mucosal irritation.
IEE augments the detection, diagnosis and treatment of esophageal SCN. Narrow-band imaging (NBI) (Olympus, Japan), Pentax I-Scan (Pentax, Japan), and the Fujinon Intelligence Colour Enhancement system (FICE, Fujinon Corporation, Japan) are frequently applied electronic-based endoscopic modalities that target the microvessels of the mucosa. Although all of the above can be utilized to detect early esophageal SCN, narrow-band imaging (NBI) is used most commonly. It uses specific blue and green wavelength light to illuminate blood vessels more distinctly in comparison to WLI. Non-magnification NBI (NM-NBI) allows endoscopists to recognize esophageal SCN as brownish areas (Figure 3). In Japan, it is standard to utilize IEE during the observation of the oropharynx and esophagus during endoscope withdrawal. As opposed to Lugol’s CE, using IEE is safe and easily performed with the push of a single button on the endoscope.
Figure 3.
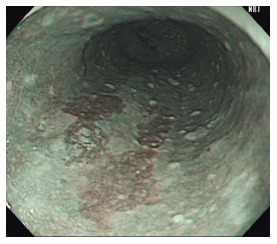
Brownish areas seen under non-magnifying narrow-band imaging can be seen with inflammation, low-grade and high-grade intraepithelial neoplasm, and squamous cell carcinoma.
White-light imaging vs image enhanced endoscopy vs Lugol’s chromoendoscopy
A multicenter, prospective, randomized, controlled trial conducted in 2010 comparing WLI and NM-NBI revealed a significantly higher detection rate of small (< 5 mm), early SCC lesions with NM-NBI when compared to WLI[27]. Nagami et al[28] compared NM-NBI and Lugol’s CE in 202 patients with risk factors for esophageal SCC. The operating characteristics of NM-NBI were superior to that of Lugol’s CE as the accuracy, sensitivity, and specificity of NM-NBI were 77.0%, 88.3%, and 75.2% respectively, compared with 68.0%, 94.2%, and 64.0% respectively for unstained areas detected by Lugol’s CE. This study was limited, however, by the fact that the authors did not incorporate the pink color sign into their assessments. Goda et al[29] conducted a randomized, non-inferiority trial comparing ME-NBI to the PC sign seen on Lugol’s CE. They found no sig_nificant differences between these two techniques. ME-NBI showed a significantly shorter examination time with similar accuracy, however was less reliable in patients with multiple Lugol’s unstained areas. As such, the optimal use of these modalities remains unclear. We recommend that a standard screening endoscopy should include WLI and NM-NBI. When mucosal changes are seen on WLI or brownish areas identified on NM-NBI, ME-NBI or Lugol’s CE should be considered for further assessment.
Lateral margin assessment
After detecting a lesion, it is important to delineate the lateral extent of the lesion in order to achieve R0 resection. This is accomplished via careful inspection with a combination of WLI, IEE, and Lugol’s CE[30-32]. Typically, the assessment of lateral extent is done with Lugol’s CE. It is important to keep in mind that if the preoperative assessment is done with Lugol’s CE, it may cause chemical esophagitis resulting in re-epithelialization thereby complicating the demarcation of tumors at the time of endoscopic treatment[25]. As such, there is a theoretical advantage to performing Lugol’s CE and endoscopic resection during the index endoscopy and/or using IEE alone (without Lugol’s CE) to examine the lateral margins of a lesion.
Depth assessment
After detecting esophageal SCN, it is of utmost importance to predict a lesion’s depth of invasion to determine if endoscopic resection is possible. Such assessment begins with WLI and the use of the Paris classification[33]. Paris 0-IIa, 0-IIb, 0-IIc lesions, which are flat or slightly elevated/depressed lesions, are generally confined to the EP and LPM. Slight color change with redness and irregular elevation/depression raise the possibility of deeper invasion into the MM and SM1. If the lesion has a large, broad-based protrusion, crater, and/or stiffened wall, the lesion likely invades deeper. A majority of protruded (Paris classification 0-I) and/or excavated (0-III) lesions usually represent invasion into the submucosa or deeper. A recent multicenter, prospective study showed that the accuracy of invasion depth using WLI alone was 71.4%. The sensitivity and specificity for MM lesions was 61.1% and 77.4% respectively[34].
Recently, ME-NBI has been utilized to assess lesion depth. This allows for the assessment of the surface capillary microvasculature or intraepithelial papillary capillary loop (IPCL), a superficial fine vascular network of the esophageal mucosa. Attention should be made to the four signs of abnormal vessels which include dilatation, tortuosity, caliber change, and non-uniformity. The IPCL is known to change morphologically depending on the severity of structural irregularities in the esophageal mucosa. The Japanese Esophageal Society has developed a simplified magnifying endoscopic classification for estimating invasion depth based on the degree of irregularity of the microvascular morphology[35]. The microvessels are classified into two main categories; Type A or B. Microvessels are considered Type B vessels when all four features of abnormal IPCL (dilatation, tortuosity, caliber change, and non-uniformity) are seen. Type B can be further sub-classified into B1, B2, and B3. B1 vessels can be seen as dot-like microvessels under NM-NBI or low magnification ME-NBI (Figure 4). B2 vessels can be recognized as stretched and markedly elongated microvessels. B3 vessels are dilated and abnormal vessels (Figure 5). When type B1, B2, B3 microvessels are identified on ME-NBI, the extent of invasion is likely into the EP-LPM, MM-SM1, and SM2 or deeper, respectively. Goda et al[36] reported that ME-NBI could differentiate intramucosal cancer from submucosal cancer with a sensitivity and specificity of 78% and 95% respectively. On the other hand, a recent multicenter, prospective study showed no additional benefit of adding ME-NBI to WLI for the assessment of invasion depth[34].
Figure 4.
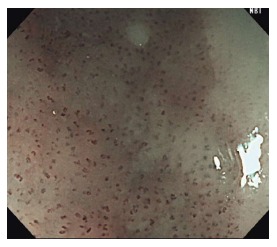
Abnormal intraepithelial papillary capillary loop, a superficial fine vascular network of the esophageal mucosa. Type B1 vessels, shown here, are identified as dot-like microvessels under non-magnifying narrow-band imaging or low magnified ME-NBI.
Figure 5.
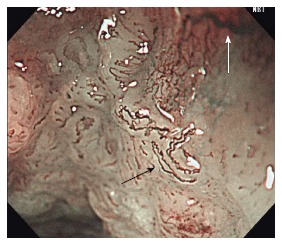
Abnormal intraepithelial papillary capillary loop. Type B2 (black arrow) can be recognized as stretched and markedly elongated microvessels vs type B3 (white arrow) microvessels which are highly dilated, abnormal vessels.
Endoscopic ultrasound (EUS) is another modality used to assess the depth of invasion of esophageal SCC. Its utility is to rule out invasion into the muscularis propria and detect regional lymphadenopathy. A meta-analysis reported that among patients with T1 disease, EUS had a pooled sensitivity in differentiating T1a and T1b lesions of 84% and 83%, and a specificity of 91% and 89%. For T4 lesions, EUS had a pooled sensitivity of 84% and specificity of 96%. The overall accuracy of EUS for T-staging was 79% and 71% for N-staging[37]. In spite of its limitations (time, expertise required, expense and resolution), EUS is currently considered a standard modality used in the evaluation of lesion extent and regional lymphadenopathy. CT scan and PET-CT scan are adjunct modalities that should be considered in the evaluation of regional lymphadenopathy[32].
Other diagnostic modalities
There are other emerging advanced diagnostic imaging modalities such as confocal laser endomicroscopy (CLE) and optical coherence tomography (OCT) that may play a role in screening and/or diagnosis of SCC. Probe-based confocal laser endomicroscopy (pCLE) allows for real-time in-vivo histologic imaging of the esophagus. Its use has been shown to improve the detection of BE-associated dysplasia when compared to WLE alone[38]. Although there are proposed diagnostic criteria for the detection of squamous epithelial cells with pCLE (irregular arrangement, increased diameter, irregular shape and long branching of the IPCL), the diagnostic accuracy of pCLE is not well known[39]. pCLE is limited by its imaging depth and field of view and thus standard imaging with WLE and chromoendoscopy is required for detecting lesions. Recently, Guo et al[40] described the diagnostic value of pCLE for esophageal SCN. The authors reported high sensitivity, specificity, and accuracy of pCLE for SCN as 94.6%, 90.7%, and 92.3% respectively. In the other recent study comparing ME-NBI and probe-based CLE, pCLE possessed higher specificity and accuracy[41].
Volumetric laser endomicroscopy, a second-generation optical coherence technology, is an advanced, non-invasive imaging modality that uses infrared light to produce real-time high-resolution cross-sectional images of the GI tract. Its resolution (10-20 μm) is extremely fine approaching that of histopathology. This technology has been applied to the detection of Barrett’s esophagus related dysplasia in clinical practice[42-44]. For esophageal SCC, it has been reported to be useful in assessing tumor invasion depth. Hatta et al[45] utilized OCT for pre-operative staging with a high overall accuracy rate. Its ability to select lesions for endoscopic resection was significantly better than EUS (94.6% vs 80.6%, P < 0.05). OCT is limited by its inability to accurately detect dysplasia and therefore requires further study, like pCLE, before it can be incorporated into clinical practice. In addition to the aforementioned limitations, we have futher concerns with respect to cost effectiveness, acquisition of expertise in image interpretation and indication standardization that may limit widespread use.
TREATMENT OF ESOPHAGEAL SQUAMOUS CELL NEOPLASIA
Traditionally, esophagectomy and lymph node dissection have been the standard of care for the treatment of esophageal cancer including early esophageal SCC. However, the paradigm has shifted to less invasive therapy with improved techniques in endoscopic resection. There are two widely accepted endoscopic resection methods: Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). These are indicated when there is minimal to no risk of lymph node metastases.
EMR
EMR has been widely accepted as a safe and effective treatment for early esophageal SCC. Cap-assisted mucosectomy (EMR-C) and EMR-ligation (EMR-L) are the two methods of performing EMR. A randomized trial comparing EMR-L with multiband mucosectomy (MBM) and EMR-C for endoscopic piecemeal resection of large SCN (2-6 cm, maximum 2/3 of esophageal circumference) has been conducted[46]. Both methods were highly effective and safe, however EMR-L with MBM was faster and less expensive. Although large lesions can be treated with piecemeal EMR, this technique is limited by its inability to achieve oncologic (R0) resections. In the aforementioned study, a high local recurrence rate was seen in lesions exceeding 2 cm and in lesions subject to piecemeal resection in five or more pieces. The optimal size for EMR is still debatable. To overcome the limitation of piecemeal resection, endoscopic submucosal dissection (ESD) has become the treatment of choice for en bloc resection of larger lesions.
ESD
ESD was initially introduced in the 1990s to allow en bloc resection with detailed pathological assessment regardless of lesion size[47]. This method spread rapidly as it optimized the rate of en bloc resection in gastric and colonic lesions. Yet, ESD is known to be more time consuming and associated with higher rates of complications such as bleeding and perforation. This is especially true in the esophagus. Esophageal ESD is technically more challenging than gastric ESD due to a narrower lumen thus limiting endoscopic maneuvers. Movement due to heartbeat and respiration are other factors making this technique more challenging. Moreover, the esophagus has a thin muscle wall and an absence of serosal fat leading to higher perforation rates. Previous literature in Japan has shown that ESD in the esophagus has a high en bloc resection rate (95%-100%), a low local recurrence rate (0%-1%), and is a relatively safe procedure with perforation occurring in 0%-6% of cases. However, most early studies were limited by small sample sizes and short follow-up. Tsujii et al[48] recently reported clinical outcomes of ESD in a multicenter, retrospective, cohort study. A total of 368 superficial esophageal neoplasms in 307 patients were treated by ESD at 11 hospitals. The en bloc resection and complete resection rates were 96.7% and 84.5% respectively. Perforation, bleeding and esophageal strictures occurred in 5.2%, 0%, and 7.1% of patients, respectively. All complications were successfully managed conservatively[48]. More recent studies show an en bloc resection rate between 83%-100%, R0 resection rate between 78%-100% and local recurrence rates of 0%-6%[49-57]. The experience of Western endoscopists with ESD in esophageal SCC was recently published. Probst et al[58] reported the outcome of 24 SCC undergoing ESD resulting in en bloc resection in 100% and R0 resection in 91.7% of the cases. Disease-specific survival was 95.8% and overall survival was 66.7% over a mean follow-up period of 38 mo[58].
EMR vs ESD
Although there is ongoing debate as to what size lesions should be resected by ESD vs EMR, the European Society of Gastrointestinal Endoscopy (ESGE) recommends endoscopic en bloc resection for superficial esophageal SCC without obvious submucosal involvement. EMR may be considered for lesions smaller than 20 mm if en bloc resection is feasible. If not, ESD should be performed. According to the Japanese Esophageal Society, absolute indications for endoscopic mucosal resection are: flat intramucosal cancers confined to the epithelium and lamina propria that occupy less than 2/3 of the circumference of the lumen of the esophagus. Relative indications include cancers involving the muscularis mucosa or < 200 μm of invasion into the submucosa, and lesions extending more than ¾ of the circumference of the lumen[59].
The literature comparing these two methods is scant. Ishihara et al[60] analyzed the outcomes of EMR and ESD in 171 superficial esophageal SCC’s less than 20 mm in diameter. For lesions less than 15 mm, there was no difference in local recurrence, R0 resection, and en bloc resection concluding that EMR is feasible for treating lesions this small. Recently, another retrospective study compared the efficacy and safety of EMR-L using MBM and ESD. They found no statistical difference in the rate of complete resection between the two methods. For lesions over 15 mm however, ESD had a significantly higher rate of en bloc (100%) and curative resection (92.3%) compared to MBM (44.8 and 41%, P < 0.05). As expected, ESD procedure times were longer (84 ± 35 min vs 38 ± 11 min). In addition, ESD had higher rate of major bleeding (16.7% vs 1.85%) and perforation (8.3% vs 0%). As such, EMR appears safe and effective for the treatment of lesions 15 mm or smaller leaving ESD as the treatment of choice for larger lesions[61].
Post-endoscopic resection strictures
One of the major complications of endoscopic resection for large SCN is esophageal stricturing, which can severely affect patients’ quality of life. Treatment related strictures requiring multiple sessions of endoscopic dilation can occur when resecting lesions involving over half of the esophageal circumference. Lesions greater than or equal to 3/4 of the circumference of the lumen are strongly associated with stricturing[62,63]. In addition, muscle layer damage and defects larger than 5 cm after circumferential ESD were significant factors associated with refractory stenosis[64]. Typically, steroids injected locally and/or taken orally are used to prevent post-ESD strictures for lesions involving over 3/4 of the esophageal circumference. There are differences in the timing of administration, route of administration (local vs oral), and the dosages among institutions. The most common strategy in Japan is to inject triamcinolone into the base of the post-ESD ulcer directly post-procedure or on post-procedure day one. With the prevalence of this complication, more studies are needed to further elucidate the ideal prevention strategies post-endoscopic resection.
Post-endoscopic resection therapy
Treatment post-endoscopic resection is determined based on the pathological evaluation of the resected specimen. No additional treatment is required for EP and LPM lesions with no lymphovascular invasion as the probability of lymph node metastases approaches 0%. Although MM and SM1 lesions have a risk of lymph node metastases, most can be predicted based on the presence of lymphovascular invasion or poor differentiation in the resected specimen. In 2011, Moriya et al[65] showed that well-differentiated MM and SM1 tumors without lymphovascular invasion had minimal risk of lymph node involvement. Therefore, close follow-up is acceptable for such lesions. Surgery and/or chemoradiation should be considered for lesions with lymphovascular invasion or SM2 or deeper involvement. The 5-year overall survival rate for patients with EP, LPM, MM, and SM cancers is 90.5%, 71.1%, and 70.8% respectively[66]. As mentioned, it is very important to consider the risk of metachronous lesions in patients with esophageal SCC and therefore, close endoscopic surveillance is needed.
Radiofrequency ablation
Radiofrequency ablation (RFA) has emerged as the ablative technique of choice for Barrett’s esophagus related dysplasia. Since a multicenter, randomized, sham-controlled study demonstrated decreased disease progression to malignancy along with high rates of dysplasia and intestinal metaplasia eradication, RFA has been used to treat BE in patients with HGD[67]. However, the role of RFA in squamous neoplasia remains unclear. RFA involves the direct application of thermal energy to the targeted area using either a balloon for circumferential treatment (HALO360; BARRX, Sunnyvale, California, United States), a probe attached to the scope (HALO90, HALO60, HALO-ultra; BARRX) or a through-the-scope catheter for focal therapy (Channel Catheter; BARRX). The clear benefit and role of radiofrequency ablation in SCN has not yet been proven. There are reports with small patient populations describing a possible role for RFA in early esophageal SCN[68-70]. The largest study consists of a total of 96 patients including 42 patients with HGIN and 9 with early SCC. At 3 and 12 mo post-RFA treatment, 73 % (70/96) and 84 % (81/96) showed an absence of dysplasia and SCC (considered a complete response). Two patients progressed in spite of RFA (MGIN to HGIN and HGIN to SCC respectively) however both were treated endoscopically and achieved complete response with additional ablations. Strictures occurred in 20 patients (21%), all of whom underwent circumferential RFA. Lugol’s CE with RFA (12 J/cm2, single application, no cleaning) was the favored baseline circumferential RFA technique. In patients with SCN, RFA appears to be associated with a high response rate and an acceptable safety profile[70].
The clear disadvantage of ablative therapy is that no tissue is obtained for histopathological assessment. Proper staging and risk stratification with various endoscopic techniques described above must be done prior to treatment to appropriately select patients thereby avoiding treatment failures. On the other hand, there may be a role for RFA in cases where endoscopic resection is challenging. The feasibility of RFA for treating early SCC on or adjacent to esophageal varices was recently reported. While this study was limited to only 8 patients (5 HGIN and 3 SCC), 6 patients achieved complete response after a single circumferential treatment. All achieved a complete response at 12 mo with further focal ablation therapy. Although there is data revealing that RFA is both safe and efficacious, it should only be considered when the lesion is deemed to be non-invasive. Currently, endoscopic resection with histological evaluation should be the first choice and we believe that we should be conservative on applying RFA to squamous cell neoplasia. We need more studies regarding safety and efficacy of RFA in squamous cell neoplasia.
Other ablative therapies such as photodynamic therapy (PDT), argon plasma coagulation (APC) and Nd: YAG laser have been discussed in the literature[71-74]. Recently, PDT was described as a palliative or less-invasive salvage treatment option for local failure after chemoradiotherapy (CRT). Retrospective analysis of 130 patients treated with PDT for local failure (T2 lesions without metastases) after CRT was performed[75]. The complete response rate, progression-free survival and the overall survival rates at 5 years after salvage PDT were 58.4%, 22.1% and 35.9% respectively. The treatment-related death rate was 1.8%. APC is another ablative therapy used to safely treat lesions that are not endoscopically resectable and to control the recurrence after endoscopic resection or chemoradiotherapy[76]. However, the literature supporting this technique is lacking.
CONCLUSION
Esophageal SCC remains one of the leading causes of cancer death. Improving the detection of early stage lesions remains of utmost importance as these lesions can be cured with endoscopic therapy. With improvements in advanced imaging modalities, we will better be able to detect, assess, and treat lesions at earlier stages. As we await improved endoscopic technologies, endoscopists everywhere must remain vigilant in their endoscopic evaluation of the esophagus during each and every endoscopy performed.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: March 7, 2017
First decision: April 17, 2017
Article in press: August 17, 2017
P- Reviewer: Goda K, Uno K S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
Contributor Information
Yuto Shimamura, Division of Gastroenterology, St. Michael’s Hospital, University of Toronto, Toronto, ON M5B1W8, Canada.
Takashi Ikeya, Department of Gastroenterology, St. Luke’s International Hospital, Tokyo 104-8560, Japan.
Norman Marcon, Division of Gastroenterology, St. Michael’s Hospital, University of Toronto, Toronto, ON M5B1W8, Canada.
Jeffrey D Mosko, Division of Gastroenterology, St. Michael’s Hospital, University of Toronto, Toronto, ON M5B1W8, Canada. moskoj@smh.ca.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Wei WQ, Chen ZF, He YT, Feng H, Hou J, Lin DM, Li XQ, Guo CL, Li SS, Wang GQ, et al. Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China. J Clin Oncol. 2015;33:1951–1957. doi: 10.1200/JCO.2014.58.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Natsugoe S, Baba M, Yoshinaka H, Kijima F, Shimada M, Shirao K, Kusano C, Fukumoto T, Mueller J, Aikou T. Mucosal squamous cell carcinoma of the esophagus: a clinicopathologic study of 30 cases. Oncology. 1998;55:235–241. doi: 10.1159/000011857. [DOI] [PubMed] [Google Scholar]
- 5.Tajima Y, Nakanishi Y, Tachimori Y, Kato H, Watanabe H, Yamaguchi H, Yoshimura K, Kusano M, Shimoda T. Significance of involvement by squamous cell carcinoma of the ducts of esophageal submucosal glands. Analysis of 201 surgically resected superficial squamous cell carcinomas. Cancer. 2000;89:248–254. doi: 10.1002/1097-0142(20000715)89:2<248::aid-cncr7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Bollschweiler E, Baldus SE, Schröder W, Prenzel K, Gutschow C, Schneider PM, Hölscher AH. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38:149–156. doi: 10.1055/s-2006-924993. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Azuma M, Nakatani K, Ishido K, Naruke A, Ryu T. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res. 2009;3:153–161. [PMC free article] [PubMed] [Google Scholar]
- 8.Hölscher AH, Bollschweiler E, Schneider PM, Siewert JR. Early adenocarcinoma in Barrett’s oesophagus. Br J Surg. 1997;84:1470–1473. [PubMed] [Google Scholar]
- 9.Stein HJ, Feith M, Mueller J, Werner M, Siewert JR. Limited resection for early adenocarcinoma in Barrett’s esophagus. Ann Surg. 2000;232:733–742. doi: 10.1097/00000658-200012000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigro JJ, DeMeester SR, Hagen JA, DeMeester TR, Peters JH, Kiyabu M, Campos GM, Oberg S, Gastal O, Crookes PF, et al. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg. 1999;117:960–968. doi: 10.1016/S0022-5223(99)70377-6. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama A, Muramatsu T, Ohmori T, Higuchi S, Hayashida M, Ishii H. Esophageal cancer and aldehyde dehydrogenase-2 genotypes in Japanese males. Cancer Epidemiol Biomarkers Prev. 1996;5:99–102. [PubMed] [Google Scholar]
- 13.Chung CS, Lee YC, Wu MS. Prevention strategies for esophageal cancer: Perspectives of the East vs. West. Best Pract Res Clin Gastroenterol. 2015;29:869–883. doi: 10.1016/j.bpg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Petrick JL, Wyss AB, Butler AM, Cummings C, Sun X, Poole C, Smith JS, Olshan AF. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer. 2014;110:2369–2377. doi: 10.1038/bjc.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardefeldt HA, Cox MR, Eslick GD. Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis. Epidemiol Infect. 2014;142:1119–1137. doi: 10.1017/S0950268814000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubuc J, Legoux J-, Winnock M, Seyrig J-, Barbier J-, Barrioz T, Laugier R, Boulay G, Grasset D, Sautereau D, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006;38:690–695. doi: 10.1055/s-2006-925255. [DOI] [PubMed] [Google Scholar]
- 17.Scherübl H, von Lampe B, Faiss S, Däubler P, Bohlmann P, Plath T, Foss HD, Scherer H, Strunz A, Hoffmeister B, et al. Screening for oesophageal neoplasia in patients with head and neck cancer. Br J Cancer. 2002;86:239–243. doi: 10.1038/sj.bjc.6600018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su YY, Chen WC, Chuang HC, Guo CS, Lin YT, Luo SD, Fang FM, Chien CY. Effect of routine esophageal screening in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139:350–354. doi: 10.1001/jamaoto.2013.46. [DOI] [PubMed] [Google Scholar]
- 19.Lao-Sirieix P, Fitzgerald RC. Screening for oesophageal cancer. Nat Rev Clin Oncol. 2012;9:278–287. doi: 10.1038/nrclinonc.2012.35. [DOI] [PubMed] [Google Scholar]
- 20.Katada C, Yokoyama T, Yano T, Kaneko K, Oda I, Shimizu Y, Doyama H, Koike T, Takizawa K, Hirao M, et al. Alcohol Consumption and Multiple Dysplastic Lesions Increase Risk of Squamous Cell Carcinoma in the Esophagus, Head, and Neck. Gastroenterology. 2016;151:860–869.e7. doi: 10.1053/j.gastro.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara R, Kanzaki H, Iishi H, Nagai K, Matsui F, Yamashina T, Matsuura N, Ito T, Fujii M, Yamamoto S, et al. Pink-color sign in esophageal squamous neoplasia, and speculation regarding the underlying mechanism. World J Gastroenterol. 2013;19:4300–4308. doi: 10.3748/wjg.v19.i27.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu Y, Omori T, Yokoyama A, Yoshida T, Hirota J, Ono Y, Yamamoto J, Kato M, Asaka M. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol. 2008;23:546–550. doi: 10.1111/j.1440-1746.2007.04990.x. [DOI] [PubMed] [Google Scholar]
- 23.Muto M. Endoscopic diagnostic strategy of superficial esophageal squamous cell carcinoma. Dig Endosc. 2013;25 Suppl 1:1–6. doi: 10.1111/den.12025. [DOI] [PubMed] [Google Scholar]
- 24.Katada C, Muto M, Tanabe S, Higuchi K, Sasaki T, Azuma M, Ishido K, Katada N, Sakuramoto S, Yamashita K, et al. Factors associated with the presence of multiple Lugol-voiding lesions in patients with esophageal squamous-cell carcinoma. Dis Esophagus. 2014;27:457–462. doi: 10.1111/j.1442-2050.2012.01429.x. [DOI] [PubMed] [Google Scholar]
- 25.Asada-Hirayama I, Ono S, Kodashima S, Niimi K, Mochizuki S, Yamamichi N, Fujishiro M, Matsusaka K, Fukayama M, Koike K. Preoperative iodine staining may complicate the demarcation of esophageal carcinoma. Gut Liver. 2013;7:492–496. doi: 10.5009/gnl.2013.7.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo H, Fukuda H, Ono H, Gotoda T, Saito D, Takahiro K, Shirao K, Yamaguchi H, Yoshida S. Sodium thiosulfate solution spray for relief of irritation caused by Lugol’s stain in chromoendoscopy. Gastrointest Endosc. 2001;53:199–202. doi: 10.1067/mge.2001.110730. [DOI] [PubMed] [Google Scholar]
- 27.Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–1572. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagami Y, Tominaga K, Machida H, Nakatani M, Kameda N, Sugimori S, Okazaki H, Tanigawa T, Yamagami H, Kubo N, et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014;109:845–854. doi: 10.1038/ajg.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goda K, Dobashi A, Yoshimura N, Kato M, Aihara H, Sumiyama K, Toyoizumi H, Kato T, Ikegami M, Tajiri H. Narrow-Band Imaging Magnifying Endoscopy versus Lugol Chromoendoscopy with Pink-Color Sign Assessment in the Diagnosis of Superficial Esophageal Squamous Neoplasms: A Randomised Noninferiority Trial. Gastroenterol Res Pract. 2015;2015:639462. doi: 10.1155/2015/639462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue H, Rey JF, Lightdale C. Lugol chromoendoscopy for esophageal squamous cell cancer. Endoscopy. 2001;33:75–79. [PubMed] [Google Scholar]
- 31.Kuwano H, Kitamura K, Baba K, Morita M, Matsuda H, Mori M, Sugimachi K. Determination of the resection line in early esophageal cancer using intraoperative endoscopic examination with Lugol staining. J Surg Oncol. 1992;50:149–152. doi: 10.1002/jso.2930500304. [DOI] [PubMed] [Google Scholar]
- 32.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 33.Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 34.Ebi M, Shimura T, Yamada T, Mizushima T, Itoh K, Tsukamoto H, Tsuchida K, Hirata Y, Murakami K, Kanie H, et al. Multicenter, prospective trial of white-light imaging alone versus white-light imaging followed by magnifying endoscopy with narrow-band imaging for the real-time imaging and diagnosis of invasion depth in superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2015;81:1355–1361.e2. doi: 10.1016/j.gie.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, Hirasawa D, Takeuchi M, Tomori A, Goda K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–112. doi: 10.1007/s10388-016-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goda K, Tajiri H, Ikegami M, Yoshida Y, Yoshimura N, Kato M, Sumiyama K, Imazu H, Matsuda K, Kaise M, et al. Magnifying endoscopy with narrow band imaging for predicting the invasion depth of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:453–460. doi: 10.1111/j.1442-2050.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 37.Thosani N, Singh H, Kapadia A, Ochi N, Lee JH, Ajani J, Swisher SG, Hofstetter WL, Guha S, Bhutani MS. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242–253. doi: 10.1016/j.gie.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A, et al. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465–472. doi: 10.1016/j.gie.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, Zuo XL, Li CQ, Zhang JN, Guo YT, Zhang TG. Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy. 2009;41:99–106. doi: 10.1055/s-0028-1119492. [DOI] [PubMed] [Google Scholar]
- 40.Guo J, Li CQ, Li M, Zuo XL, Yu T, Liu JW, Liu J, Kou GJ, Li YQ. Diagnostic value of probe-based confocal laser endomicroscopy and high-definition virtual chromoendoscopy in early esophageal squamous neoplasia. Gastrointest Endosc. 2015;81:1346–1354. doi: 10.1016/j.gie.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 41.Prueksapanich P, Pittayanon R, Rerknimitr R, Wisedopas N, Kullavanijaya P. Value of probe-based confocal laser endomicroscopy (pCLE) and dual focus narrow-band imaging (dNBI) in diagnosing early squamous cell neoplasms in esophageal Lugol’s voiding lesions. Endosc Int Open. 2015;3:E281–E288. doi: 10.1055/s-0034-1391903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trindade AJ, Vamadevan AS, Sejpal DV. Finding a needle in a haystack: use of volumetric laser endomicroscopy in targeting focal dysplasia in long-segment Barrett’s esophagus. Gastrointest Endosc. 2015;82:756; discussion 757. doi: 10.1016/j.gie.2015.03.1984. [DOI] [PubMed] [Google Scholar]
- 43.Trindade AJ, George BJ, Berkowitz J, Sejpal DV, McKinley MJ. Volumetric laser endomicroscopy can target neoplasia not detected by conventional endoscopic measures in long segment Barrett’s esophagus. Endosc Int Open. 2016;4:E318–E322. doi: 10.1055/s-0042-101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leggett CL, Gorospe E, Owens VL, Anderson M, Lutzke L, Wang KK. Volumetric laser endomicroscopy detects subsquamous Barrett’s adenocarcinoma. Am J Gastroenterol. 2014;109:298–299. doi: 10.1038/ajg.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatta W, Uno K, Koike T, Iijima K, Asano N, Imatani A, Shimosegawa T. A prospective comparative study of optical coherence tomography and EUS for tumor staging of superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2012;76:548–555. doi: 10.1016/j.gie.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Zhang YM, Boerwinkel DF, Qin X, He S, Xue L, Weusten BL, Dawsey SM, Fleischer DE, Dou LZ, Liu Y, et al. A randomized trial comparing multiband mucosectomy and cap-assisted endoscopic resection for endoscopic piecemeal resection of early squamous neoplasia of the esophagus. Endoscopy. 2016;48:330–338. doi: 10.1055/s-0034-1393358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 48.Tsujii Y, Nishida T, Nishiyama O, Yamamoto K, Kawai N, Yamaguchi S, Yamada T, Yoshio T, Kitamura S, Nakamura T, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. 2015;47:775–783. doi: 10.1055/s-0034-1391844. [DOI] [PubMed] [Google Scholar]
- 49.Kanzaki H, Ishihara R, Ohta T, Nagai K, Matsui F, Yamashina T, Hanafusa M, Yamamoto S, Hanaoka N, Takeuchi Y, et al. Randomized study of two endo-knives for endoscopic submucosal dissection of esophageal cancer. Am J Gastroenterol. 2013;108:1293–1298. doi: 10.1038/ajg.2013.161. [DOI] [PubMed] [Google Scholar]
- 50.Kawahara Y, Hori K, Takenaka R, Nasu J, Kawano S, Kita M, Tsuzuki T, Matsubara M, Kobayashi S, Okada H, et al. Endoscopic submucosal dissection of esophageal cancer using the Mucosectom2 device: a feasibility study. Endoscopy. 2013;45:869–875. doi: 10.1055/s-0033-1344229. [DOI] [PubMed] [Google Scholar]
- 51.Fujinami H, Hosokawa A, Ogawa K, Nishikawa J, Kajiura S, Ando T, Ueda A, Yoshita H, Sugiyama T. Endoscopic submucosal dissection for superficial esophageal neoplasms using the stag beetle knife. Dis Esophagus. 2014;27:50–54. doi: 10.1111/dote.12039. [DOI] [PubMed] [Google Scholar]
- 52.Yamashina T, Ishihara R, Uedo N, Nagai K, Matsui F, Kawada N, Oota T, Kanzaki H, Hanafusa M, Yamamoto S, et al. Safety and curative ability of endoscopic submucosal dissection for superficial esophageal cancers at least 50 mm in diameter. Dig Endosc. 2012;24:220–225. doi: 10.1111/j.1443-1661.2011.01215.x. [DOI] [PubMed] [Google Scholar]
- 53.Repici A, Hassan C, Carlino A, Pagano N, Zullo A, Rando G, Strangio G, Romeo F, Nicita R, Rosati R, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010;71:715–721. doi: 10.1016/j.gie.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Chaves DM, Moura EG, Milhomem D, Arantes VN, Yamazaki K, Maluf F, Albuquerque W, Conrado AC, Araújo JC, Uejo PH, et al. Initial experience of endoscopic submucosal dissection in Brazil to treat early gastric and esophagheal cancer: a multi-institutional analysis. Arq Gastroenterol. 2013;50:148–152. doi: 10.1590/s0004-28032013000200025. [DOI] [PubMed] [Google Scholar]
- 55.Teoh AY, Chiu PW, Yu Ngo DK, Wong SK, Lau JY, Ng EK. Outcomes of endoscopic submucosal dissection versus endoscopic mucosal resection in management of superficial squamous esophageal neoplasms outside Japan. J Clin Gastroenterol. 2010;44:e190–e194. doi: 10.1097/MCG.0b013e3181ce52fb. [DOI] [PubMed] [Google Scholar]
- 56.Park JS, Youn YH, Park JJ, Kim JH, Park H. Clinical Outcomes of Endoscopic Submucosal Dissection for Superficial Esophageal Squamous Neoplasms. Clin Endosc. 2016;49:168–175. doi: 10.5946/ce.2015.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Zhao Y, Zhao X, Shi R. Clinical Outcomes of Endoscopic Submucosal Dissection for Early Esophageal Squamous Cell Neoplasms: A Retrospective Single-Center Study in China. Gastroenterol Res Pract. 2016;2016:3741456. doi: 10.1155/2016/3741456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Probst A, Aust D, Märkl B, Anthuber M, Messmann H. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy. 2015;47:113–121. doi: 10.1055/s-0034-1391086. [DOI] [PubMed] [Google Scholar]
- 59.Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066–1072. doi: 10.1016/j.gie.2008.03.1114. [DOI] [PubMed] [Google Scholar]
- 61.Jin XF, Chai TH, Gai W, Chen ZS, Guo JQ. Multiband Mucosectomy Versus Endoscopic Submucosal Dissection for Treatment of Squamous Intraepithelial Neoplasia of the Esophagus. Clin Gastroenterol Hepatol. 2016;14:948–955. doi: 10.1016/j.cgh.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 62.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–866. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 63.Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T, Zhou JM, Chen TY, Zhong YS. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. 2014;46:640–644. doi: 10.1055/s-0034-1365648. [DOI] [PubMed] [Google Scholar]
- 64.Miwata T, Oka S, Tanaka S, Kagemoto K, Sanomura Y, Urabe Y, Hiyama T, Chayama K. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc. 2016;30:4049–4056. doi: 10.1007/s00464-015-4719-3. [DOI] [PubMed] [Google Scholar]
- 65.Moriya H, Ohbu M, Kobayashi N, Tanabe S, Katada N, Futawatari N, Sakuramoto S, Kikuchi S, Okayasu I, Watanabe M. Lymphatic tumor emboli detected by D2-40 immunostaining can more accurately predict lymph-node metastasis. World J Surg. 2011;35:2031–2037. doi: 10.1007/s00268-011-1143-2. [DOI] [PubMed] [Google Scholar]
- 66.Yamashina T, Ishihara R, Nagai K, Matsuura N, Matsui F, Ito T, Fujii M, Yamamoto S, Hanaoka N, Takeuchi Y, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013;108:544–551. doi: 10.1038/ajg.2013.8. [DOI] [PubMed] [Google Scholar]
- 67.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 68.Wang WL, Chang IW, Chen CC, Chang CY, Mo LR, Lin JT, Wang HP, Lee CT. A case series on the use of circumferential radiofrequency ablation for early esophageal squamous neoplasias in patients with esophageal varices. Gastrointest Endosc. 2017;85:322–329. doi: 10.1016/j.gie.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 69.Wang WL, Chang IW, Chen CC, Chang CY, Mo LR, Lin JT, Wang HP, Lee CT. Radiofrequency Ablation Versus Endoscopic Submucosal Dissection in Treating Large Early Esophageal Squamous Cell Neoplasia. Medicine (Baltimore) 2015;94:e2240. doi: 10.1097/MD.0000000000002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He S, Bergman J, Zhang Y, Weusten B, Xue L, Qin X, Dou L, Liu Y, Fleischer D, Lu N, et al. Endoscopic radiofrequency ablation for early esophageal squamous cell neoplasia: report of safety and effectiveness from a large prospective trial. Endoscopy. 2015;47:398–408. doi: 10.1055/s-0034-1391285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okunaka T, Kato H, Conaka C, Yamamoto H, Bonaminio A, Eckhauser ML. Photodynamic therapy of esophageal carcinoma. Surg Endosc. 1990;4:150–153. doi: 10.1007/BF02336594. [DOI] [PubMed] [Google Scholar]
- 72.Tajiri H, Oguro Y. Laser endoscopic treatment for upper gastrointestinal cancers. J Laparoendosc Surg. 1991;1:71–78. doi: 10.1089/lps.1991.1.71. [DOI] [PubMed] [Google Scholar]
- 73.Savary JF, Grosjean P, Monnier P, Fontolliet C, Wagnieres G, Braichotte D, van den Bergh H. Photodynamic therapy of early squamous cell carcinomas of the esophagus: a review of 31 cases. Endoscopy. 1998;30:258–265. doi: 10.1055/s-2007-1001252. [DOI] [PubMed] [Google Scholar]
- 74.Tanaka T, Matono S, Nagano T, Murata K, Sueyoshi S, Yamana H, Shirouzu K, Fujita H. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc. 2011;73:1–6. doi: 10.1016/j.gie.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 75.Hatogai K, Yano T, Kojima T, Onozawa M, Daiko H, Nomura S, Yoda Y, Doi T, Kaneko K, Ohtsu A. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest Endosc. 2016;83:1130–1139.e3. doi: 10.1016/j.gie.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 76.Tahara K, Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Nakatani K, Naruke A, Kim M, et al. Argon plasma coagulation for superficial esophageal squamous-cell carcinoma in high-risk patients. World J Gastroenterol. 2012;18:5412–5417. doi: 10.3748/wjg.v18.i38.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]


