Abstract
Background
Identification of mechanisms promoting neutrophil trafficking to the lungs of patients with cystic fibrosis (CF) is a challenge for next generation therapeutics. Cholesterol, a structural component of neutrophil plasma membranes influences cell adhesion, a key step in transmigration. The effect of chronic inflammation on neutrophil membrane cholesterol content in patients with CF (PWCF) remains unclear. To address this we examined neutrophils of PWCF to evaluate the cause and consequence of altered membrane cholesterol and identified the effects of lung transplantation and ion channel potentiator therapy on the cellular mechanisms responsible for perturbed membrane cholesterol and increased cell adhesion.
Methodology
PWCF homozygous for the ΔF508 mutation or heterozygous for the G551D mutation were recruited (n = 48). Membrane protein expression was investigated by mass spectrometry. The effect of lung transplantation or ivacaftor therapy was assessed by ELISAs, and calcium fluorometric and μ-calpain assays.
Findings
Membranes of CF neutrophils contain less cholesterol, yet increased integrin CD11b expression, and respond to inflammatory induced endoplasmic reticulum (ER) stress by activating μ-calpain. In vivo and in vitro, increased μ-calpain activity resulted in proteolysis of the membrane cholesterol trafficking protein caveolin-1. The critical role of caveolin-1 for adequate membrane cholesterol content was confirmed in caveolin-1 knock-out mice. Lung transplant therapy or treatment of PWCF with ivacaftor, reduced levels of circulating inflammatory mediators and actuated increased caveolin-1 and membrane cholesterol, with concurrent normalized neutrophil adhesion.
Interpretation
Results demonstrate an auxiliary benefit of lung transplant and potentiator therapy, evident by a reduction in circulating inflammation and controlled neutrophil adhesion.
Abbreviations: CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; PWCF, patients with CF; CD11b, integrin alpha-M; MβCD, methyl-β-cyclodextrin; CXCL, C-X-C Motif Chemokine Ligand; TNF-α, tumor necrosis factor alpha; sTNFR1, soluble TNF receptor 1; Cav−/−, caveolin-1 knock-out mice
Keywords: Cystic fibrosis, Neutrophils, Cholesterol, Lung transplant therapy, Ivacaftor therapy
Highlights
-
•
This study explored neutrophil adhesion in cystic fibrosis.
-
•
Altered membrane cholesterol lead to increased adhesion.
-
•
Circulating inflammatory mediators caused increased calpain activity and reduced membrane cholesterol content.
In patients with cystic fibrosis (CF), chronic inflammation in the circulation, in part originating from the pulmonary compartment, leads to decreased membrane cholesterol in circulating neutrophils, resulting in increased cell adhesion. The mechanism of action involves proteolytic down-regulation of the cholesterol trafficking protein caveolin-1. The overall effect of lung transplant therapy, or CFTR potentiator treatment, was to significantly diminish the circulating inflammatory burden thereby permitting caveolin-1 expression, with concomitant decreased CF cell adhesion and significant clinical improvement.
1. Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CF transmembrane conductance regulator (CFTR) gene (Riordan et al., 1989), resulting in altered chloride ion (Cl−) transport, and airways disease characterized by chronic bacterial colonization, bronchiectasis and progressive lung destruction. One common characteristic of CF lung disease is sustained neutrophil recruitment, with neutrophils accounting for in excess of 70% of the inflammatory cell population (Hartl et al., 2006). The involvement of neutrophils in the initiation and perpetuation of CF lung disease is an area of immense interest and occurs early in life with secretion of proteases associated with early bronchiectasis (Sly et al., 2013). Neutrophil dysfunction in CF appears to be governed not only by the genetic defect and a lack of CFTR function (Painter et al., 2006, Zhou et al., 2013, Pohl et al., 2014), but is also provoked by chronic bacterial infiltration and inflammation (Taggart et al., 2000, Alexis et al., 2006, Hayes et al., 2011). Our interest in CF was further fuelled by the availability of novel therapeutics in CF such as lung transplant and CFTR potentiators for class III defects including the G551D mutation which encodes a CFTR protein localized at the plasma membrane containing a primary defect in channel gating. Ivacaftor (VX-770) is one such drug and has been shown to improve Cl− secretion in vitro (Ley et al., 2007), and in PWCF with at least one G551D-CFTR mutation demonstrated a marked improvement in patient lung function (Accurso et al., 2010), decreased sweat Cl− concentration to the normal range (Ramsey et al., 2011) and normalized neutrophil cytosolic ion levels for improved extracellular bacterial killing (Pohl et al., 2014).
Upon responding to chemoattractant stimuli, neutrophil recruitment commences and involves a sequence of steps encompassing selectin-dependent rolling, integrin dependent adhesion and transmigration (Kolaczkowska and Kubes, 2013). Persistent neutrophil adhesion and migration is supported by increased membrane CD11b (integrin alpha-M) expression, as has been reported in type 2 diabetes resulting in vascular dysfunction and increased inflammation (Oostrom et al., 2004). Similarly, neutrophils isolated from patients with psoriasis are more adherent, influencing the accumulation of neutrophils within the lesional psoriatic skin (Wetzel et al., 2006). Of major significance, increased neutrophil migratory capacity has been documented in CF (Brennan et al., 2001), and with this in mind, we investigated changes that alter the adhesion properties of circulating CF neutrophils. The present study has identified a pathological mechanism triggered by altered membrane cholesterol levels leading to increased CD11b expression and neutrophil adhesion in PWCF. These results provide strong insight into a mechanism for the ancillary therapeutic benefit of lung transplant and ivacaftor therapy, involving anti-inflammatory and anti-adhesive effects on circulating neutrophils.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals and reagents were of the highest purity obtainable, endotoxin free, and purchased from Sigma-Aldrich ®, unless otherwise specified.
2.2. Patient Selection and Recruitment
This study was approved by the Beaumont Hospital Ethics Committee (REC # 14/98) and informed written consent was obtained from all patient cohorts. Healthy control (HC) volunteers (Table 1, n = 38, age 30.4 ± 0.82 years) recruited for this study possessed a FEV1 percentage of 99.8 ± 0.6% predicted and had no evidence of lung disease. Clinically stable PWCF homozygous for the ΔF508 mutation or heterozygous G551D/ΔF508 were recruited (Table 1, n = 48, age 29.3 ± 4.34 years) with a FEV1 of 62.58 ± 3.62% predicated. PWCF receiving 150 mg ivacaftor twice daily (Table 1, n = 6, age 26.42 ± 2.38 years) with an FEV1 of 41.0 ± 4.75% predicated were recruited. For patients receiving ivacaftor therapy, a blood samples was taken before commencement of treatment, and 4 and 12 weeks post therapy. Ivacaftor therapy continued indefinitely thereafter. Transplant patients (Table 1, n = 6, age 31.33 ± 1.73 years) recruited for this study were clinically stable and had a FEV1 percentage predicted of 65.17 ± 7.62.
Table 1.
Characteristics of HC, PWCF, PWCF receiving ivacaftor therapy for 12 weeks and PWCF post lung transplant used in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 6 and 7 and Supplementary Figs. S1–3 and S5.
| HC | CF | Ivacaftor therapy | Lung transplant therapy | |
|---|---|---|---|---|
| No. of subjects | 38 | 48 | 6 | 6 |
| Age, years (mean ± SEM) | 30.4 ± 0.82 | 29.3 ± 4.34 | 26.42 ± 2.38 | 31.33 ± 1.73 |
| Gender (female/male) | 20/18 | 20/25 | 2/4 | 1/5 |
| FEV1 (% predicted) (± SEM) | 99.8 ± 0.60 | 61.58 ± 3.62 | Pre-therapy 32 ± 2.8 Post-therapy 42.83 ± 6.6 P = 0.04 |
Pre-therapy 20.33 ± 2.7 Post-therapy 65.17 ± 7.62 P = 0.001 |
| BMI (kg/m2) (± SEM) | 24.81 ± 0.48 | 22.01 ± 0.73 | Pre-therapy 20.01 ± 1.07 Post-therapy 20.57 ± 1.01 P = 0.15 |
Pre-therapy 19.62 ± 0.97 Post-therapy 22.04 ± 0.4 P = 0.02 |
Definition of abbreviations: SEM = standard error of mean; FEV1 = forced expiratory volume in the first second; BMI = body mass index. Significant treatment effect calculated by Paired Student's t-test.
2.3. Isolation of Plasma and Quantification of Circulating Proinflammatory Mediators and Cholesterol Levels
Blood collected in lithium heparin tubes (Sarstedt Monovette ®) was centrifuged at 500 × g (Hettich Zentrifugen EBA 20) for 5 min at room temperature (RT). Collected plasma was aliquoted and stored immediately at − 80 °C until required. Quantification of CXCL8 was carried out by ELISA as per the manufacturer's instructions (R&D Systems Ltd., MN, USA, product codes MAB208 and BAF208). CXCL7 (RayBiotech ®, GA, USA, product code ELH-NAP2-001) and sTNFR1 (R&D Systems Ltd., product code DY225-05) studies were also carried out by ELISA using standards ranging from 0 to 1000 pg/ml and antibodies outlined in Table S1 in the online data supplement. Measurements were recorded at 405 nm for CXCL8 and 450 nm for CXCL7 and sTNFR1 using the VictorTM X3 2030 Multilabel Reader, PerkinElmer.
Fasting lipids were measured by standard clinical lab testing using the Beckman Coulter AU5800/5400. Cholesterol was measured in mmol/l. The normal range for total cholesterol is ˂5 mmol/l, LDL is ˂3 mmol/l, HDL is ˃1 mmol/l and triglycerides is ˂2 (Irish Heart Foundation, 2011).
2.4. Purification and Fractionation of Neutrophils
Human peripheral blood neutrophils were isolated using dextran sedimentation and LymphoPrep (Axis-Shield Poc AS, Oslo, Norway) centrifugation as previously described (Reeves et al., 2002). The purified neutrophils were resuspended in phosphate buffered saline (PBS) (pH 7.4) containing 5 mM glucose and used immediately. Purity of isolated neutrophils was validated by flow cytometric analysis using a monoclonal antibody against CD16b and was > 96% (O'Dwyer et al., 2015). Neutrophil viability was assessed by Trypan Blue exclusion assay and analysis of apoptotic and dead cells was evaluated by using an Annexin V-FITC Apoptosis Kit (BioVision Inc. Milpitas, CA, USA). In a subset of experiments, isolated neutrophils (2 × 107/ml) remained untreated or were incubated in the presence of 0.1, 1.0 or 10 mM MβCD at 37 °C for 1 h (Bergin et al., 2010). In a further set of experiments, HC neutrophils (5 × 106/ml) were treated with CFTR-inhibitor 172 (10 μM), 0.1% (v/v) dimethyl sulfoxide (DMSO) (vehicle control) (Pohl et al., 2014), thapsigargin (100 nM) or TNF-α (10 ng/2 × 107 cells) for indicated time points at 37 °C (Hurley et al., 2014).
Plasma membranes were isolated from neutrophils using sucrose density gradients as previously described (Bergin et al., 2010, Simons et al., 1983). Briefly, neutrophils were suspended in ice cold 10% (w/w) sucrose containing 1 × Lamberth's Break Buffer (LBB) (10 mM potassium chloride (KCl), 3 mM sodium chloride (NaCl), 4 mM magnesium chloride (MgCl2), 10 mM piperazine-N,N′-bis (2-ethanesulfonic acid) (PIPES) with protease inhibitors; 20 μg/ml phenylmethane sulfonyl fluoride (PMSF), 10 μg/ml Na-tosyl-l-lysine chloromethyl ketone hydrochloride (TLCK), 10 μg/ml pepstatin A and 10 μg/ml leupeptin hemisulfate, and subjected to sonication. After centrifugation (8,00 × g, 10 min, 4 °C) the resulting post nuclear supernatant (PNS) was layered on top of sucrose gradients; 60%, 35%, 17.5% (w/w), all prepared with 1 × LBB containing protease inhibitors. Ultracentrifugation was carried out at 273,620 × g for 1 h at 4 °C (SW41 Ti Rotor Beckman Coulter®). The cytosol was removed from the top 10% (w/w) sucrose layer, and the membranes were collected from the interface between the 17.5% (w/w) and the 35% (w/w) sucrose layer. The 1 ml membrane fraction was diluted 1:4 in 1 × LBB with protease inhibitors. This was centrifuged (Sorvall® RC M120EX ultracentrifuge, S100At5-0011 rotator) at 137,391 × g for 30 min at 4 °C to form a pellet of purified membranes. Proteomic analysis of membranes samples was performed as previously described (Pohl et al., 2014). In further experiments membrane pellets was stored in 2 × sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer or 1 × reaction buffer for cholesterol analysis (5 × reaction buffer: 0.5 M potassium phosphate, pH 7.4, 0.25 M NaCl, 25 mM cholic acid, 0.5% Triton® X-100, from Amplex® Red Cholesterol Assay Kit) at − 80 °C. Lipid rafts were isolated from neutrophils using a detergent free method as previously described (Bergin et al., 2010, Song et al., 1996) and fractions were stored at − 80 °C.
For the isolation of neutrophils from mice, experiments were performed on Caveolin-1 knock-out (−/−) mice on a B6129SF2/J background (stock number 004585; The Jackson Laboratory, Bar Harbor, ME, USA) with B6129SF2/J (stock number 101045; The Jackson Laboratory) used as wild-type (WT) controls. Animals were bred in a pathogen-free barrier facility at State University of New York Downstate Medical Center (Brooklyn, New York, USA). Eight-week-old mice were used and each experiment had at least 8 animals per group. Neutrophils were purified from whole blood using LymphoPrep gradient centrifugation. Isolated neutrophils were enumerated and cell fractions isolated as already described. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and Institutional Animal Care and Use Committee guidelines. State University of New York Downstate Medical Center's Institutional Animal Care and Use Committee approved the protocol.
2.5. Neutrophil Adhesion Assays
A black 96 well plate was coated with fibronectin solution (10% (v/v) 1 mg/ml of BSA, 1% (v/v) collagen, 1% (v/v) 1 mg/ml human fibronectin in LHC basal medium) for 2 h at RT, and then blocked with 1% (w/v) BSA for 30 min before LTB4 (100 nM) or U-75302 (1 μM) were added. Isolated neutrophils (4 × 106/ml) were resuspended in calcein-AM dye (5 μM; Life technologies) in DPBS-glucose (5 mM) for 30 min at 37 °C in the dark and were then washed (PBS (2 ×)) by centrifugation at 470 × g for 5 min at RT. Neutrophils (4 × 105/100 μl) containing calcein-AM dye were then loaded onto the plate and incubated 37 °C for 30 min. Fluorescence was recorded at excitation 485 nm and emission 535 nm.
2.6. HL-60 Cell Culture and Treatments
The HL-60 cell line was obtained from the American Type Culture Collection; CCL-240. HL-60 cells were sub-cultured with complete RPMI 1640 and 2 mM glutamine supplemented with 1% (v/v) penicillin/streptomycin and 10% (v/v) fetal bovine serum (FBS). Cells were sub-cultured at 5 × 106/ml in 5% CO2 at 37 °C every two to three days for a total of six weeks. Fully differentiated HL-60 cells were prepared by treating 2 × 105/ml with complete RPMI 1640 and 2 mM glutamine supplemented with 1% (v/v) penicillin/streptomycin, 10% (v/v) FBS, and 1.25% (v/v) DMSO for a total of 5 days, with the media being replenished on day 3. For treatments, HL-60 cells (2 × 106/ml) were exposed to fresh 20% (v/v) HC or CF pooled plasma for 24 h in triplicate. In further experiments, HL-60 cells (2 × 106/ml) were serum starved for 2 h prior to exposure to a combination of CXCL8 (200 pg/ml), 10 μg/ml of CXCL7(10 μg/ml) and TNF-α (400 pg/ml) for 24 h and 72 h in triplicate. Cells were harvested and cytosols and membranes were isolated by methods already described. To confirm that the concentration of TNF-α used did not affect cell viability, a MTS Assay Kit was used, which is a colorimetric method for sensitive quantification of viable cells (Abcam, catalogue # ab197010).
2.7. SDS-PAGE and Western Blot Analyses
SDS-PAGE was carried out using the Laemmli method (Laemmli, 1970), under denaturing conditions. After electrophoresis, protein bands were visualized by staining gels with either Coomassie blue R250, silver stain (SilverSNAP® Stain Kit 2, Pierce), or alternatively proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Roche) by Western blotting. Transfer was carried out at 30 V for 60 min. Efficient transfer of PVDF membrane was determined by staining membrane in Ponceau S solution. Membranes were then blocked in blocking buffer containing 3% (w/v) dry milk marvel and 1% (w/v) bovine serum albumin (BSA) in PBS-Tween (0.01 M PBS, 0.5% (v/v) Tween®, and 1 L deionised water) for 1 h. Membranes were then placed in relevant primary antibody (Table S1). Horseradish peroxidase (HRP)-linked anti-mouse or anti-rabbit IgG (Table S1) antibodies were used as secondary antibodies. Protein bands were detected using Immobilon Western Chemiluminescent HRP Substrate solution (Millipore), and developed using the chemiluminescence on the G:BOX SynGene machine (Synoptics, U.K.). Densitometry was carried out using the GeneSnap SynGene Programme (Synoptics, U.K.).
2.8. Membrane Cholesterol Assays
Amplex® red cholesterol assay kit (Invitrogen, CA, USA, product code A12216) was used to quantify total cholesterol content in whole cell, plasma membrane and lipid raft fractions as previously described (Solomkin et al., 2007). Reactions were prepared as per manufacturer's instructions. Fluorescence was read with excitation 530 nm and emission detection 590 nm (VictorTM X3 2030 Multilabel Reader, PerkinElmer). Cholesterol levels were normalized to cell number. MarkerGene™ Membrane Fluidity Kit (Marker Gene Technologies, Inc., OR, USA, Product code M0271) was used to determine lateral membrane fluidity of neutrophil plasma membranes (Zhang et al., 2011). Reaction was prepared as per manufactures instructions. Fluorescence was recorded with emission 470 nm and excitation 350 nm. Fluidity was normalized to protein concentration as determined by BCA.
2.9. Calcium Quantification and Calpain Activity Assays
Calcium (Ca2 +) quantification assay kit (Abcam Ltd., England, product code ab112115) was used to quantify Ca2 + levels in neutrophil cytosol fractions. Reagents were prepared as per the manufacturer's instructions with fluorescence intensity at excitation 540 nm and emission at 590 nm recorded. Kinetic analysis of calpain activity was quantified in neutrophil cytosols using Calpain-Glo™ Protease Assay (Promega, WI, USA, product code G8501) (Reeves et al., 2013). Reagents were prepared and the protocol performed as per the manufacturer's instructions. Human erythrocytes μ-calpain (Calbiochem, product code 208713) was used as a standard prepared in calpain buffer (10 mM HEPES pH 7.2, 10 mM DTT, and 1 mM EDTA). Calpain Glo ™ Reagent consisting of the luciferin detection reagent, Suc-LLYY-Glo™ substrate and 2 mM of calcium chloride (CaCl2) was added to each well. Luminescence was record using the VictorTM X3 2030 Multilabel Reader, PerkinElmer.
2.10. In Vitro Digestion of Caveolin-1
Recombinant caveolin-1 protein (Cusabio, product code CSB-EP004571DO) at 0.1 μg was incubated with increasing quantities of μ-calpain (0–600 U) in calpain buffer for 2 h at 37 °C. In a further set of experiments, isolated neutrophil cytosols prepared in the absence of protease inhibitors and either remained untreated or exposed to calpain (2 μM) or Ca2 + (0.02 mM) in calpain buffer for 0, 5, 10, 20, and 40 min at 37 °C. All reactions were stopped with the addition of 2 × SDS-PAGE Sample Buffer and boiled for 3 min.
2.11. Statistical Analysis
All data investigated in this report was analysed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA). Results are expressed as mean ± SEM of either independent experiments or technical replicates as stated in each figure legend. Statistical significance was calculated using a Student's t-test when comparisons were made between two groups, and data sets were n < 6. When comparisons of larger data sets (n > 6) were made, the D'Agostino and Pearson omnibus normality test was carried out, and when normally distributed Student's t-test determined significance. Non-normal data was analysed by the Mann-Whitney U test. One-way ANOVA was used to determine statistical significance when comparing three or more groups. A P value ≤ 0.05 was deemed statistically significant. For proteomic analysis, differential expression was defined as > 1.5 fold difference with P < 0.05 or a 1.2-fold difference with P < 0.02.
3. Results
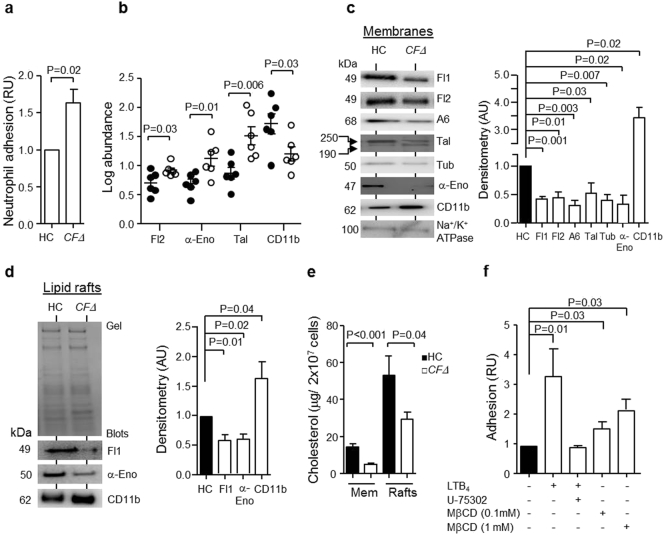
3.1. Protein Expression and Cholesterol Content of Neutrophil Membranes is Altered in Patients with CF
In light of a previous publication demonstrating elevated serum levels of adhesion molecules in CF (De Rose et al., 1998), we assessed levels of CF neutrophil adhesion in vitro. Adherence of Calcein-AM loaded HC neutrophils and cells of individuals homozygous for the ΔF508 mutation (CFΔ) to fibronectin coated surfaces was assessed after 30 min incubation at 37 °C. Results demonstrated that CFΔ neutrophils exhibited a significantly increased level of adhesion compared to HC cells (P = 0.02) (Fig. 1a). Proteomic analysis was subsequently carried out to evaluate the altered expression of adhesion molecules and membrane proteins on CFΔ cells. The 6 patients with CFΔ selected for proteomic analysis were representative of the whole group (same degree of lung disease, mean % FEV1 37% predicted) and controls included membrane samples from 6 HC donors. In total, ~ 750 protein spots were detected on 2-D analytical gels by DeCyder software. When comparing the membrane protein spots on the gels from CFΔ and HC membranes, 36 spots showed differential expression. Those unknown spots were excised from the gels and were identified by LC-MS/MS, with 9 proteins demonstrating at least a 1.5-fold differential expression. All 9 proteins were recognized as key membrane lipid raft associated proteins with 8 downregulated and 1 upregulated (Table 2). The average of 6 different samples is illustrated in Fig. 1b and demonstrates sustained down-regulation of flotillin-2 (P = 0.03), α-enolase (P = 0.01) and talin (P = 0.006), yet up-regulation of CD11b (P = 0.03) in CFΔ membrane fractions compared to HC samples. Proteomic and LC-MS/MS results were verified by Western blotting, and densitometry of immuno-bands confirmed significantly reduced membrane expression levels of flotillin-1 and flotillin-2 (P = 0.001 and P = 0.01), annexin-6 (P = 0.003), talin (P = 0.03), tubulin (P = 0.007) and α-enolase (P = 0.02) (Fig. 1c). In contrast, increased levels of the adhesion molecule CD11b in CFΔ neutrophil plasma membranes were detected compared to HC membranes (P = 0.02) (Fig. 1c). Lipid rafts are focused regions of the neutrophil plasma membrane implicated in neutrophil adhesion (Solomkin et al., 2007). Analysis of purified lipid raft domains by immune-blotting confirmed significantly decreased levels of flotillin-1 (P = 0.01) and α-enolase (P = 0.02), yet a 60% increase in levels of CD11b in CFΔ lipid rafts when compared to HC samples (P = 0.04) (Fig. 1d).
Fig. 1.
Changes in the proteome and cholesterol content of CF neutrophil membranes and lipid rafts.
(a) Neutrophils of ΔF508 homozygous PWCF (CF ∆) were significantly more adherent than cells of HC (P = 0.02, Student's t-test, N = 6). (b) Comparative analysis of proteins extracted from membranes of CF ∆ and HC. The log protein abundance illustrates persistent down-regulation of flotillin-2 (Fl2, P = 0.03), α-enolase (α-Eno, P = 0.01) and talin (Tal, P = 0.006) and up-regulation of CD11b (P = 0.03) on CF ∆ (●) compared to HC (○) (Student's t-test, n = 6 subjects per group). (c) Neutrophil membrane fractions from HC and CF ∆ were subjected to SDS-PAGE and Western blot analysis for flotillin-1 (Fl1), Fl2, annexin-6 (A6), Tal, tubulin (Tub), α-Eno and CD11b. Band intensity for all proteins was quantified by densitometry and normalized to Na+/K+ ATPase. Significantly decreased levels of Fl1 (P = 0.001), Fl2 (P = 0.01), A6 (P = 0.003), Tal (P = 0.03), Tub (P = 0.007) and α-Eno (P = 0.02), yet increased levels of CD11b (P = 0.02), were detected on CF membranes (n = 6 subjects per group, Student's t-test). (d) Lipid raft fractions were isolated from HC or CF ∆ and subjected to SDS-PAGE analyses. Western blot and densitometry analysis determined significantly decreased Fl1 (P = 0.01) and α-Eno (P = 0.02) and increased CD11b (P = 0.04) in CF ∆ samples (n = 9 subjects per group, Student's t-test). (e) Neutrophil plasma membranes (Mem) and lipid rafts (Rafts) were analysed for cholesterol content using an Amplex ® red cholesterol assay kit. Cholesterol content of samples from CFΔ (P < 0.001 and P = 0.04, respectively, n = 9 subjects per group) were significantly reduced compared to HC membranes (Student's t-test). (f) HC neutrophils (5 × 106 cell/ml) remained untreated or were treated with MβCD and then loaded with calcein-AM dye. LTB4 (100 nM) and U-75302 were employed as positive and negative controls, respectively. Concentrations of 0.1 mM and 1 mM MβCD (P = 0.03) significantly increased cell adhesion (Student's t-test, n = 5). Each measurement is the mean ± SEM. Coomassie blue stained gel in (d) demonstrates equal protein loading.
Table 2.
Membrane proteins with altered expression in CF neutrophil membranes.
| No. | Identified protein (minimum of 1.5 fold change) |
Coverage (%) | Accession # | Mass (kDa) |
|---|---|---|---|---|
| 1 | Flotillin-1 ↓ | 39.58 | gi26006960 | 47.36 |
| 2 | Flotillin-2 ↓ | 50.64 | gi254763294 | 47.03 |
| 3 | Annexin-6 ↓ | 52.15 | gi113962 | 75.82 |
| 4 | α-enolase ↓ | 55.95 | gi119339 | 55.99 |
| 5 | Vinculin ↓ | 31.04 | gi21903479 | 123.72 |
| 6 | Heat shock cognate 71 kDa protein ↓ | 18.58 | gi123648 | 70.85 |
| 7 | Tubulin β-1 chain ↓ | 30.13 | gi135474 | 41.36 |
| 8 | Talin ↓ | 36.91 | gi81175200 | 269.59 |
| 9 | Integrin αβ ↑ | 58.26 | gi2405646 | 84.72 |
Up or downregulation of protein expression is indicated by the arrows (↑ and ↓, respectively).
Cholesterol is a crucial structural component of lipid rafts, and diminished cholesterol levels give rise to disorganized lipid raft structure (Bodin and Welch, 2005). For this reason, quantification of cholesterol levels in HC and CFΔ neutrophils was performed. Initial experiments confirmed that freshly isolated HC and CFΔ cells contained similar levels of total cholesterol (Fig. S1a), however, results revealed a 65% reduction in the cholesterol content of CFΔ membranes compared to HC samples (P < 0.001) (Fig. 1e). In parallel experiments, a 45% reduction in the cholesterol content of purified CF lipid rafts compared to HC fractions was recorded (P = 0.04) (Fig. 1e). CD11b is increased upon fusion of internal secretory vesicles with the outer plasma membrane, and to exclude this fusion event as a cause for altered membrane cholesterol content, HC neutrophil membrane cholesterol content was assessed post exposure to the degranulation inducer TNF-α (10 ng/2 × 107 cells). Results revealed no significant difference in membrane cholesterol content post TNF-α challenge (Fig. S1b). Furthermore, previous reports have demonstrated that reduced membrane cholesterol influences membrane fluidity (Le Grimellec et al., 1992), and in agreement, CF neutrophils demonstrated increased membrane fluidity ex vivo (P = 0.04) (Fig. S1c). To gain insight into the potential negative impact of reduced cholesterol content on lipid raft structure, we employed the cholesterol depleting drug methyl-β-cyclodextrin (MβCD) (10 mM). Quantification of cholesterol and lipid raft proteins in untreated and MβCD treated HC cells revealed significantly less cholesterol (P = 0.01), flotillin-1 (P < 0.0001) and α-enolase (P = 0.04), yet a 2-fold increase in CD11b (P = 0.006) in lipid rafts of drug treated cells when compared to untreated neutrophils (Fig. S1b and S1d). Moreover, the consequence of reduced cholesterol on cell responsiveness was explored by comparing the level of neutrophil adherence between MβCD treated and untreated cells. In this set of experiments the potent inducer of cell adhesion LTB4 (100 nM), along with its receptor antagonist U-75302 (1 μM), were employed for comparison. Results demonstrated that MβCD (0.1 or 1 mM) treated neutrophils exhibited a significantly increased level of adhesion compared to untreated cells (P = 0.03) (Fig. 1f), confirming the previously described increased adhesive response of cholesterol depleted neutrophils. Collectively these results demonstrate altered composition of the neutrophil membrane and lipid raft proteome in PWCF as a result of reduced cholesterol content, thereby leading to enhanced neutrophil adhesion.
3.2. Endoplasmic Reticulum Stress in CF Neutrophils Gives Rise to Decreased Caveolin-1 Levels
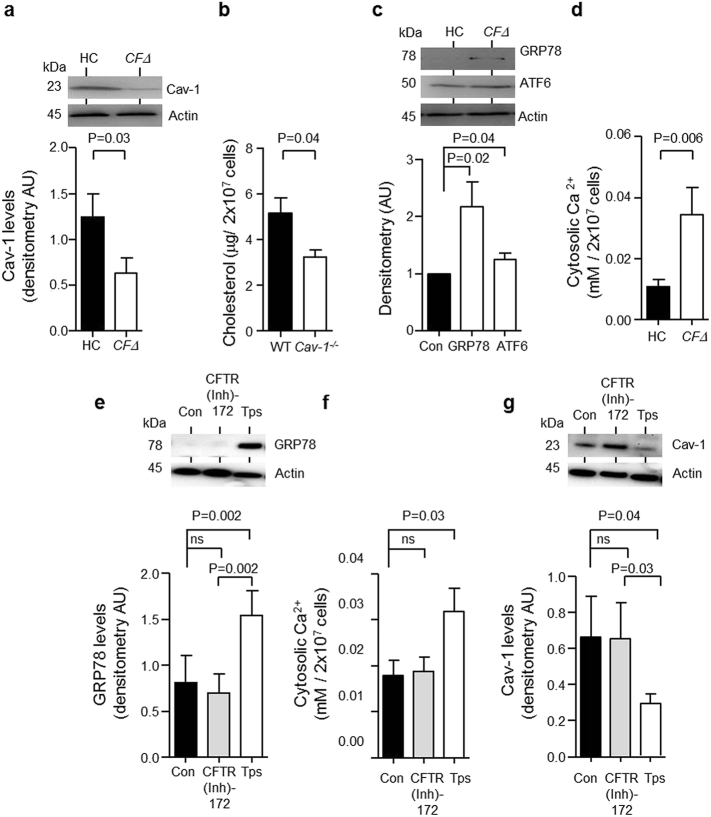
The mechanism leading to decreased membrane cholesterol in CF neutrophils was next explored. As approximately 80–90% of PWCF present with exocrine pancreatic insufficiency (The Cystic Fibrosis Registry, 2013), to rule out hypocholesterolemia as a potential cause for lower membrane cholesterol in circulating CF neutrophils, serum levels of total cholesterol, triglycerides, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were analysed (Fig. S2). No significant difference was recorded and the mean values for the four major cholesterol types in PWCF were within the normal range (Irish Heart Foundation, 2011). Moreover, no link between altered expression of neutrophil membrane LDL receptor (LDLR) that mediates LDL endocytosis, or cytosolic Rab7 and Rab9 involved in intracellular trafficking of cholesterol (Choudhury et al., 2002) as a cause for this defect in CF neutrophils was observed (Fig. S3). In contrast, caveolin-1 which plays a direct role in trafficking newly synthesized cholesterol from the endoplasmic reticulum (ER) to the plasma membrane (Frank et al., 2006) was shown to be significantly reduced in CFΔ neutrophils compared to HC cells (P = 0.03) (Fig. 2a). To confirm that caveolin-1 plays a role in maintaining the membrane cholesterol content of circulating neutrophils, neutrophils were isolated from wild-type and caveolin-1 knock-out mice (Cav−/−). Cav−/− neutrophils expressed no caveolin-1 protein yet contained similar levels of total cholesterol compared to wild-type mice (Fig. S4a and S4b in the online data supplement). However, results revealed a 40% reduction in the cholesterol content of Cav−/− plasma membranes compared to wildtype neutrophil fractions (P = 0.04) (Fig. 2b).
Fig. 2.
Reduced protein abundance of Cav-1 in neutrophil cytosols of individuals with CF.
(a) HC and CFΔ purified neutrophil cytosols were analysed by immunoblotting for protein expression of Cav-1. For comparative analysis immunoband intensity was quantified by densitometry with CF values calculated relative to HC. Decreased levels of Cav-1 in cytosols of CF cells were recorded (P = 0.03, n = 6 subjects per group, Student's t-test). (b) Neutrophils were isolated from wild-type (WT) and caveolin-1 null (Cav-1−/−) mice. A deficiency in caveolin-1 is sufficient to reduce neutrophil membrane cholesterol content (P = 0.04, n = 4 mice per group, Student's t-test). (c) Western blot and densitometry analysis of GRP78 (P = 0.02, n = 9 subjects per group, Student's t-test) and cleaved ATF6 (P = 0.04, n = 9 subjects per group, Student's t-test) expression in neutrophil cytosols of HC compared to CFΔ. GRP78 and ATF6 were significantly elevated in cytosols of CFΔ. (d) Ca2 + was significantly elevated in CFΔ neutrophil cytosols compared to HC samples (P = 0.006, n = 9 subjects per group, Student's t-test). (e–g) Neutrophil cytosols were purified from HC cells that were untreated (Con) or exposed to the CFTR inhibitor CFTR(Inh)-172 (10 μM) or the ER stress inducer thapsigargin (Tps, 100 nM). GRP78 protein expression (e), Ca2 + levels (f), and Cav-1 levels (g) were quantified. CFTR(Inh)-172 had no effect (P = ns, n = 8 independent experiments, Student's t-test). GRP78 (P = 0.002), and Ca2 + (P = 0.03) were significantly elevated, while Cav-1 (P = 0.03) was significantly reduced after treatment of neutrophils with Tps (n = 8 independent experiments, Student's t-test). For data in (a), (c), (e) and (g) actin was used as a loading control and CFΔ values were calculated relative to HC. Each measurement is the mean ± SEM.
Previous studies have suggested that disturbance in the normal function of the ER impacts upon expression levels of cytosolic proteins (Jimbo et al., 2003). We therefore sought to determine if ER stress was occurring in CFΔ neutrophils and investigated the impact of ER stress on caveolin-1 levels. Firstly, the protein expression of the ER stress associated chaperone protein GRP78 and transcription factor ATF6 in cell cytosols isolated from CFΔ neutrophils was compared to HC (Fig. 2c). Western blot results demonstrate that both the active form of GRP78 and ATF6 (cleaved ATF6) protein expression are increased in CFΔ neutrophils compared to control cells ex vivo (P = 0.02 and P = 0.04, respectively). Moreover, ER stress is accompanied by alterations in Ca2 + homeostasis resulting in increased cytosolic Ca2 + levels (Thastrup et al., 1990). In the present study, significantly increased Ca2 + in CFΔ cytosols compared to HC samples was recorded (P = 0.006) (Fig. 2d).
To investigate a potential link between ER stress and a lack of CFTR function, HC neutrophils were treated with the CFTR inhibitor CFTR(inh)-172 (10 μM). In contrast to the effect of thapsigargin (100 nM) a known inducer of ER stress and the unfolded protein response, CFTR inhibition did not result in a significant increase in cytosolic GRP78 (Fig. 2e) or Ca2 + levels (Fig. 2f), confirming that ER stress in CFΔ neutrophils was independent of CFTR dysfunction. Crucially, while CFTR inhibition by CFTR(inh)-172 had no effect on caveolin-1 protein expression (Fig. 2g), thapsigargin treatment significantly increased GRP78 (Fig. 2e P = 0.002) and Ca2 + levels (Fig. 2f P = 0.03), and decreased cytosolic caveolin-1 levels (Fig. 2g P = 0.04). Taken together these results demonstrate that ER stress, rather than intrinsic impairment of CFTR function in neutrophils of PWCF, results in reduced cytosolic caveolin-1 protein levels.
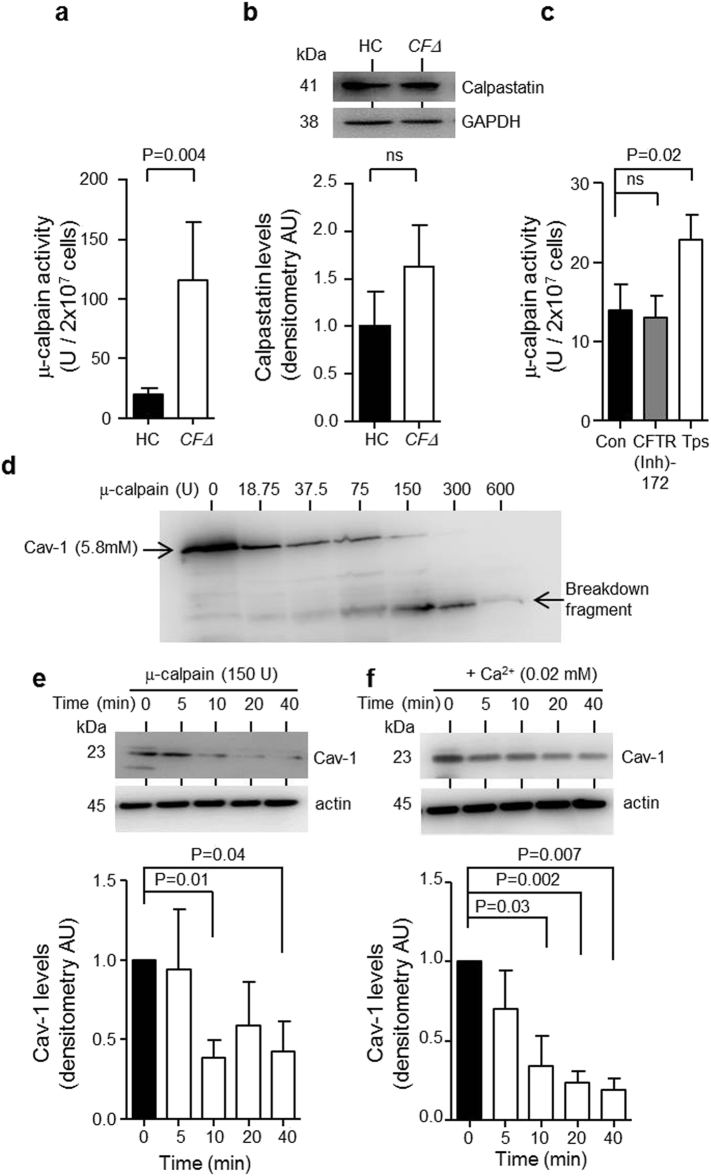
3.3. Increased μ-Calpain Activity in CF Neutrophils Triggers Decreased Caveolin-1 Levels
In light of results indicating increased ER stress markers in CFΔ neutrophils coupled with increased cytosolic Ca2 + levels and reduced levels of the μ-calpain target talin (Franco et al., 2004) (Table 2), subsequent studies investigated activity levels of the Ca2 +-dependent cysteine proteinase μ-calpain in CF cells. To this end, a significant 6-fold increase in μ-calpain activity in CF neutrophil cytosols compared to HC samples was detected (P = 0.004) (Fig. 3a). Moreover, the possibility that decreased levels of cytosolic calpastatin, a highly specific intrinsic inhibitor of calpain was the cause of increased μ-calpain activity was excluded based on Western blot results demonstrating equal expression levels in CFΔ and HC cells (Fig. 3b). In addition, results revealed that induction of ER stress in control neutrophils by exposure to thapsigargin significantly increased μ-calpain activity compared to untreated cells (P = 0.02) (Fig. 3c), an effect not observed by pharmacological inhibition of CFTR by inclusion of CFTR(inh)-172, confirming that ER stress in CFΔ neutrophils contributes to increased μ-calpain activity.
Fig. 3.
Increased μ-calpain activity in neutrophil cytosols of individuals with CF cleaves Cav-1. (a) μ-calpain activity was measured using the calpain GloTM protease assay kit and was found to be significantly elevated in CFΔ neutrophil cytosols compared to HC samples (P = 0.004, n = 4 subjects per group, Student's t-test). (b) Western blot and densitometry analysis of calpastatin in HC and CFΔ neutrophil cytosols revealed no significant difference (P = ns, n = 5 subjects per group, Student's t-test). (c) Neutrophil cytosols were purified from HC cells that were untreated (Con) or exposed to CFTR(Inh)-172 (10 μM) or Tps (100 nM). μ-calpain activity was unaffected by CFTR(Inh)-172 (P = ns, n = 4 independent experiments, Student's t-test). μ-calpain activity was significantly increased post treatment with Tps (P = 0.02, n = 3 independent experiments, Student's t-test). (d) Rh-Cav-1 (5.8 mM) was incubated with increasing concentrations of μ-calpain (0–600 U) for 2 h at 37 °C. Cav-1 degradation was analysed by Western blotting. (e) Western blot and densitometry of Cav-1 in neutrophil cytosols exposed to μ-calpain (150 U). Endogenous levels of Cav-1 were significantly reduced after 10 (P = 0.01, n = 4 independent experiments, Student's t-test) and 40 min incubation (n = 3 independent experiments, P = 0.04, Student's t-test). (f) Neutrophils were suspended in PBS supplemented with Ca2 + (0.02 mM) and incubated at 37 °C. Purified cytosols were subjected to Western blot analysis for Cav-1. Cav-1 was significantly reduced after 10, 20 and 40 min incubation (P = 0.03, P = 0.002 and P = 0.007, respectively, n = 4 independent experiments, Student's t-test). Each measurement is the mean ± SEM.
In order to confirm that μ-calpain cleaves caveolin-1, in vitro studies were performed. A dose-response experiment was carried out with physiologically relevant activity levels of μ-calpain (18.75–600 U). Western blot analysis of samples showed that μ-calpain degrades caveolin-1 in vitro, resulting in almost complete cleavage of full length caveolin-1 protein with the appearance of a breakdown product produced by 150 U of μ-calpain (Fig. 3d). Next the ability of exogenous μ-calpain (150 U) to cleave native caveolin-1 expressed in HC cytosols prepared from 2 × 107 cells was investigated. A time-course experiment revealed that μ-calpain significantly degraded native caveolin-1 after 10 min (P = 0.01) (Fig. 3e). Moreover, by increasing the Ca2 + levels of HC cell cytosols to within the range found in CF neutrophils (0.02 mM) we confirmed cleavage of caveolin-1, as significantly reduced levels of the cholesterol transport protein were detected after 10 min incubation at 37 °C (P = 0.03) (Fig. 3f). Overall this set of results confirmed the ability of μ-calpain to degrade caveolin-1 both in vitro and in vivo.
3.4. Inflammation Induces ER Stress in Neutrophils Resulting in Caveolin-1 Cleavage
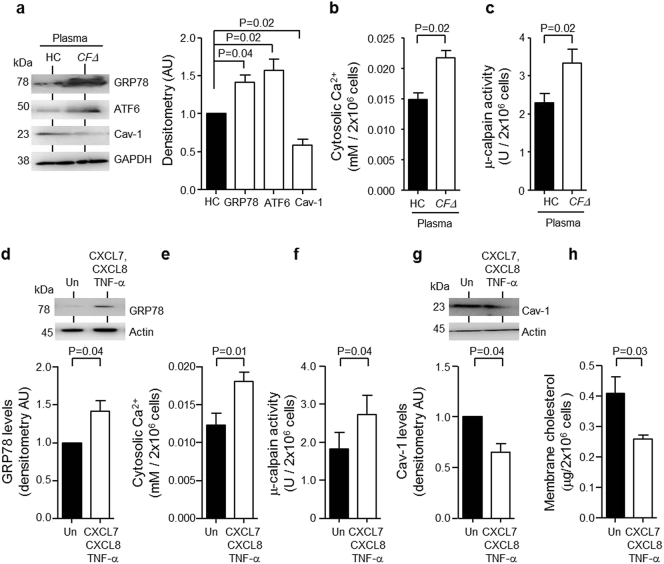
As chronic inflammation is a hallmark of CF lung disease (Greally et al., 1993, Mcelvaney et al., 1992, Corvol et al., 2003), the ability of inflammatory cytokines to affect ER stress in the neutrophil like human promyelocytic HL-60 cell line was examined. HL-60 cells (2 × 106/ml) were incubated with 20% (v/v) fresh CFΔ or HC plasma for 24 h. Compared to HC plasma, CFΔ plasma treatment significantly increased abundance of GRP78 (P = 0.04) and ATF6 (P = 0.02), and reduced levels of caveolin-1 (P = 0.02) (Fig. 4a). Similarly, compared to HC plasma, CFΔ plasma significantly increased cytosolic Ca2 + levels (P = 0.02) (Fig. 4b) and raised μ-calpain activity levels (P = 0.02) (Fig. 4c).
Fig. 4.
Inflammatory induced ER stress results in degradation of Cav-1.
HL-60 cells (2 × 106) were cultured for 24 h in 20% (v/v) pooled plasma from HC (plasma pool of n = 3 individuals) or patients with CF homozygous for the ΔF508 mutation (CFΔ, plasma pool of n = 3 patients). (a) Treated HL-60 cells were lysed and purified cytosols subjected to Western blot analysis for GRP78, ATF6 or Cav-1. Significantly increased GRP78 and ATF6, yet significantly decreased Cav-1 protein levels were detected (P = 0.04, P = 0.02 and P = 0.02 respectively, n = 3 independent experiments, Student's t-test). (b and c) Significantly increased cytosolic Ca2 + and μ-calpain activity were detected in cytosols of HL-60 cells exposed to CFΔ plasma compared to HC plasma (P = 0.02, n = 3 independent experiments, Student's t-test). (d-h) HL-60 cells (2 × 106) were exposed to a combination of CXCL7 (10 μg/ml), CXCL8 (200 pg/ml) and TNF-α (400 pg/ml) for 24 or 72 h and cytosols and membranes were purified. (d) Western blot and densitometry analysis revealed significantly increased cytosolic GRP78 (P = 0.04, n = 3 independent experiments, Student's t-test) in cells exposed to CXCL7, CXCL8 and TNFα for 24 h. (e and f) HL-60 cells exposed to pro-inflammatory mediators had significantly elevated cytosolic Ca2 + and μ-calpain activity (P = 0.01 and P = 0.04 respectively, n = 3 independent experiments, Student's t-test) at 24 h. (g) Cytosolic Cav-1 (P = 0.04, n = 3 independent experiments, Student's t-test) and (h) membrane cholesterol content (P = 0.03, n = 3 independent experiments, Student's t-test) were significantly reduced in HL-60 cells treated with CXCL7, CXCL8 and TNFα for 24 and 72 h, respectively. Each measurement in the mean ± SEM.
Ensuing experiments investigated the inflammatory mediators present in CFΔ plasma responsible for ER stress activation. Previously published data on levels of circulating inflammatory molecules (Reeves et al., 2010), and cytokine antibody array results of the present study (Fig. S5), supported the application of CXCL7, CXCL8 and TNF-α (10 μg, 0.2 ng and 0.4 ng/2 × 106/ml respectively) in in vitro cell culture experiments. When used individually, none of the three inflammatory mediators induced ER stress, alterations in Ca2 + levels or μ-calpain activity in HL-60 cells (Fig. S6). In contrast, in response to a combination of CXCL7, CXCL8 and TNF-α Western blot results of cell cytosols revealed significantly increased expression of the active form of GRP78 protein compared to untreated cell samples (P = 0.04) (Fig. 4d). Furthermore, results revealed significantly increased cytosolic Ca2 + levels in HL-60 cells exposed to the three inflammatory mediators compared to untreated cell cytosols (P = 0.01) (Fig. 4e), a result mirrored by significantly increased μ-calpain activity (P = 0.04) (Fig. 4f). Importantly, CXCL7, CXCL8 and TNF-α treatment diminished cytosolic caveolin-1 levels, as measured by Western blot analyses (P = 0.04) (Fig. 4g), and caused a 30% reduction in plasma membrane cholesterol content (P = 0.03) (Fig. 4h). Collectively, these results indicate that chronic inflammation can exert a negative impact on caveolin-1 expression, with downstream consequences directing lower plasma membrane cholesterol content.
3.5. Altered Plasma Membrane Cholesterol is Associated with Chronic Lung Disease
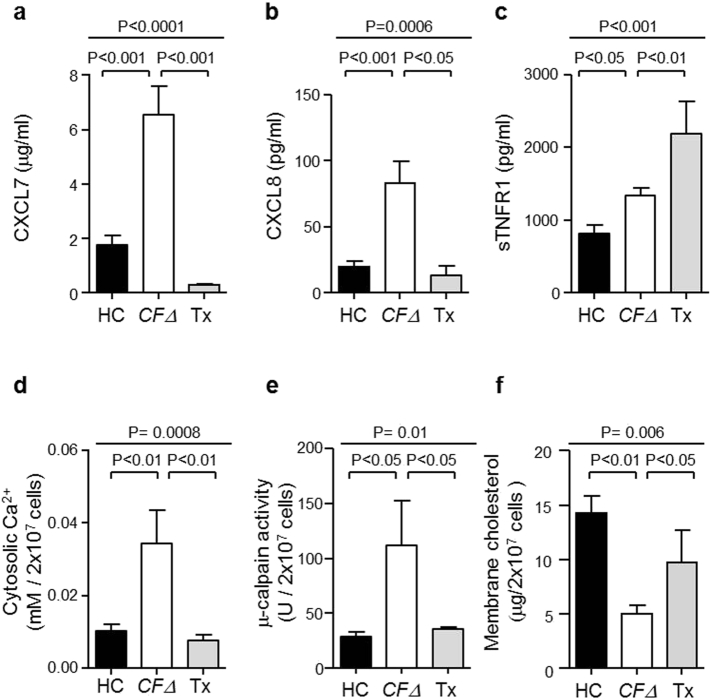
CF lung disease is characterized by significant chronic inflammation in the circulation, largely originating from the pulmonary compartment (Cypel et al., 2017). To confirm that caveolin-1 and cholesterol deficiency in CFΔ circulating neutrophils was caused by chronic systemic inflammation leaked from the airways, neutrophil samples were collected from CFΔ patients 12 months post lung transplantation (n = 6). ELISA analyses of CXCL7 in plasma revealed a 3-fold increase in CFΔ compared to HC individuals (P < 0.001), and a significant decrease in CFΔ patients who had received a transplant (P < 0.001) (Fig. 5a). Similarly, analysis of CXCL8 levels revealed that transplant treatment caused an 85% decrease in CXCL8 in CFΔ patients when compared to plasma isolated from CFΔ patients who had never received a transplant (P < 0.05) (Fig. 5b). Moreover, soluble TNFR1 (sTNFR1) is recognized as a marker of TNF-α activation and in the present study the concentration of sTNFR1 detected in plasma was increased in CFΔ individuals compared to HC (P < 0.05), but was increased further in CFΔ post-transplant therapy (P < 0.01) (Fig. 5c). As TNF-α applied to HL-60 cells on its own failed to affect membrane cholesterol content (Fig. S6a); elevated TNF-α post-transplant may not affect neutrophil physiology. In concurrence, post lung transplant therapy significantly reduced CFΔ neutrophil cytosolic Ca2 + concentrations (P < 0.01) (Fig. 5d) and μ-calpain activity were recorded (P < 0.05) (Fig. 5e), a result allied to significantly increased plasma membrane cholesterol content (P < 0.05) (Fig. 5f). Overall these results indicate that altered plasma membrane cholesterol content of circulating neutrophils is positively associated with chronic inflammation, and further provides evidence that the origin of inflammation is from the pulmonary compartment and not directly related to intrinsic CFTR dysfunction.
Fig. 5.
Lung transplant treatment reduced levels of circulating inflammatory mediators.
(a–c) ELISA analysis for CXCL7, CXCL8 and sTNFR1 demonstrated significantly increased plasma levels in CFΔ compared to HC (P < 0.001 and P < 0.05, respectively, n = 6 subjects per group, Student's t-test). Significantly decreased plasma CXCL7 and CXCL8 levels, yet increased levels of sTNFR1 detected post-transplant (Tx) when compared to CFΔ (P < 0.001, P < 0.05 and P < 0.01, respectively, n = 6 subjects per group, Student's t-test). (d) Analyses of neutrophil cytosols for Ca2 + revealed significantly increased levels in CFΔ compared to HC cells (P < 0.01, n = 6 subjects per group, Student's t-test), and significantly decreased levels in Tx (P < 0.01, n = 6 subjects per group, Student's t-test). (e) μ-calpain activity was significantly elevated in CFΔ cytosols compared to HC samples (P < 0.05, n = 6 subjects per group, Student's t-test) and significantly decreased in TX (P < 0.05, n = 6). (f) Plasma membrane cholesterol content was significantly reduced in CFΔ compared to HC samples (P < 0.01, n = 9 subjects per group, Student's t-test) and significantly increased in Tx (P < 0.05, n = 4 subjects per group, Student's t-test). Each measurement is the mean ± SEM.
3.6. Ivacaftor Treatment in PWCF Decreases Levels of Proinflammatory Mediators and Restores Membrane Cholesterol in CF Neutrophils
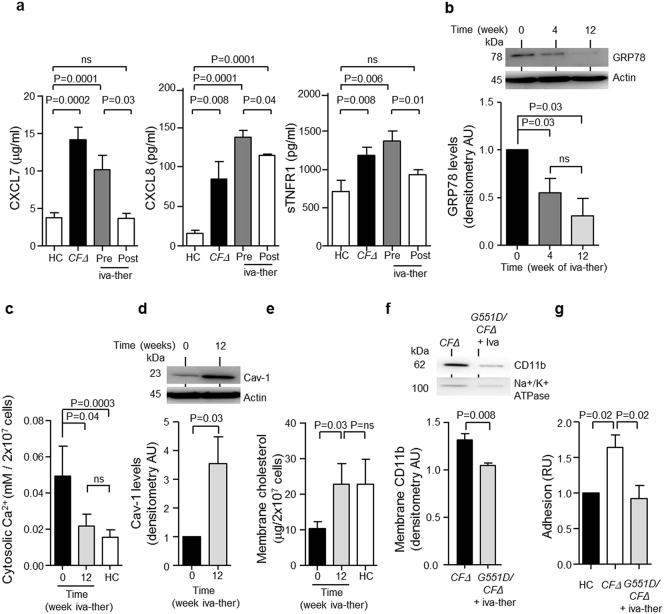
As improvements in lung function after treatment with ivacaftor have been reported (Ramsey et al., 2011), we investigated the influence of ivacaftor on plasma levels of circulating pro-inflammatory mediators. While the concentrations of CXCL7, CXCL8 and sTNFR1 were significantly increased in CFΔ (P = 0.0002, P = 0.008 and P = 0.008, respectively) and G551D/CFΔ (P = 0.0001 and P = 0.006) plasma compared to HC plasma (Fig. 6a), statistical analysis revealed that plasma donated by G551D/∆F508 heterozygote patients on ivacaftor therapy had reduced levels of CXCL7 and sTNFR1, similar to HC plasma levels (Fig. 6a). Moreover, significantly decreased CXCL8 levels were detected in plasma of patients receiving ivacaftor therapy compared to untreated patients (P = 0.04) (Fig. 6a).
Fig. 6.
Treatment with the ion channel potentiator ivacaftor reduced inflammatory mediators and normalized neutrophil membrane cholesterol content in PWCF.
(a) Plasma of patients with CF homozygous for the ΔF508 mutation (CFΔ) or heterozygous for the G551D mutation before receiving ivacaftor therapy (iva-ther) Pre- and 12 weeks post-treatment (Post). ELISA analysis for circulating inflammatory mediators demonstrated significantly reduced levels of CXCL7, (P = 0.03, Post, n = 4 subjects per group, Student's t-test), CXCL8 (P = 0.04, Post, n = 4 subjects per group, Student's t-test) and sTNFR1 (P = 0.01, Post, n = 4 subjects per group, Student's t-test). (b) Western blot and densitometry analysis of GRP78 expression in neutrophil cytosols from patients with CF (G551D/CFΔ) before receiving iva-ther (0) and 4 or 12 weeks post treatment. GRP78 was significantly decreased at 4 and 12 weeks (P = 0.03, n = 4 subjects per group, Student's t-test). (c) Analyses of cytosolic Ca2 + demonstrated that iva-ther significantly decreased Ca2 + levels 12 weeks post-treatment (P = 0.04, n = 4 subjects per group, Student's t-test). (d) Western blot and densitometry analysis of neutrophil cytosols demonstrated significantly increased Cav-1 abundance 12 weeks post ivacaftor therapy (P = 0.03, n = 4 subjects per group, Student's t-test). (e) Measurements of membrane cholesterol content demonstrated significantly increased cholesterol 12 weeks post ivacaftor therapy compared to pre-treatment (0) (n = 5 subjects per group, P = 0.03 Student's t-test). (f) Neutrophil plasma membranes were isolated from patients with CF homozygous for the ΔF508 mutations (CFΔ) or patients receiving ivacaftor therapy (G551D/CFΔ + iva-ther). Analyses of CD11b expression by Western blot revealed significantly decreased CD11b levels in G551D/CFΔ + iva-ther membrane samples (P = 0.008, n = 4 subjects per group, Student's t-test). (g) CFΔ neutrophils demonstrated significantly increased levels of adhesion compared to HC (P = 0.02, n = 5 subjects per group, Student's t-test). G551D/CFΔ + iva-ther demonstrated significantly decreased adhesion compared to CFΔ (P = 0.02, n = 4 subjects per group, Student's t-test). Actin or Na+/K+ ATPase was employed as a loading control for experiments in (b, d or f). Each measurement is the mean ± SEM.
Ensuing experiments were designed to determine whether ivacaftor therapy decreased ER stress and reduced cytosolic Ca2 + levels. In G551D/∆F508 patients receiving ivacaftor therapy cytosolic levels of GRP78 protein expression significantly decreased 2-fold after 4 weeks treatment, and 4-fold after 12 weeks therapy (P = 0.03) (Fig. 6b). In addition, a significant decrease in the level of cytosolic Ca2 + was detected post-ivacaftor treatment compared to cytosolic levels pre-therapy (P = 0.04) (Fig. 6c). Moreover, post-therapy Ca2 + concentrations were similar to HC levels (Fig. 6c). Western blot analysis of cytosols from isolated neutrophils revealed that ivacaftor therapy significantly increased caveolin-1 levels after 12 weeks treatment (P = 0.03) (Fig. 6d), which in turn led to a significant 2-fold increase in membrane cholesterol content (P = 0.03) (Fig. 6e). Furthermore, analysis of membrane CD11b expression by Western blotting demonstrated significantly reduced levels of the adhesion molecule in neutrophil membranes isolated from G551D/∆F508 patients receiving ivacaftor therapy compared to samples from patients not receiving therapy (P = 0.008) (Fig. 6f). Finally, results demonstrated that neutrophils isolated from G551D/∆F508 patients post-ivacaftor therapy had significantly reduced cell adhesion compared to untreated patients (P = 0.02) (Fig. 6g). Overall these results establish that ivacaftor therapy can reduce circulating levels of inflammatory mediators, reduce ER stress and increase membrane cholesterol content, an immunomodulatory property reducing cell adhesion.
4. Discussion
In CF, neutrophils may act as mediators of tissue destruction and are thus considered an important target for the development of new therapies that disrupt the sustained recruitment associated with chronic inflammation. In the present study we report significant changes in neutrophils of PWCF as a result of inflammatory induced ER stress. As a result of the latter, Ca2 +-dependent regulation of μ-calpain activity is considerably perturbed, leading to proteolysis of caveolin-1 and reduced membrane and lipid-raft cholesterol content. Indeed, a number of studies have identified alterations in cholesterol processing associated with CF (Cui et al., 2007, Manson et al., 2008), however, contrary to murine studies demonstrating increased membrane cholesterol content in Cftr−/− and ΔF508 nasal epithelium (White et al., 2007), in the present study we observed significantly decreased plasma membrane cholesterol content in neutrophils of patients with the ΔF508 and G551D mutations; trafficking and gating CFTR defects, respectively. The consequence of reduced membrane cholesterol content involved increased membrane expression of CD11b and adherence of CF neutrophils, findings supported by in vitro reports linking reduced cholesterol content to increased expression of cell surface adhesion molecules (Solomkin et al., 2007, Tuluc et al., 2003).
Cholesterol levels are a key factor in determining lipid raft stability with reduced cholesterol content associated with structural changes (Rossy et al., 2009). In support of this concept, levels of raft-associated proteins including the scaffolding proteins flotillin and talin were significantly decreased in CF samples. Lipid profiling studies to date have focused on increased levels of ceramide in primary CF cells (Teichgraber et al., 2008) and reduced plasma levels of ceramide in PWCF (Guilbault et al., 2008). So far only a small number of studies have focused on circulating cholesterol content in PWCF (Figueroa et al., 2002), and here we show that triglycerides, HDL and LDL in the adult CF population are within the normal range for both homozygous ΔF508 and heterozygous G551D/ΔF508 patients (Irish Heart Foundation, 2011). Furthermore, previous studies have suggested that free cholesterol accumulates within CF epithelial cells and in the late endosome and lysosome of ΔF508 CF Chinese hamster ovary cells (Gentzsch et al., 2007). Both of the above-mentioned reports employed cell lines expressing the ΔF508 mutation, however, ex vivo experiments of the present study demonstrated similar quantities of total cholesterol in HC and CF neutrophil samples indicating that cholesterol is not altered at a cellular level. Interestingly, the intracellular trafficking protein caveolin-1 was significantly reduced in ΔF508 CF neutrophils, and in concurrence, a study of macrophages isolated from PWCF demonstrated reduced cytosolic caveolin-1 (Zhang et al., 2013). In the aforementioned study the low level of caveolin-1 was associated with impaired CFTR function (Zhang et al., 2013), however this may not be the full explanation as in asthmatic patients where CFTR presence and function is not altered, caveolin-1 expression is significantly reduced in bronchial epithelial cells and monocytes (Bains et al., 2012). In the present study we demonstrate proteolysis of caveolin-1 by the Ca2 + dependent protease μ-calpain and in support of our findings, increased calpain activity has been reported in peripheral blood mononuclear cells isolated from PWCF (Averna et al., 2011). Importantly, the authors concluded that the increase in calpain activity was a result of reduced calpastatin levels; however, we could not find a marked reduction in the levels of this regulatory protein in neutrophils potentially indicating cell-type specific regulation of μ-calpain expression.
In the present study, we report ER stress in circulating CF neutrophils as a result of inflammation. CXCL8 and TNF-α have been associated with chronic inflammatory disorders including CF, but to the best of our knowledge, to date no research on the involvement of CXCL7 has been performed. Within this study, HL-60 cells exposed to either CF plasma or a combination of CXCL8, TNF-α and CXCL7, demonstrated significantly increased levels of ER stress and reduced caveolin-1 and membrane cholesterol abundance. Furthermore, reduced circulating levels of CXCL8, TNF-α and CXCL7 were found in plasma isolated from PWCF post lung transplant, strongly indicating that the source of inflammation is the whole lung or cells in the lung (Cypel et al., 2017). Moreover, although these findings do not establish that these inflammatory molecules are the only mediators present in CF plasma responsible for induction of ER stress, ivacaftor therapy significantly reduced circulating CXCL8, TNF-α and CXCL7 levels and ER stress, while simultaneously increasing caveolin-1 and plasma membrane cholesterol levels. Both these interventions, lung transplant and ivacaftor therapy, with their subsequent anti-inflammatory effects and influence on neutrophil membrane cholesterol, would suggest that the membrane cholesterol deficiency we are reporting is not an intrinsic CFTR defect but rather due to the significant inflammation seen in CF. This part of the study specifically highlights the need for further studies focused on evaluating the role of ivacaftor in maintaining low-level systemic inflammation. Indeed reports are emerging on the potential benefits of ivacaftor in reducing CXCL8 levels in nasal lavage fluid (Mainz et al., 2016), increasing clearance of mucosal bacteria (Rowe et al., 2014) and reducing CD11b expression (Bratcher et al., 2016); effects not directly related to the drug's action as a potentiator.
In summary, there are a number of reports demonstrating impaired neutrophil activity in PWCF due to a lack of CFTR function. Our study demonstrates exaggerated adhesion of neutrophils; an impairment not intrinsic to CF but rather related to chronic inflammation. The secretion of micro-particles can contribute to the removal of cholesterol from membranes of granulocytes (Porro et al., 2010) and mononuclear leukocytes (Hafiane and Genest, 2017), however, results of the present study indicate that reduced CF neutrophil membrane cholesterol and cell adhesion involves inflammatory induced ER stress and caveolin-1 proteolysis. Furthermore, we show that lung transplant or potentiator therapy for PWCF reduced the circulating inflammatory burden, thereby mechanistically preventing ER stress and the exaggerated neutrophil response.
The following are the supplementary data related to this article.
List of antibodies used for Western blot, Flow cytometry and ELISA.
Supplementary material
Conflict of Interest Disclosure
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript.
Author Contributions
Conception: MMW, EH, NGM and EPR; analysis and interpretation of experimental results: MMW, PG, EH, SC, WL, BA, GML, JK, OJM, PM, MH, MC RF, CG, NGM and EPR; Drafting of manuscript: MMW, NGM and EPR.
Acknowledgments
Acknowledgments
Preparation of this article was supported in part by grants from Science Foundation Ireland (11/RFP/BMT/3094), the US Cystic Fibrosis Foundation (MCELVA1210), the US Alpha-1 Foundation, Flight Attendant Medical Research Institute (YCSA 113380) and the Program for Research in Third Level Institutes (PRTLI) administered by the Higher Education Authority. The funders of this study had no role in the study design, data collection, data analysis, interpretation or writing of the manuscript.
We are grateful to Dr. Fatma Gargoum, Dr. Cormac McCarthy, Dr. Emmet O'Brien, Dr. Padraig Hawkins, Dr. Johan Meurling, Dr. Killian Hurley, and Dr. Kevin Molloy for recruitment of patients with cystic fibrosis from the CF Clinic, Beaumont Hospital and Dr. Xian-Cheng Jiang for the use of equipment at SUNY Downstate. Finally, the authors would like to thank all patients with cystic fibrosis who participated in this study.
References
- Accurso F.J., Rowe S.M., Clancy J.P., Boyle M.P., Dunitz J.M., Durie P.R., Sagel S.D., Hornick D.B., Konstan M.W., Donaldson S.H., Moss R.B., Pilewski J.M., Rubenstein R.C., Uluer A.Z., Aitken M.L., Freedman S.D., Rose L.M., Mayer-Hamblett N., Dong Q., Zha J., Stone A.J., Olson E.R., Ordonez C.L., Campbell P.W., Ashlock M.A., Ramsey B.W. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis N.E., Muhlebach M.S., Peden D.B., Noah T.L. Attenuation of host defense function of lung phagocytes in young cystic fibrosis patients. J. Cyst. Fibros. 2006;5:17–25. doi: 10.1016/j.jcf.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averna M., Stifanese R., De Tullio R., Minicucci L., Cresta F., Palena S., Salamino F., Pontremoli S., Melloni E. Evidence for alteration of calpain/calpastatin system in PBMC of cystic fibrosis patients. BBA Mol. Basis Dis. 2011;1812:1649–1657. doi: 10.1016/j.bbadis.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Bains S.N., Tourkina E., Atkinson C., Joseph K., Tholanikunnel B., Chu H.W., Riemer E.C., Martin R., Hoffman S. Loss of Caveolin-1 from bronchial epithelial cells and monocytes in human subjects with asthma. Allergy. 2012;67:1601–1604. doi: 10.1111/all.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin D.A., Reeves E.P., Meleady P., Henry M., Mcelvaney O.J., Carroll T., Condron C., Chotirmall S.H., Clynes M., Neill S.J., Mcelvaney N.G. α-1 antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J. Clin. Invest. 2010:12. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin S., Welch M.D. Plasma membrane organization is essential for balancing competing pseudopod- and uropod-promoting signals during neutrophil polarization and migration. Mol. Biol. Cell. 2005;16:5773–5783. doi: 10.1091/mbc.E05-04-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratcher P.E., Rowe S.M., Reeves G., Roberts T., Szul T., Harris W.T., Tirouvanziam R., Gaggar A. Alterations in blood leukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J. Cyst. Fibros. 2016;15:67–73. doi: 10.1016/j.jcf.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S., Cooper D., Sly P.D. Directed neutrophil migration to IL-8 is increased in cystic fibrosis: a study of the effect of erythromycin. Thorax. 2001;56:62–64. doi: 10.1136/thorax.56.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A., Dominguez M., Puri V., Sharma D.K., Narita K., Wheatley C.L., Marks D.L., Pagano R.E. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 2002;109:1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol H., Fitting C., Chadelat K., Jacquot J., Tabary O., Boule M., Cavaillon J.-M., Clement A., Chade-, K. & Distinct, A. C. Distinct cytokine production by lung and blood neutrophils from children with cystic fibrosis. Am. J. Phys. Lung Cell. Mol. Phys. 2003;284:997–1003. doi: 10.1152/ajplung.00156.2002. [DOI] [PubMed] [Google Scholar]
- Cui L., Aleksandrov L., Chang X.B., Hou Y.X., He L., Hegedus T., Gentzsch M., Aleksandrov A., Balch W.E., Riordan J.R. Domain interdependence in the biosynthetic assembly of CFTR. J. Mol. Biol. 2007;365:981–994. doi: 10.1016/j.jmb.2006.10.086. [DOI] [PubMed] [Google Scholar]
- Cypel M., Waddell T., Singer L.G., Del Sorbo L., Fan E., Binnie M., Ferguson N.D., Keshavjee S. Bilateral pneumonectomy to treat uncontrolled sepsis in a patient awaiting lung transplantation. J. Thorac. Cardiovasc. Surg. 2017;153:e67–e69. doi: 10.1016/j.jtcvs.2016.11.031. [DOI] [PubMed] [Google Scholar]
- De Rose O.A., Messore B., Grosso B., Mollar C., Pozzi E. Circulating adhesion molecules in cystic fibrosis. Am. J. Respir. Crit. Care Med. 1998;157:1234–1239. doi: 10.1164/ajrccm.157.4.9704134. [DOI] [PubMed] [Google Scholar]
- Figueroa V., Milla C., Parks E.J., Schwarzenberg S.J., Moran A. Abnormal lipid concentrations in cystic fibrosis. Am. J. Clin. Nutr. 2002;75:1005–1011. doi: 10.1093/ajcn/75.6.1005. [DOI] [PubMed] [Google Scholar]
- Franco S.J., Rodgers M.A., Perrin B.J., Han J., Bennin D.A., Critchley D.R., Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Frank P.G., Cheung M.W.-C., Pavlides S., Llaverias G., Park D.S., Lisanti M.P. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H677–H686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- Gentzsch M., Choudhury A., Chang X.-B., Pagano R.E., Riordan J.R. Misassembled mutant ΔF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J. Cell Sci. 2007;120:447–455. doi: 10.1242/jcs.03350. [DOI] [PubMed] [Google Scholar]
- Greally P., Hussein M.J., Cook A.J., Sampson A.P., Piper P.J., Price J.F. Sputum tumour necrosis factor-alpha and leukotriene concentrations in cystic fibrosis. J. Cell Sci. 1993;68:389–392. doi: 10.1136/adc.68.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbault C., De Sanctis J.B., Wojewodka G., Saeed Z., Lachance C., Skinner T.A.A., Vilela R.M., Kubow S., Lands L.C., Hajduch M., Matouk E., Radzioch D. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2008;38:47–56. doi: 10.1165/rcmb.2007-0036OC. [DOI] [PubMed] [Google Scholar]
- Hafiane A., Genest J. ATP binding cassette A1 (ABCA1) mediates microparticle formation during high-density lipoprotein (HDL) biogenesis. Atherosclerosis. 2017;257:90–99. doi: 10.1016/j.atherosclerosis.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Hartl D., Griese M., Kappler M., Zissel G., Reinhardt D., Rebhan C., Schendel D.J., Krauss-Etschmann S. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J. Allergy Clin. Immunol. 2006;117:204–211. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Hayes E., Pohl K., Mcelvaney N.G., Reeves E.P. The cystic fibrosis neutrophil: a specialized yet potentially defective cell. Arch. Immunol. Ther. Exp. 2011;59:97–112. doi: 10.1007/s00005-011-0113-6. [DOI] [PubMed] [Google Scholar]
- Hurley K., Lacey N., O'dwyer C.A., Bergin D.A., Mcelvaney O.J., O'brien M.E., Mcelvaney O.F., Reeves E.P., Mcelvaney N.G. Alpha-1 antitrypsin augmentation therapy corrects accelerated neutrophil apoptosis in deficient individuals. J. Immunol. 2014;193:3978–3991. doi: 10.4049/jimmunol.1400132. [DOI] [PubMed] [Google Scholar]
- Irish Heart Foundation 2011. http://irishheart.ie/wp content/uploads/2017/01/a_healthy_cholesterol_2011__final.pdf
- Jimbo A., Fujita E., Kouroku Y., Ohnishi J., Inohara N., Kuida K., Sakamaki K., Yonehara S., Momoi T. ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp. Cell Res. 2003;283:156–166. doi: 10.1016/s0014-4827(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Grimellec C., Friedlander G., Yandouzi E.H.E., Zlatkine P., Giocondi M.-C. Membrane fluidity and transport properties in epithelia. Kidney Int. 1992;40:825–836. doi: 10.1038/ki.1992.357. [DOI] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Mainz J.G., Hentschel J., Hünniger K., Hipler U., Lehmann T., Rabanser B., Kurzai O., Beck J.F., Ellemunter H., Arnold C. Significant reduction of IL-6 and IL-8 in nasal lavage of CF patients with G551D mutation receiving a new therapy with ivacaftor. Pediatr. Pulmonol. 2016;51:S194–S485. [Google Scholar]
- Manson M.E., Corey D.A., White N.M., Kelley T.J. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-pick type C cells. Am. J. Phys. Lung Cell. Mol. Phys. 2008;295:L809–19. doi: 10.1152/ajplung.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelvaney N.G., Nakamura H., Birrer P., Hébert C.A., Wong W.L., Alphonso M., Baker J.B., Catalano M.A., Crystal R.G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J. Clin. Invest. 1992;90:1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'dwyer C.A., O'brien M.E., Wormald M.R., White M.M., Hurley K., Mccarthy C. The BLT1 antagonistic function of alpha-1 antitrypsin augmentation therapy disrupts leukotriene B2. J. Immunol. 2015;195:3628–3641. doi: 10.4049/jimmunol.1500038. [DOI] [PubMed] [Google Scholar]
- Oostrom A.J.V., Wijk J.P.V., Sijmonsma T.P., Rabelink T.J., Cabezas M.C. Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Neth. J. Med. 2004;62:320–325. [PubMed] [Google Scholar]
- Painter R.G., Valentine V.G., Lanson N.A., Jr., Leidal K., Zhang Q., Lombard G., Thompson C., Viswanathan A., Nauseef W.M., Wang G. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl K., Hayes E., Keenan J., Henry M., Meleady P., Molloy K., Jundi B., Bergin D.A., Mccarthy C., Mcelvaney O.J., White M.M., Clynes M., Reeves E.P., Mcelvaney N.G. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124:999–1009. doi: 10.1182/blood-2014-02-555268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro C., Lepore S., Trotta T., Castellani S., Ratclif L., Battaglino A., Di Gioia S., Martinez M.C., Conese M., Maffione A.B. Isolation and characterization of microparticles in sputum from cystic fibrosis patients. Respir. Res. 2010;11:94. doi: 10.1186/1465-9921-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey B.W., Davies J., Mcelvaney N.G., Tullis E., Bell S.C., Drevinek P., Griese M., Mckone E.F., Wainwright C.E., Konstan M.W., Moss R., Ratjen F., Sermet-Gaudelus I., Rowe S.M., Dong Q., Rodriguez S., Yen K., Ordonez C., Elborn J.S. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves E.P., Lu H., Jacobs H.L., Messina C.G.M., Bolsover S., Gabella G., Potma E.O., Warley A., Roes J., Segal A.W. Killing activity of neutrophils is mediated through activation of proteases by K + flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Reeves E.P., Williamson M., Byrne B., Bergin D.A., Smith S.G., Greally P., O'kennedy R., O'neill S.J., Mcelvaney N.G. IL-8 dictates glycosaminoglycan binding and stability of IL-18 in cystic fibrosis. J. Immunol. 2010;184:1642–1652. doi: 10.4049/jimmunol.0902605. [DOI] [PubMed] [Google Scholar]
- Reeves E.P., Banville N., Ryan D.M., O'reilly N., Bergin D.A., Pohl K., Molloy K., Mcelvaney O.J., Alsaleh K., Aljorfi A., Kandalaft O., O'flynn E., Geraghty P., O'neill S.J., Mcelvaney N.G. Intracellular secretory leukoprotease inhibitor modulates inositol 1,4,5-triphosphate generation and exerts an anti-inflammatory effect on neutrophils of individuals with cystic fibrosis and chronic obstructive pulmonary disease. Biomed. Res. Int. 2013;2013:560141. doi: 10.1155/2013/560141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J.R., Rommens J.M., Kerem B.-S., Alon N., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.-L., Drumm L., Iannuzzi M.C., Collins F.S., Tsui L.-C., Alon N.O.A., Grzelczak R.R., Piavsic N., Drumm M.L. Identification of the cystic fibrosis gene : cloning and characterization of complementary DNA. Science AAAS. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rossy J., Schlicht D., Engelhardt B., Niggli V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS One. 2009;4:e5403. doi: 10.1371/journal.pone.0005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe S.M., Heltshe S.L., Gonska T., Donaldson S.H., Borowitz D., Gelfond D., Sagel S.D., Khan U., Mayer-Hamblett N., Van Dalfsen J.M., Joseloff E., Ramsey B.W. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons E.R., Borregaard N., Heiple J.M., Clark R.A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase. J. Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly P.D., Gangell C.L., Chen L., Ware R.S., Ranganathan S., Mott L.S., Murray C.P., Stick S.M., Investigators A.C. Risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- Solomkin J.S., Robinson C.T., Cave C.M., Ehmer B., Lentsch A.B. Alterations in membrane cholesterol cause mobilization of lipid rafts from specific granules and prime human neutrophils for enhanced adherence-dependent oxidant production. Shock. 2007;28:334–338. doi: 10.1097/shk.0b013e318047b893. [DOI] [PubMed] [Google Scholar]
- Song K.S., Li S., Okamoto T., Quilliam L.A., Sargiacomo M., Lisanti M.P. Co-purification and direct interaction of ras with caveolin, an integral membrane protein of caveolae microdomains: detergent-free purification of caveolae membranes. J. Biol. Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Taggart C., Coakley R.J., Greally P., Canny G., O'neill S.J., Mcelvaney N.G. Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-alpha and interleukin-8. Am. J. Phys. Lung Cell. Mol. Phys. 2000;278:L33–41. doi: 10.1152/ajplung.2000.278.1.L33. [DOI] [PubMed] [Google Scholar]
- Teichgraber V., Ulrich M., Endlich N., Riethmuller J., Wilker B., De Oliveira-Munding C.C., Van Heeckeren A.M., Barr M.L., Von Kurthy G., Schmid K.W., Weller M., Tummler B., Lang F., Grassme H., Doring G., Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 2008;14:382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P.J., Drøbak B.K., Hanley M.R., Dawson A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2 + stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. U. S. A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cystic Fibrosis Registry The cystic fibrosis regisrty of Ireland Annual Report (2013) 2013. https://www.cfri.ie/docs/annual_reports/CFRI2013.pdf
- Tuluc F., Meshki J., Kunapuli S.P. Membrane lipid microdomains differentially regulate intracellular signaling events in human neutrophils. Int. Immunopharmacol. 2003;3:1775–1790. doi: 10.1016/j.intimp.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Wetzel A., Wetzig T., Haustein U.F., Sticherling M., Anderegg U., Simon J.C., Saalbach A. Increased neutrophil adherence in psoriasis: role of the human endothelial cell receptor Thy-1 (CD90) J. Invest. Dermatol. 2006;126:441–452. doi: 10.1038/sj.jid.5700072. [DOI] [PubMed] [Google Scholar]
- White N.M., Jiang D., Burgess J.D., Bederman I.R., Previs S.F., Kelley T.J. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am. J. Phys. Lung Cell. Mol. Phys. 2007;292:L476–86. doi: 10.1152/ajplung.00262.2006. [DOI] [PubMed] [Google Scholar]
- Zhang X., Hurng J., Rateri D.L., Daugherty A., Schmid-Schönbein G.W., Shin H.Y. Membrane cholesterol modulates the fluid shear stress response of polymorphonuclear leukocytes via its effects on membrane fluidity. Am. J. Phys. Cell Phys. 2011;301:C451–C460. doi: 10.1152/ajpcell.00458.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.-X., Murray T.S., Villella V.R., Ferrari E., Esposito S., D'souza A., Raia V., Maiuri L., Krause D.S., Egan M.E., Bruscia E.M. Reduced caveolin-1 promotes hyperinflammation due to abnormal heme oxygenase-1 localization in lipopolysaccharide-challenged macrophages with dysfunctional cystic fibrosis transmembrane conductance regulator. J. Immunol. 2013;190:5196–5206. doi: 10.4049/jimmunol.1201607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Song K., Painter R.G., Aiken M., Reiser J., Stanton B.A., Nauseef W.M., Wang G. Cystic fibrosis transmembrane conductance regulator recruitment to phagosomes in neutrophils. J. Innate Immun. 2013;5:219–230. doi: 10.1159/000346568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of antibodies used for Western blot, Flow cytometry and ELISA.
Supplementary material