Abstract
Background
There is currently no anti-fibrotic drug therapy available to treat hepatitis C virus (HCV) cirrhosis. The aim of this study was to assess the safety, tolerability, and anti-fibrotic effect of PRI-724, a small-molecule modulator of Wnt signaling, in patients with HCV cirrhosis.
Methods
In this single-center, open-label, phase 1 trial, we sequentially enrolled patients with HCV cirrhosis who were classified as Child-Pugh (CP) class A or B. PRI-724 was administered as a continuous intravenous infusion of 10, 40, or 160 mg/m2/day for six cycles of 1 week on and 1 week off. The primary endpoints were frequency and severity of adverse events. The secondary endpoint was efficacy of PRI-724 in treating cirrhosis based on CP score and liver biopsy. This study is registered with ClinicalTrials.gov (no. NCT02195440).
Findings
Between Sept 3, 2014 and May 2, 2016, 14 patients were enrolled: CP class A:B, 6:8; median age, 62 (range: 43 to 74) years; male:female, 10:4. Twelve of the 14 patients completed six cycles of treatment; one was withdrawn from the study due to possible study drug-related liver injury (grade 3) in the 160 mg/m2/day dose cohort and one withdrew for personal reasons. Serious adverse events occurred in three patients [21% (3/14)], one of which was possibly related to PRI-724. The most common adverse events were nausea [29% (4/14)] and fatigue [21% (3/14)].
After PRI-724 administration, the CP scores worsened by 1 point in two patients in the 10 mg/m2/day cohort, improved in three patients at 1, 1, and 2 points in the 40 mg/m2/day cohort, and improved in one patient by 3 points in the 160 mg/m2/day cohort. The histology activity index scores of the liver tissue improved in two patients and exacerbated in two patients in the 10 mg/m2/day cohort, and improved in one patient in the 40 mg/m2/day cohort.
Interpretation
This study showed that administration of 10 or 40 mg/m2/day intravenous PRI-724 over 12 weeks was well-tolerated by patients with HCV cirrhosis; however, liver injury as a possible related serious adverse event was observed in the 160 mg/m2/day cohort.
Funding Source
AMED.
Keywords: PRI-724, Liver cirrhosis, HCV, Wnt inhibitor
Abbreviations: ALT, alanine aminotransferase; ALB, albumin; AUC, area under the concentration curve; CBP, CREB binding protein; CREB, cAMP-response element-binding protein; Cmax, maximum concentration; CP, Child-Pugh; HAI, histology activity index; HCV, hepatitis C virus; HTLV, human T-cell leukemia virus; PT, prothrombin time; Tmax, time of maximum observed serum
Highlights
-
•
Safety, tolerability, pharmacokinetics, and anti-fibrotic effect of PRI-724 were assessed in patients with HCV cirrhosis.
-
•
PRI-724 was well tolerated, suggesting that the treatment is relatively safe against HCV liver cirrhosis.
-
•
Significant fibrosis reduction in hepatic lobules 12 weeks after PRI-724 treatment (40 mg/m2/day) in 3 CP class B patients.
Liver cirrhosis is one of the leading causes of morbidity and mortality in developed countries and is the 14th most common cause of death in adults worldwide. However, there is currently no anti-fibrotic drug therapy available to treat hepatitis C virus (HCV) cirrhosis. This study showed that intravenous PRI-724 at 10 or 40 mg/m2/day over 12 weeks was well-tolerated by patients with HCV cirrhosis and resulted in an improvement of liver histology and CP class in several patients.
1. Introduction
Liver cirrhosis is one of the causes of morbidity and mortality in developed countries and is the 14th most common cause of death in adults worldwide (Tsochatzis et al., 2014). Liver cirrhosis results from several mechanisms of liver injury (i.e., viral hepatitis, alcohol, metabolic) that lead to fibrogenesis. Fibrosis is an excessive wound healing response that occurs in most forms of chronic liver damage and results in the accumulation of excessive extracellular matrix (Schuppan and Afdhal, 2008). Fibrosis may progress to cirrhosis as a result of continuous liver damage. Cirrhosis is characterized by diffuse nodular regeneration surrounded by dense fibrotic septa with subsequent parenchymal extinction and collapse of liver structure (Hernandez-Gea and Friedman, 2011).
Current antiviral therapies for chronic hepatitis C can retard or prevent liver fibrosis, and direct acting antivirals improve liver function even in decompensated liver cirrhosis (Elsharkawy et al., 2017) (Curry et al., 2015, Manns et al., 2016), but none of these therapies treat the fibrosis directly. Liver cirrhosis is a major predictor of liver-related morbidity due to various complications (i.e., ascites, esophageal varices, encephalopathy) and a risk factor for hepatocellular carcinoma (Lee et al., 2015). Thus, there is an urgent need to develop anti-fibrotic treatments that can prevent and improve liver cirrhosis.
Activated Wnt-β-catenin signaling has been implicated in the fibrosis of a number of organ systems, including the lung, kidney, skin, and liver (Edeling et al., 2016, Monga, 2015). Modulation of the small molecule Wnt has proven to be extremely effective in murine models of lung, kidney, and liver fibrosis (Emami et al., 2004, Henderson et al., 2010). Specific inhibition of the CBP-β-catenin interaction was shown to not only ameliorate, but also to reverse late stage fibrotic injury of the lung and kidney in murine models (Kahn, 2014). Recently, we have reported that the selective CBP-β-catenin antagonist PRI-724 has anti-fibrotic effects in liver fibrosis murine models and promoted resolution of liver fibrosis due to hepatic stellate cell inactivation and macrophage migration (Osawa et al., 2015). These findings suggested that PRI-724 has a therapeutic potential in human liver cirrhosis.
In this phase 1 trial of PRI-724, we aimed to assess the overall safety, tolerability, and preliminary efficacy of escalating doses in a cohort of patients with HCV liver cirrhosis.
2. Methods
2.1. Study Design and Patients
In this single-center, open-label, phase I, cohort dose-escalation trial, we sequentially enrolled patients with HCV liver cirrhosis treated at the Cancer and Infectious diseases center of Tokyo Metropolitan Komagome Hospital, Tokyo, Japan. The study was approved by the Institutional Review Board of the Komagome Hospital (approval number: 14-005) and was done in compliance with Good Clinical Practice guidelines, the Declaration of Helsinki, and regulatory requirements. Prism Pharma provided PRI-724, related safety information, and scientific advice.
Eligible participants were between 18 and 75 years of age with HCV infection (positive for serum HCV RNA) and a definitive diagnosis of liver cirrhosis established by liver biopsy performed during the screening period [histological activity index (HAI) score: Grade IV-D]. They were classified as CP class A (a score of 5 to 6 on a scale ranging from 5 to 15) or CP class B (a score of 7 to 9). Patients who had a history of or requirement of treatment for esophageal and gastric varices were not eligible. We excluded patients with human immunodeficiency virus (HIV), human T-lymphotropic virus (HTLV), syphilis, and hepatitis B virus infection. We also excluded patients with a history of primary hepatocellular carcinoma due to a complication of cirrhosis. The full eligibility criteria are included in the appendix. We obtained signed informed consent from all participants at the time of screening.
2.2. Procedures
The patients were divided into three cohorts that were administered 10, 40, or 160 mg/m2/day PRI-724 (Prism Pharma, Yokohama, Japan), respectively. PRI-724 was administered for six cycles, where each cycle consisted of 1 week of continuous IV administration of PRI-724, followed by a 1-week observation period. The administration of the second cycle was not started until the plasma concentrations of PRI-724 and C-82 (an active metabolite of PRI-724) on day 1 and day 2 in cycle 1 were examined.
In the original plan, CP class A patients were then escalated to the next dose when the following criteria were met: all three patients within each CP class A cohort had completed all cycle 1 treatments; the plasma concentrations of PRI-724 and C-82 on day 1, 2 and on day 8, 9 in cycle 1, as well as the safety of patients in the cohort were confirmed; and when it was determined to be safe to proceed to the next dose. Class B patients were moved to the next dose following the same procedure as class A patients.
We planned to enroll 18 patients (6 per cohort), with each cohort consisting of three CP class A and three CP class B patients; however, the final number of subjects was only 14. We extended the registration period in an effort to enroll four more patients, but had to close registration owing to limitation of public funds. PRI-724 was administered to these 14 patients according to the above procedure for six cycles (total: 12 weeks). Each patient was then observed for 28 days after the last day of administration. We recorded adverse events and clinical laboratory results throughout the study and graded adverse events according to the Common Terminology Criteria for Adverse Events version 4·0. Investigators regularly assessed safety and tolerability, including serious adverse events, especially those associated with treatment discontinuation.
Liver biopsy samples taken at screening were used as baseline data. Samples were also taken within 2 weeks of the final treatment. Three independent central pathologists examined all biopsy slides at baseline and at 12 weeks in a blind manner. Biopsy slides were evaluated using the Knodell scoring system, and fibrosis was staged according to the Ishak modified HAI. Patients were assessed for histological improvement (HAI score: ≥ 2 points reduction in score) at 12 weeks. To evaluate whether the study drug had an anti-fibrotic effect, we measured fibrosis in hepatic lobules by standardized computer-assisted image analysis. The independent pathologist blindly selected five Sirius red-stained parenchyma spots in all biopsy samples and automatically calculated the Sirius red-positive area using HistoQuant software (3DHISTECH, Budapest, Hungary). We carried out pharmacokinetic assessments of plasma from blood samples collected before treatment and at specified time points after PRI-724 treatment on days 1 and 7 of cycle 1. We measured the plasma concentrations of PRI-724 and C-82 (an active metabolite of PRI-724) in patient blood samples taken from all patients on days 1 and 7.
2.3. Outcomes
The primary endpoints were safety, tolerability, and dose-limiting toxicities of multiple escalating doses of PRI-724 when administered via intravenous infusion; safety was measured by analyzing the frequency and severity of adverse events. The other major primary goal was determination of PRI-724 pharmacokinetics in vivo. The secondary endpoint was efficacy for liver cirrhosis treatment. To evaluate efficacy, we examined CP scores and histopathology of liver biopsies before and after treatment. The CP score was determined before initiation of PRI-724 administration by measuring serum albumin (ALB), serum bilirubin, and prothrombin time (PT), and by diagnosing ascites and hepatic encephalopathy via biopsy within 7 days before the start of PRI-724 administration. Similarly, in accordance with the study schedule, CP scores were determined on day 1, before study drug administration, and on day 15 in cycles 4 and 6. The liver biopsies examined the specimens for fibrosis by staining the liver tissues with silver, Masson-Noguchi, Hematoxylin-Eosin (HE), and Sirius red. Staging and grading were performed using HAI to assess treatment efficacy. Final PRI-724 efficacy was determined by the Treatment Effect Assessment Committee, which comprises liver pathologists.
2.4. Statistical Analysis
All patients were included in the analyses of safety, tolerability, and anti-fibrotic effect of PRI-724. All statistics were descriptive and calculated for each treatment group. Data are expressed as the mean ± SD. Differences between groups were analyzed by the 2-tailed Student's t-test. A P value < 0.05 was considered an indication of statistical significance. Clinical safety and pharmacokinetic data were included in the safety analysis. We performed pre-specified analyses of changes in CP score from baseline to post treatment on day 8 in cycle 4 and on day 15 in cycle 6. We also did a pre-specified secondary analysis of change from baseline in histological scores; it focused on patients with biopsy samples from baseline and 12 weeks after PRI-724 treatment. When the data for a subsequent assessment was missing, it was replaced with the immediately preceding data obtained by the LOCF (last-observation-carried-forward) method, and analysis was performed at the end of the last cycle. However, when the data of day 1 for cycle 2 or subsequent cycles were missing, the data on day 1 of the preceding cycle was used. When the data in cycle 1 were missing, the measurements in the screening period were used.
All analyses were performed with SAS (version 9·2) software. This trial is registered with ClinicalTrials.gov, number NCT02195440.
3. Results
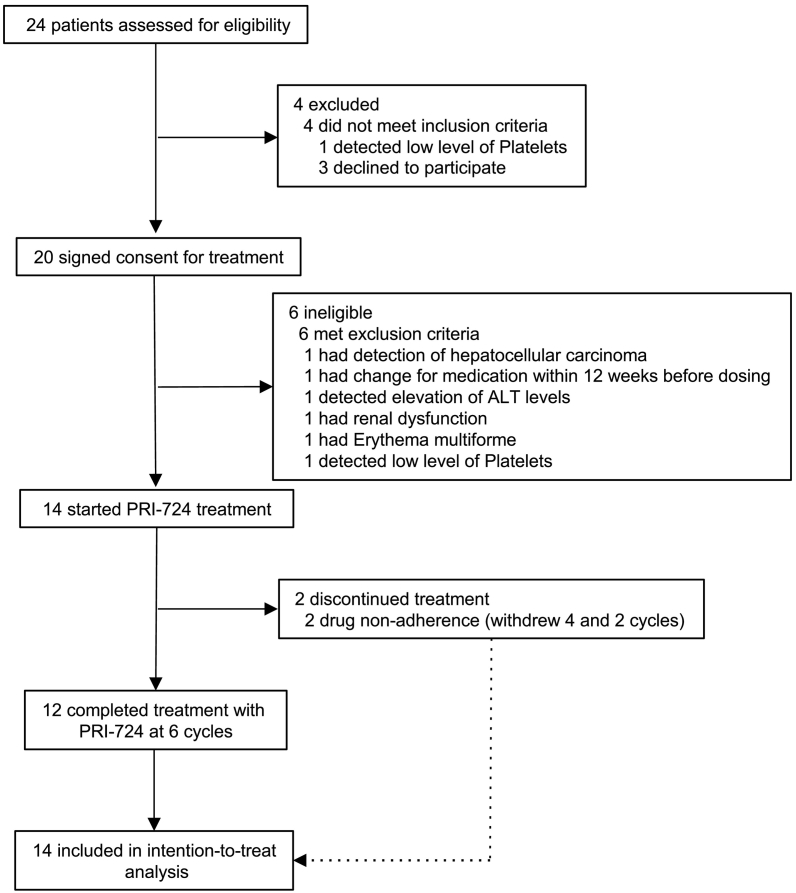
Between Aug 11, 2014 and Aug 8, 2016, we screened 24 patients and enrolled 20 patients (Fig. 1). Of those, 14 patients were treated with PRI-724: six patients entered the 10 mg/m2/day cohort and six patients entered the 40 mg/m2/day dose cohort. Only two patients were enrolled in the 160 mg/m2/day dose cohort. We extended the registration period in an effort to enroll four more patients, but had to close registration owing to limitation of public funds. Baseline patient characteristics are shown in Table 1. No dose-limiting toxicities were observed. PRI-724 was generally well-tolerated, with most adverse events being of grade 1 or 2 (Table 2). Most of the observed adverse events relating to PRI-724 were mild, such as reaction at the injection site [64% (9/14)] and gastrointestinal symptoms [nausea (29% (4/14)), vomiting (14% (2/14)), and constipation (14% (2/14))]. We observed three serious adverse events in three of the 14 patients (one patient from each cohort). We concluded that two of the serious adverse events were not related to the study drug: prolonged hospitalization due to hemorrhage after liver biopsy (10 mg/m2/day cohort) and bacillemia caused by infection at the infusion site (40 mg/m2/day cohort). The other adverse event was possibly related to the study drug (160 mg/m2/day cohort). When the patient (C3-01) was administered antibiotics (Cefaclor) for suppurative dermatitis, an elevated serum alanine aminotransferase (ALT) level (98 IU/mL) was observed. Antibiotic treatment was interrupted, and the patient received intensive therapy for drug-induced liver injury. After the patient's serum ALT level returned to approximately the baseline level (44 IU/mL), the patient started cycle 5 of PRI-724 treatment. However, hyperbilirubinemia (3.8 mg/dL) was observed and the patient's total serum bilirubin level reached a peak at 5.1 mg/dL. Based on this laboratory data, we concluded this case to be possibly related to the study drug and discontinued the study drug at the fifth cycle.
Fig. 1.
Trial profile.
Table 1.
Baseline characteristics.
| Characteristics | 12 weeks of PRI-724 (n = 14) |
|---|---|
| Age (years) | 62 (43–74) |
| Sex | |
| Women | 4 (29%) |
| Men | 10 (71%) |
| Race | |
| Asian | 14 (100%) |
| White | 0 |
| Other | 0 |
| HCV genotype | |
| 1a | 0 |
| 1b | 11 (79%) |
| 2a | 1 (7%) |
| 2b | 2 (14%) |
| HCV RNA | |
| Mean-log IU/mL | 5.65 (4.8–6.9) |
| CP score - no. (%) | |
| ≤ 6 | 6 (43%) |
| 7 | 4 (29%) |
| 8 | 3 (21%) |
| 9 | 1 (7%) |
| ≥ 10 | 0 |
| HAI score - no. (%) | |
| < 10 | 1 (7%) |
| 10–15 | 10 (71%) |
| ≥ 16 | 3 (21%) |
| Ascites - no. (%) | |
| None | 11 (79%) |
| Mild or moderate | 3 (21%) |
| Severe | 0 |
| Treatment experienced | |
| No | 7 (50%) |
| Yes | 7 (50%) |
| Protease inhibitor regimen | 0 |
| Peginterferon plus ribavirin | 5 (36%) |
| Peginterferon or interferon | 2 (14%) |
Table 2.
Adverse events and laboratory abnormalities.
| 10 mg/m2/day (n = 6) |
40 mg/m2/day (n = 6) |
160 mg/m2/day (n = 2) |
||||
|---|---|---|---|---|---|---|
| Events | Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 |
| Common adverse events | ||||||
| Fatigue | 1 (17%) | 0 | 1 (17%) | 0 | 1 (50%) | 0 |
| Nausea | 3 (50%) | 0 | 1 (17%) | 0 | 0 | 0 |
| Vomiting | 2 (33%) | 0 | 0 | 0 | 0 | 0 |
| Headache | 1 (17%) | 0 | 0 | 0 | 0 | 0 |
| Pruritus | 2 (33%) | 0 | 1 (17%) | 0 | 0 | 0 |
| Constipation | 1 (17%) | 0 | 2 (33%) | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 1 (50%) | 0 |
| Insomnia | 2 (33%) | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 |
| Cough | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscle spasm | 0 | 0 | 0 | 0 | 0 | 0 |
| Rash | 1 (17%) | 0 | 0 | 0 | 0 | 0 |
| Irritability | 0 | 0 | 0 | 0 | 0 | 0 |
| Vertigo | 1 (17%) | 0 | 1 (17%) | 0 | 0 | 0 |
| Fever | 3 (50%) | 0 | 2 (33%) | 0 | 2 (100%) | 0 |
| Bleeding | 3 (50%) | 0 | 0 | 0 | 0 | 0 |
| Laboratory abnormality | ||||||
| Alanine aminotransferase | 3 (50%) | 0 | 1 (17%) | 0 | 0 | 0 |
| Aspartate aminotransferase | 3 (50%) | 0 | 1 (17%) | 0 | 0 | 0 |
| Alkaline phosphatase | 0 | 0 | 0 | 0 | 0 | 0 |
| Total bilirubin | 2 (33%) | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 2 (33%) | 2 (33%) | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 1 (17%) | 0 | 0 | 0 |
| Leukopenia | 2 (33%) | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 1 (17%) | 0 | 1 (17%) | 0 | 1 (50%) | 0 |
| Bacteremia | 0 | 0 | 0 | 1 (17%) | 0 | 0 |
| Drug-induced hepatotoxicity | 0 | 0 | 0 | 0 | 0 | 1 (50%) |
The frequent grade 1-2 laboratory abnormality observed during the treatment period was elevation in total bilirubin level, which occurred in two of 14 patients [14% (2/14)]. These two patients had post-baseline elevation in ALT level of at least grade 1 during treatment or within 30 days after the end of treatment. In three patients [21% (3/14)], we observed an elevation in ALT levels of less than grade 1 after the end of treatment with the study drug. These events may be explained by a rebound phenomenon after discontinuing treatment with the study drug, since these elevated levels returned to the baseline within 2 weeks after the last treatment. The most common grade 3 adverse events regardless of PRI-724 association that occurred in more than one patient were thrombocytopenia [2 of 14 patients (14% (2/14)] and bacteremia, as mentioned above (Table 2).
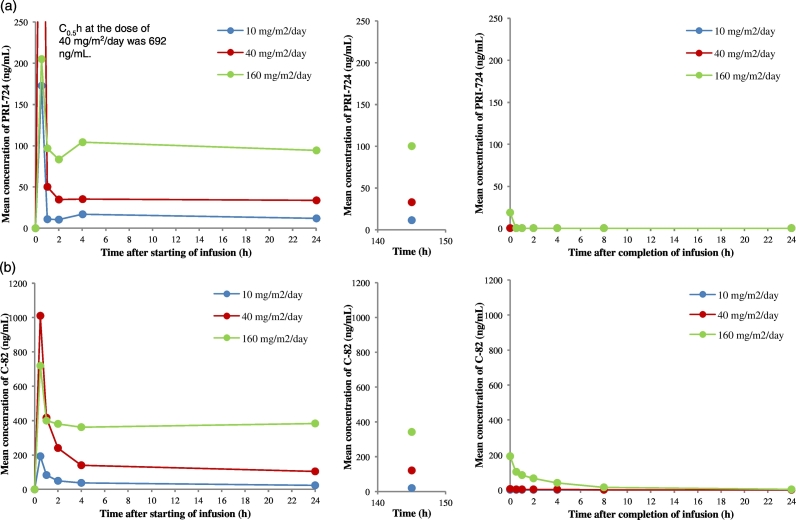
We measured the maximum concentration (Cmax), area under the concentration curve (AUC) with the last concentration extrapolated on the basis of constant elimination, and time of maximum observed serum (Tmax) (Table 3). The intravenous infusion of PRI-724 allowed for immediate and full absorption into systemic circulation, as measured by the detection of the active forms of PRI-724 and C-82. Blood samples from each patient were obtained at six time points within 24 h after PRI-724 infusion, 1 point between 140 and 150 h after infusion, and seven points within 24 h following day 7 of cycle 1. The mean concentration of PRI-724 in the plasma of each cohort group is shown in Fig. 2. PRI-724 concentration in plasma reached Cmax 0·5 h after infusion for all patients except one (C1-01; 10 mg/m2/day). The mean Cmax in the 10, 40, and 160 mg/m2/day cohorts was 177 ± 117, 692 ± 418, and 205 ± 86 ng/mL (mean ± SD), respectively. Thereafter, 2 and 4 h later until 140–150 h after infusion, the concentration of PRI-724 in plasma was stable. The mean Cmax at day 7 in the 10, 40, and 160 mg/m2/day cohorts was 11.4 ± 1.8, 32.7 ± 7.2, and 100 ± 41 ng/mL (mean ± SD), respectively. These results were related to the treatment doses. The concentration of C-82 in plasma showed the same kinetics as PRI-724. The Cmax of C-82 in all patients was detected 0.5 h after infusion. The mean Cmax in the 10, 40, and 160 mg/m2/day cohorts was 193 ± 23, 1010 ± 420, and 720 ± 308 ng/mL (mean ± SD), respectively. The mean Cmax at day 7 in the 10, 40, and 160 mg/m2/day cohorts was 18.6 ± 5.5119 ± 29, and 342 ± 8 ng/mL(mean ± SD), respectively. These results were related to the treatment doses. In addition, we examined the correlation between CP class and concentrations of PRI-724 and C-82 in plasma in each cohort. We concluded that there is no relationship between CP class and levels of PRI-724 and C-82 in plasma.
Table 3.
Pharmacokinetic parameters for PRI-724 and C82 after PRI-724 infusion.
| PRI-724 |
C82 |
|||||
|---|---|---|---|---|---|---|
| Cmax day 1 (ng/mL) | AUC0-24 h day 1 (ngxh/mL) | Tmax day 1 (h) | Cmax day 1 (ng/mL) | AUC0-24 h day 1 (ngxh/mL) | Tmax day 1 (h) | |
| 10 mg/m2/day (n = 6) | 177 (117) | 411 (168) | 1.1 (1·4) | 193 (23) | 877 (348) | 0.5 (0) |
| 40 mg/m2/day (n = 6) | 692 (418) | 1160 (450) | 0.5 (0) | 1010 (420) | 3750 (1690) | 0.5 (0) |
| 160 mg/m2/day (n = 2) | 205 (86) | 2380 (80) | 0.5 (0) | 720 (308) | 9000 (2260) | 0.5 (0) |
| Cmax day 8 (ng/mL) | AUC0-24h day 8 (ngxh/mL) | Tmax day 8 (h) | Cmax day 8 (ng/mL) | AUC0-24 h day 8 (ngxh/mL) | Tmax day 8 (h) | |
| 10 mg/m2/day (n = 6) | BLQ | NC | NC | 1.26 (0.94) | 9.18 (8.43) | 0.1 (0.2) |
| 40 mg/m2/day (n = 6) | 0.018 (0.044) | 0.0045 (0.110) | NC | 6.03 (4.53) | 47.7 (41.7) | 0.3 (0.8) |
| 160 mg/m2/day (n = 2) | 18.8 (26.4) | 4.71 (6.61) | 0 | 192 (76) | 575 (134) | 0 |
BLQ: Below limit of quantification (< 0.1 ng/mL).
NC: Not calculated.
Fig. 2.
Pharmacokinetics of PRI-724 and C-82 after PRI-724 intravenous infusion.
(a) Mean concentration profiles of PRI-724 in the plasma of patients with liver cirrhosis caused by hepatitis C virus during continuous intravenous infusion of PRI-724. (b) Mean plasma concentration profiles of C-82, a metabolite of PRI-724, in the same patients.
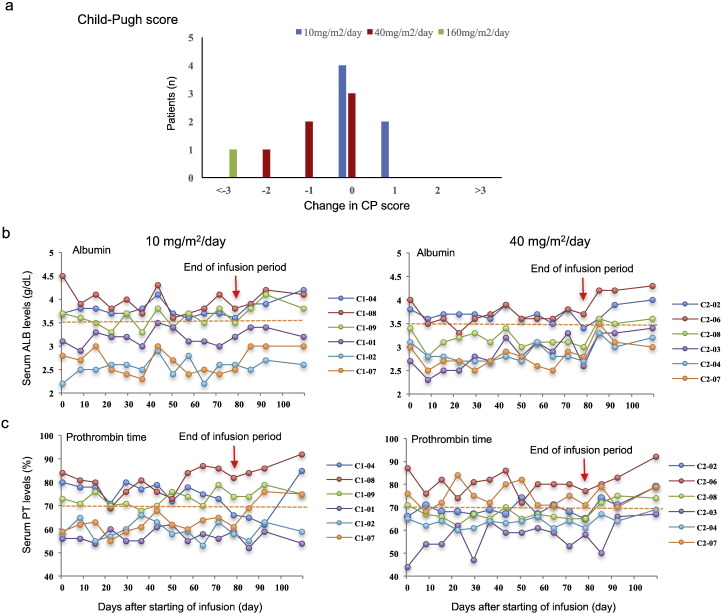
We examined the changes in CP score and HAI score to evaluate whether PRI-724 improved liver function and has the potential to be used as an anti-fibrotic drug in patients with HCV liver cirrhosis. Since one patient (C3-03) completed only two cycles of PRI-724 treatment, 7 out of eight CP class B patients were evaluable. Two patients (C2-07, C3-01) who were diagnosed as CP class B at baseline improved to CP class A after six cycles of PRI-724 treatment (Fig. 3). The serum PT levels of these patients increased and ascites were not detectable although they were present at baseline. We found that in another patient (C1-07), the serum PT time improved from 58 to 78% within 28 days after final PRI-724 treatment. Furthermore, serum PT level in another CP class B patient (C2-03) improved from 43 to 73% at day 45 after final treatment. However, of the 12 patients, two showed deterioration by 1 point. Thus, PRI-724 treatment increased serum ALB and PT levels for several CP class B patients, implying that this study drug has the potential to improve protein synthesis (Fig. 3).
Fig. 3.
Effects of PRI-724 infusion on liver function.
(a) Change in Child-Pugh (CP) score from baseline to post-treatment following six cycles of PRI-724. The CP score ranges from 5 to 15, with higher values indicating more advanced liver cirrhosis. There were six patients each in the 10 and 40 mg/m2/day treatment cohorts, and one patient received 160 mg/m2/day for four cycles. Changes in (b) serum albumin and (c) prothrombin time before, during, and after six cycles of treatment with PRI-724. The dotted yellow line indicates a borderline value in CP score (ALB: 3.5 g/dL; PT: 70%).
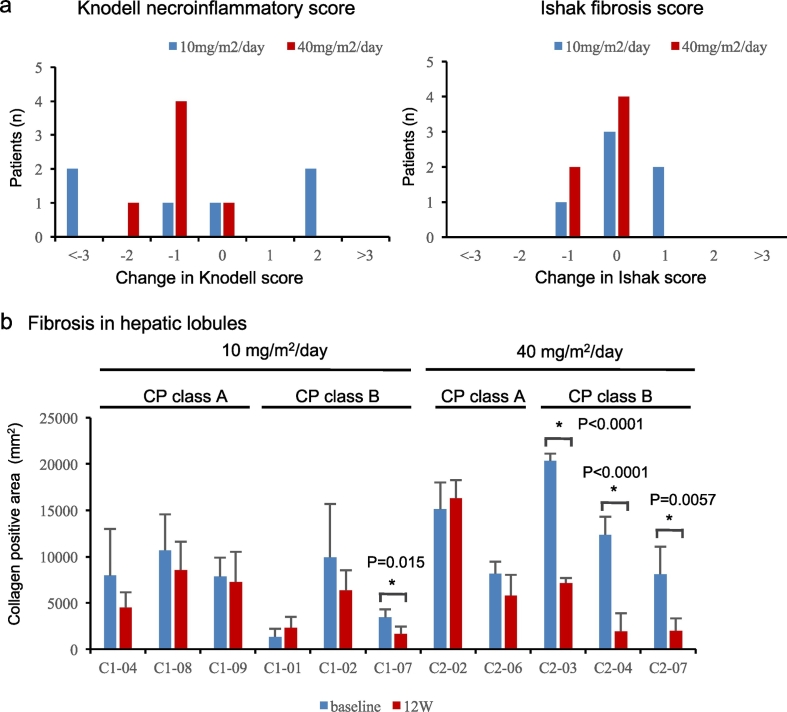
Twelve patients [86% (12/14)] had liver biopsy results at both baseline and 12 weeks after PRI-724 treatment. PRI-724 treatment was associated with histological improvement (≥ 2 points reduction in HAI score) in three of the 12 patients (Fig. 4). However, of the 12 patients, two showed deterioration by 2 points. Furthermore, histological analysis with silver and Sirius Red stains showed that PRI-724 reduced fibrosis (especially reticular fiber) in the hepatic lobules in a dose-dependent manner. To support these findings, we quantified the degree of fibrosis in hepatic lobules by standardized computer-assisted image analysis. As mentioned above, independent liver pathologists randomly selected five views in the liver parenchyma and fibrosis was measured by automated digital imaging microscopy. Among the three class B patients treated with 10 mg/m2/day PRI-724, one had a significant reduction in fibrosis. On the other hand, all the three CP class B patients treated with 40 mg/m2/day PRI-724 had a significant reduction in fibrosis, but no reduction was observed in CP class A patients (Fig. 4).
Fig. 4.
Histological analysis after PRI-724 infusion.
Change from baseline to post-treatment following six cycles of PRI-724 in (a) histology active index (HAI) score. (b) Fibrosis in hepatic lobules. The independent pathologist blindly selected five Sirius red-stained parenchyma spots in all biopsy samples and automatically calculated the Sirius red-positive areas using HistoQuant software. The average of the measured collagen positive areas at baseline was compared with the average at 12 weeks after PRI-724 infusion. Statistical analyses were performed using the paired t-test.
Lower leg edema worsened in one patient in the 10 mg/m2/day cohort, whereas all six patients in the 40 mg/m2/day dose cohort showed no change. In the 160 mg/m2/day dose cohort, no edema was observed in one patient starting at the base line, while the other showed no change.
4. Discussion
We herein reported the results of the first clinical proof-of-concept study targeting CBP-β-catenin by administering the small-molecule compound PRI-724 to patients with HCV cirrhosis. Our results show that overall, PRI-724 was well-tolerated when administered as a continuous intravenous infusion in a regimen of six cycles of 1 week on and 1 week off. In addition, PRI-724 shows encouraging anti-fibrotic activity in HCV cirrhosis, for which no currently approved treatment exists. Two patients in the 160 mg/m2/day dose cohort discontinued treatment in this study; however, only one of these cases was possibly related to adverse events caused by the study drug. No treatment-related death was recorded. Among nine events in three patients (21%) with drug suspension, the adverse events were drug-induced hepatotoxicity, neutropenia, gout, and elevated uric acid in blood. Serious adverse events occurred in three patients (21%); however, two of these events were not related to the study drug. The other serious adverse event was hepatotoxicity, which was possibly related to the study drug. In this event, hyperbilirubinemia was detected, but it resolved with intensive therapy. We also found that the serum bilirubin level in this patient recovered from 2.2 mg/dL at baseline to 1.2 mg/dL at the end of four cycles of treatment.
On the basis of clinical data on another Wnt inhibitor vantictumab (anti-Fzd7) (Bahrami et al., 2017), special attention was given to potential risks of osteoporosis or osteopenia. Given that the Wnt signaling pathway has been shown to be crucial in bone development (Canalis, 2013), we monitored serum parathyroid hormone, bone specific alkaline phosphatase, osteocarcin, and type I collagen C-terminal telopeptide to evaluate whether PRI-724 influences bone metabolism (see supplementary data). We found that these parameters were within normal limits, indicating that PRI-724 treatment did not influence bone-related blood markers. In addition, we did not observe bone-related adverse events during the 12 weeks after infusion. Patients who had been treated with PRI-724 were eligible to enroll in a registry study (ClinicalTrials.gov number, NCT02828254) designed to evaluate incidences of adverse events for 12 months after completion of this study.
Three patients who received 40 mg/m2/day of PRI-724 had a significant reduction of fibrosis in hepatic lobules at 12 weeks; in contrast, only one patient who received 10 mg/m2/day had a reduction in fibrosis. In addition, we found that PRI-724 reduced fibrosis in CP class B patients, but did not reduce fibrosis in CP class A patients. In general, analysis of histological findings should take into account the potential limiting factors including variability in interpretation between observers and sampling error associated with needle biopsy (Manning and Afdhal, 2008). In this study, an independent pathologist reviewed all biopsy samples in a blind manner, unaware of the treatment outcome or patient background. Furthermore, all biopsy samples collected before and after the study drug administration were stained simultaneously with Sirius red, silver, and H.E at a different hospital, thereby minimizing the potential for analytical error.
We measured the HAI score and the area of reticulum fibers in the hepatic lobules using liver biopsy tissue samples to determine the therapeutic anti-fibrotic effect. In some patients, the degree of improvement of HAI score and the area of reticulum fiber were not necessarily consistent with each other. For example, in the case of patient C1-04, HAI score was decreased by 5 points, but no significant decrease was observed in the area of fibrosis. Similarly, in the case of two patients (C2-04 and C2-07), the fibrosis area was significantly decreased, but the HAI score was only decreased by one point. This discrepancy could be due to the fact that the modified HAI scores were determined by considering both inflammatory and fibrosis results. However, it was expected that histological improvement of the thick fibers that have progressed to cirrhosis would take time. Furthermore, histological improvement is difficult to evaluate in the PRI-724 administration period as short as 3 months. Therefore, we focused on the degree of improvement of thin fibers (reticulum fibers) within the hepatic lobule. In the present study, as few as 12 patients could be included; the accuracy and objectivity of this anti-fibrotic analysis method will be further examined in the future.
Although limited to six patients in the dose-escalation phase of the study, the safety profile of the 40 mg/m2/day dose was favorable and did not lead to any treatment discontinuations due to adverse events, supporting the tolerability of this dose. On the basis of its tolerable safety profile, the pharmacokinetics data, and dose-dependent anti-fibrotic effect, 40 mg/m2/day of PRI-724 was determined to be the recommended phase 2 dose.
In preclinical studies, PRI-724 inhibited the activation of isolated primary mouse quiescent hepatic stellate cells and caused the migration of Ly6Clow CD11b+ macrophages into the liver during the fibrosis resolution period. Fibrotic resolution was accompanied by increased matrix metalloproteinase expression in intrahepatic leukocytes (Osawa et al., 2015). Even though the levels of serum HCV RNA were almost same in all patients irrespective of PRI-724 treatment (Supplementary data), it is noteworthy that serum ALB and PT levels improved in several patients only after 12 weeks of treatment. We speculate that these increased concentrations in the blood of liver sinusoids occurred as a result of fiber regression; further investigation with respect to the mechanism is required. The role of hepatocytes is of particular interest because Wnt/β-catenin signaling is essential for hepatocyte proliferation and differentiation (Colletti et al., 2009).
One of the limitations of this study was the small sample size, particularly after the participants were divided into different dose cohorts. Furthermore, two patients in the 160 mg/m2/day had to leave the trial before completing the six cycles of treatment owing to serious adverse events or personal reasons, resulting in inadequate data to evaluate the safety and tolerability of PRI-724 in the maximum dose cohort. Recruitment of patients for this phase 1 study was difficult because the trial involved an experimental drug that had not been tested in cirrhosis patients before. Furthermore, the enrollment period was limited because a research fund expired.
Recently, the development of anti-HCV direct-acting antivirals has led to high rate of viral eradication and improvement in liver function in patients with HCV-decompensated cirrhosis (Curry et al., 2015, Manns et al., 2016). However, based on the fact that the occurrence of liver cancer is not reduced in effectively treated cirrhotic patients (Conti et al., 2016) and because a significant period of time is required for liver fibrosis to resolve (Schuppan and Kim, 2013), there is a need to develop anti-fibrotic drugs to improve the quality of life for convalescent patients and decrease their risk of liver cancer. Development of therapeutic drugs that dissolve fibers in established liver cirrhosis has been a long-standing task. Initially, after a report was published that IFN dissolves fibers (Inagaki et al., 2003), a clinical trial of IFN for HCV cirrhosis was conducted, but adequate effectiveness was not demonstrated from the side effects (Di Bisceglie et al., 2008). In addition, clinical trials of ACE inhibitors (losartan) (Torres et al., 2011), recombinant IL-10 (Nelson et al., 2003), and anti-TNF-α antibodies (Lebrec et al., 2010) were also conducted, but efficacy could not be confirmed. Recently, pilot studies on autologous bone marrow transplantation (Terai et al., 2006) and mesenchymal stem cell (MSC) administration (Suk et al., 2016) have been attempted, but there is room for improvement in terms of patient burden and safety profiles. Thus, although it has been very difficult to develop anti-fibrotic drugs against cirrhosis so far, our report suggests that the control of transcription factors called CBP-β-catenin can be a target for fibrosis treatment in humans. From the preclinical data, PRI-724 has confirmed effectiveness in non-alcoholic steatohepatitis (NASH) and HBV cirrhosis model, and there is a possibility that it can be adapted and expanded for use in non-HCV cirrhosis in the future.
Several promising clinical trials for NASH-induced fibrosis that focus on drugs that target the anti-LOXL-2 antibody, ASK inhibitor, and CCR2/5 antagonist are in progress (Ikenaga et al., 2017, Lee et al., 2015, Torok et al., 2015, Townsend and Newsome, 2016). Given that a variety of mechanisms can cause liver fibrosis, combination therapy including drugs with different targets might be an effective approach to halting and resolving liver fibrosis.
Preclinical studies have revealed that ICG-001, which is a prototype compound of PRI-724, attenuates bleomycin-induced lung fibrosis (Henderson et al., 2010) and renal interstitial fibrosis induced by unilateral ureteral obstruction (Hao et al., 2011). These results, combined with those of our study, suggest that PRI-724 could be a useful therapeutic drug for fibrotic diseases in a variety of organs.
Finally, we conducted this clinical trial by intravenous administration of PRI-724 injection formulation. By taking practicality and versatility into consideration, we think that it is necessary to develop oral delivery formulations in the future. Currently, we are planning to develop oral preparations.
In conclusion, PRI-724 administered by intravenous injection to patients with HCV cirrhosis at doses of 10 and 40 mg/m2/day appeared to be safe, provided dose-dependent plasma exposure of the drug, and resulted in an improvement in liver histology and CP scores in several patients. Further studies to confirm the safety and efficacy of the drug in patients with decompensated HCV cirrhosis are now in the planning stage.
Conflicts of Interest
There are no conflicts of interests.
Funding Sources
This study was funded by the Research Program on Hepatitis (15fk0210025h003), Development the Translational Research Network Program (16lm0103008j0005), and Acceleration Transformative Research for Medical Innovation (ACT-M) (16im0210105h0001) from the Japan Agency for Medical Research and Development (AMED). None of the funders played any role in the design of the study, data analysis, or interpretation.
Author Contributions
KK, AI, MS, KT, YN, MK, and MM designed the study, and collected, analyzed and interpreted the data. KK and AI wrote the manuscript and designed the figures. SS, JI, YO, MK, KN, and TK collected and interpreted the data. KH, MS, and TH collected the data and interpreted the histological analysis data. TO, SM, and KI interpreted the data and organized the study logistics.
Acknowledgments
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.08.016.
Appendix A. Supplementary Data
Supplementary material
References
- Bahrami A. Therapeutic potential of targeting Wnt/beta-catenin pathway in treatment of colorectal cancer: rational and progress. J. Cell. Biochem. 2017;18(10):3028–3033. doi: 10.1002/jcb.25903. [DOI] [PubMed] [Google Scholar]
- Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat. Rev. Endocrinol. 2013;9(10):575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- Colletti M. Convergence of Wnt signaling on the HNF4alpha-driven transcription in controlling liver zonation. Gastroenterology. 2009;137(2):660–672. doi: 10.1053/j.gastro.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Conti F. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016;65(4):727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Curry M.P. Sofosbuvir and Velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie A.M. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N. Engl. J. Med. 2008;359(23):2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeling M. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 2016;12(7):426–439. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsharkawy A. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J. Gastroenterol. Hepatol. 2017 doi: 10.1111/jgh.13758. [DOI] [PubMed] [Google Scholar]
- Emami K.H. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc. Natl. Acad. Sci. U. S. A. 2004;101(34):12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2011;22(9):1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W.R., Jr. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2010;107(32):14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gea V., Friedman S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- Ikenaga N. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017 doi: 10.1136/gutjnl-2016-312473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38(4):890–899. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13(7):513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrec D. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138(5):1755–1762. doi: 10.1053/j.gastro.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Lee Y.A., Wallace M.C., Friedman S.L. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64(5):830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning D.S., Afdhal N.H. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134(6):1670–1681. doi: 10.1053/j.gastro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Manns M. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect. Dis. 2016;16(6):685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- Monga S.P. Beta-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148(7):1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.R. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38(4):859–868. doi: 10.1053/jhep.2003.50427. [DOI] [PubMed] [Google Scholar]
- Osawa Y. Inhibition of cyclic adenosine monophosphate (cAMP)-response element-binding protein (CREB)-binding protein (CBP)/beta-catenin reduces liver fibrosis in mice. EBioMedicine. 2015;2(11):1751–1758. doi: 10.1016/j.ebiom.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371(9615):838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Kim Y.O. Evolving therapies for liver fibrosis. J. Clin. Invest. 2013;123(5):1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk K.T. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology. 2016;64(6):2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- Terai S. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24(10):2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- Torok N.J. Strategies and endpoints of antifibrotic drug trials: summary and recommendations from the AASLD Emerging Trends Conference, Chicago, June 2014. Hepatology. 2015;62(2):627–634. doi: 10.1002/hep.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres D.M. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open-label trial. Hepatology. 2011;54(5):1631–1639. doi: 10.1002/hep.24558. [DOI] [PubMed] [Google Scholar]
- Townsend S.A., Newsome P.N. Non-alcoholic fatty liver disease in 2016. Br. Med. Bull. 2016;119(1):143–156. doi: 10.1093/bmb/ldw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material