Abstract
Osteoarthritis (OA) is a slowly progressive disease where cartilage of the synovial joint degenerates. It is most common in the elderly where patients experience pain and reduce physical activity. In combination with lack of conventional treatment, patients are often left with no other choices than arthroplasty. Over the last years, multipotent stromal cells have been used in efforts to treat OA. Mesenchymal stem/progenitor cells (MSCs) are stromal cells that can differentiate into bone, fat, and cartilage cells. They reside within bone marrow and fat. MSCs can also be found in synovial joints where they affect the progression of OA. They can be isolated and proliferated in an incubator before being applied in clinical trials. When it comes to treatment, emphasis has hitherto been on autologous MSCs, but allogenic cells from healthy donors are emerging as another source of the cells. The first adaptations of MSCs revolved in the use of cell-rich matrix, delivered as invasive surgical procedure, which resulted in production of hyaline cartilage and fibrocartilage. However, the demand for less invasive delivery of cells has prompted the use of direct intra-articular injections, wherein a large amount of suspended cells are implanted in the cartilage defect.
Keywords: Intra-articular injection, Mesenchymal stem cells, Osteoarthritis, Regeneration, Cartilage
Core tip: There are several published reviews of the role of multipotent stromal cells in osteoarthritis (OA) of the knee. However, there is also need for additional current therapeutic options and clinical trials of multipotent stromal cells for OA. We review additional therapeutic potentials of mesenchymal stem cells in knee OA using either autogenous or allogenic cells. Direct intra-articular injections of cells in suspension become a delivery method, being both relatively simple and cost effective compared to major surgical procedures. The amount of cells injected is a critical factor; higher numbers of cells resulting in greater pain reduction and increased cartilage volume.
INTRODUCTION
Increases in life expectancies and an ageing population results in rise of chronic and degenerative diseases which are becoming a major public health concern. Osteoarthritis (OA) is characterized by degeneration of articular cartilage, leading to articular cartilage damage, subchondral cysts, joint space narrowing, subchondral sclerosis, osteophyte formation at the joint margin, and synovitis[1]. Known risk factors include advancing age, genetics, obesity, mechanical stress and joint trauma[2]. Predominantly occurring in the elderly, OA can afflict any joints even nonweight-bearing ones. Major weight-bearing joints or joints under repetitive stress, including hands, hips and knees are in particular risk of developing OA[3]. Conservative treatment options do not stop the onset of OA and are mainly focused on pain control. They include physiotherapy, rehabilitation, pain relief with acetaminophen and non-steroidal anti-inflammatory agents, as well as intra-articular injection of hyaluronic acid (IA-HA). Although these therapeutic strategies may be helpful in reducing symptoms, they are no longer considered effective. Due to lack of compelling medical treatments, advance-stage OA patients often undergo total joint arthroplasty. Surgical procedures come with risks of failure and infection as well as the cost of hospital care, physiotherapy, and rehabilitation. The lack of conventional treatment, combined with risks and high costs of joint replacement surgery has driven researchers towards application of multipotent stromal cells for the repair of full thickness articular cartilage.
Mesenchymal stem/progenitor cells (MSCs) are multipotent stromal cells first identified and described in 1966 by Alexander Fridenstein[4]. MSCs from bone marrow (BM) have been demonstrated to exhibit differentiation potentials for mesodermal cell lineage, including osteo-, adipo-, chondro- and myogenic potentials[5]. MSCs are partly responsible for the maintenance and healing of connective tissues and Karp et al[6] indicated that MSCs could migrate from their reservoirs following tissue injury or inflammation.
MSCs are adult stem cells present in various parts of our body, for instance BM, peripheral blood, umbilical cord blood, fatty tissues, skeletal and cardiac muscles, Wharton’s Jelly of umbilical cord, facet joints, interspinous ligaments, and ligamentum flavum[7-10]. They are primarily isolated from the BM, with major harvesting sites being the iliac crest, tibia and femur. MSCs are then separated from the rest of the marrow cells and expanded to obtain sufficient amounts. Adipose tissues are also rich in MSCs, even more so than BM with up to 1000 times more MSCs per gram of fatty tissues contrasted with that of BM[11]. Therefore, a sufficient amount of cells can be obtained from adipose tissues without the need of culture expansion, resulting in minimum manipulation of cells. MSCs grown under standard culture conditions feature a fibroblast like phenotype, exhibit plastic adherent property and are capable of giving rise to a cell colony derived from a single cells called colony-forming fibroblast unit[12], nonetheless only a fraction of the population remains clonogenic. The plasticity of MSCs has made them a promising candidate for various tissue engineering applications to treat several diseases including immune-mediated disorders, genetic abnormalities, and OA[13].
The publications reviewed here were collected manually from NCBI PubMed (https://www.ncbi.nlm.nih.gov/pubmed) from 1966 until December 2016. The following keywords or combination of keywords were used in the search engine to achieve the articles presented in this review: MSCs, OA, intra-articular injection, tissue engineering, and regeneration. Initial selection was done by screening titles and abstracts. No special screening process was applied, even though restrictions were utilized to select exclusively human investigation and the English language. Preference was given to clinical studies. Articles featuring pioneering methods or vast improvements of existing methods according to the author opinions were selected for this review. Figure 1 illustrates the flowchart and the assortment step.
Figure 1.
Flow chart representing the search and selection of articles for review.
MSCs AND OA
Synovial joints consist of number of different tissues and MSCs have been isolated from most of them although their functions within the joints are not yet fully understood. It has been suggested that they act as a source of cells which are able to be stimulated for the repair and reconstruction or regeneration of the joints. Moreover, they might also be involved in the suppression of T-cells to reduce inflammation within the joint[14]. Although chondrocytes are the predominant cell type within cartilage, MSCs have also been found to have no capability for cartilage regeneration after articular cartilage damage. Since MSCs are progenitors of young and mature chondrocytes, chondrogenic progenitor cells may help restore the superficial zone with lubricating glycoprotein to lessen friction in the articular cartilage (Figure 2)[15].
Figure 2.
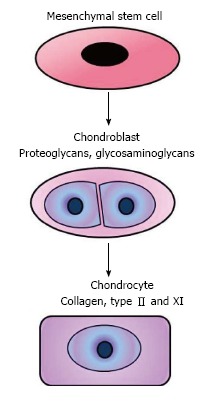
The way in which mesenchymal stem cells contribute to cartilage repair still remains unknown. Paracrine signalling and chondrogenic differentiation most likely play a crucial role.
Murphy et al[16] revealed that MSCs isolated from end-stage OA patients showed impaired differentiation capacity as well as reduced proliferation activity in vitro. They compared BM-derived MSCs (BMSCs) from patients who underwent total knee arthroplasty (TKA) with cells isolated from healthy individuals. They reported that a significantly lower amount of MSCs could be isolated from OA patients and that they had reduced proliferation activity. Interestingly, they also observed the MSCs having altered differentiation profiles, favouring osteogenic differentiation, whilst having reduced adipogenic and chondrogenic potentials. Another study found that patients’ age and stage of OA also affected the differentiation capability and expression of stemness genes of localized adipose-derived MSCs[17]. More shockingly, recent study reported that synovial fluid from OA patients inhibited the in vitro chondrogenic differentiation in MSC cultures of healthy donors[18]. Albeit, these functional deficiencies can be ameliorated by culture media supplementation with growth factors[19].
CELL BASED THERAPIES FOR CARTILAGE REPAIR
Cell based tissue engineering for OA have been used with varying results for over two decades. Autologous chondrocyte implantation (ACI) is a cell based technology wherein the cultured chondrocytes are applied on the injured area for regenerating and repairing cartilage[20]. This method has evinced promising outcomes in subjects with a variety of articular cartilage lesions that had responded poorly to prior treatments. However, the results have been conflicting[21] and the self-renewal rate and activity of chondrocytes is low, leading to slow an inadequate healing. Additionally, arthroscopy is required to obtain healthy cartilage from unaffected non-weight bearing joints and implanted during an open knee surgery, resulting in high costs, morbidity at donor sites and increased stress for patients. Reductions in pain, improvements in the joint function and the formation of functional hyaline cartilage have all been reported. The destruction of articular cartilage is irreversible in OA and does not relieve symptoms. Patients are often left with no other choice than total joint replacement. Consequently, autologous chondrocyte implantation may not be a favourable surgical procedure for subjects with OA. In addition, this method faces challenges with cartilage tissues having a limited capacity for repair. Accordingly, MSCs are emerging as a potential substitute or alternate cellular materials for more durable treatment of cartilage defects.
MICROFRACTURE AND MSCs
Microfracture is a well-established and studied surgery technique that stimulates migration of cells from the BM to cartilage lesions. Small holes are drilled at the target site into the subchondral BM which then bleeds into the lesion and forms a clot of marrow cells, including BMSCs[22]. This technique has given good results on small lesions but comes with limitations. BMSCs are only a portion of total cells within the BM and a relatively few BMSCs are recruited through microfracture being inadequate treatment for bigger cartilage lesions. Researchers have focused on isolating and expanding cells before applying them to the target area and a number of studies illustrated successful BMSC treatment for repairing focal chondral defects. Knowledge on MSC extraction, chondrogenic capacity, and tissue engineering has encouraged scientists and surgeons to test clinical potentials of MSCs to stop and reverse onset of OA, as well using their plastic adherent ability to resurface large cartilage lesions and facilitate osteochondral integration within the affected joints (Figure 3).
Figure 3.
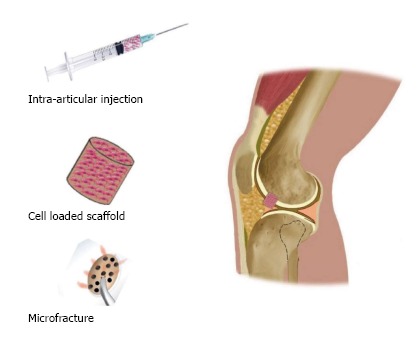
There are 3 major methods in which mesenchymal stem cells can be applied: Intra-articular injection of mesenchymal stem cell suspension, implantation of cell-mixed composite scaffold, and microfracture through articular cartilage and subchondral bone.
Buda et al[23] established a one-step surgical procedure which employs BMSCs to heal osteochondral lesions of the knee. The procedure was completed in a single setting where BM aspiration and concentration were accomplished in the same operating room as knee arthroscopy. The BM aspirate concentrate was fixed in hyaluronic acid blended membranes before being transplanted to the site where it was applied with autogenous platelet-rich-fibrin glue, providing growth factors to further stimulate the healing process. All of the patients treated with this technique showed significant improvements for parameters measuring the severity of OA and pain scores. Moreover, magnetic resonance imaging (MRI) showed that the cartilage lesion was fully covered in at least 70% of the subjects. This method was both feasible and effective for treating most of the patients enrolled and the whole process could be completed within a day. Furthermore, it did not require any cell expansion or manipulation, reducing costs and risks involved. Nevertheless, as only a fraction of BM cells are MSCs, one may speculate that the quantity of MSCs recruited this way was somewhat unsatisfactory. Another major contributing factor might have been growth factors embedded in the platelet-rich-fibrin glue produced from the peripheral blood of the subjects. Gobbi et al[24,25] reported a similar “one-step cartilage repair” technique in 2011 and 2014. The advantage of the one-step approach is that it only requires one surgery and it involves no long-term cell expansion, resulting in fewer visits to the clinic. However, it is nearly impossible to estimate the number of MSCs recruited in this process making standardized treatment with reproducible results challenging.
INTRA-ARTICULAR INJECTION OF MSCs
In 2008, Centeno et al[26,27] reported two male patients with knee OA. MSCs were harvested from the posterior superior iliac spine and simultaneously venous blood was harvested to prepare platelet lysate (PL) containing growth factors to facilitate the repair. The first subject received an injection of one milliliter of phosphate-buffered saline (PBS) consisting of 2.2 × 107 MSCs. The second subject received 4.6 × 107 MSCs suspended in one milliliter PBS. Both cases then received one milliliter of 10% PL in PBS one and 2 wk after the initial injections. Additionally, after one week, the subjects obtained intra-articular injection of dexamethasone as low-dose dexamethasone treatment has been demonstrate to stimulate chondrogenic differentiation[12]. Modified visual analogue scale (VAS) scores have been utilized to estimate pain followed by MRI to evaluate cartilage volume. Both subjects showed improvements in VAS scores and cartilage volume following 3 and 6 mo follow-up with up to 28.6% cartilage volume increase. While a decrease was also observed in the latter patient, it was not considered significant since it was below the measurement error. These studies revealed that increased cartilage volume and reduction in pain could be achieved with minimum invasive measures and is a promising treatment for OA. However, a major weakness in these cases was the short follow up time consisting of 6 mo for the first and 3 mo for the second patient. Besides, long-term follow-up results are unavailable.
In recent years, Wong et al[28] examined the outcomes of BMSC injection following a microfracture and medial opening-wedge high tibial osteotomy (HTO). The study was a randomised controlled trial that included 56 patients with unicompartmental osteoarthritic knees. The participants were classified into two groups, all received HTO combined with microfracture, and 3 wk later the intervention group received an intra-articular injection of 1.5 × 107 autologous BMSCs suspended in hyaluronic acid, while the control group obtained exclusively hyaluronic acid. The observed time spanned two years, during which both groups showed improvements in the International Knee Documentation Committee scores as well as Tegner and Lysholm scores. Interestingly, the intervention group showed significantly better improvements in all parameters. Moreover, MRI performed 1 year following injections, found cartilage thickness to be significantly greater in the intervention group. In this study, MSCs were recruited for cartilage repair by two means, such as microfracture in both groups and intra-articular injections in the intervention group. In conclusion, the postoperative injection of culturally expanded MSCs, significantly improved the treatment outcome and rate of cartilage regeneration. Additionally, the MSCs could be collected during operation and proliferated in culture to achieve sufficient numbers before the patients return to the hospital. Although microfracture and HTO are an effective means to treat OA, they are still major surgical procedures with high cost and risk.
DETERMINATION OF THE OPTIMAL DOSE
In 2014, Jo et al[29] investigated the dose effects of MSCs in OA treatment. Their phases I/II clinical trial consisted of 18 patients suffering from knee OA who received injections of adipose-derived MSCs. In phase I, patients were divided into 3 groups receiving either low dose (1.0 × 107), mid dose (5.0 × 107) or high dose (1.0 × 108) injections of MSCs. Whereas in phase II, the high dose treatment was given to 9 patients. The MSCs were harvested from abdominal subcutaneous fats via liposuction before being suspended in 3 mL of saline and injected into the knee joints. During the 6 mo follow up period, the high dose group showed a significant reductions in Western Ontario and McMaster Universities Arthritis Index scores, VAS pain scores and improvements were also observed in the low and mid dose groups. Although, pain improvements were observed in the low dose group, the size of the cartilage defect increased, whereas in the mid and high dose groups it showed significant reduction in size. Arthroscopy and histological staining further revealed the presence of hyaline-like cartilage covering the site in the high dose group. No adverse effects were observed during the follow-up time and the researchers concluded that the high dose treatment reduced pain, was safe and improved the knees function by forming hyaline-like cartilage.
ALLOGENIC MSCs FOR TREATMENT
The first randomised, double blinded control study was reported in 2014 when Vangsness et al[30] treated 55 patients with allogenic MSCs. Furthermore, it was the first study investigating the safety, effectiveness and clinical outcome of intra-articular injections of non-matched human leukocyte antigen (HLA) allogenic BMSCs. Patients were blindly divided into 3 groups, and all groups underwent partial medial meniscectomy. Afterwards, group A received 5.0 × 107 cells and group B 1.5 × 108 cells in 5 mL injections of HA, whereas the control group received only HA. Patients were evaluated before and 2 years post-treatment using MRI to measure cartilage volume and VAS scores for measuring level of pain. The MRI revealed a significant increase in cartilage volume, with group A showing the best outcome. Pain reduction was observed for groups A and B, whilst no pain reduction was recorded in the control group. Even though cartilage growth was not observed in all patients, the study confirmed the effectiveness and safety of using non-matched HLA allogenic BMSCs. Several reports have since demonstrated the successful treatment of cartilage regeneration using MSCs in OA (Table 1). Recent evidences have highlighted the importance of MSCs from development to postnatal joint homeostasis and OA[31]. Possible mechanisms of MSCs in the treatment of OA may be attributed to the ability of MSCs to initiate the repair process by promoting cartilage regeneration[32]. Further research efforts will be needed to better understand the exact role of MSCs in the treatment of OA.
Table 1.
Summary of studies using mesenchymal stem cells for cartilage regeneration in osteoarthritis
| Ref. | Type of study | Type of cells | Significance |
| Wakitani et al[33] | Case and control study | Autologous BMSCs from Iliac crest | Autologous BMSCs cell implants are effective for treating OA cartilage defects in humans, producing hyaline like cartilage |
| Centeno et al[26] | Case study | Autologous BMSCs from Iliac crest | Autologous BMSCs can be introduced by intra-articular injections into an osteoarthritic knee promotes cartilage regeneration and reduction pain |
| Buda et al[23] | Case series | Autologous BMSCs from Iliac crest | One-step repair technique utilising bone-marrow concentrate is a simple and time-efficient way to treat large chondral defects |
| Koh et al[34] | Case and control study | Autologous AMSCs from infrapatellar fat pad | Intra-articular injections of AMSCs are a safe treatment option, reducing pain and improving the function of knee OA patients |
| Wong et al[28] | Randomized control trial | Autologous BMSCs from Iliac crest | Postoperative intra-articular injections of autologous BMSCs improves the MOCART outcomes of patients with varus knees undergoing HTO and microfracture |
| Vangsness et al[30] | Randomized, double-blind, controlled study | Allogenic BMSCs from 18-30 years old donors | Postoperative intra-articular injections of allogenic BMSCs contribute to meniscus regeneration and reduction in pain following medial meniscectomy |
| Jo et al[29] | Cohort study | Autologous AMSCs from abdominal subcutaneous fats | Cartilage regeneration and pain reduction is significantly improved when high amounts of AMSCs are injected into OA knees compared with low amounts |
| Sekiya et al[35] | Therapeutic study | Autologous synovial MSCs | Localized synovial MSCs can be used to treat cartilage defects resulting in hyaline like cartilage and improved pain scores |
AMSCs: Adipose-derived MSCs; BMSCs: Bone marrow-derived MSCs; HTO: High tibial osteotomy; MOCART: Magnetic Resonance Observation of Cartilage Repair Tissue; MSCs: Mesenchymal stem cells; OA: Osteoarthritis.
CONCLUSION
In summary, these studies show that MSCs can be employed successfully to treat mild to moderate OA through various ways. They provide alternative treatment options and treatment can start early during progression of OA. The traditional major surgeries used to treat late stages are expensive and come with risks. The less invasive techniques outlined in this minireview have revealed good outcomes, but the field merits further investigation. Superior outcome was evident with greater quantity of MSCs injected. Allogenic cells from healthy young donors can also be utilized. These findings have further empowered researchers to investigate the potentials of MSCs for tissue engineering and a number of clinical trials are now underway. Most of the emphasis on minimally invasive therapeutic alternatives including intraarticular injections of MSCs, aim to cut out cost and risks of major surgeries. Additional investigations are warranted to validate the safety and efficiency of different application before a standardized treatment regimen can be established.
ACKNOWLEDGMENTS
The authors would like to thank Professor Henry Wilde for reviewing the manuscript and the National Research University Project, Office of Higher Education Commission through the Aging Society Cluster, Chulalongkorn University, and the National Science and Technology Development Agency (RES5829130016).
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with coauthors contributed their efforts in this manuscript.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: February 28, 2017
First decision: May 9, 2017
Article in press: July 3, 2017
P- Reviewer: Anand A, Chow KC, Liu K, Sancheti P, Peng BG, Zhen P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Contributor Information
Baldur Kristjánsson, Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok 10330, Thailand.
Sittisak Honsawek, Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok 10330, Thailand; Department of Orthopaedics, Vinai Parkpian Orthopaedic Research Center, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok 10330, Thailand. sittisak.h@chula.ac.th.
References
- 1.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 2.Haq SA, Davatchi F, Dahaghin S, Islam N, Ghose A, Darmawan J, Chopra A, Yu ZQ, Dans LF, Rasker JJ. Development of a questionnaire for identification of the risk factors for osteoarthritis of the knees in developing countries. A pilot study in Iran and Bangladesh. An ILAR-COPCORD phase III study. Int J Rheum Dis. 2010;13:203–214. doi: 10.1111/j.1756-185X.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58:150–167. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 5.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Kristjánsson B, Limthongkul W, Yingsakmongkol W, Thantiworasit P, Jirathanathornnukul N, Honsawek S. Isolation and Characterization of Human Mesenchymal Stem Cells From Facet Joints and Interspinous Ligaments. Spine (Phila Pa 1976) 2016;41:E1–E7. doi: 10.1097/BRS.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 9.Chin S, Furukawa K, Ono A, Asari T, Harada Y, Wada K, Tanaka T, Inaba W, Mizukami H, Motomura S, et al. Immunohistochemical localization of mesenchymal stem cells in ossified human spinal ligaments. Biochem Biophys Res Commun. 2013;436:698–704. doi: 10.1016/j.bbrc.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen YT, Wei JD, Wang JP, Lee HH, Chiang ER, Lai HC, Chen LL, Lee YT, Tsai CC, Liu CL, et al. Isolation of mesenchymal stem cells from human ligamentum flavum: implicating etiology of ligamentum flavum hypertrophy. Spine (Phila Pa 1976) 2011;36:E1193–E1200. doi: 10.1097/BRS.0b013e3182053f58. [DOI] [PubMed] [Google Scholar]
- 11.Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 12.Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375–392. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 13.Farini A, Sitzia C, Erratico S, Meregalli M, Torrente Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int. 2014;2014:306573. doi: 10.1155/2014/306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9:584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 15.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 16.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 17.Chua KH, Zaman Wan Safwani WK, Hamid AA, Shuhup SK, Mohd Haflah NH, Mohd Yahaya NH. Retropatellar fat pad-derived stem cells from older osteoarthritic patients have lesser differentiation capacity and expression of stemness genes. Cytotherapy. 2014;16:599–611. doi: 10.1016/j.jcyt.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Centeno CJ, Schultz JR, Cheever M, Robinson B, Freeman M, Marasco W. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2010;5:81–93. doi: 10.2174/157488810790442796. [DOI] [PubMed] [Google Scholar]
- 19.Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Bühring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25:3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- 20.Vasiliadis HS, Wasiak J. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2010;10:CD003323. doi: 10.1002/14651858.CD003323.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaszkin-Bettag M. Is autologous chondrocyte implantation (ACI) an adequate treatment option for repair of cartilage defects in paediatric patients? Drug Discov Today. 2013;18:740–747. doi: 10.1016/j.drudis.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29:1579–1588. doi: 10.1016/j.arthro.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92 Suppl 2:2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 24.Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions: Results at 2-Year Follow-up. Cartilage. 2011;2:286–299. doi: 10.1177/1947603510392023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648–657. doi: 10.1177/0363546513518007. [DOI] [PubMed] [Google Scholar]
- 26.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 27.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses. 2008;71:900–908. doi: 10.1016/j.mehy.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Wong KL, Lee KB, Tai BC, Law P, Lee EH, Hui JH. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29:2020–2028. doi: 10.1016/j.arthro.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 29.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 30.Vangsness CT, Farr J, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 31.De Bari C, Kurth TB, Augello A. Mesenchymal stem cells from development to postnatal joint homeostasis, aging, and disease. Birth Defects Res C Embryo Today. 2010;90:257–271. doi: 10.1002/bdrc.20189. [DOI] [PubMed] [Google Scholar]
- 32.Gupta PK, Das AK, Chullikana A, Majumdar AS. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 2012;3:25. doi: 10.1186/scrt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 34.Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19:902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees With Cartilage Defects. Clin Orthop Relat Res. 2015;473:2316–2326. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


