Abstract
For patients recovering from acute pancreatitis, the development of a pancreatic fluid collection (PFC) predicts a more complex course of recovery, and introduces difficult management decisions with regard to when, whether, and how the collection should be drained. Most PFCs resolve spontaneously and drainage is indicated only in pseudocysts and walled-off pancreatic necrosis when the collections are causing symptoms and/or local complications such as biliary obstruction. Historical approaches to PFC drainage have included surgical (open or laparoscopic cystgastrostomy or pancreatic debridement), and the placement of percutaneous drains. Endoscopic drainage techniques have emerged in the last several years as the preferred approach for most patients, when local expertise is available. Lumen-apposing metal stents (LAMS) have recently been developed as a tool to facilitate potentially safer and easier endoscopic drainage of pancreatic fluid collections, and less commonly, for other indications, such as gallbladder drainage. Physicians considering LAMS placement must be aware of the complications most commonly associated with LAMS including bleeding, migration, buried stent, stent occlusion, and perforation. Because of the patient complexity associated with severe pancreatitis, management of pancreatic fluid collections can be a complex and multidisciplinary endeavor. Successful and safe use of LAMS for patients with pancreatic fluid collections requires that the endoscopist have a full understanding of the potential complications of LAMS techniques, including how to recognize and manage expected complications.
Keywords: Pancreatic fluid collection, Lumen apposing metal stent, Endoscopic necrosectomy, Cystgastrostomy
Core tip: Pancreatic fluid collections (PFC) are a recognized complication of pancreatitis, trauma or surgical injury to the pancreas. Over the years, management has included surgical, radiologic or endoscopic intervention. Endoscopic interventions are now at the forefront for management of PFCs, and development of lumen apposing metal stents (LAMS) have made endoscopic drainage more accessible and easy. It is important for practitioners to understand the risks of LAMS including bleeding, stent migration, buried stent, stent occlusion, and perforation, as well as proper management approaches to these complications.
INTRODUCTION
Acute pancreatitis (AP) leads to 275000 admissions annually and $2.5 billion in healthcare costs worldwide[1]. Pancreatic fluid collections (PFCs) are a burdensome, sometimes devastating complication of AP. PFCs can arise after interstitial or necrotizing pancreatitis. Acute peripancreatic fluid collections occur commonly in interstitial AP, generally resolve, and require no intervention. When an acute PFC does not resolve, a wall forms around the collection, signaling the evolution into a pancreatic pseudocyst. Pseudocysts require intervention only if they are symptomatic. Necrotizing pancreatitis that fails to resolve spontaneously can develop into walled-off necrosis (WON). As with pseudocysts, WON warrants intervention only when symptomatic or if there is clear evidence of infection[2]. Infected pancreatic necrosis, which may occur before or after a collection becomes walled-off, carries a mortality rate of 30%, compared to only 2% of all AP and 10% of patients with sterile necrosis[1].
Historically, PFCs were drained or debrided surgically or percutaneously; however, these techniques have a high risk of complications and morbidity. Endoscopic cystgastrostomy and necrosectomy have emerged as an alternative to these approaches in many cases. An endoscopic approach requires close contact between the GI lumen and PFC and is not an appropriate alternative for surgical indications other than drainage, such as bleeding or perforation[3]. For pseudocysts, endoscopic cystgastrostomy has been demonstrated to be as effective as surgical cystgastrostomy when it is technically feasible, but with shorter hospital stays, lower costs and higher patient physical and mental health scores[4]. A 2016 Cochrane review of randomized control trial data for management of pancreatic pseudocysts concluded that endoscopic ultrasound (EUS)-guided drainage leads to better short term healthcare quality of life and lower costs compared to surgical drainage[5]. For necrotic collections, the rate of surgical complication is as high as 72%[6,7]. When it can be utilized, endoscopic necrosectomy has been shown to result in lower rates of major complications and death[3]. While there is strong data now supporting lower morbidity/mortality for endoscopic approaches over surgical approaches in PFCs, there is limited data comparing percutaneous approaches and endoscopic approaches. The main limitations of percutaneous approaches are: (1) the need for external drainage catheters; (2) the potential development of a drain tract/fistula; and (3) diameter limitations of external drainage catheters which can limit their effectiveness for WON.
Early techniques for endoscopic management of PFCs involved radiologic and endoscopic identification of an area of extrinsic gastric or duodenal compression, creation of a cystgastrostomy with a needle-knife sphincterotome, wire passage, and use of a Seldinger technique to balloon dilate the tract and place double pigtail plastic stents to maintain its patency and allow drainage[8]. Over the ensuing years, techniques and technologies have evolved. Endoscopic ultrasound guidance is now almost universally used for confirmation of PFC location and identification of an avascular path for drainage[5]. While plastic stents continue to be used commonly for simple pseudocysts, self-expanding metal biliary and esophageal stents have also been used for PFC drainage, as these have the advantage of a larger lumen, which is particularly useful in the setting of WON and in some cases to enable endoscopic necrosectomy[9]. Traditional self-expanding metal stents (SEMS) are designed to anchor in place in a stricture; however, when used for PFC management, there is a significant migration risk, as the size and shape of available biliary and esophageal SEMS are not specifically designed for management of pancreatic collections[10].
The first clinical experiences with LAMS were described in 2012, and over the last several years LAMS have been increasingly preferred by many endoscopists for cystgastrostomy and pancreatic necrosectomy procedures[11,12]. LAMS address the need for a larger lumen for drainage and endoscopist entry into the cyst cavity, and are designed with a “dumbbell” shape with flanged ends to oppose the gastrointestinal and cyst lumens in order to reduce stent migration[11]. There are numerous studies showing safety and efficacy of LAMS[10-15]. There is also data showing the superiority of LAMS to double pigtail stents (DPS) for drainage of WON[16].
In early studies of both WON and pseudocysts, the use of LAMS has been associated with higher rates of clinical success, fewer required endoscopic sessions, shorter procedure times, shorter hospital stays and lower costs compared to DPS[16-20]. In the United States, the Axios stent (Boston Scientific) is used most commonly, but internationally, other LAMS stent designs including the Spaxus stent (TaeWoong Medical) and Nagi stent (TaeWoong Medical) are available, among others[21].
COMPLICATIONS OF LAMS
While much has been written regarding the technical advantages of LAMS, there has been more limited discussion of their complications. Reported complication rates vary widely. Several studies report complication rates under 10%[22-24]. Conversely, one group recently published their experience of conducting an interim audit during a randomized controlled trial and ultimately changing their study protocol and clinical practice due to a higher than expected complication rate of 50% in the LAMS arm of their trial[25]. The variations may be at least partially attributable to different definitions of complications in the studies. Given that these series represent the experiences of earlier adopters of the technology who are therapeutic endoscopists at high volume centers, broader use of these devices may either decrease complication rates, as technical experience and approaches evolve, or may increase complication rates, as these procedures become more commonly performed in centers with less clinical experience and expertise. The most common complications encountered in PFC drainage with LAMS include bleeding, stent migration/dislodgement, buried stents, stent occlusion, and perforation (Table 1). While these complications are not unique to LAMS placement, the prevalence and management of these complications is somewhat different than for prior endoscopic drainage techniques.
Table 1.
Complication rates in lumen apposing metal stents case series
| Author | Year | Single/multi-center | n | PFC type | Bleeding | Perforation | Migration | Buried stent | Failure to deploy |
| Itoi et al[11] | 2012 | Single | 15 | 6 WON, 9 PC2 | 0% | 0% | 7% | 0% | 0% |
| Yamamoto et al[12] | 2013 | Multi | 9 | 4 WON, 5 PC | 11% | 0% | 11% | 0% | 0% |
| Chandran et al[14] | 2015 | Multi | 54 | 9 WON, 39 PC | 6% | 0% | 19% | 6% | 2% |
| Shah et al[10] | 2015 | Multi | 33 | 11 WON, 18 PC | 0% | 0% | 3% | 0% | 9% |
| Walter et al[39] | 2015 | Multi | 61 | 46 WON, 15 PC | 0% | 2% | 10% | 0% | 2% |
| Rinninella et al[24] | 2015 | Multi | 93 | 4 APFC, 37 PC, 52 WON | 1% | 2% | 1% | 0% | 1% |
| Mukai et al[40] | 2015 | Single | 21 | 19 WON, 2 PC | 10% | 0% | 19% | 0% | 0% |
| 1Mukai et al[17] | 2015 | Single | 43 | WON | 0% | 2% | 5% | 0% | 0% |
| 1Siddiqui et al[19] | 2016 | Multi | 86 | WON | 7% | 3% | 0% | 3% | 2% |
| 1Bang et al[25] | 2016 | Single | 12 | WON | 25% | 0% | 0% | 17% | - |
| 1Lang et al[26] | 2016 | Single | 19 | 9 WON 10 PC | 21% | 0% | 0% | 0% | - |
| 1Bapaye et al[18] | 2017 | Single | 72 | WON | 3% | 0% | 3% | 0% | 0% |
| 1Ang et al[16] | 2017 | Multi | 12 | 8 WON, 8 PC3 | 0% | 0% | 8% | 0% | - |
| Lakhtakia et al[37] | 2017 | Single | 205 | WON | 3% | 1% | 1% | 1% | 1% |
Study includes multiple stent types, only LAMS cases are included here;
PFC type not explicitly defined, inferred based on description of cyst contents;
Study includes patients with multiple stent types. Number of subjects is not equal to sum of PFC types. LAMS: Lumen-apposing metal stents; WON: Walled off necrosis; PC: Pseudocyst; APFC: Acute pancreatic fluid collection.
Bleeding
Cystgastrostomy, and indeed any transenteric procedure, carries a risk of hemorrhage. This can include acute bleeding, at the time of initial access and tract creation, and delayed bleeding, which can occur due to a variety of mechanisms, weeks or months after the initial procedure[25]. A major advantage of EUS guidance is that Doppler ultrasound helps identify an avascular path, which should reduce procedural bleeding risk. Acute bleeding or oozing at the site of mucosal entry can still occur, and can often be managed by tissue tamponade from either balloon dilation or stent placement and radial expansion. The more serious bleeding complication of endoscopic cystgastrostomy and endoscopic necrosectomy is bleeding within the PFC. The reported rate of bleeding can be as high as 25%, but is lower in most studies (Table 1). Acute or delayed bleeding within the PFC is often not endoscopically manageable, in part due to limited visualization. Rapid bleeding, whether acute or delayed, may require immediate referral to angiographic embolization or surgery (Figure 1). Pseudoaneurysm development and variceal formation are expected complications in patients with severe pancreatitis and may increase the risk of bleeding during PFC management (Figure 2).
Figure 1.
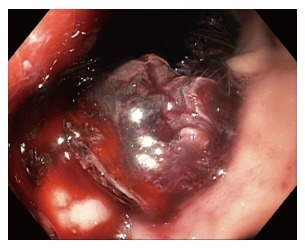
Delayed bleeding after lumen apposing metal stent placement, which required angiographic embolization.
Figure 2.
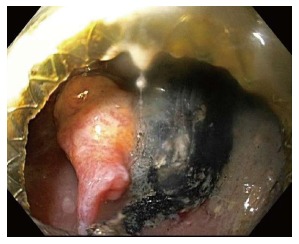
Pulsatile pseudoaneurysm seen on left of screen after endoscopic entry into cyst cavity, several weeks after initial lumen apposing metal stent placement for walled off necrosis.
A comparison of LAMS and DPS in PFC drainage found that while the two treatment groups had similar rates of PFC resolution at 6 mo, there were significantly higher rates of bleeding (19% vs 1%, P = 0.0003) in the LAMS group compared to the DPS group. The bleeding events in the LAMS group included a splenic artery pseudoaneurysm, 2 collateral vessel bleeds, and an intracavitary variceal bleed; whereas the single bleeding event in the DPS group was an erosion of the stent into the gastric wall[26].
In a second trial comparing LAMS to DPS for management of WON, three major bleeding events requiring transfusion and ICU admission among the first 12 patients randomized to the LAMS arm were reported. All three events occurred in a delayed fashion, at 3 wk (n = 1) and 5 wk (n = 2) from LAMS placement. As a result of this experience, the authors changed their study protocol and clinical practice and now perform a CT scan 3 wk after LAMS placement to assess for PFC resolution, rather than 6 wk as is their practice for DPS and their original study protocol[25]. Similarly, another comparison between LAMS and DPS in WON describes erosion of the LAMS into the splenic artery as the cause of 2 bleeding complications, both of which were delayed (Table 1)[18].
The primary concept that has been proposed to explain the increased bleeding risk reported in the early LAMS literature is that the more rapid collapse of the collection which may occur due to the large diameter of LAMS, may lead to direct impingement of the stent on blood vessels on the cyst wall, leading to risk of pseudoaneurysm and hemorrhage. Furthermore, plastic stents are softer and more flexible, and may be less likely to cause bleeding if they encounter a vascular structure. The data regarding bleeding risk should become clearer as a larger experience develops with LAMS use. Based on the available evidence, our approach is to perform short term CT imaging after LAMS placement, typically within 3-4 wk, with the plan to endoscopically remove the stent after cyst collapse is demonstrated. In recurrent pseudocysts for which long-term drainage is desired, use of plastic stents may be preferable based on our current understanding. We also strongly recommend that physicians considering LAMS placement should have experienced angiography and surgical teams available to help manage bleeding when it occurs. This is in keeping with recommended approaches for all patients with pancreatic fluid collections, which should typically involve multidisciplinary care involving a gastroenterologist, surgeon, and radiologist.
Migration
While the LAMS flanged ends are intended to anchor the stent in place, there remains some risk of stent migration after placement. Migration rates of up to 19% have been reported (Table 1). Migration can occur either into the cyst cavity, or back into the gut lumen. Migration can occur immediately due to improper deployment of the stent, but may also occur spontaneously, weeks after stent placement, and also due to subsequent manipulation of the stent during endoscopic debridement procedures[27]. While some endoscopists prefer to place a double pigtail stent through the LAMS to “stabilize” the LAMS, it is currently unknown whether this approach may reduce the risk of LAMS migration, and we do not currently recommend this practice.
In the case of migration into the gastric lumen, the stent may either remain in the stomach (Figure 3), or pass spontaneously. Small bowel obstruction has been reported in association with LAMS migration, requiring surgical exploration for stent retrieval[28]. Migration of the stent into the cyst cavity can be more problematic as the cystgastrostomy tract may partially or completely close.
Figure 3.
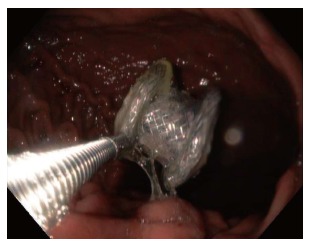
Endoscopic removal of lumen apposing metal stent that migrated into gastric lumen.
If stent migration is recognized during routine imaging or endoscopy, endoscopic removal of the stent should be pursued urgently. In the case of migration into the lumen, retrieval is straightforward if the stent is in the stomach or proximal small bowel. More distal migration of the stent may be managed with a deep enteroscopy attempt at removal, or conservatively with serial abdominal X-rays to confirm passage, and prompt surgical management if bowel obstruction occurs. Migration of the stent into the cyst cavity requires re-establishing the cyst gastrostomy tract with wire passage, dilation, and subsequent re-introduction of the endoscope into the cavity for stent retrieval using a snare or forceps. If stent retrieval is not possible endoscopically, then surgical removal is indicated.
Buried stent
The term “buried stent” refers to the situation when gastric or enteric mucosa grows over the flanged end of the LAMS. This complication may occur with LAMS due to the tight apposition of the gastric and PFC lumen and the relatively low stent profile. Buried stent has been reported in up to 17% of cases in reported series (Table 1). The management of this issue has been described in several case reports[29-31].
Given that buried stent is a relatively uncommon occurrence, the specific risk factors for this event are not clear. Some have proposed that placing the stent across the gastric antrum (rather than the gastric body), may increase the risk of buried stent because of the significant motility of the gastric antrum[32].
Techniques for removing buried stents have included the use of a needle knife device and argon plasma coagulation to partially uncover the enteric side of stent, prior to removal with a snare or forceps. Dilation of the stent and tract may also facilitate removal. When adequate exposure of the buried enteric side of the stent is not feasible, an alternative approach is to dilate the stent, enter the cavity with the endoscope through the stent, and subsequently capture the internal flange of the stent in order to facilitate removal[31]. Aggressive attempts at removal of a buried stent may increase the risk of bleeding, and potentially separation of the cyst cavity from the enteric wall.
Perforation
The risk of perforation when performing an upper GI endoscopy including EUS is low, at less than 0.05%[33]. The reported risk of perforation in endoscopic drainage of PFCs is less than 5% (Table 1)[34]. Peritonitis or pneumoperitoneum caused by gastric perforation or separation of the cyst wall and stomach are perhaps the most feared complications of endoscopic cystgastrostomy and similar techniques. These challenges are not specific to the use of LAMS and can occur in any endoscopic PFC drainage procedures.
While LAMS are designed to make endoscopic access into PFCs easier, and potentially safer, initial data does not suggest that LAMS use eliminates the risk of perforation. In one study comparing DPS, SEMS and LAMS, 3 cases of perforation were reported in the LAMS group (n = 86), all resulting from stent maldeployment. One was fixed endoscopically with an “over the scope” clip (OTSC) and two required surgical repair. In the DPS and SEMS groups (n = 227), there were only 2 such perforations[19].
Patients in whom a perforation is not immediately recognized and closed endoscopically may present immediately post procedure with abdominal/chest pain, hemodynamic instability or in a more delayed fashion with worsening clinical status including sepsis. Because patients recovering from severe pancreatitis may have pre-procedural abdominal pain, and, in some situations, features of systemic inflammatory response syndrome, post-procedural perforation may not be immediately obvious. Physicians must be alert to the risk of perforation, and initiate clinical investigation early if there is evidence of clinical deterioration or increased abdominal pain after LAMS placement. Initial management includes imaging studies such as a CT scan with contrast to assess extent. Conservative, nonsurgical management with nothing by mouth, nasogastric/nasoduodenal tube placement and intravenous antibiotics can be considered in clinically stable patients. A small perforation/leak after successful stent placement may lead to fluid or air in the peritoneum, and can potentially be followed conservatively. However, the surgical team should be consulted at time of presentation to evaluate the role of surgical intervention.
When a perforation is recognized, the endoscopist should describe the size and location of the perforation in clear terms. Endoscopic treatment of the perforation can include endoscopic clip placement, OTSC placement, endoscopic suturing, or a combination of these tools (Figure 4)[35]. For gastric perforations less than 1cm, monotherapy with these tools should be considered; however, for gastric perforations of 1-3 cm, a combination of techniques may be required[35]. If it is clear that the stent has been deployed outside of the PFC, either from incorrect deployment or separation of the cavities, the stent should be removed and the site closed endoscopically with standard clips, OTSC, or endoscopic suturing.
Figure 4.
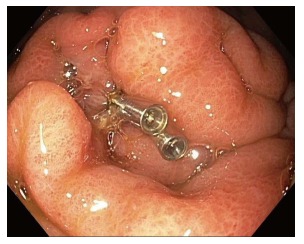
Closure of a perforation in the stomach after lumen apposing metal stents maldeployment and removal (a second gastric location was subsequently chosen for successful lumen apposing metal stents placement).
For PFCs, perforation/pneumoperitoneum risk can be minimized by choosing a site with clear wall apposition (with ideally less than 1cm of distance) between the gut lumen and cyst wall on EUS. Carbon dioxide is also strongly preferred for all LAMS placements as this may lower the risk of tension-pneumothorax, pneumomediastinum, pneumopericardium, or abdominal compartment syndrome[35].
Stent occlusion
LAMS placement is intended to allow fluid and debris to flow out of the cyst cavity, and also to permit digestive juices to flow in, which may facilitate clearance of the cavity. When the stent lumen becomes occluded, either with food debris (Figure 5), or with cyst contents (Figure 6), drainage is impaired[36]. The prevalence of stent occlusion has not been clearly reported in the available literature, as this has not been included in the described list of complications in most available reports. While some physicians advocate placing double pigtail plastic stents through the LAMS at the time of initial placement in order to reduce the likelihood of stent occlusion, the benefit of this approach, if any, has not been evaluated in clinical trials.
Figure 5.
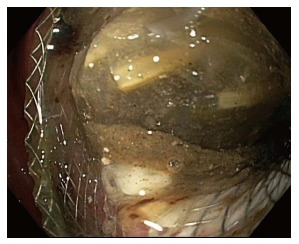
Food debris occluding lumen apposing metal stent lumen.
Figure 6.
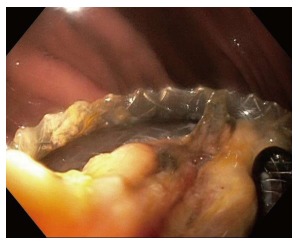
Necrotic debris occluding lumen apposing metal stent lumen.
LAMS occlusion is important to recognize as it may slow the rate of cyst resolution, and lead to early “closure” of a partially drained cyst may also increase the risk of infection. Stent occlusion should be considered in patients who have a sudden clinical worsening (for instance new abdominal pain or fever), after initial improvement or stability following LAMS placement, and also in patients where follow-up clinical imaging demonstrates lack of improvement in cyst cavity size. Management of stent occlusion is generally straightforward, endoscopically, requiring standard techniques of debris removal, with a forceps or retrieval net, in order to re-establish the stent lumen.
An active, step-up approach to managing WON has been proposed, which employs early assessment for stent occlusion and clearing stent debris as a first re-intervention step for cases that do not resolve after LAMS placement[37]. If resolution still does not occur, the algorithm employs nasocystic tube with hydrogen peroxide and saline lavage, and, ultimately direct endoscopic necrosectomy for the most persistent cases.
CONCLUSION
LAMS technology is a welcome and important addition to the armamentarium of gastrointestinal endoscopists managing PFCs. While these devices represent an important leap forward with regard to ease of rapid endoscopic drainage, promoting lumen apposition and limiting stent migration compared to off-label use of other stent designs, LAMS do not eliminate the risk of complications of endoscopic cystgastrostomy and endoscopic necrosectomy and may even carry some of their own unique risks. The continued study of these devices as they become more commonly used will be critical to more precisely characterize their risks and the best techniques to avoid them.
Specific questions which will be important to address in future research will include: (1) what is the appropriate/safe duration between LAMS placement and removal? (2) what are the ideal intervals of radiologic and endoscopic follow-up to reduce the risk of stent migration and buried stent? and (3) does the use of double pigtail stents placed through a LAMS have any effect on the risk migration, occlusion, or other complications? The consistent application of deliberately developed and refined protocols should help drive down the rate of LAMS complications, and will allow for safer application of these important devices as their clinical usage broadens over time[38].
Footnotes
Conflict-of-interest statement: Dr. Berzin is a consultant and speaker for Boston Scientific.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 26, 2017
First decision: May 17, 2017
Article in press: July 23, 2017
P- Reviewer: Bilir C, Fiori E, Kumar A, Khan MA S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
Contributor Information
Michael L DeSimone, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States. mdesimon@bidmc.harvard.edu.
Akwi W Asombang, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
Tyler M Berzin, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, United States.
References
- 1.Forsmark ChE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2017;376:598–599. doi: 10.1056/NEJMc1616177. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Hines OJ, Donald GW. Endoscopic transgastric necrosectomy for infected necrotizing pancreatitis. JAMA. 2012;307:1084–1085. doi: 10.1001/jama.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–590.e1. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Gurusamy KS, Pallari E, Hawkins N, Pereira SP, Davidson BR. Management strategies for pancreatic pseudocysts. Cochrane Database Syst Rev. 2016;4:CD011392. doi: 10.1002/14651858.CD011392.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raraty MG, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans J, Sutton R, Neoptolemos JP. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251:787–793. doi: 10.1097/SLA.0b013e3181d96c53. [DOI] [PubMed] [Google Scholar]
- 7.Parekh D. Laparoscopic-assisted pancreatic necrosectomy: A new surgical option for treatment of severe necrotizing pancreatitis. Arch Surg. 2006;141:895–902; discussion 902-903. doi: 10.1001/archsurg.141.9.895. [DOI] [PubMed] [Google Scholar]
- 8.Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- 9.Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video) Gastrointest Endosc. 2008;68:1199–1203. doi: 10.1016/j.gie.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Shah RJ, Shah JN, Waxman I, Kowalski TE, Sanchez-Yague A, Nieto J, Brauer BC, Gaidhane M, Kahaleh M. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13:747–752. doi: 10.1016/j.cgh.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 11.Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–876. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto N, Isayama H, Kawakami H, Sasahira N, Hamada T, Ito Y, Takahara N, Uchino R, Miyabayashi K, Mizuno S, et al. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013;77:809–814. doi: 10.1016/j.gie.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, Yan L, Laique SN, Kowalski T, Loren DE, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos) Gastrointest Endosc. 2016;83:699–707. doi: 10.1016/j.gie.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Chandran S, Efthymiou M, Kaffes A, Chen JW, Kwan V, Murray M, Williams D, Nguyen NQ, Tam W, Welch C, et al. Management of pancreatic collections with a novel endoscopically placed fully covered self-expandable metal stent: a national experience (with videos) Gastrointest Endosc. 2015;81:127–135. doi: 10.1016/j.gie.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Sharaiha RZ, Tyberg A, Khashab MA, Kumta NA, Karia K, Nieto J, Siddiqui UD, Waxman I, Joshi V, Benias PC, et al. Endoscopic Therapy With Lumen-apposing Metal Stents Is Safe and Effective for Patients With Pancreatic Walled-off Necrosis. Clin Gastroenterol Hepatol. 2016;14:1797–1803. doi: 10.1016/j.cgh.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Ang TL, Kongkam P, Kwek AB, Orkoonsawat P, Rerknimitr R, Fock KM. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc Ultrasound. 2016;5:320–327. doi: 10.4103/2303-9027.191659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukai S, Itoi T, Baron TH, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, et al. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: a retrospective single-center series. Endoscopy. 2015;47:47–55. doi: 10.1055/s-0034-1377966. [DOI] [PubMed] [Google Scholar]
- 18.Bapaye A, Dubale NA, Sheth KA, Bapaye J, Ramesh J, Gadhikar H, Mahajani S, Date S, Pujari R, Gaadhe R. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig Endosc. 2017;29:104–110. doi: 10.1111/den.12704. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui AA, Kowalski TE, Loren DE, Khalid A, Soomro A, Mazhar SM, Isby L, Kahaleh M, Karia K, Yoo J, et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc. 2017;85:758–765. doi: 10.1016/j.gie.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Lee BU, Song TJ, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Newly designed, fully covered metal stents for endoscopic ultrasound (EUS)-guided transmural drainage of peripancreatic fluid collections: a prospective randomized study. Endoscopy. 2014;46:1078–1084. doi: 10.1055/s-0034-1390871. [DOI] [PubMed] [Google Scholar]
- 21.Mangiavillano B, Pagano N, Baron TH, Arena M, Iabichino G, Consolo P, Opocher E, Luigiano C. Biliary and pancreatic stenting: Devices and insertion techniques in therapeutic endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography. World J Gastrointest Endosc. 2016;8:143–156. doi: 10.4253/wjge.v8.i3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoi T, Nageshwar Reddy D, Yasuda I. New fully-covered self-expandable metal stent for endoscopic ultrasonography-guided intervention in infectious walled-off pancreatic necrosis (with video) J Hepatobiliary Pancreat Sci. 2013;20:403–406. doi: 10.1007/s00534-012-0551-5. [DOI] [PubMed] [Google Scholar]
- 23.Dhir V, Teoh AY, Bapat M, Bhandari S, Joshi N, Maydeo A. EUS-guided pseudocyst drainage: prospective evaluation of early removal of fully covered self-expandable metal stents with pancreatic ductal stenting in selected patients. Gastrointest Endosc. 2015;82:650–657; quiz 718.e1-e5. doi: 10.1016/j.gie.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 24.Rinninella E, Kunda R, Dollhopf M, Sanchez-Yague A, Will U, Tarantino I, Gornals Soler J, Ullrich S, Meining A, Esteban JM, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video) Gastrointest Endosc. 2015;82:1039–1046. doi: 10.1016/j.gie.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Bang JY, Hasan M, Navaneethan U, Hawes R, Varadarajulu S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. 2016 doi: 10.1136/gutjnl-2016-312812. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang GD, Fritz C, Bhat T, Murad F, Edmundowicz S, Early DS, Kushnir V, Mullady D. 721 Comparing the Efficacy and Complication Rates of EUS-Guided Drainage of Peri-Pancreatic Fluid Collections Using Lumen-Apposing Covered Self-Expanding Metal Stents and Double Pigtail Stents. Gastrointest Endosc [Internet] 2016;83:AB171–AB172. [Google Scholar]
- 27.Guarner-Argente C, Colán-Hernández J, Concepción-Martín M, Martínez-Guillén M, Soriano G, Sainz S, Guarner C. Replacement of the same lumen-apposing metallic stent for multiple necrosectomy sessions. Endoscopy. 2015;47 Suppl 1 UCTN:E447–E448. doi: 10.1055/s-0034-1392788. [DOI] [PubMed] [Google Scholar]
- 28.Razzak A, Larsen M, Irani S, Gan SI, Ross A. Small-bowel obstruction due to a migrated lumen-apposing metal stent. Gastrointest Endosc. 2016;84:867–869. doi: 10.1016/j.gie.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 29.Fabbri C, Luigiano C, Marsico M, Cennamo V. A rare adverse event resulting from the use of a lumen-apposing metal stent for drainage of a pancreatic fluid collection: “the buried stent”. Gastrointest Endosc. 2015;82:585–587. doi: 10.1016/j.gie.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues-Pinto E, Grimm IS, Baron TH. Removal of buried gastroduodenal stents after drainage of pancreatic fluid collections: Silence of the LAMS (with video) Gastrointest Endosc. 2016;83:853–854. doi: 10.1016/j.gie.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Seerden TC, Vleggaar FP. Endoscopic removal of buried lumen-apposing metal stents used for cystogastrostomy and cholecystogastrostomy. Endoscopy. 2016;48 Suppl 1:E179. doi: 10.1055/s-0042-107073. [DOI] [PubMed] [Google Scholar]
- 32.Irani S, Kozarek RA. 926 The Buried Lumen Apposing Metal Stent (LAMS): Is this a Stent Problem, a Location Problem or Both. A Case Series. Gastrointest Endosc [Internet] 2016;83:AB186–AB187. doi: 10.1016/j.vgie.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ASGE Standards of Practice Committee. Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Dominitz JA, Cash BD. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707–718. doi: 10.1016/j.gie.2012.03.252. [DOI] [PubMed] [Google Scholar]
- 34.Fabbri C, Luigiano C, Maimone A, Polifemo AM, Tarantino I, Cennamo V. Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J Gastrointest Endosc. 2012;4:479–488. doi: 10.4253/wjge.v4.i11.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BP, Vezakis A, Gonzalez JM, Turino SY, Tsiamoulos ZP, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46:693–711. doi: 10.1055/s-0034-1377531. [DOI] [PubMed] [Google Scholar]
- 36.Capone P, Petrone MC, Dabizzi E, Mariani A, Arcidiacono PG. Endoscopic ultrasound-guided drainage of a pancreatic fluid collection using a novel lumen-apposing metal stent complicated by stent occlusion. Endoscopy. 2016;48 Suppl 1:E203. doi: 10.1055/s-0042-108572. [DOI] [PubMed] [Google Scholar]
- 37.Lakhtakia S, Basha J, Talukdar R, Gupta R, Nabi Z, Ramchandani M, Kumar BVN, Pal P, Kalpala R, Reddy PM, et al. Endoscopic “step-up approach” using a dedicated biflanged metal stent reduces the need for direct necrosectomy in walled-off necrosis (with videos) Gastrointest Endosc. 2017;85:1243–1252. doi: 10.1016/j.gie.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 38.Thompson CC, Kumar N, Slattery J, Clancy TE, Ryan MB, Ryou M, Swanson RS, Banks PA, Conwell DL. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology. 2016;16:66–72. doi: 10.1016/j.pan.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter D, Will U, Sanchez-Yague A, Brenke D, Hampe J, Wollny H, López-Jamar JM, Jechart G, Vilmann P, Gornals JB, et al. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47:63–67. doi: 10.1055/s-0034-1378113. [DOI] [PubMed] [Google Scholar]
- 40.Mukai S, Itoi T, Sofuni A, Tsuchiya T, Gotoda T, Moriyasu F. Clinical evaluation of endoscopic ultrasonography-guided drainage using a novel flared-type biflanged metal stent for pancreatic fluid collection. Endosc Ultrasound. 2015;4:120–125. doi: 10.4103/2303-9027.156738. [DOI] [PMC free article] [PubMed] [Google Scholar]


