Abstract
AIM
To evaluate the clinical and radiographic results of patients with complicated infectious spondylitis treated with single-stage anterior debridement and reconstruction using tantalum mesh cage (TaMC) followed by immediate instrumentation.
METHODS
Single-stage radical debridement and subsequent reconstruction with TaMC instead of autograft or allograft were performed to treat 20 patients with spinal deformity or instability due to complicated infectious spondylitis. Clinical outcomes were assessed by careful physical examination and regular serological tests to determine the infection control. In addition, the visual analog score (VAS), neurologic status, length of vertebral body reconstruction, and the correction of sagittal Cobb angle on radiography were recorded and compared before and after surgery. The conditions of the patients were evaluated based on the modified Brodsky’s criteria.
RESULTS
The average VAS score significantly decreased after the surgery (from 7.4 ± 0.8 to 3.3 ± 0.8, P < 0.001). The average Cobb angle correction was 14.9 degrees. The neurologic status was significantly improved after the surgery (P = 0.003). One patient experienced refractory infection and underwent additional debridement. Eighteen patients achieved good outcome based on the modified Brodsky’s criteria and significant improvement after the surgery (P < 0.001). No implant breakage or TaMC dislodgement was found during at least 24 mo of follow-up.
CONCLUSION
Single-stage anterior debridement and reconstruction with TaMC followed by immediate instrumentation could be an alternative method to manage the patients with spinal deformity or instability due to complicated infectious spondylitis.
Keywords: Anterior reconstruction, Complicated infectious spondylitis, Instrumentation, Spinal deformity, Tantalum mesh cage
Core tip: Complicated infectious spondylitis is a rare infection with vertebral pathological fracture and severe spinal destruction that require anterior reconstruction. The use of metallic implants for vertebral body stabilization and reconstruction following debridement at the lesion of infection remains controversial. In the present study a series of 20 patients with complicated infectious spondylitis were treated with single-stage anterior debridement and reconstruction using tantalum mesh cage (TaMC) followed by immediate instrumentation. The results demonstrated that good functional outcome and low complication rate could be achieved by a single-stage anterior debridement and reconstruction with TaMC.
INTRODUCTION
Spinal infections continue to be a challenge for clinical physicians and surgeons because of their initial vague symptoms with varied manifestations, and subsequent complex progression[1-6]. A delay or failure of diagnosis and treatment can lead to structural instability, spinal deformity, neurologic impairment, sepsis, and even death. Tuberculous spondylitis is common in almost all developing and underdeveloped regions of the world and is resurgent in developed nations. Neurologic involvement is usually gradual in onset and typically results from a kyphotic deformity with secondary spinal cord compression. Pyogenic spondylitis, either referred to as spondylodiscitis or vertebral osteomyelitis, generally results from arterial or venous hematogenous seeding, which can occur from infection in the urinary or respiratory tract, soft tissue, or elsewhere. The spine also can be seeded from infection related to the diagnostic and therapeutic procedures, or intravenous drug abuse[7-10]. Infectious spondylitis may be acute, subacute, or chronic. The virulence of the offending pathogens and the host condition are major determinants of clinical presentations.
Complicated infectious spondylitis indicated for anterior reconstruction is a spinal infection with vertebral pathologic fracture and severe spinal destruction. Various biological and mechanical spacers, including autograft, allograft, and titanium mesh cage (TiMC), are used to reconstruct the anterior column after corpectomy. Previously, the use of metallic implants for vertebral body stabilization and reconstruction following debridement at the lesion of infection is controversial. Recently, several reports of patients with vertebral osteomyelitis treated with cages for anterior reconstruction have been published. The results showed that TiMC did not increase the rate of recurrent or persistent infection[11-13]. Furthermore, a direct comparison between autograft and cages showed no difference in clinical and imaging outcomes[14]. Tantalum components are reported to be associated with an even lower incidence of subsequent infection when used in patients with periprosthetic joint infection[15]. However, no study has investigated the use of tantalum mesh cage (TaMC) for anterior reconstruction in the treatment of complicated infectious spondylitis. Therefore, this study aimed to evaluate the clinical and radiographic results of 20 patients with complicated infectious spondylitis treated with single-stage anterior debridement and reconstruction using TaMC followed by immediate instrumentation and followed-up at least 2 years.
MATERIALS AND METHODS
A total of 20 patients (7 women and 13 men) who underwent single-stage combined extensive debridement and anterior reconstruction using tantalum mesh cages at our university hospital between January 2012 and December 2014 were included in the study. This study was approved by the ethical committee in our institution. The patients’ average age was 58.4 years (range, 39 to 73 years). Their medical records including outpatient and emergency room notes, admission notes, inpatient progress and nursing notes, discharge summaries, procedure notes, surgical reports, radiology reports, pathology reports, and microbiology laboratory results were reviewed. Infectious spondylitis was diagnosed based on clinical examinations including positive physical or neurological presentations, elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values, and radiographic and magnetic resonance imaging findings. Complicated infectious spondylitis was defined as at least one-level vertebral osteomyelitis with pathological fracture or severe bony destruction and adjacent discitis, based on imaging studies. All 20 patients with complicated infectious spondylitis enrolled in this study met the surgical indications of failed conservative treatment or debridement procedure, neurological compromise, and spinal instability or kyphotic deformity. These patients wore a rigid orthosis for protection at least 3 mo after surgery. Radiographic assessment was performed before and after surgery, and at the 3-, 6-, and 12-mo visit after discharge and every year thereafter. Systemic antibiotics or anti-tuberculous drugs were administered based on sensitivity studies for identified pathogens. A 6-wk full course of intravenous antibiotics was prescribed for pyogenic spondylitis, and a 12-mo full course of antimicrobial chemotherapy for tuberculous spondylitis. All 20 enrolled patients were followed-up for at least 24 mo after undergoing single-stage anterior debridement and reconstruction surgery.
Surgical technique
All patients underwent single-stage anterior debridement and reconstruction surgery carried out by our spinal surgery team. Three patients had cervical spine infection, and 17 patients had thoracolumbar spine infection. The patients were operated under general anesthesia with endotracheal intubation. During the operation, the vital signs of the patients, including heart rhythm, blood pressure, and pulse oxygenation levels, were continually monitored by anesthesiologists. A Smith-Robinson approach was used for patients with cervical spine lesions. An anterior transthoracic or retroperitoneal approach was performed for patients with thoracolumbar spine lesions. The infected lesion was debrided radically, and all destroyed tissues were removed. The spinal cord and neural elements were also decompressed completely. Smear, bacterial, and tuberculous cultures were all performed. The local area was irrigated thoroughly with diluted povidone-iodine and normal saline solution. The length of the defect after extensive debridement was determined, and a TaMC (Zimmer, NJ, United States) was then introduced into the space between the healthy vertebral bodies for anterior support and axial loading. The cervical locked plate was used for immediate stability after anterior reconstruction in patients with cervical spine lesions. Posterior transpedicular screw instrumentation was performed in patients with thoracolumbar spine lesion. The sequence of anterior or posterior surgery depended on the spinal stability and neurological status of the individual. The wound was closed with drain insertion.
Outcome assessment
The severity of the neurological status was evaluated using the Frankel scale before and after surgery, and regular follow-up visits. Radiographic examination images were used to compare the correction of the sagittal Cobb angle before and after surgery. The Cobb angle, defined as the angle between the superior endplate of the vertebrae above the implanted TaMC and the inferior endplate of the vertebrae below, was measured on plain lateral radiographs. Clinical outcomes were assessed by asking patients to qualify their pain on a visual analog scale (VAS) using a scale of 0-10 (0 = no pain and 10 = worst possible pain) and by careful physical examination and regular serological tests during admission and before discharge to determine the modified Brodsky criteria scores. Data of outcome assessment are presented as mean values with standard deviations or median values with interquartile ranges. The Frankel scale, sagittal Cobb angle, and VAS before and after surgery were compared and analyzed using the Wilcoxon signed-rank test. Nonparametric statistics were used because some variables did not have normally distributed data. SPSS 13.0 software (SPSS Inc., Chicago, United States) was used for data analysis. A value of P < 0.05 was considered statistically significant.
RESULTS
Seventeen patients with thoracolumbar infection and 3 patients with cervical infection were enrolled in this study. Sixteen patients underwent anterior debridement with 1-level corpectomy and adjacent discectomies, 3 patients with 2 levels, and 1 patient with 3 levels. Sixteen patients underwent 2 levels above and 2 levels below instrumentation of anterior reconstruction using TaMCs after extensive debridement and corpectomy, 3 patients underwent 1 level above and 1 level below, and 1 patient underwent 3 levels above and 3 levels below (Figures 1-7). The average number of segments of resected vertebrae was 1.25 (4 in the cervical region, 6 in the thoracic region, and 15 in the lumbar region). The average length of the TaMC for anterior reconstruction was 36.9 mm. Kyphotic deformity reduced in all patients, with an average angle correction of 14.9° (Table 1).
Figure 1.
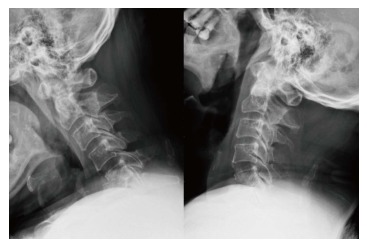
A 51-year-old man experienced intractable neck pain and neurologic deficit. Dynamic radiograph showed pathological fractures with kyphotic deformity of 5th and 6th cervical vertebrae.
Figure 7.
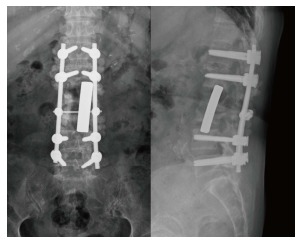
Single-stage anterior radical debridement and tantalum mesh cage implantation followed by supplemental posterior pedicle screw instrumentation were performed to treat infectious spondylitis and correct kyphotic deformity. The antibiotic beads were also deposited for infection control. Postoperative radiograph showed better lordotic alignment after single-stage combined anterior-posterior surgery for complicated infectious spondylitis.
Table 1.
Patient demographic data
| Case no | Age (yr) | Gender | Infection level | Instrumented level | Length of mesh cage | Pathogen | Cobb angle correction |
| 1 | 62 | M | L2 and adjacent discs | T12L1 to L3L4 | 38 mm | OSSA | 15° |
| 2 | 50 | M | L1 and adjacent discs | T11T12 to L2L3 | 35 mm | ORSA | 18° |
| 3 | 39 | M | T11-L1 and adjacent discs | T8T9T10 to L2L3L4 | 56 mm | MT | 25° |
| 4 | 55 | M | L2 and adjacent discs | T12L1 to L3L4 | 41 mm | SV | 19° |
| 5 | 48 | F | L3 and adjacent discs | L1L2 to L4L5 | 35 mm | PA | 6° |
| 6 | 51 | M | C5C6 and adjacent discs | C4 to C7 | 44 mm | ORSA | 29° |
| 7 | 70 | F | L3 and adjacent discs | L1L2 to L4L5 | 33.5 mm | OSSA | 12° |
| 8 | 72 | F | T8T9 and adjacent discs | T6T7 to T10T11 | 38 mm | MT | 20° |
| 9 | 69 | F | C4 and adjacent discs | C3 to C5 | 24.5 mm | OSSA | 22° |
| 10 | 42 | M | L1 and adjacent discs | T11T12 to L2L3 | 38 mm | OSSA | 15° |
| 11 | 52 | M | L1 and adjacent discs | T11T12 to L2L3 | 38 mm | EF | 9° |
| 12 | 60 | F | T12 and adjacent discs | T10T11 to L1L2 | 32 mm | No growth | 8° |
| 13 | 65 | M | L1 and adjacent discs | T11T12 to L2L3 | 33.5 mm | ORSA | 8° |
| 14 | 59 | M | L4 and adjacent discs | L2L3 to L5S1 | 41 mm | PA | 10° |
| 15 | 73 | F | L2L3 and adjacent discs | T12L1 to L4L5 | 62 mm | EC | 23° |
| 16 | 58 | M | T11 and adjacent discs | T9T10 to T12L1 | 29 mm | MT | 15° |
| 17 | 55 | F | L1 and adjacent discs | T10T11 to L2L3 | 29 mm | OSSA | 14° |
| 18 | 64 | M | C4 and adjacent discs | C3 to C5 | 23 mm | EC | 12° |
| 19 | 68 | M | L1 and adjacent discs | T11T12 to L2L3 | 32 mm | ORSA | 7° |
| 20 | 56 | M | L2 and adjacent discs | T12L1 to L3L4 | 35 mm | OSSA | 11° |
F: Female; M: Male; T: Thoracic spine; L: Lumbar spine; S: Sacrum; OSSA: Oxacillin-sensitive staphylococcus aureus; ORSA: Oxacillin-resistant staphylococcus aureus; MT: Mycobacterium tuberculosis; SV: Streptococcus viridans; PA: Pseudomonas aeruginosa; EF: Enterococcus faecalis; EC: Escherichia coli.
Figure 2.
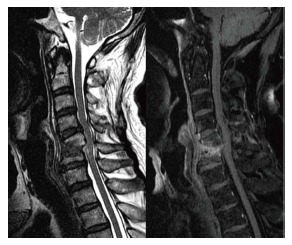
Sagittal T2-weighted and contrast-enhanced magnetic resonance imaging revealed C5-C6 infectious spondylitis with epidural abscess accumulation and spinal cord compression.
Figure 3.
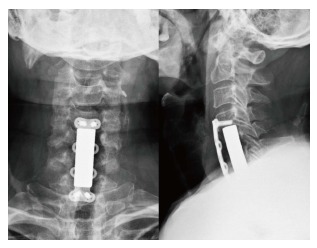
Single-stage anterior radical debridement and tantalum mesh cage implantation followed by supplemental anterior locked plate instrumentation were performed to treat infectious spondylitis and correct kyphotic deformity. Postoperative radiograph showed better alignment after single-stage anterior surgery for complicated infectious spondylitis.
Figure 4.
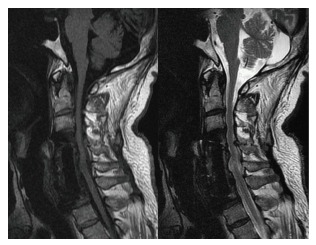
The follow-up sagittal T1- and T2-weighted magnetic resonance imaging demonstrated good implant position and no evidence of recurrent infection.
Figure 5.
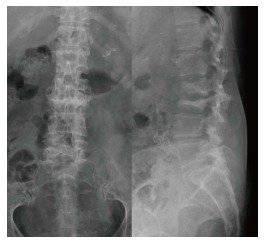
A 73-year-old woman with end-stage renal disease sustained severe back pain and intermittent high fever. Radiograph showed endplate erosion and destruction of the 2nd and 3rd lumbar vertebrae, and loss of lumbar lordotic alignment.
Figure 6.
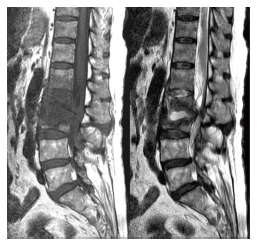
Sagittal T1- and T2-weighted magnetic resonance imaging revealed L2-L3 infectious spondylitis with epidural abscess accumulation.
Neurologic deficits were much improved, from the median of Frankel D before surgery to Frankel D before discharge (P = 0.003), and to Frankel E at the 1-year follow-up visit (P = 0.001). No patient experienced neurologic deterioration after the operation. Severe back pain related to infectious spondylitis significantly decreased from the average VAS of 7.4 ± 0.8 (range, 6 to 9) before surgery to VAS of 3.3 ± 0.8 (range, 2 to 5) after surgery (P < 0.001), and to VAS of 2.2 ± 0.9 (range, 1 to 4) at the 1-year follow-up visit (P < 0.001). Eight patients could achieve excellent outcomes. Ten patients have good outcomes based on the modified Brodsky’s criteria, and the improvement showed significant differences 1 year after surgery (P < 0.001) (Table 2).
Table 2.
Comparison of clinical outcomes and radiographic findings before surgery and after surgery
| Preop | Postop | 1 yr later | P (a/b) | |
| VAS | 7.4 ± 0.81 | 3.3 ± 0.81 | 2.2 ± 0.91 | < 0.001/< 0.001 |
| FS | D (C,E)2 | D (D,E)2 | E (E,E)2 | = 0.003/= 0.001 |
| MBC | F (P,F)2 | G (G,G)2 | G (G,E)2 | < 0.001/< 0.001 |
mean ± SD;
median (25th percentile, 75th percentile). Preop: Preoperative; Postop: Postoperative; a: Postop vs preop with Wilcoxon signed-rank test; b: Postop 1 year vs preop with Wilcoxon signed-rank test. VAS: Visual analog scale: 0 means no pain and 10 means the most pain possible. MBC: Modified Brodsky criteria: P = poor, F = fair, G = good, E = excellent; FS: Frankel scale: A = complete paralysis; B = sensory function only below the injury level; C = incomplete motor function below injury level; D = fair to good motor function below injury level; E = normal function.
Causative bacteria were isolated in 19 (95%) of 20 biopsy cultures through either preoperative or intraoperative procedure. Ten of the 20 patients were infected with Staphylococcus aureus, including 6 with the oxacillin-sensitive strain and 4 with the oxacillin-resistant strain. Three patients had Mycobacterium tuberculosis infection, 2 had Pseudomonas aeruginosa infection, 2 had Escherichia coli infection, and 2 had Streptococcus viridans and Enterococcus faecalis infections. Intravenous antibiotic therapy was continuously administered for a minimum of 6 wk postoperatively based on the specific microbial sensitivities and the identified pathogenic organism. Oral antibiotics were not routinely used after discharge. At outpatient clinics, anti-tuberculous chemotherapy was given for 12 mo or longer. A full course of broad-spectrum antibiotics was administered for the patient with negative culture results. Both elevated CRP and ESR values returned to normal limits at the 1-year follow-up visit (Figure 8).
Figure 8.

Percentage changes in serological values before and after single-stage anterior and/or posterior surgery in patients with complicated infectious spondylitis.
No implant breakage or tantalum mesh cage dislodgement was found. One patient who was receiving regular hemodialysis experienced refractory infection and underwent additional debridement with antibiotic bead deposition. No recurrent infections were found among the patients during the postoperative follow-up.
DISCUSSION
Infectious spondylitis has generally been regarded as medical disease. Effective antibiotic therapy is the mainstay of successful nonsurgical treatment. Surgery has historically been recommended in several circumstances: Cases refractory to appropriate conservative management, spinal cord compression resulting in neurologic deficit, progressive instability due to significant destruction, severe scoliosis or kyphosis caused by chronic infection[16,17]. All patients in the present study sustained complicated infectious spondylitis with pathological fracture and adjacent discitis. Extensive destruction of the vertebral body resulted in a large amount of epidural abscess accumulation, progressive scoliotic or/and kyphotic deformity, severe back pain, spinal instability, and neurological impairment. A single-stage anterior or combined anterior-posterior procedure, including an anterior surgery for initially radical debridement and subsequent TaMC reconstruction, was performed to treat these patients An additional posterior surgery was used for immediate pedicle screw instrumentation to keep physiologic alignment and secure spinal stability in patients with thoracolumbar spine infection. Anterior instrumentation using locked plate/screw one above and one below was used for patients with cervical spine infection.
Various biological and mechanical spacers, including fibular autograft, fibular allograft, and even antibiotic-impregnated methylmethacrylate, are used to reconstruct the anterior column after corpectomy[18-21]. Antibiotic-impregnated methylmethacrylate is sometimes formed into antibiotic beads to salvage deep wound infection in our clinical practice. The defect after corpectomy is usually repaired by an autologous bone graft from the fibula or the iliac crest, which may cause a 25% increase in permanent morbidity of the donor site[22-25]. The advantages of allograft bone include the elimination of the harvesting surgical site, the related postoperative pain, and the added expense of a second operative procedure. However, there is still a slight chance of disease transmission using allograft. The other concern is that the sources or sufficient allograft is usually unavailable in most hospitals. The use of metallic implants for vertebral body reconstruction and stabilization following extensive debridement at the site of infection is controversial. Only few retrospective cohort studies including few cases have been previously published. Recent studies have focused on the usefulness, stability, and safety with minimal recurrence of internal fixation of metallic implants in eradicating an active spinal infection[26-29]. In an observational cohort study at 5 tertiary care hospitals in South Korea, 153 patients with spinal infection requiring surgical management were enrolled. Among these patients, 94 (61.4%) underwent non-instrumented surgery and 59 (38.6%) underwent instrumented surgery[30]. Clinical outcomes were evaluated using the following measures: Infection-related death, primary failure, recurrence, and sequelae. The results showed that placement of spinal instrumentation did not adversely affect the clinical outcomes. The authors concluded that concerns about infection recurrence and complications should not prevent the use of instrumentation in the management of vertebral osteomyelitis where spinal stability is necessary.
Despite the fact that there is evidence for bacterial adhesion to metal implants, the strong immunity of the highly vascularized cancellous vertebral body bone is unique, even in the presence of infection. In an in vitro cell culture experimental study, S. aureus and Staphylococcus epidermidis were used to evaluate qualitatively and quantitatively bacterial adherence to metallic implants including tantalum, tantalum-coated stainless steel, titanium, titanium alloy, and grit-blasted and polished stainless steel. The results showed that pure tantalum presents with a lower or similar bacterial adhesion when compared with commonly used materials in orthopedic implants[31]. Schildhauer et al[32] compared the functions and cytokine response of human leukocytes and equally sized solid orthopedic metal implant materials (pure titanium, titanium alloy, stainless steel, pure tantalum, and tantalum-coated stainless steel) toward porous tantalum foam biomaterial. The results indicated that leukocyte activation at the surface of tantalum material induces a microenvironment, which promotes local host defense mechanism with increased phagocytosis, chemotaxis, and whole blood S. aureus killing rate. In a clinical study, Tokarski et al compared the use of tantalum and titanium acetabular components in revision total hip arthroplasty[15]. They concluded that tantalum components are associated with a lower incidence of subsequent infection when used in patients with periprosthetic joint infection.
TaMC derived from trabecular metal (TM) Technology has an advanced fixation surface designed for orthopedic implants. With a high coefficient of friction (0.98), it provides excellent initial scratch fit. In contrast to coatings and other surfaces, TM material has up to 80% porosity, which enhances the potential for bone ingrowth and soft tissue vascularization[33]. Sinclair et al[34] reported an in vivo assessment of polyetheretherketone (PEEK) and porous tantalum cervical interbody fusion devices in a goat model. The result showed that bone growth into and around the TM implant margins was better than the PEEK devices. Ordway et al[35] examined the implant-endplate interface using a cyclic fatigue loading protocol to model the subsidence observed in vivo. The TM construct demonstrated comparable axial stability and subsidence to a fibular allograft. Given the above-mentioned reasons, it could be an alternative material for anterior reconstruction in spine surgery.
A single-stage combined anterior-posterior approach, as opposed to 2-stage surgery, has advantages of a shorter anesthesia time, less anxiety for the patient and family, less blood loss, and earlier mobilization. In the current study, nineteen out of twenty (95%) patients achieved excellent outcome without complication, which was better than the previous reports. Additional anterior debridement and antibiotic bead deposition successfully treated the residual patient with refractory infection and anterior wound dehiscence. All 20 patients who received this combined surgery for their complicated infectious spondylitis recovered from the illness uneventfully. Tantalum with adequate length was used for anterior column reconstruction after radical debridement. All patients had significant improvement of neurologic function and back pain. Good recovery of the sagittal alignment was achieved with an average 14.9° correction of the Cobb angle. No complications related to the single-stage combined surgery were noted. Tokarski et al[15] analyzed 10 clinical series involving 106 patients undergoing a single-stage procedure for their spinal infection. Deep wound infections were reported in 6.6% of patients, with most treated by debridement and secondary granulation. Superficial wound infections were found in 2.8% of patients. Patients undergoing spinal instrumentation for spinal disorders other than infection treatment had a similar incidence of postoperative infection. An incidence as high as 20% in instrumented spinal surgery was even reported in previous studies[36,37]. Therefore, if a cooperative surgical team and care unit are available, a single-stage combined anterior-posterior surgery may be a good alternative in consideration of complications of staged surgeries.
This study has several limitations. First, only 20 cases were examined. Second, this retrospective study did not include patients receiving variant treatment strategies for comparison, and lacked randomization. A large patient population with prospectively controlled comparison groups may be required to evaluate the benefit and feasibility of this single-stage combined anterior-posterior procedure using tantalum reconstruction for complicated infectious spondylitis. Third, most of the patients in this study had received different types of treatment, either open surgery or conservative antibiotics, and then were admitted to our institute due to progressive infection at their original hospital and/or previous failed treatment. After careful examination and evaluation, a primary or revision surgery was performed in these eligible patients, which might cause selective and therapeutic bias. However, uncontrolled infection and difficulty in bony incorporation did not occur in the patients with either single-stage surgery or anterior reconstruction using tantalum, based on the clinical results of this small patient population.
In conclusion, single-stage anterior debridement and reconstruction combined with anterior instrumentation for cervical spine and posterior instrumentation for thoracolumbar spine can be recommended to treat patients with complicated infectious spondylitis. Anterior radical debridement and TaMC implantation and associated supplemental instrument fixation provide immediate secure stability, successful infection management, neurologic impairment recovery, better physiological alignment, satisfactory pain relief, and significant improvement of daily activities. TaMC can be considered an alternative for anterior column reconstruction, which can provide biomechanical support, avoid donor site morbidity, proceed to bony incorporation, and appear to be protective against infection. However, an administration of a full course of offending pathogens specific intravenous antibiotics or chemotherapy is mandatory to maintain good long-term outcomes and eliminate the risk of recurrent infection.
COMMENTS
Background
Spinal infections challenge clinical physicians because of their varied presentation and complicated course. The use of metallic implants for vertebral body stabilization and reconstruction following debridement at the lesion of infection is controversial. Previously, no study has investigated the use of tantalum mesh cage (TaMC) for anterior reconstruction in the treatment of complicated infectious spondylitis. The primary aim of this study was to evaluate the efficacy of TaMC in patients with Complicated infectious spondylitis.
Research frontiers
The authors demonstrated that TaMC could be a good alternative for anterior reconstruction.
Innovations and breakthroughs
Single-stage anterior debridement and TaMC implantation followed by immediate instrumentation provided immediate stability, successful infection control, neurologic deficit recovery, more satisfactory alignment, adequate pain relief, and better improvement of daily activities.
Applications
Single-stage anterior debridement and TaMC implantation followed by immediate instrumentation was useful for patients with complicated infectious spondylitis. This method could avoid the complications derived from various biological spacers such as autograft and allograft.
Terminology
TaMC derived from Trabecular Metal (TM) Technology has an advanced fixation surface designed for orthopedic implants. TM material has up to 80% porosity, which enhances the potential for bone ingrowth and soft tissue vascularization.
Peer-review
This is a good study, which evaluates clinical and radiological results of patients with complicated infectious spondylitis treated with single-stage anterior debridement and reconstruction using tantalum mesh cage followed by immediate instrumentation.
Footnotes
Institutional review board statement: This retrospective study was undertaken using data from medical records. This study was approved by the ethical committee of the E-DA hospital (EMRP: 105-064).
Informed consent statement: Our retrospective study contained data from medical records only. The study was registered based on the data protection agency of our institute.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest. No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 14, 2017
First decision: April 14, 2017
Article in press: May 31, 2017
P- Reviewer: Garip Y, Pan HC, Wang F S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
Contributor Information
Shih-Chieh Yang, Department of Orthopedic Surgery, E-Da Hospital, I-Shou University, Kaohsiung 82445, Taiwan. ed102777@edah.org.tw.
Hung-Shu Chen, Department of Orthopedic Surgery, E-Da Hospital, I-Shou University, Kaohsiung 82445, Taiwan.
Yu-Hsien Kao, Department of Orthopedic Surgery, E-Da Hospital, I-Shou University, Kaohsiung 82445, Taiwan.
Yuan-Kun Tu, Department of Orthopedic Surgery, E-Da Hospital, I-Shou University, Kaohsiung 82445, Taiwan.
References
- 1.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27–33. doi: 10.1097/01.blo.0000203452.36522.97. [DOI] [PubMed] [Google Scholar]
- 2.Bonfiglio M, Lange TA, Kim YM. The Classic: Pyogenic vertebral osteomyelitis: disk space infections. 1973. Clin Orthop Relat Res. 2006;444:4–8. doi: 10.1097/01.blo.0000201172.64764.bb. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Yan CG, Xiang HY, Xing T, Wang NS. The significance of platelet activation in ankylosing spondylitis. Clin Rheumatol. 2008;27:767–769. doi: 10.1007/s10067-008-0847-7. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Zhao B, Wang N. Significance of sacroiliac joint aerocele in diagnosis of ankylosing spondylitis. Shanghai Jiaotong Daxue Xuebao (Ziran Kexue Ban) 2011;16:636–640. [Google Scholar]
- 5.Cahill DW, Love LC, Rechtine GR. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74:878–886. doi: 10.3171/jns.1991.74.6.0878. [DOI] [PubMed] [Google Scholar]
- 6.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874–880. doi: 10.2106/00004623-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;69:26–35. doi: 10.1302/0301-620X.69B1.3818728. [DOI] [PubMed] [Google Scholar]
- 8.Maiuri F, Iaconetta G, Gallicchio B, Manto A, Briganti F. Spondylodiscitis. Clinical and magnetic resonance diagnosis. Spine (Phila Pa 1976) 1997;22:1741–1746. doi: 10.1097/00007632-199708010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Perronne C, Saba J, Behloul Z, Salmon-Céron D, Leport C, Vildé JL, Kahn MF. Pyogenic and tuberculous spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis. 1994;19:746–750. doi: 10.1093/clinids/19.4.746. [DOI] [PubMed] [Google Scholar]
- 10.Rohde V, Meyer B, Schaller C, Hassler WE. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine (Phila Pa 1976) 1998;23:615–620. doi: 10.1097/00007632-199803010-00016. [DOI] [PubMed] [Google Scholar]
- 11.Korovessis P, Vardakastanis K, Fennema P, Syrimbeis V. Mesh cage for treatment of hematogenous spondylitis and spondylodiskitis. How safe and successful is its use in acute and chronic complicated cases? A systematic review of literature over a decade. Eur J Orthop Surg Traumatol. 2016;26:753–761. doi: 10.1007/s00590-016-1803-x. [DOI] [PubMed] [Google Scholar]
- 12.Talia AJ, Wong ML, Lau HC, Kaye AH. Safety of instrumentation and fusion at the time of surgical debridement for spinal infection. J Clin Neurosci. 2015;22:1111–1116. doi: 10.1016/j.jocn.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Zeng K, Yin X, Huang J, Tang M, Guo C. Debridement, internal fixation, and reconstruction using titanium mesh for the surgical treatment of thoracic and lumbar spinal tuberculosis via a posterior-only approach: a 4-year follow-up of 28 patients. J Orthop Surg Res. 2015;10:150. doi: 10.1186/s13018-015-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang ZX, Li T, Hao DJ. Single-stage Treatment of Osteomyelitis of the Cervical Spine Using Anterior Instrumentation and Titanium Mesh Cages. Spine (Phila Pa 1976) 2016;41:E949–E954. doi: 10.1097/BRS.0000000000001515. [DOI] [PubMed] [Google Scholar]
- 15.Tokarski AT, Novack TA, Parvizi J. Is tantalum protective against infection in revision total hip arthroplasty? Bone Joint J. 2015;97-B:45–49. doi: 10.1302/0301-620X.97B1.34236. [DOI] [PubMed] [Google Scholar]
- 16.Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;44:1018–1025;. doi: 10.1097/00006123-199905000-00047. [DOI] [PubMed] [Google Scholar]
- 17.Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg. 2001;94:1–7. doi: 10.3171/spi.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 18.Dietze DD, Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg. 1997;86:981–989. doi: 10.3171/jns.1997.86.6.0981. [DOI] [PubMed] [Google Scholar]
- 19.Graziano GP, Sidhu KS. Salvage reconstruction in acute and late sequelae from pyogenic thoracolumbar infection. J Spinal Disord. 1993;6:199–207. doi: 10.1097/00002517-199306030-00003. [DOI] [PubMed] [Google Scholar]
- 20.Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926–933. doi: 10.1097/00006123-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Chung TC, Yang SC, Chen HS, Kao YH, Tu YK, Chen WJ. Single-stage anterior debridement and fibular allograft implantation followed by posterior instrumentation for complicated infectious spondylitis: report of 20 cases and review of the literature. Medicine (Baltimore) 2014;93:e190. doi: 10.1097/MD.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurz LT, Garfin SR, Booth RE. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine (Phila Pa 1976) 1989;14:1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255–265. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 24.Schnee CL, Freese A, Weil RJ, Marcotte PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976) 1997;22:2222–2227. doi: 10.1097/00007632-199710010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 26.Jin D, Qu D, Chen J, Zhang H. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13:114–121. doi: 10.1007/s00586-003-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shetty AP, Aiyer SN, Kanna RM, Maheswaran A, Rajasekaran S. Pyogenic lumbar spondylodiscitis treated with transforaminal lumbar interbody fusion: safety and outcomes. Int Orthop. 2016;40:1163–1170. doi: 10.1007/s00264-015-3063-5. [DOI] [PubMed] [Google Scholar]
- 28.Robinson Y, Tschoeke SK, Finke T, Kayser R, Ertel W, Heyde CE. Successful treatment of spondylodiscitis using titanium cages: a 3-year follow-up of 22 consecutive patients. Acta Orthop. 2008;79:660–664. doi: 10.1080/17453670810016687. [DOI] [PubMed] [Google Scholar]
- 29.Linhardt O, Matussek J, Refior HJ, Krödel A. Long-term results of ventro-dorsal versus ventral instrumentation fusion in the treatment of spondylitis. Int Orthop. 2007;31:113–119. doi: 10.1007/s00264-006-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park KH, Cho OH, Lee YM, Moon C, Park SY, Moon SM, Lee JH, Park JS, Ryu KN, Kim SH, et al. Therapeutic outcomes of hematogenous vertebral osteomyelitis with instrumented surgery. Clin Infect Dis. 2015;60:1330–1338. doi: 10.1093/cid/civ066. [DOI] [PubMed] [Google Scholar]
- 31.Schildhauer TA, Robie B, Muhr G, Köller M. Bacterial adherence to tantalum versus commonly used orthopedic metallic implant materials. J Orthop Trauma. 2006;20:476–484. doi: 10.1097/00005131-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Schildhauer TA, Peter E, Muhr G, Köller M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J Biomed Mater Res A. 2009;88:332–341. doi: 10.1002/jbm.a.31850. [DOI] [PubMed] [Google Scholar]
- 33.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Sinclair SK, Konz GJ, Dawson JM, Epperson RT, Bloebaum RD. Host bone response to polyetheretherketone versus porous tantalum implants for cervical spinal fusion in a goat model. Spine (Phila Pa 1976) 2012;37:E571–E580. doi: 10.1097/BRS.0b013e318240f981. [DOI] [PubMed] [Google Scholar]
- 35.Ordway NR, Rim BC, Tan R, Hickman R, Fayyazi AH. Anterior cervical interbody constructs: effect of a repetitive compressive force on the endplate. J Orthop Res. 2012;30:587–592. doi: 10.1002/jor.21566. [DOI] [PubMed] [Google Scholar]
- 36.Mok JM, Guillaume TJ, Talu U, Berven SH, Deviren V, Kroeber M, Bradford DS, Hu SS. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine (Phila Pa 1976) 2009;34:578–583. doi: 10.1097/BRS.0b013e31819a827c. [DOI] [PubMed] [Google Scholar]
- 37.Rechtine GR, Bono PL, Cahill D, Bolesta MJ, Chrin AM. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15:566–569. doi: 10.1097/00005131-200111000-00006. [DOI] [PubMed] [Google Scholar]


