Abstract
The proteolytic processing of collagen (collagenolysis) is critical in development and homeostasis, but also contributes to numerous pathologies. Mammalian interstitial collagenolytic enzymes include members of the matrix metalloproteinase (MMP) family and cathepsin K. While MMPs have long been recognized for their ability to catalyze the hydrolysis of collagen, the roles of individual MMPs in physiological and pathological collagenolysis are less defined. The use of knockout and mutant animal models, which reflect human diseases, has revealed distinct collagenolytic roles for MT1-MMP and MMP-13. A better understanding of temporal and spatial collagen processing, along with the knowledge of the specific MMP involved, will ultimately lead to more effective treatments for cancer, arthritis, cardiovascular conditions, and infectious diseases.
Keywords: Arthritis, Collagen, Matrix metalloproteinase, Metastasis, Skeletal defects, Wound healing
1. Introduction
The matrix metalloproteinases (MMPs) are a family of Zn2+-dependent endopeptidases. MMPs were first identified as enzymes capable of catalyzing the hydrolysis of collagen [1]. MMP-mediated collagenolysis has long been implicated in the physiological remodeling of tissues and embryonic development as well as the progression of disease pathologies. Inhibition of MMP collagenolytic activity has been extensively pursued [2–4], but with little success in the clinic [5–7]. One of the limitations of previous inhibitor development was the lack of recognition that some MMPs have host beneficial functions that should not be modulated if possible [8–11]. Systems biology approaches have allowed for a more global view of MMP activities [12–18], and insights into the MMP collagenolytic mechanism [19–21] has revealed possibilities for selective inhibition of collagenolytic MMPs. The role of specific MMPs in collagenolysis, and the relationship between collagenolysis and disease, has been better defined through the use of knockout and mutant animal models.
2. Structural organization and assembly of interstitial collagens
Collagens are the most abundant proteins in the human body and the main components of the extracellular matrix (ECM). The collagen family is made up of at least 28 members [22–24]. Collagens are composed of three α chains of primarily repeating Gly-Xaa-Yaa triplets, which induce each α chain to adopt a left-handed polyPro II helix. Three chains then intertwine, staggered by one residue and coiled, to form a right-handed superhelix [25, 26]. Triple-helical structure provides collagens with exceptional mechanical strength, broad resistance to the proteolytic enzymes, and a distinct topology for protein-protein interactions [27].
Collagens have been classified according to their α chains. Homotrimeric collagens (i.e., types II and III) have three α chains of identical sequence. Heterotrimeric collagens have two α chains of identical sequence (designated α1) and one α chain of differing sequence (designated α2) (i.e., type I), or three α chains with different sequences (designated α1, α2, and α3) (i.e., type VI) [28]. Collagens are further classified into subfamilies, based on their quaternary structure. These subfamilies include fibrillar, fibril associated with interrupted triple-helices, short chain, basement membrane, multiplexins, and membrane associated with interrupted triple-helices [28]. The most common collagens (types I, II, III, V, and XI) have fibrillar structures [29].
Types I, II, and III collagen compose the interstitial collagen subfamily. Interstitial collagens are so named because of their proximity to cells in the extracellular space. Type I collagen, the most profuse and ubiquitous of the collagens, is found in the majority of connective and embryonic tissues [28, 30]. Type II collagen is found in cartilage and the vitreous humor [30]. Its expression also occurs during embryogenesis. Type III collagen is found in visceral and cardiovascular tissues [30], as well as in numerous tissues characterized by high type I collagen content. Type V collagen is found associated with type I collagen, while type XI collagen is associated with type II collagen [31, 32].
The triple-helical domains of types I, II, and III collagen span 1014–1023 residues. Each of these collagens also initially possess N- and C-terminal non-triple-helical regions (propeptides). Following synthesis, but before interstitial procollagen can be properly folded, a series of post-translational modifications must occur on the central (Gly-Xaa-Yaa)n domain, including hydroxylation of most Pro and some Lys residues in the Yaa position (by prolyl 4-hydroxylase, prolyl 3-hydroxylase, and lysyl 5-hydroxylases) followed by glycosylation of selective 5-hydroxylysine residues [33–36]. Glycosylation also occurs on some Asn residues in the C-terminal propeptides. Disulfide bonds between the propeptides are rearranged by protein disulfide isomerase and isomerization of Pro and 4-hydroxy-L-proline (Hyp) from cis to trans takes place [37–39]. Assembly and correct folding of procollagen occurs within the endoplasmic reticulum [40]. Hsp47 stabilizes the folded triple-helix [41–43].
The C-terminal propeptides mediate interaction between three α chains and hold these chains in place, nucleating triple-helical formation. Lateral association of triple-helices occurs in the Golgi [40]. The triple-helical molecules are then secreted from the cell and the N- and C-terminal propeptides that flank the central (Gly-Xaa-Yaa)n domain are removed. The resulting tropocollagen contains short N- and C-terminal telopeptides and the central triple-helical domain. A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAMTS-2) removes the N-terminal propeptide from types I, II, and III procollagens [44]. ADAMTS-3 processes the N-terminal propeptide from type II procollagen, while ADAMTS-14 processes the N-terminal propeptide from type I procollagen [44]. Procollagen C-proteinase-2/bone morphogenetic protein-1 cleaves the C-terminal propeptides from types I, II, and III procollagens [45, 46]. Meprins α and β also cleave procollagen III N- and C-propeptides, releasing the mature protein which then assembles into fibrils [47]. Cleavage by meprins is at the same site as procollagen C-proteinase-2. Oxidation of Lys residues by lysyl oxidases (LOXs) allows for the formation of intermolecular crosslinks, which stabilize higher order structures such as fibrils and fibers [48].
3. Am I a collagenase? MMPs that catalyze interstitial collagen catabolism
Hydrolysis of interstitial collagens occurs by a limited number of proteases. The scientific literature contains numerous examples of proteases deemed “collagenolytic,” but this is often obscured by the lack of criteria by which an enzyme is classified to efficiently catalyze the hydrolysis of an intact triple-helix. A collagenolytic enzyme should be considered one that processes a triple-helix under conditions by which that triple-helix is intact. One standard test for triple-helical integrity is susceptibility to trypsin hydrolysis [49]. Some collagens (type III) have more “flexible” potential cleavage sites than others (type I), and thus are more susceptible to hydrolysis by a variety of proteases [50–52]. A collagenolytic enzyme should thus process the triple-helix efficiently, i.e. with a reasonable kcat/KM value for soluble collagen or specific activity for fibrillar collagen [53]. The fibrillar form of collagen is more resistant to general proteolysis [54], and MMP hydrolysis of fibrillar collagen has a higher activation energy than for soluble collagen [55]. Collagenolytic activities between enzymes can also be directly compared to determine relative efficiencies of proteolysis.
Interstitial collagens have long been recognized as being hydrolyzed by the “classic” collagenases, MMP-1, MMP-8, and MMP-13, into ¾ and ¼ length fragments (Table 1 and Fig. 1) [53, 56–62]. All three of these enzymes catalyze collagen hydrolysis efficiently (Table 2), but their relative activities towards interstitial collagens differ. MMP-1 has greater catalytic activity on type III collagen as a substrate. At 25 °C, the MMP-1 collagen preference is III > I ≫ II [63]. MMP-8 preferentially cleaves type I collagen over types II and III collagen at 25 °C [63]. MMP-13 cleaves type II collagen 5- and 6-times faster than types I and type III collagen, respectively, at 25 °C [53].
Table 1.
Representative MMP and cathepsin K cleavage sites within collagen triple-helical domains.
| Enzyme | Collagen Chain (collagen type) | Sequencea |
|---|---|---|
| MMP-1, -2, -8, -9, -12, -13, MT1-MMP | α1(I) | Pro-Gln-Gly775~Ile776-Ala-Gly |
| MMP-1, -2, -8, -9, -12, -13, MT1-MMP | α2(I) | Pro-Gln-Gly775~Leu776-Leu-Gly |
| MMP-1, -8, -13, MT1-MMP | α1(II) | Pro-Gln-Gly775~Leu776-Ala-Gly |
| MMP-1, -8, -9, -12, -13, MT1-MMP, MT3-MMP | α1(III) | Pro-Leu-Gly775~Ile776-Ala-Gly |
| MMP-9 | α1(V) | Pro-Pro-Gly439~Val440-Val-Gly |
| MMP-9 | α2(V) | Pro-Pro-Gly445~Leu446-Arg-Gly |
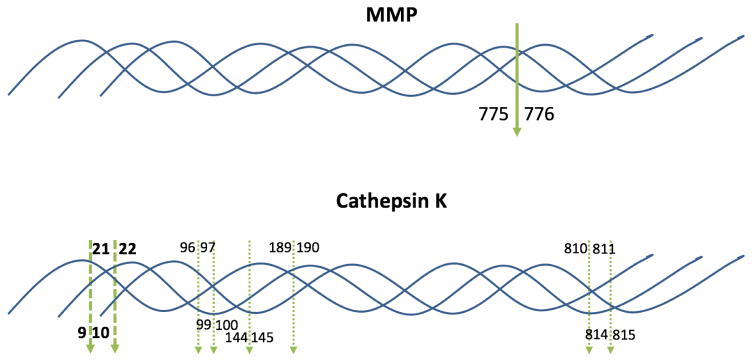
| Cathepsin K | α1(I) | Gly-Pro-Arg9~Gly10-Leu-Pro |
| Cathepsin K | α1(I) | Gly-Pro-Gln21~Gly22-Phe-Gln |
| Cathepsin K | α1(I) | Gly-Leu-Asp96~Gly97-Ala-Lys |
| Cathepsin K | α1(I) | Gly-Pro-Gln189~Gly190-Val-Arg |
| Cathepsin K | α1(I) | Gly-Pro-Ser810~Gly811-Ala-Ser |
| Cathepsin K | α2(I) | Gly-Pro-Arg9~Gly10-Pro-Pro |
| Cathepsin K | α2(I) | Gly-Pro-Gln21~Gly22-Phe-Gln |
| Cathepsin K | α2(I) | Gly-Leu-Lys99~Gly100-Pro-Gln |
| Cathepsin K | α2(I) | Gly-Ala-Arg144~Gly145-Ser-Asp |
| Cathepsin K | α2(I) | Pro-Pro-Gly814~Ala815-Arg-Gly |
| Cathepsin K | α1(II) | Lys-Pro-Gly61~Lys62-Ser-Gly |
Numbering begins at the N-terminus of the triple-helical region of each collagen.
Fig. 1.
Schematic representation of MMP and cathepsin K cleavage sites in type I collagen. The bold, solid green arrow indicates the known MMP cleavage site (bond 775–776) aligned in all three chains in the triple-helix. The bold, dashed green arrows indicate the cathepsin K cleavage sites where all three chains in the triple-helix align (bonds 9–10 and 21–22; see Table 1). The dashed green arrows indicate the cathepsin K cleavage sites in individual collagen chains that do not align within the triple-helix (see Table 1). For cleavage by cathepsin K within individual chains, sites in the α1(I) chain are noted above the triple-helix, while cleavage sites in the α2(I) chain are noted below the triple-helix.
Table 2.
Kinetic parameters for human collagen hydrolysis by MMPs.
| Collagen type | Enzyme | kcat/KM (sec−1M−1) | KM (μM) | kcat (sec−1) | Assay T (°C) | Reference |
|---|---|---|---|---|---|---|
| I | MMP-1 | 14,600 | 0.82 | 0.012 | 30 | [56] |
| I | MMP-1 | 18,750 | 0.80 | 0.015 | 30 | [56] |
| I | MMP-1 | 525,000 | 0.9 | 0.472 | 37 | [243] |
| Ia | MMP-1 | 742,000 | 0.31 | 0.23 | 27 | [244] |
| Ib | MMP-1 | 4,740 | 1.3 | 0.00617 | 27 | [74] |
| Ic | MMP-1 | 4,610 | 1.0 | 0.00461 | 25 | [64] |
| Ic | MMP-2 | 529 | 8.5 | 0.00450 | 25 | [64] |
| I | MMP-8 | 2,540 | 0.7 | 0.00178 | 25 | [63] |
| Ia | MMP-8 | 2,260,000 | 0.21 | 0.470 | 37 | [245] |
| Ib | MT1-MMP | 680 | 2.9 | 0.00197 | 27 | [74] |
| II | MMP-1 | 132 | 2.1 | 0.00028 | 25 | [246] |
| II | MMP-8 | 593 | 1.1 | 0.00065 | 25 | [63] |
| III | MMP-1 | 112,000 | 1.4 | 0.157 | 25 | [50] |
| III | MMP-1 | 118,000 | 1.3 | 0.153 | 25 | [50] |
| IIId | MMP-1 | 93,000 | 15 | 1.4 | 25 | [247] |
| IIIe | MMP-1 | 59,000 | 1.3 | 0.08 | 25 | [52] |
| III | MMP-8 | 131 | 1.8 | 0.00024 | 25 | [63] |
| IIId | MMP-13 | 22,600 | 14 | 0.32 | 25 | [247] |
| III | MT1-MMP | 366 | 0.95 | 0.00035 | 27 | [82] |
| III | MT3-MMP | 1,926 | 0.45 | 0.00087 | 27 | [82] |
Bovine type I collagen.
Guinea pig type I collagen.
Rat type I collagen.
Human type III collagen sequence inserted into bacterial collagen.
Recombinant type III collagen expressed in P. pastoris.
There is some ambiguity as to the collagenolytic activity of the gelatinase members of the MMP family, MMP-2 and MMP-9. MMP-2 has been reported to cleave types I, II, and III collagen [64–66], although other reports have brought into question how robust the type I collagenolytic activity of MMP-2 is [67, 68]. Recombinant MMP-9 (0.5 μg) was found to cleave type I collagen (27 μg) at 37 °C after 72 h [69]. MMP-9 also digested type III collagen at 25 °C following 98 h treatment [69]. For MMP-2 and MMP-9, the interstitial collagen cleavage site is the same as the classic collagenases (Table 1). For both MMP-2 and MMP-9, type III collagen is a preferred substrate compared with types I and II [65, 69]. Most likely, MMP-2 and MMP-9 do not contribute significantly to interstitial collagen turnover in vivo, but instead produce collagen fragments following the action of MMP-13 or MT1-MMP (see below).
The ability of MMP-9 to cleave the intact triple-helix of type V collagen has been reported [70–72]. Conditions were 438–876 nM MMP-9, 2 μg human type V collagen for 30 h at 30 °C [71], which resulted in near complete digestion of the collagen, or 22–43 nM MMP-9, 1 μg/μL human type V collagen for 18 h at 30 °C [72], which resulted in more moderate digestion of the collagen. Although the cleavage sites within type V collagen was identified by treatment at 30 °C for 16 h, the sites were slightly out of alignment within the [α1(V)]2α2(V) heterotrimeric triple-helix (Table 1) [70].
Transfection of two membrane type-MMPs (MT-MMPs), MT1-MMP/MMP-14 and MT2-MMP/MMP-15, allowed invasion-incompetent cells to penetrate type I collagen matrices [73]. MT1-MMP processes types I–III collagen at the same site as the classic collagenases (Table 1) [74]. MT1-MMP prefers type I collagen, as activity against type I collagen was estimated to be 4 times that of type II collagen and 6.5 times that of type III collagen [74].
Some studies indicated a requirement of MT1-MMP homodimerization through the hemopexin-like (HPX) domain for efficient collagenolysis [75, 76]. Alternatively, deletion of the HPX domain did not inhibit collagen invasion modulated by cell surface-bound MT1-MMP [77, 78]. In solution, MT1-MMP was not found as a dimer [21, 79] and the MT1-MMP HPX domain alone did not form a dimer [80]. The low level of collagenolytic activity observed with an MT1-MMP HPX domain mutant [81] may have been due to disruption of favorable MT1-MMP interaction with the cell surface rather than dimer disruption [79]. The conflicting results in prior studies may result from different MT1-MMP constructs being utilized. When MT1-MMP residues 336-535 were deleted (the resulting enzyme contained the CAT domain, the linker, and 18 residues from the HPX domain), collagenolysis was inhibited [76]. In this construct Cys318 is present; in the full-length MT1-MMP, Cys318 forms a disulfide bond with Cys507. When MT1-MMP residues 318-535 were deleted (the resulting enzyme contained the CAT domain and the linker), collagenolyis was still observed [77]. In this construct there are no unpaired Cys residues. Ultimately, recent studies have concluded that dimerization is not necessary for MT1-MMP catalyzed collagenolysis [21, 79], and that collagenolysis can occur without the HPX domain when the enzyme is cell-surface bound [77, 78].
MT3-MMP/MMP-16 was found to cleave type III collagen at the classic site (Table 1), and was more efficient at processing type III collagen than MT1-MMP (Table 2) [82]. Conversely, MT3-MMP did not cleave types I and II collagen within their triple-helical domains [82]. Transfection of MT3-MMP either did not allow or only weakly allowed invasion-incompetent cells to penetrate type I collagen matrices [73, 83]. Similar behavior was observed with MT3-MMP-expressing WM852 melanoma cells [84]. However, in complete contrast, Shi et al. found MT3-MMP to efficiently process types I and II collagen films [85]. The differences in observed MT3-MMP collagenolytic activities may originate from the constructs or cell types (MDCK cells [73, 83], WM852 melanoma [84], and Cos-7 cells [85]) used.
MT6-MMP/MMP-25 was initially reported to have little or no collagenolytic activity [86, 87], but subsequently was found to cleave types I and II collagen (albeit at 37 °C) [88] and a triple-helical peptide model of the classic collagenase cleavage site in interstitial collagen [89]. The MT6-MMP cleavage sites in the α1 and α2 chains of type I collagen did not align, and many sites were located in the non-triple-helical C-terminal telopeptide region [88]. This indicates that MT6-MMP is not aa truly collagenolytic protease.
The CAT domain of MMP-12 processes types I and III collagens at 33 °C, where hydrolysis occurs at the classic cleavage site and at numerous other sequences [90]. The classic collagenase cleavage site seemed to be the most sensitive to MMP-12 (Table 1). However, we found that MMP-12 could not cleave type I collagen efficiently under conditions comparable to other collagenases (Fig. 2). The observed hydrolysis reported previously was most likely due to the combination of high concentration of enzyme and substrate (10 μg/mL of enzyme with 1 mg/mL substrate), temperature (33 °C), and time (24 h). In similar fashion, although the MMP-12 catalytic domain has been reported to cleave the triple-helix of type V collagen [91], we found that it could not cleave type V collagen efficiently (Fig. 3). The prior study used 0.2 μg of enzyme and 10 μg of type V collagen at room temperature for 16 h.
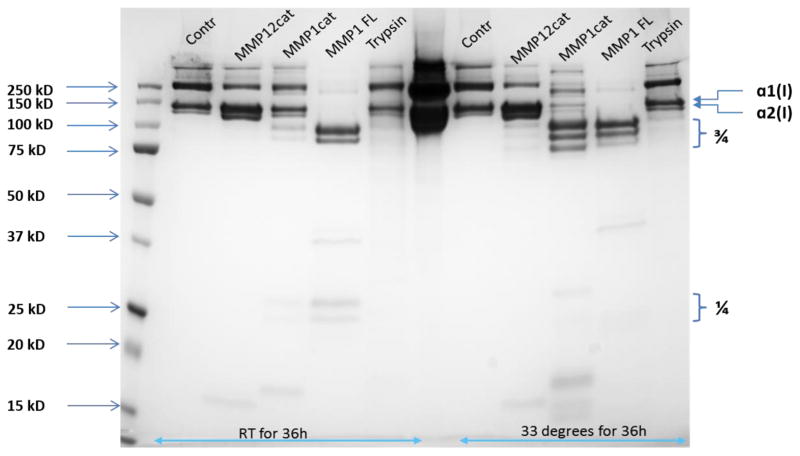
Fig. 2.
Cleavage of type I collagen by MMP-1 CAT domain, full-length MMP-1, MMP-12 CAT domain, and trypsin. Type I collagen (10 μg) was treated with 200 ng of enzyme in 50 mM Tris•HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij35, 1 μM ZnCl2 for 36 h at either room temperature or 33 °C. Full-length MMP-1 (MMP1 FL) cleaved type I collagen at room temperature, resulting in the characteristic ¾ and ¼ fragments, while MMP-1 CAT domain (MMP1cat) showed a low level of hydrolysis and MMP-12 CAT domain (MMP12cat) did not cleave the collagen. At 33 °C, increased hydrolysis by MMP-1 CAT domain and a low level of hydrolysis by MMP-12 CAT domain was observed. Trypsin showed minimal collagen hydrolysis at either temperature. “Contr” is type I collagen alone.
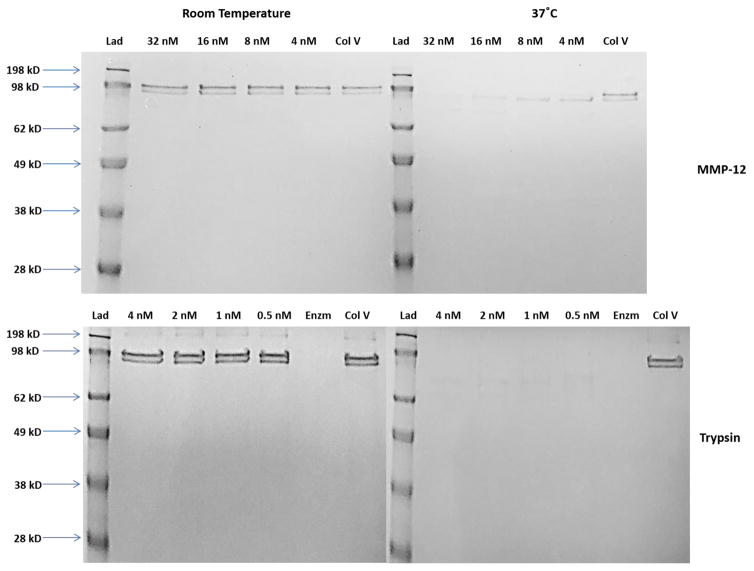
Fig. 3.
Cleavage of type V collagen by MMP-12 CAT domain (top) and trypsin (bottom). Type V collagen (333 nM) was treated with 4–32 nM of MMP-12 or 0.5–4 nM of trypsin in 50 mM Tris•HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij35, 1 μM ZnCl2 overnight at either room temperature (gels on the left) or 37 °C (gels on the right). MMP12 CAT domain or trypsin catalyzed the hydrolysis of type V collagen at 37 °C, but not at room temperature. “Lad” is the molecular weight ladder, “Col V” is type V collagen alone, and “Enzm” is trypsin alone.
MMP-3 binds to type I collagen, but does not cleave the native triple-helix [92, 93]. However, the MMP-3 catalytic (CAT) domain can cleave collagen when the triple-helix is destabilized by catalytically inactive MMP-1 [94]. Thus, MMP-3 is entirely competent to cleave type I collagen, but does not. Based on the MMP collagenolysis mechanism, the linker needs to be able to properly orient the CAT and HPX domains [19–21]. Large domain movements based on the flexible linker have been observed for MMP-1, MMP-9, and MMP-12 [20, 95–98]. Gly272 is critical for the collagenolytic activity of MMP-1, with its role proposed to be the linker-bending motion that allows the HPX domain to present collagen to the CAT domain [99, 100]. MMP-1 and MMP-8 linkers are considerably shorter than the MMP-3 linker, while MT1-MMP linker is very long (33 residues), with significant and heterogeneous O-glycosylation [101]. Thus, linker length per se is not the ultimate criteria for efficient collagenolysis. A chimeric MMP-8 whose linker region (16 residues) was replaced with the corresponding MMP-3 sequence (25 residues) lost activity towards collagen [102]. In similar fashion, MMP-1/MMP-3 chimeras possessing the MMP-3 linker are not active towards collagen [93, 103]. The linker appears critical for proper alignment of the CAT and HPX domains during collagenolysis. Ultimately, there may be negative regulation of collagenolytic activity due to (mis)alignment of the CAT and HPX domains in the case of MMP-3 and other non-collagenolytic MMPs.
The intact triple-helix of interstitial collagen is cleaved efficiently by the cysteine protease cathepsin K under acidic conditions (optimum pH 5.0) [104–106]. Five distinct sites of cathepsin K hydrolysis of type I collagen have been identified, as well as one in type II collagen (Table 1 and Fig. 1) [105, 107].
To determine “am I a collagenase?”, the most prudent approach is to compare an enzyme to a known collagenase (such as MMP-1) and a non-collagenolytic protease (such as trypsin) using gel-based analysis of collagen degradation (as shown in Figs. 2 and 3). One can readily monitor the disappearance of the intact collagen chains over time to evaluate kinetic parameters. Active enzyme concentrations should be comparable on a molar basis, and an appropriate temperature used whereby there is no collagenolysis by the non-collagenolytic protease. For cell surface-bound enzymes, comparisons to MT1-MMP-producing or -transfected cells can be performed for invasion of collagen matrices or processing of collagen films. In lieu of titrating the amount of active enzyme on the cell surface (which can be quite difficult), total protein concentration of the enzyme and MT1-MMP should be comparable.
4. The role of collagen catabolism in normal physiology
The proteolysis of collagen is integral for numerous physiological functions including morphogenesis, tissue remodeling, and wound healing. Determining which MMPs participate in collagenolysis is difficult, based on the fact that MMPs have multiple activities beyond collagenolysis. For example, MT1-MMP participates in collagenolysis, shedding of cell surface biomolecules, hydrolysis of serum proteins, cytokines, fibrillar amyloid β-protein, fibronectin, Notch1, and the laminin-5 γ2 chain, and activation of proMMP-2 and the pro-αv integrin subunit [108–125]. MT1-MMP is also active intracellularly, processing centrosomal breast cancer type 2 susceptibility gene (BRCA2) and pericentrin, where the latter event leads to chromosomal instability [126, 127]. In addition, collagen hydrolysis by MMPs has other effects, such as directly disrupting the fibronectin binding site [128] and revealing cryptic binding sites within collagen chains [129–132]. Bulk collagenolysis may be performed by several MMPs in a redundant and compensatory fashion [78]. However, the ultimate products of collagenolysis and their effects on cellular behaviors differ based on the specific MMP [132]. Precise roles for collagen catabolism have been ascertained from MMP knockout mice or mutant collagen mice.
There are several pathways that have been considered for mammalian collagen catabolism [62]. One pathway involves initial extracellular MMP hydrolysis of collagen fibrils, followed by the large collagen fragments undergoing urokinase plasminogen activator receptor-associated protein (uPARAP)/Endo180-mediated (on mesenchymal cells) and mannose receptor-mediated (on macrophages) endocytosis, lysosomal delivery, and cathepsin catalyzed degradation [133–136]. The initial collagen proteolysis has been ascribed to MT1-MMP [133, 137].
Knockout studies showed that MT1-MMP has a variety of roles in skeletal development, as aberrant cranial bone formation was observed at birth in MT1-MMP knockout mice, and over time osteopenia increased and bone mass decreased [138]. These effects were attributed to a lack of interstitial collagenolytic activity of MT1-MMP [138, 139], as the knockout mice exhibited increasing fibrosis in tendons, ligaments, synovial capsules, musculotendinal junctions, and septal/fascial structures and persistence of parietal cartilage [138]. The skeletal defects may also have contributions from the lack of other proteolytic activities of MT1-MMP, or indirect effects of decreased collagenolysis, such as the lack of regulation of fibronectin binding to collagen (see above). A mutation in the signal peptide (Thr17Arg) results in decreased production of active MT1-MMP in Winchester Syndrome [140]. The mutation is hypothesized to affect MT1-MMP transport to the cell membrane [140]. Winchester Syndrome is characterized by osteolysis, or “vanishing bone” syndrome, whose skeletal phenotype parallels that observed in the MT1-MMP knockout mouse [140].
MT3-MMP also contributes to skeletal development [85]. MT1-MMP/MT3-MMP double deficiency mice have severe craniofacial dysmorphism and shortening of cortical bone beyond that observed in MT1-MMP knockout mice. These contributions of MT3-MMP are proposed to be a result of the collagenolytic activity of the enzyme (see above) [85].
Knockout studies indicated that MMP-13 functions in skeletal growth plate development (the transition from cartilage to bone) [141]. More specifically, in the knockout mice growth plates had a lengthened hypertrophic chondrocyte zone and trabecular bone was increased over time [141]. The lack of MMP-13 to process cartilage type II collagen was key to these effects [139, 141]. A mutation in the propeptide of MMP-13 (Phe56Ser) results in the Missouri variant of spondyloepimetaphyseal dysplasia (SEMD), a human disorder [142]. The mutant MMP-13 is degraded intracellularly [142]. SEMD is characterized by abnormalities in development and growth of endochondral skeletal components [141, 142]. An Arg792Gly mutation in type II collagen results in SEMD congenita [143]. This mutation has been suggested to negatively effect the MMP HPX domain interaction with the P17’ subsite of collagen [144] and hence decrease collagen turnover. Alternatively, it has also been proposed that the mutation results in increased binding of type II collagen to fibronectin and poor ECM assembly [145].
MT1-MMP knockout mice have arrested tendon development around the time of birth [146]. The knockout mouse tendons had collagen fibrils of ~50 nm diameter that were retained by fibripositors (actin-dependent invaginations of the plasma membrane). It was determined that collagenolysis by MT1-MMP was not essential for tendon development, but MT1-MMP processing of fibronectin was, resulting in the release of fibrils from fibripositors [146].
Substitution of Pro for Gln774 and Ala777 in the Col1a-1 gene results in the production of type I collagen resistant to MMP-1, MMP-8, and MT1-MMP processing [147–149]. Introduction of this MMP resistant type I collagen in mice did not affect development to young adulthood [150]. MMP-13 cleaved the N-terminal telopeptide region of the resistant type I collagen [151, 152]. The relatively mild effects of the mutant collagen on development to young adulthood may be due to release of triple-helices from fibrils by aminotelopeptidase activity [150], denaturation of the isolated triple-helices at body temperature [153], and general proteolysis of isolated chains. However, after 3–6 months of age, mice displayed thickened skin with dermal fibrosis [150]. Additionally, postpartum involution of the uterus was impaired in female mice bearing the mutant collagen [150]. The uteri were filled with nodules consisting of primarily type I collagen fibers [150]. Thus, it was proposed that cleavage in the N-terminal telopeptide region contributed to remodeling of type I collagen during development to young adulthood, but cleavage within the triple-helix was needed for remodeling during adulthood [151]. This may be due to collagen cross-linking over time.
Skeletal remodeling was altered in the collagen mutant mice, with increased calvarial periosteal and tibial/femoral endosteal bone deposition observed at 3–12 months of age [154]. Osteocyte/osteoblast apoptosis occurred in the collagen mutant mice starting at 2 weeks of age [154]. It has been proposed that MMP-derived collagen cleavage products are anti-apoptotic [131, 141, 155]. This may be the reason that parathyroid hormone induction of osteoclastic bone resorption is greatly reduced in the collagen mutant mice [156]. Failure to degrade type I collagen impaired hepatic stellate cell apoptosis and may prevent the effective restoration of hepatocyte mass in liver fibrosis [157]. Wound healing, reepithelization, and contraction were delayed in the first 2 weeks after injury in type I collagen mutant mice [158]. The number of contractile myofibroblasts in the wound was decreased, and thus differentiation of fibroblasts to myofibroblasts was impaired [158]. The signal to produce α smooth muscle actin to generate tensile force to contract the tissue was not received [158]. It is possible that apoptosis, as described above, may be the reason why wound healing is impaired [158]. In addition, MMP-1 processing of type I collagen has been shown to promote keratinocyte migration during reepithelialization [159, 160].
For the mutant collagen studies, the precise MMP involved was not identified. As described above, specific roles for MT1-MMP and MMP-13 collagenolysis have been identified. MT1-MMP also contributes to postnatal vascular development and skin homeostasis by cleaving type I collagen [139, 161]. MT1-MMP does not appear to be the critical collagenase for wound repair [161].
Cathepsin K deficiency resulted in pycnodysostosis, a bone-sclerosing dysplasia [162]. Undigested collagen fibrils are observed in osteoblasts and fibroblasts during pycnodysostosis [163, 164]. Patients treated with the cathepsin K inhibitor balicatib exhibited skin hardening, which was correlated to thickened collagen bundles and a hypocellular and hypovascular dermis [165].
5. The role of collagen catabolism in disease
The proteolysis of collagen has been recognized as a contributing factor to multiple pathologies, including tumor cell spreading (metastasis), arthritis, glomerulonephritis, periodontal disease, tissue ulcerations, cardiovascular disease, and neurodegenerative diseases [166–171].
It has long been demonstrated that tumor extracts can possess collagenolytic activity [61, 171, 172]. MT1-MMP is the dominant pericellular collagenase operative in vivo enabling cells to migrate through connective tissue matrices where collagens exist as insoluble fibers [121, 173–175]. MT1-MMP appears to play a significant role in tumor metastasis [173, 176, 177]. Interestingly, even though MT1-MMP is an interstitial collagenase, in similar fashion to several secreted MMPs (MMP-1, MMP-8, and MMP-13), the activity of MT1-MMP, but not of secreted collagenases, is critical for transmigration of tumor cells, endothelial cells, and fibroblasts through collagen matrices [73, 78, 178–183]. Tumor cell invasion through type I collagen is dependent upon MT1-MMP activity [182]. Collagen degradation by MT1-MMP results in cryptic Arg-Gly-Asp sites being revealed and binding to the αvβ3 integrin. Integrin ligation then activates ERK through c-Src, which in turn causes tumor cell proliferation [184]. MT1-MMP collagenolysis has been correlated to metastasis in vivo [176]. Additional roles for collagenolysis in tumor progression have been described [185], including participation of MMP-1 collagenolytic activity in metastasis [186].
Homotrimeric type I collagen is produced by a variety of tumor cells but not cancer-associated fibroblasts [187]. Homotrimeric type I collagen is highly resistant to collagenolytic MMPs [188], and wild type fibroblasts degraded heterotrimeric type I collagen matrices but not homotrimeric type I collagen matrices [187]. Homotrimeric type I collagen enhances tumor cell proliferation and migration compared with heterotrimeric type I collagen. It has been suggested that tumor cells might use MMP-resistant homotrimeric type I collagen fibers as “roadways” for invasion [187].
Matrix stiffness has been implicated in tumor progression, with collagen considered a significant contributor to changes in the cellular mechanical microenvironment [189, 190]. Increased orientation of interstitial and fibrillar collagens, and increased stiffness, is seen in the invasive front of human breast cancer [190]. Transforming growth factor β (TGF-β) enhances collagen deposition in breast and pancreatic cancers [190, 191], and TGF-β can be activated by MMP-2, MMP-9, and MT1-MMP [192–195]. Increased matrix tension due to LOX crosslinking of collagen induces integrin signaling [196]. In turn, inhibition of LOX activity impedes breast tumor progression [196]. Mechanotransduction and oncogenic signaling pathways may be synergistic in promoting tumorigenicity [189], and there is mechanical heterogeneity within tumors [190]. While intact collagen is required for signaling and matrix stiffness, MMP degradation of collagen facilitates tumorigenesis [189]. LOX and MMPs most likely collaborate to create a dynamic collagen-based microenvironment [189].
Osteoarthritis (OA), the most common form of arthritis, is characterized by the destruction of articular cartilage. The main constituents of articular or joint cartilage are type II collagen and various proteoglycans, such as aggrecan, chondroitin sulfate, and hyaluronan [197]. Tensile strength of articular cartilage is due to the triple-helical structure of type II collagen [198]. In native joint cartilage, type II collagen fibrils are protected from cleavage by tight association with molecles of aggrecan [199]. In arthritic cartilage, aggrecan is hydrolyzed by ADAMTS-1, ADAMTS-4, and ADAMTS-5, collectively known as aggrecanases [200]. Aggrecanolysis removes aggrecan molecules from type II collagen fibrils, which makes collagenolysis possible.
MMP-13 has been shown to be the main collagenase responsible for degradation of articular cartilage during OA [201, 202]. Under normal circumstances, MMP-13 is constitutively produced in human chondrocytes, but is rapidly endocytosed and degraded [203, 204]. MMP-13 is specifically expressed in the cartilage of human OA patients and is not present in normal cartilage. MMP-13 synovial fluid levels correlate to human OA severity [205]. Furthermore, transgenic animal models indicate that overexpression of MMP-13 induces joint abnormalities characteristic of human OA [206]. More specifically, mice expressing an inducible transgene of spontaneously active MMP-13 had increased cartilage collagen cleavage and OA progression [202]. Studies with semi-selective and selective MMP-13 inhibitors demonstrated that MMP-13 inhibition renders protection to human and bovine cartilage cultures as well as providing chondroprotective effects in vivo [206, 207]. Cathepsin K has also been implicated in fibroblast-mediated degradation of type II collagen in cartilage [208].
Osteoporosis (OP) is a chronic skeletal disease that is predicted to affect nearly 61 million women over the age of 50 in the United States by the year 2020 [209]. The skeletal density is dependent on constant bone remodeling events, which are regulated by the balance of osteoblast bone building and osteoclast resorptive actions. Bone is primarily comprised of type I collagen which is mineralized via the deposition of apatite during its synthesis by osteoblasts. Estrogen deficiency increases osteoclast formation by increasing the levels of available pro-osteoclastogenic cytokines [210, 211]. Osteoblastic cells have been shown to secrete multiple MMPs, including MMP-2, MMP-3, MMP-8, MMP-9, MMP-13, and MT1-MMP, while MMP-9 is mainly expressed by osteoclasts [212]. These MMPs have been shown to be capable of degradation of the osteoid that covers the bone trabeculae and to initiate or activate bone remodeling in mice, rats, and humans [213, 214]. MMP-13 is mainly associated with mineralized bone matrix, is thought to be essential for osteoclastogenesis, and plays an important role in degradation of type I collagen in bone matrix in concert with cathepsin K and MMP-9 [212]. One of the mechanisms of estrogen deficiency-induced bone loss is ascribed to the abnormal expression of multiple MMPs in osteoblastic cells, as estrogen inhibits bone resorption and reduces bone turnover rate by down-regulating the expression of MMP-13 in osteoblastic cells [214].
The selection of MMP-13, as opposed to other proteases, as a target in OA and OP is well justified. For example, cartilage degradation is reversible in the presence of aggrecanase activity, but not once type II collagen degradation has proceeded [215]. Inhibition of cathepsin K, which has been pursued for OP, may indiscriminately prevent normal collagen turnover outside of the skeletal system [216].
During pathological vessel remodeling, neointimal lesions and subsequent occlusive events found in atherosclerosis and postangioplasty restenosis result from MT1-MMP activity [217]. Vascular smooth muscle cells use MT1-MMP to degrade and infiltrate three-dimensional collagenous barriers including the arterial wall (which is rich in type I collagen) [217]. Amongst several causes, atherosclerotic plaque vulnerability (rupture) has been postulated to result from processing of interstitial collagens in the fibrous cap of the plaque [218]. It is presently not clear which collagenase (MMP-1, MMP-8, and/or MMP-13) contributes to plaque instability [218].
Increased collagen synthesis over catabolism can result in myocardial fibrosis, leading to ventricular hypertrophy and diastolic dysfunction [219]. In contrast, increased collagen catabolism over synthesis can lead to ventricular dilatation and systolic dysfunction [219]. MT1-MMP myocardial levels are increased post-myocardial infarction (MI) and coincident with adverse left ventricular remodeling [220, 221]. MT1-MMP is the dominant collagenase within myocardial tissues [221]. Following MI, MT1-MMP+/− mice have a survival advantage over MT1-MMP+/+ mice, while post-MI survival is reduced when MT1-MMP is overexpressed [220]. Survival has been correlated to decreased collagenolytic potential of cardiac fibroblasts (preservation of myocardial type I collagen network) [221]. Liberation of collagen fragments and subsequent processing by MMP-9 can help or hinder left ventricle remodeling post-MI, depending upon the timing and extent of MMP-9 action [222, 223].
Pulmonary fibrosis occurs following repeated bouts of lung injury, as observed in cystic fibrosis, usual interstitial pneumonitis (UIP)/idiopathic pulmonary fibrosis (IPF), and acute respiratory distress syndrome (ARDS). Pulmonary fibrosis corresponds to excess collagen production compared with degradation. [224]. Fibrosis may be the result of a change in collagen composition, resulting in decreased degradation, or an increase in the production of protease inhibitors. A greater proportion of type I collagen compared with type III collagen is observed in lung fibrotic tissue compared to normal lung tissue. Fibrotic tissue also has increased amounts of collagen binding biomolecules, such as fibronectin and proteoglycans, increased proportion of hydroxylated Lys residues within the collagen, and an increase in collagen crosslinking via lysyl oxidase. An increased tissue inhibitor of metalloproteinase (TIMP) to MMP ratio and decreased collagenolysis in the lung is found in human UIP/IPF patients. Knockout studies have implicated MT1-MMP and cathepsin K as key collagenases in fibrosis [224].
Inducible deletion of MT1-MMP in stromal fibroblasts was used to examine the role of this enzyme in skin fibrosis [161]. Deletion of MT1-MMP resulted in increased type I collagen accumulation in skin due to a lack of collagen degradation, and a subsequent continuous increase in skin thickness and stiffness. Fibrosis was entirely due to the lack of collagen turnover, as collagen fibril diameters did not increase [161].
MT1-MMP also contributes to tissue damage and mortality in infectious diseases. Tuberculosis, once the leading cause of death in the U.S., remains a global threat due to limited treatment options, high percentage of infection transmission, and increasing Mycobacterium tuberculosis resistance [225]. The interaction between the Mycobacterium tuberculosis bacteria and the host immune response (macrophage infection) evokes inflammation and breakdown of the pulmonary ECM leading to formation of granulomas, the hallmark of the disease [226]. Granulomas, formed by aggregates of lung epithelial and immune cells, were once thought to curtail the spread of the disease by encasing Mycobacterium tuberculosis. However, recently it has been shown that infected macrophages shuttle between granulomas and the lung surface in order to recruit uninfected macrophages. Upon arrival in the granulomas, newly attracted macrophages become infected by the bacteria and further propagate the infection [225].
MT1-MMP expression is upregulated in Mycobacterium tuberculosis-infected macrophages [227]. MT1-MMP is significantly upregulated in patients with pulmonary tuberculosis, expressed throughout granulomas, upregulated by monocyte-monocyte networks, and is functionally active [226]. This upregulation was correlated to local tissue degradation (including collagen destruction) and leukocyte recruitment to the granuloma, contributing to the disease pathology [226].
The lung epithelial barriers, supported by the ECM scaffold and functioning as the first line of defense against pathogens, are severely damaged during viral infections.[228, 229] Uncontrolled immune-mediated ECM-remodeling events act as a double-edged sword, allowing multiple immune cells to infiltrate the infection focus while causing devastating collateral damage that promotes acute respiratory failure. Global genomics analysis of lung tissue derived from an H1N1 influenza mouse model detected extremely elevated ECM remodeling collagenase genes (mostly MT1-MMP) without a corresponding increase in tissue inhibitor of metalloproteinase-2 [230]. Follow-up experiments, including fluorescence-correlated electron microscopy of intact tissues [231], global mass spectrometry, immune staining, and tissue zymography, revealed dramatic morphologic and compositional ECM changes in influenza-infected lungs, including depletion of fibrillar collagens [230]. The majority of the MT1-MMP-expressing cells during the infection were immune cells of myeloid origin. Remarkably, mice receiving Tamiflu exhibited a devastating ECM phenotype, despite having extremely low viral titers [230]. Mice treated with an anti-MT1-MMP Fab fragment [232] showed tissue recovery, both at the level of morphology and composition (including improved collagen component abundance), and therapeutic effects [230]. The two mouse models used were influenza A infection and influenza A co-infected with Streptococcus pneumoniae [230]. Treatment with anti-MT1-MMP Fab fragment significantly increased the ability of virally infected mice to fight off secondary Streptococcus pneumoniae bacterial infection over control. This was demonstrated by the finding that 50% of the mice receiving the anti-MT1-MMP Fab fragment survived the double-infection, whereas 100% of the mock treated mice died. Mice that did not receive the inhibitor exhibited bacteremia and dissemination of Streptococcus pneumoniae bacteria into the spleen and liver, whereas the infection of treated mice remained confined within the lungs, with no systemic bacterial dissemination [230]. The results suggested that the ECM damage is caused by infiltrating immune cells contributing to the lethal outcome from influenza infection. Immune cell invasion and respiratory failure depended on tissue damage, presumably by MT1-MMP. Blocking MT1-MMP dysregulated collagenolytic activity in vivo and prompted a therapeutic effect in both primary and co-infected disease stages/models.
Mutations of type I collagen genes have been identified in osteogenesis imperfecta (OI) [22, 28, 32, 39, 233, 234]. OI dominant-negative mutations can occur in either gene that encode the α chains of type I collagen and are typically missense mutations that change the Gly codons in the triple-helical motifs. Gly substitutions result in different effects on helix stability, depending on their location and the newly substituted amino acid. Mutations near the MMP cleavage site, particularly in the α1(I) chain, often result in severe forms of the disease [235]. This may be due to rendering the cleavage site susceptible to proteases that are normally inhibited by triple-helical structure. We have found that decreasing the thermal stability of the MMP cleavage site renders it more susceptible to MMP-2 and MMP-9 hydrolysis [236].
MMP processing of type I collagen can ultimately result in the production of numerous, distinct fragments (see prior discussion). One fragment resulting from collagenolysis, CO1-764/C1M (Gly-Ser-Pro-Gly-Lys-Asp-Gly-Val-Arg-Gly586; numbering based on the α1(I) chain triple-helical region), is generated by the action of MMP-13, MMP-2, and/or MMP-9 [237]. It is presumed that a collagenase first cleaves type I collagen at the 775–776 bond (see Table 1), followed by MMP-13, MMP-2, and/or MMP-9 action at the 586–587 bond of the denatured α1(I) chain. CO1-764/C1M was proposed as a biomaker for liver fibrosis [237]. Serum levels of C1M were found to be predictive of increased mortality in women up to 9 years prior to death, while no correlation was observed for a cathpesin K generated type I collagen fragment (CTX-1) and mortality [238]. The most prevalent primary causes of death in the study were cancer and cardiovascular disease [238]. Increased C1M levels in the serum has been associated with chronic inflammation [238], and recent studies have focused on the interrelationships between chronic inflammation and numerous diseases, including cancer and vascular diseases, as well as the role of MMPs in inflammation [11, 194, 239].
The peptide Pro-Gly-Pro is generated by the initial processing of type I or II collagen by neutrophil MMP-8, followed by the proteolytic action of MMP-9 and prolyl endopeptidase [240, 241]. Pro-Gly-Pro is a neutrophil chemoattractant and induces production of superoxide [242]. Pro-Gly-Pro has been implicated in neutrophilic inflammation in lung diseases such as cystic fibrosis and chronic obstructive pulmonary disease (COPD) [240, 242].
6. Summary
The role of specific MMPs in collagenolysis has been better defined through the use of animal models and the correlation of animal studies with human diseases. The processing of collagen by MT1-MMP is now associated with metastasis and progression of tuberculosis and influenza to the lungs. In turn, MMP-13-mediated collagenolysis contributes significantly to osteoarthritis as well as normal bone development, while collagen turnover by MMP-1 is a contributor to wound healing. As we improve our understanding of temporal and spatial collagen processing, along with the knowledge of the specific MMP involved, we will ultimately be able to design more effective treatments for cancer, arthritis, cardiovascular conditions, and infectious diseases.
Supplementary Material
Highlights.
Collagenolysis is critical in numerous developmental processes.
MT1-MMP, MT3-MMP, and MMP-13 contribute to bone development.
MT1-MMP-mediated collagenolysis facilitates numerous pathologies.
Collagenolysis generates bioactive peptides.
Acknowledgments
We gratefully acknowledge the National Institutes of Health (CA98799, AR063795, MH078948, and NHLBI contract 268201000036C), the Multiple Sclerosis National Research Institute, the Robert A. Welch Foundation, and the Lustgarten Foundation for support of our laboratory’s research on MMPs. We thank Dr. Anna Knapinska for creating Fig. 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc Natl Acad Sci USA. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker M, Floyd CD, Brown P, Gearing AJH. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev. 1999;99:2735–2776. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
- 3.Breuer E, Frant J, Reich R. Recent non-hydroxamate matrix metalloproteinase inhibitors. Expert Opin Ther Patents. 2005;15:253–269. [Google Scholar]
- 4.Mohan V, Talmi-Frank D, Arkadash V, Papo N, Sagi I. Matrix metalloproteinase protein inhibitors: Highlighting a new beginning for metalloproteinases in medicine. Metalloproteinases in Medicine. 2016;3:31–47. [Google Scholar]
- 5.Fox S. European roundup: No anticancer benefit in trials of marimastat. Gen Eng News. 2000;20(4):30. [Google Scholar]
- 6.Bloomston M, Zervos EE, Rosemurgy AS., II Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9:668–674. doi: 10.1007/BF02574483. [DOI] [PubMed] [Google Scholar]
- 7.Li NG, Shib ZH, Tang YP, Duan JA. Selective matrix metalloproteinase inhibitors for cancer. Curr Med Chem. 2009;16:3805–3827. doi: 10.2174/092986709789178037. [DOI] [PubMed] [Google Scholar]
- 8.Overall CM, Kleifeld O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 9.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 10.Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: Protective roles in cancer. J Cell Mol Med. 2011;15:1254–1265. doi: 10.1111/j.1582-4934.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharm Sci. 2013;34:233–242. doi: 10.1016/j.tips.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 13.Butler GS, Overall CM. Proteomic validation of protease drug targets: Pharmacoproteomics of matrix metalloproteinase inhibitor drugs using isotope-coded affinity tag labelling and tandem mass spectrometry. Curr Pharm Design. 2007;13:263–270. doi: 10.2174/138161207779313524. [DOI] [PubMed] [Google Scholar]
- 14.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 15.Butler GS, Overall CM. Updated biological roles for matrix metalloproteinases and new “intracellular” substrates revealed by degradomics. Biochemistry. 2009;48:10830–10845. doi: 10.1021/bi901656f. [DOI] [PubMed] [Google Scholar]
- 16.Cauwe B, Opdenakker G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2010;45:351–423. doi: 10.3109/10409238.2010.501783. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Fortelny N, Cox JH, Kappelhoff R, Starr AE, Lange PF, Pavlidis P, Overall CM. Network analyses reveal pervasive functional regulation between proteases in the human protease web. PLoS Biol. 2014;12:e1001869. doi: 10.1371/journal.pbio.1001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertini I, Fragai F, Luchinat C, Melikian M, Toccafondi M, Lauer JL, Fields GB. Structural Basis for Matrix Metalloproteinase 1 Catalyzed Collagenolysis. J Am Chem Soc. 2012;134:2100–2110. doi: 10.1021/ja208338j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerofolini L, Fields GB, Fragai M, Geraldes CFGC, Luchinat C, Parigi G, Ravera E, Svergun DI, Teixeira JMC. Examination of matrix metalloproteinase-1 (MMP-1) in solution: A preference for the pre-collagenolysis state. J Biol Chem. 2013;288:30659–30671. doi: 10.1074/jbc.M113.477240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Marcink T, Gari RRS, Marsh BP, King GM, Stawikowska R, Fields GB, Van Doren SR. Transient Collagen Triple Helix Binding to a Key Metalloproteinase in Invasion and Development. Structure. 2015;23:257–269. doi: 10.1016/j.str.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myllyharju J, Kivirikko KI. Collagen and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DMA, Iwatsubo T. CLAC: A novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 2002;21:1524–1534. doi: 10.1093/emboj/21.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bächinger HP, Mizuno K, Vranka JA, Boudko SP. Collagen Formation and Structure. In: Mander L, Liu H-W, editors. Comprehensive Natural Products II. Chemistry and Biology. Elsevier Science; 2010. pp. 469–530. [Google Scholar]
- 25.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 Å resolution. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 26.Holmgren SK, Taylor KM, Bretscher LE, Raines RT. Code for collagen’s stability deciphered. Nature. 1998;392:666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]
- 27.Saffarian S, Collier IE, Marmer BL, Elson EL, Goldberg G. Interstitial collagenase is a Brownian rachet driven by proteolysis of collagen. Science. 2004;306:108–111. doi: 10.1126/science.1099179. [DOI] [PubMed] [Google Scholar]
- 28.Cole WG. Collagen genes: Mutations affecting collagen structure and expression. Prog Nucl Acid Res Mol Biol. 1994;47:29–80. doi: 10.1016/s0079-6603(08)60249-4. [DOI] [PubMed] [Google Scholar]
- 29.Herr AB, Farndale RW. Structural insights into the interactions between platelet receptors and fibrillar collagen. J Biol Chem. 2009;284:19781–19785. doi: 10.1074/jbc.R109.013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linsenmayer TF. Collagen. In: Hay ED, editor. Cell Biology of Extracellular Matrix. Plenum Press; 1991. pp. 7–44. [Google Scholar]
- 32.Prockop DJ, Kivirikko KI. Collagens: Molecular biology, diseases, and potentials for therapy. Ann Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 33.Kivirikko KI, Myllylä R, Pihlajaniemi T. Hydroxylation of proline and lysine residues in collagens and other animal and plant proteins. In: Harding JJ, Crabbe MJC, editors. Post-translational Modifications of Proteins. CRC Press; 1992. pp. 1–51. [Google Scholar]
- 34.Heikkinen J, Risteli M, Wang C, Latvala J, Rossi M, Valtavaara M, Myllylä R. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J Biol Chem. 2000;275:36158–36163. doi: 10.1074/jbc.M006203200. [DOI] [PubMed] [Google Scholar]
- 35.Takaluoma K, Lantto J, Myllyharju J. Lysyl hydroxylase 2 is a specific telopeptide hydroxylase, while all three isoenzymes hydroxylate collagenous sequences. Matrix Biol. 2007;26:396–403. doi: 10.1016/j.matbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Schegg B, Hülsmeier AJ, Rutschmann C, Maag C, Hennet T. Core glycosylation of collagen is initiated by two β(1-O)galactosyltransferases. Mol Cell Biol. 2009;29:943–952. doi: 10.1128/MCB.02085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bächinger HP, Bruckner P, Timpl R, Engel J. The role of cis-trans isomerization of peptide bonds in the coil <=> triple helix conversion of collagen. Eur J Biochem. 1978;90:605–613. doi: 10.1111/j.1432-1033.1978.tb12641.x. [DOI] [PubMed] [Google Scholar]
- 38.Bächinger HP, Bruckner P, Timpl R, Prockop DJ, Engel J. Folding mechanism of the triple helix in type-III collagen and type-III pN-collagen. Eur J Biochem. 1980;106:619–632. doi: 10.1111/j.1432-1033.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 39.Baum J, Brodsky B. Folding of peptide models of collagen and misfolding in disease. Curr Opin Struct Biol. 1999;9:122–128. doi: 10.1016/s0959-440x(99)80016-5. [DOI] [PubMed] [Google Scholar]
- 40.Persikov AV, Brodsky B. Unstable molecules form stable tissues. Proc Natl Acad Sci USA. 2002;99:1101–1103. doi: 10.1073/pnas.042707899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makareeva E, Leikin S. Procollagen triple helix assembly: an unconventional chaperone-assisted folding paradigm. PLoS One. 2007;2:e1029. doi: 10.1371/journal.pone.0001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono T, Miyazaki T, Ishida Y, Uehata M, Nagata K. Direct in vitro and in vivo evidence for interaction between Hsp47 protein and collagen triple helix. J Biol Chem. 2012;287:6810–6818. doi: 10.1074/jbc.M111.280248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widmer C, Gebauer JM, Brunstein E, Rosenbaum S, Zaucke F, Drögemüller C, Leeb T, Baumann U. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc Natl Acad Sci USA. 2012;109:13243–13247. doi: 10.1073/pnas.1208072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tortorella MD, Malfait F, Barve RA, Shieh H-S, Malfait A-M. A review of the ADAMTS family, pharmaceutical targets of the future. Curr Pharm Design. 2009;15:2359–2374. doi: 10.2174/138161209788682433. [DOI] [PubMed] [Google Scholar]
- 45.Li S-W, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci USA. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sieron AL, Tretiakova A, Jameson BA, Segall ML, Lund-Katz S, Khan MT, Li S-w, Stöcker W. Structure and function of procollagen C-proteinase (mTolloid) domains determined by protease digestion, cicular dichroism, binding to procollagen type I, and computer modeling. Biochemistry. 2000;39:3231–3239. doi: 10.1021/bi992312o. [DOI] [PubMed] [Google Scholar]
- 47.Kronenberg D, Bruns BC, Moali C, Vadon-Le Goff S, Sterchi EE, Traupe H, Böhm M, Hulmes DJ, Stöcker W, Becker-Pauly C. Processing of procollagen III by meprins: new players in extracellular matrix assembly? J Invest Dermatol. 2010;130:2727–2735. doi: 10.1038/jid.2010.202. [DOI] [PubMed] [Google Scholar]
- 48.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruckner P, Prockop DJ. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981;110:360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- 50.Welgus HG, Burgeson RE, Wootton JAM, Minor RR, Fliszar C, Jeffrey JJ. Degradation of monomeric and fibrillar type III collagens by human skin collagenase. J Biol Chem. 1985;260:1052–1059. [PubMed] [Google Scholar]
- 51.Minond D, Lauer-Fields JL, Cudic M, Overall CM, Pei D, Brew K, Visse R, Nagase H, Fields GB. The roles of substrate thermal stability and P2 and P1’ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J Biol Chem. 2006;281:38302–38313. doi: 10.1074/jbc.M606004200. [DOI] [PubMed] [Google Scholar]
- 52.Williams KE, Olsen DR. Matrix metalloproteinase-1 cleavage site recognition and binding in full-length human type III collagen. Matrix Biol. 2009;28:373–379. doi: 10.1016/j.matbio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Knäuper V, López-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 54.Birkedal-Hansen H, Taylor RE, Bhown AS, Katz J, Lin H-Y, Wells BR. Cleavage of bovine skin type III collagen by proteolytic enzymes. J Biol Chem. 1985;260:16411–16417. [PubMed] [Google Scholar]
- 55.Welgus HG, Jeffrey JJ, Eisen AZ. Human skin fibroblast collagenase: Assessment of activation energy and deuterium isotope effect with collagenous substrates. J Biol Chem. 1981;256:9516–9521. [PubMed] [Google Scholar]
- 56.Fields GB. A model for interstitial collagen catabolism by mammalian collagenases. J Theor Biol. 1991;153:585–602. doi: 10.1016/s0022-5193(05)80157-2. [DOI] [PubMed] [Google Scholar]
- 57.Freije JMP, Diez-Itza T, Balbin M, Sanchez LM, Blasco R, Tolivia J, Lopez-Otin C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed] [Google Scholar]
- 58.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stolow MA, Bauzon DD, Li J, Sedgwick T, Liang VC, Sang QA, Shi YB. Identification and characterization of a novel collagenase in Xenopus laevis: Possible roles during frog development. Mol Biol Cell. 1996;7:1471–1483. doi: 10.1091/mbc.7.10.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang M, Kurkinen M. Cloning and characterization of a novel matrix metalloproteinase (MMP), CMMP, from chicken embryo fibroblasts. J Biol Chem. 1998;273:17893–17900. doi: 10.1074/jbc.273.28.17893. [DOI] [PubMed] [Google Scholar]
- 61.Ala-aho R, Kähäri VM. Collagenases in cancer. Biochimie. 2005;87:273–286. doi: 10.1016/j.biochi.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Fields GB. Interstitial collagen catabolism. J Biol Chem. 2013;288:8785–8793. doi: 10.1074/jbc.R113.451211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasty KA, Jeffrey JJ, Hibbs MS, Welgus HG. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987;262:10048–10052. [PubMed] [Google Scholar]
- 64.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 65.Konttinen YT, Ceponis A, Takagi M, Ainola M, Sorsa T, Sutinen M, Salo T, Ma J, Santavirta S, Seiki M. New collagenolytic enzymes/cascade identified at the pannus-hard tissue junction in rheumatoid arthritis: destruction from above. Matrix Biol. 1998;17:585–601. doi: 10.1016/s0945-053x(98)90110-x. [DOI] [PubMed] [Google Scholar]
- 66.Patterson ML, Atkinson SJ, Knäuper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503:158–162. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 67.Seltzer JL, Adams SA, Grant GA, Eisen AZ. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981;256:4662–4668. [PubMed] [Google Scholar]
- 68.Collier IE, Legant W, Marmer B, Lubman O, Saffarian S, Wakatsuki T, Elson E, Goldberg GI. Diffusion of MMPs on the Surface of Collagen Fibrils: The Mobile Cell Surface - Collagen Substratum Interface. PLoS One. 2011;6:e24029. doi: 10.1371/journal.pone.0024029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bigg HF, Rowan AD, Barker MD, Cawston TE. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J. 2007;274:1246–1255. doi: 10.1111/j.1742-4658.2007.05669.x. [DOI] [PubMed] [Google Scholar]
- 70.Niyibizi C, Chan R, Wu J-J, Eyre D. A 92 kDa gelatinase (MMP-9) cleavage site in native type V collagen. Biochem Biophys Res Commun. 1994;202:328–333. doi: 10.1006/bbrc.1994.1931. [DOI] [PubMed] [Google Scholar]
- 71.O’Farrell TJ, Pourmotabbed T. The fibronectin-like domain is required for the type V and XI collagenolytic activity of gelatinase B. Arch Biochem Biophys. 1998;354:24–30. doi: 10.1006/abbi.1998.0662. [DOI] [PubMed] [Google Scholar]
- 72.O’Farrell TJ, Pourmotabbed T. Identification of structural elements important for matrix metalloproteinase type V collagenolytic activity as revealed by chimeric enzymes. J Biol Chem. 2000;275:27964–27972. doi: 10.1074/jbc.M003936200. [DOI] [PubMed] [Google Scholar]
- 73.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type I matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 75.Lehti K, Lohi J, Juntunen MM, Pei D, Keski-Oja J. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J Biol Chem. 2002;277:8440–8448. doi: 10.1074/jbc.M109128200. [DOI] [PubMed] [Google Scholar]
- 76.Itoh Y, Ito N, Nagase H, Evans RD, Bird SA, Seiki M. Cell surface collagenolysis requires homodimerization of the membrane-bound collagenase MT1-MMP. Mol Biol Cell. 2006;17:5390–5399. doi: 10.1091/mbc.E06-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X-Y, Ota I, Yana I, Sabeh F, Weiss SJ. Molecular dissection of the structural machinery underlying the tissue-invasive activity of membrane type-1 matrix metalloproteinase. Mol Biol Cell. 2008;19:3221–3233. doi: 10.1091/mbc.E08-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabeh F, Li X-Y, Saunders TL, Rowe RG, Weiss SJ. Secreted versus membrane-anchored collagenases: Relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009;284:23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cerofolini L, Amar S, Lauer JL, Martelli T, Fragai M, Luchinat C, Fields GB. Bilayer Membrane Modulation of Membrane Type 1 Matrix Metalloproteinase (MT1-MMP) Structure and Proteolytic Activity. Nat Sci Rep. 2016;6:29511. doi: 10.1038/srep29511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tam EM, Wu YI, Butler GS, Stack MS, Overall CM. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. J Biol Chem. 2002;277:39005–39014. doi: 10.1074/jbc.M206874200. [DOI] [PubMed] [Google Scholar]
- 81.Tochowicz A, Goettig P, Evans R, Visse R, Shitomi Y, Palmisano R, Ito N, Richter K, Maskos K, Franke D, Svergun D, Nagase H, Bode W, Itoh Y. The dimer interface of the membrane type 1 matrix metalloproteinase hemopexin domain: crystal structure and biological functions. J Biol Chem. 2011;286:7587–7600. doi: 10.1074/jbc.M110.178434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimada T, Nakamura H, Ohuchi E, Fujii Y, Murakami Y, Sato H, Seiki M, Okada Y. Characterization of a truncated recombinant form of human membrane type 3 matrix metalloproteinase. Eur J Biochem. 1999;262:907–914. doi: 10.1046/j.1432-1327.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 83.Jiang A, Pei D. Distinct roles of catalytic and pexin-like domains in membrane-type matrix metalloproteinase (MMP)-mediated pro-MMP-2 activation and collagenolysis. J Biol Chem. 2003;278:38765–38771. doi: 10.1074/jbc.M306618200. [DOI] [PubMed] [Google Scholar]
- 84.Tatti O, Arjama M, Ranki A, Weiss SJ, Keski-Oja J, Lehti K. Membrane-type-3 matrix metalloproteinase (MT3-MMP) functions as a matrix composition-dependent effector of melanoma cell invasion. PLoS One. 2011;6:e28325. doi: 10.1371/journal.pone.0028325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi J, Son MY, Yamada S, Szabova L, Kahan S, Chrysovergis K, Wolf L, Surmak A, Holmbeck K. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev Biol. 2008;313:196–209. doi: 10.1016/j.ydbio.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang T, Yi J, Guo A, Wang X, Overall CM, Jiang W, Elde R, Borregaard N, Pei D. Subcellular distribution and cytokine- and chemokine-regulated secretion of leukolysin/MT6-MMP/MMP-25 in neutrophils. J Biol Chem. 2001;276:21960–21968. doi: 10.1074/jbc.M007997200. [DOI] [PubMed] [Google Scholar]
- 87.Radichev IA, Remacle AG, Shiryaev SA, Purves AN, Johnson SL, Pellecchia M, Strongin AY. Biochemical characterization of the cellular glycosylphosphatidylinositol-linked membrane type-6 matrix metalloproteinase. J Biol Chem. 2010;285:16076–16086. doi: 10.1074/jbc.M110.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Starr AE, Bellac CL, Dufour A, Goebeler V, Overall CM. Biochemical characterization and N-terminomics analysis of leukolysin, the membrane-type 6 matrix metalloprotease (MMP25) J Biol Chem. 2012;287:13382–13395. doi: 10.1074/jbc.M111.314179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amar S, Fields GB. Production and characterization of matrix metalloproteinases implicated in multiple sclerosis. In: Kokotos G, Constantinou-Kokotou V, Matsoukas J, editors. Peptides 2012 (Proceedings of the Thirty-Second European Peptide Symposium) European Peptide Society; 2012. pp. 102–103. [Google Scholar]
- 90.Taddese S, Jung MC, Ihling C, Heinz A, Neubert RHH, Schmelzer CEH. MMP-12 catalytic domain recognizes and cleaves at multiple sites in human skin collagen type I and type III. Biochim Biophys Acta. 2010;1804:731–739. doi: 10.1016/j.bbapap.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 91.Fu JY, Lyga A, Shi H, Blue ML, Dixon B, Chen D. Cloning, expression, purification, and characterization of rat MMP-12. Protein Expr Purif. 2001;21:268–274. doi: 10.1006/prep.2000.1376. [DOI] [PubMed] [Google Scholar]
- 92.Allan JA, Hembry RM, Angal S, Reynolds JJ, Murphy G. Binding of latent and high Mr forms of stromelysin to collagen is mediated by the C-terminal domain. J Cell Sci. 1991;99:789–795. doi: 10.1242/jcs.99.4.789. [DOI] [PubMed] [Google Scholar]
- 93.Murphy G, Allan JA, Willenbrock F, Cockett MI, O’Connell JP, Docherty AJP. The role of the C-terminal domain in collagenase and stromelysin specificity. J Biol Chem. 1992;267:9612–9618. [PubMed] [Google Scholar]
- 94.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23:3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenblum G, Van den Steen PE, Cohen SR, Grossmann JG, Frenkel J, Sertchook R, Slack N, Strange RW, Opdenakker G, Sagi I. Insights into the structure and domain flexibility of full-length pro-matrix metalloproteinase-9/gelatinase B. Structure. 2007;15:1227–1236. doi: 10.1016/j.str.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 96.Bertini I, Calderone V, Fragai M, Jaiswal R, Luchinat C, Melikian M, Mylonas E, Svergun DI. Evidence of reciprocal reorientation of the catalytic and hemopexin-like domains of full-length MMP-12. J Am Chem Soc. 2008;130:7011–7021. doi: 10.1021/ja710491y. [DOI] [PubMed] [Google Scholar]
- 97.Bertini I, Fragai M, Luchinat C, Melikian M, Mylonas E, Sarti N, Svergun DI. Interdomain flexibility in full-length matrix metalloproteinase-1 (MMP-1) J Biol Chem. 2009;284:12821–12828. doi: 10.1074/jbc.M809627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenblum G, Van den Steen PE, Cohen SR, Bitler A, Brand DD, Opdenakker G, Sagi I. Direct visualization of protease action on collagen triple helical structure. PLoS One. 2010;5:e11043. doi: 10.1371/journal.pone.0011043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsukada H, Pourmotabbed T. Unexpected crucial role of residue 272 in substrate specificity of fibroblast collagenase. J Biol Chem. 2002;277:27378–27384. doi: 10.1074/jbc.M201367200. [DOI] [PubMed] [Google Scholar]
- 100.Fasciglione GF, Gioia M, Tsukada H, Liang J, Iundusi R, Tarantino U, Coletta M, Pourmotabbed T, Marini S. The collagenolytic action of MMP-1 is regulated by the interaction between the catalytic domain and the hinge region. J Biol Inorg Chem. 2012;17:663–672. doi: 10.1007/s00775-012-0886-z. [DOI] [PubMed] [Google Scholar]
- 101.Shuo T, Koshikawa N, Hoshino D, Minegishi T, Ao-Kondo H, Oyama M, Sekiya S, Iwamoto S, Tanaka K, Seiki M. Detection of the heterogeneous O-glycosylation profile of MT1-MMP expressed in cancer cells by a simple MALDI-MS method. PLoS One. 2012;7:e43751. doi: 10.1371/journal.pone.0043751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirose T, Patterson C, Pourmotabbed T, Mainardi CL, Hasty KA. Structure-function relationship of human neutrophil collagenase: Identification of regions responsible for substrate specificity and general proteinase activity. Proc Natl Acad Sci USA. 1993;90:2569–2573. doi: 10.1073/pnas.90.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chung L, Shimokawa K, Dinakarpandian D, Grams F, Fields GB, Nagase H. Identification of the RWTNNFREY(183–191) region as a critical segment of matrix metalloproteinase 1 for the expression of collagenolytic activity. J Biol Chem. 2000;275:29610–29617. doi: 10.1074/jbc.M004039200. [DOI] [PubMed] [Google Scholar]
- 104.Brömme D, Okamoto K, Wang BB, Biroc S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. J Biol Chem. 1996;271:2126–2132. doi: 10.1074/jbc.271.4.2126. [DOI] [PubMed] [Google Scholar]
- 105.Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaisse J-M. The collagenolytic acitivity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273:32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 106.Aguda AH, Panwar P, Du X, Nguyen NT, Brayer GD, Brömme D. Structural basis of collagen fiber degradation by cathepsin K. Proc Natl Acad Sci USA. 2014;111:17474–17479. doi: 10.1073/pnas.1414126111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kafienah W, Brömme D, Buttle DJ, Croucher LJ, Hollander AP. Human cathepsin K cleaves native type I and II collagens at the N-terminal end of the triple helix. Biochem J. 1998;331:727–732. doi: 10.1042/bj3310727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 109.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 110.Ellerbroek SM, Stack MS. Membrane associated matrix metalloproteinases in metastasis. BioEssays. 1999;21:940–949. doi: 10.1002/(SICI)1521-1878(199911)21:11<940::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 111.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metallproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deryugina EI, Ratnikov BI, Postnova TI, Rozanov DV, Strongin AY. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J Biol Chem. 2002;277:9749–9756. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- 113.Hwang IK, Park SM, Kim SY, Lee S-T. A proteomic approach to identify substrates of matrix metalloproteinase-14 in human plasma. Biochim Biophys Acta. 2004;1702:79–87. doi: 10.1016/j.bbapap.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 114.Tam EM, Morrison CJ, Wu YI, Stack MS, Overall CM. Membrane protease proteomics: Isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc Natl Acad Sci USA. 2004;101:6917–6922. doi: 10.1073/pnas.0305862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aoki T, Sato D, Li Y, Takino T, Miyamori H, Sato H. Cleavage of apolipoprotein E by membrane-type matrix metalloproteinase-1 abrogates suppression of cell proliferation. J Biochem. 2005;137:95–99. doi: 10.1093/jb/mvi009. [DOI] [PubMed] [Google Scholar]
- 116.Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J Biol Chem. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- 117.Egawa N, Koshikawa N, Tomari T, Nabeshima K, Isobe T, Seiki M. Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J Biol Chem. 2006;281:37576–37585. doi: 10.1074/jbc.M606993200. [DOI] [PubMed] [Google Scholar]
- 118.Takino T, Watanabe Y, Matsui M, Miyamori H, Kudo T, Seiki M, Sato H. Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp Cell Res. 2006;312:1381–1389. doi: 10.1016/j.yexcr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 119.Itoh Y. MT1-MMP: A key regulator of cell migration in tissue. IUBMB Life. 2006;58:589–596. doi: 10.1080/15216540600962818. [DOI] [PubMed] [Google Scholar]
- 120.Butler GS, Dean RA, Tam EM, Overall CM. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–4914. doi: 10.1128/MCB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim Biophys Acta. 2010;1803:133–141. doi: 10.1016/j.bbamcr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu G, Atteridge CL, Wang X, Lundgren AD, Wu JD. The membrane type matrix metalloproteinase MMP14 mediates constitutive shedding of MHC class I chain-related molecule A independent of A disintegrin and metalloproteinases. J Immunol. 2010;184:3346–3350. doi: 10.4049/jimmunol.0903789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liao MC, Van Nostrand WE. Degradation of soluble and fibrillar amyloid beta-protein by matrix metalloproteinase (MT1-MMP) in vitro. Biochemistry. 2010;49:1127–1136. doi: 10.1021/bi901994d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pahwa S, Stawikowski MJ, Fields GB. Monitoring and inhibiting MT1-MMP during cancer initiation and progression. Cancers. 2014;6:416–435. doi: 10.3390/cancers6010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thakur V, Bedogni B. The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis. Pharmacol Res. 2016;111:17–22. doi: 10.1016/j.phrs.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 126.Golubkov VS, Strongin AY. Proteolysis-driven oncogenesis. Cell Cycle. 2007;6:147–150. doi: 10.4161/cc.6.2.3706. [DOI] [PubMed] [Google Scholar]
- 127.Wali N, Hosokawa K, Malik S, Saito H, Miyaguchi K, Imajoh-Ohmi S, Miki Y, Nakanishi A. Centrosomal BRCA2 is a target protein of membrane type-1 matrix metalloproteinase (MT1-MMP) Biochem Biophys Res Commun. 2014;443:1148–1154. doi: 10.1016/j.bbrc.2013.12.103. [DOI] [PubMed] [Google Scholar]
- 128.Dzamba BJ, Wu H, Jaenisch R, Peters DM. Fibronectin binding site in type I collagen regulates fibronectin fibril formation. J Cell Biol. 1993;121:1165–1172. doi: 10.1083/jcb.121.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis GE. Affinity of integrins for damaged extracellular matrix: alphavbeta3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 130.Berman A, Morozevich G, Karmansky I, Gleiberman A, Bychkova V. Adhesion of mouse hepatocytes to type I collagen: Role of supramolecular forms and effect of proteolytic degradation. Biochem Biophys Res Commun. 1993;194:351–357. doi: 10.1006/bbrc.1993.1827. [DOI] [PubMed] [Google Scholar]
- 131.Montgomery AMP, Reisfeld RA, Cheresh DA. Integrin alphavbeta3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Solomonov I, Zehorai E, Talmi-Frank D, Wolf SG, Shainskaya A, Zhuravlev A, Kartvelishvily E, Visse R, Levin Y, Kampf N, Jaitin DA, David E, Amit I, Nagase H, Sagi I. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proc Natl Acad Sci USA. 2016;113:10884–10889. doi: 10.1073/pnas.1519676113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee H, Overall CM, McCulloch CA, Sodek J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol Biol Cell. 2006;17:4812–4826. doi: 10.1091/mbc.E06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH, Holmbeck K. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol. 2007;27:6309–6322. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem. 2007;282:27037–27045. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- 136.Madsen DH, Ingvarsen S, Jürgensen HJ, Melander MC, Kjoller L, Moyer A, Honoré C, Madsen CA, Garred P, Burgdorf S, Bugge TH, Behrendt N, Engelholm LH. The non-phagocytic route of collagen uptake: A distinct degradation pathway. J Biol Chem. 2011;286:26996–27010. doi: 10.1074/jbc.M110.208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee H, Sodek KL, Hwang Q, Brown TJ, Ringuette M, Sodek J. Phagocytosis of collagen by fibroblasts and invasive cancer cells in mediated by MT1-MMP. Biochem Soc Trans. 2007;35:704–706. doi: 10.1042/BST0350704. [DOI] [PubMed] [Google Scholar]
- 138.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 139.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Evans BR, Mosig RA, Lobl M, Martignetti CR, Camacho C, Grum-Tokars V, Glucksman MJ, Martignetti JA. Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am J Hum Genet. 2012;91:572–576. doi: 10.1016/j.ajhg.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kennedy AM, Christie PT, Harding B, Pannett AAJ, Dearlove A, Whyte MP, Thakker RV. Matrix metalloproteinase 13 (MMP13) mutation causes spondyloepimetaphyseal dysplasia (SEMD), Missouri variant. Endocr Abstr. 2003;5:OC40. [Google Scholar]
- 143.Sulko J, Czarny-Ratajczak M, Wozniak A, Latos-Bielenska A, Kozlowski K. Novel amino acid substitution in the Y-position of collagen type II causes spondyloepimetaphyseal dysplasia congenita. Am J Med Genet A. 2005;137A:292–297. doi: 10.1002/ajmg.a.30881. [DOI] [PubMed] [Google Scholar]
- 144.Subramanian SR, Singam ER, Berinski M, Subramanian V, Wade RC. Identification of an Electrostatic Ruler Motif for Sequence-Specific Binding of Collagenase to Collagen. J Phys Chem B. 2016;120:8580–8589. doi: 10.1021/acs.jpcb.6b02573. [DOI] [PubMed] [Google Scholar]
- 145.Ito H, Rucker E, Steplewski A, McAdams E, Brittingham RJ, Alabyeva T, Fertala A. Guilty by association: some collagen II mutants alter the formation of ECM as a result of atypical interaction with fibronectin. J Mol Biol. 2005;352:382–395. doi: 10.1016/j.jmb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 146.Taylor SH, Yeung CY, Kalson NS, Lu Y, Zigrino P, Starborg T, Warwood S, Holmes DF, Canty-Laird EG, Mauch C, Kadler KE. Matrix metalloproteinase 14 is required for fibrous tissue expansion. eLIFE. 2015;4:e09345. doi: 10.7554/eLife.09345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu H, Byrne MH, Stacey A, Goldring MB, Birkhead JR, Jaenisch R, Krane SM. Generation of collagenase-resistant collagen by site-directed mutagenesis of murine pro a1(I) collagen gene. Proc Natl Acad Sci USA. 1990;87:5888–5892. doi: 10.1073/pnas.87.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hasty KA, Wu H, Byrne M, Goldring MB, Seyer JM, Jaenisch R, Krane SM, Mainardi CL. Susceptibility of type I collagen containing mutated α1(I) chains to cleavage by human neutrophil collagenase. Matrix. 1993;13:181–186. doi: 10.1016/s0934-8832(11)80001-6. [DOI] [PubMed] [Google Scholar]
- 149.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 150.Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Krane SM, Byrne MH, Lemaitre V, Henriet P, Jeffrey JJ, Witter JP, Liu X, Wu H, Jaenisch R, Eeckhout Y. Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem. 1996;271:28509–28515. doi: 10.1074/jbc.271.45.28509. [DOI] [PubMed] [Google Scholar]
- 152.Lemaitre V, Jungbluth A, Eeckhout Y. The recombinant catalytic domain of mouse collagenase-3 depolymerizes type I collagen by cleaving its aminotelopeptides. Biochem Biophys Res Commun. 1997;230:202–205. doi: 10.1006/bbrc.1996.5924. [DOI] [PubMed] [Google Scholar]
- 153.Leikina E, Mertts MV, Kuznetsova N, Leikin S. Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci USA. 2002;99:1314–1318. doi: 10.1073/pnas.032307099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao W, Byrne MH, Wang Y, Krane SM. Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen. J Clin Invest. 2000;106:941–949. doi: 10.1172/JCI10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Montgomery AM, Mueller BM, Reisfeld RA, Taylor SM, DeClerck YA. Effect of tissue inhibitor of the matrix metalloproteinases-2 expression on the growth and spontaneous metastasis of a human melanoma cell line. Cancer Res. 1994;54:5467–5473. [PubMed] [Google Scholar]
- 156.Zhao W, Byrne MH, Boyce BF, Krane SM. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest. 1999;103:517–524. doi: 10.1172/JCI5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, Iredale J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47–49. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 158.Beare AH, O’Kane S, Krane SM, Ferguson MW. Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol. 2003;120:153–163. doi: 10.1046/j.1523-1747.2003.12019.x. [DOI] [PubMed] [Google Scholar]
- 159.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44–46:113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 161.Zigrino P, Brinckmann J, Niehoff A, Lu Y, Giebeler N, Eckes B, Kadler KE, Mauch C. Fibroblast-Derived MMP-14 Regulates Collagen Homeostasis in Adult Skin. J Invest Dermatol. 2016;136:1575–1583. doi: 10.1016/j.jid.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gelb BD, Shi G-P, Chapman HA, Desnick RJ. Pycnodysostosis, a Lysosomal Disease Caused by Cathepsin K Deficiency. Science. 1996;273:1236–1238. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- 163.Everts V, Aronson DC, Beertsen W. Phagocytosis of bone collagen by osteoclasts in two cases of pycnodysostosis. Calcif Tissue Int. 1985;37:25–31. doi: 10.1007/BF02557674. [DOI] [PubMed] [Google Scholar]
- 164.Everts V, Hou WS, Rialland X, Tigchelaar W, Saftig P, Brömme D, Gelb BD, Beertsen W. Cathepsin K deficiency in pycnodysostosis results in accumulation of non-digested phagocytosed collagen in fibroblasts. Calcif Tissue Int. 2003;73:380–386. doi: 10.1007/s00223-002-2092-4. [DOI] [PubMed] [Google Scholar]
- 165.Rünger TM, Adami S, Benhamou C-L, Czerwinski E, Farrerons J, Kendler DL, Mindeholm L, Realdi G, Roux C, Smith V. Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol. 2012;66:e89–e96. doi: 10.1016/j.jaad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 166.Liotta LA. Cancer cell invasion and metastasis. Scientific American. 1992;266(2):54–63. doi: 10.1038/scientificamerican0292-54. [DOI] [PubMed] [Google Scholar]
- 167.Birkedal-Hansen H, Moore WGI, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 168.Nagase H. Matrix metalloproteinases. In: Hooper NM, editor. Zinc Metalloproteases In Health and Disease. Taylor & Francis; 1996. pp. 153–204. [Google Scholar]
- 169.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Nat Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 170.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 171.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: Biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 172.Labrosse KR, Liener IE, Hargrave PA. A sensitive assay for collagenolytic activity using tritiated collagen. Anal Biochem. 1976;70:218–223. doi: 10.1016/s0378-5173(83)90103-5. [DOI] [PubMed] [Google Scholar]
- 173.Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- 174.Gingras D, Beliveau R. Emerging concepts in the regulation of membrane-type 1 matrix metalloproteinase activity. Biochim Biophys Acta. 2010;1803:142–150. doi: 10.1016/j.bbamcr.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 175.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin Cell Dev Biol. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Szabova L, Chrysovergis K, Yamada SS, Holmbeck K. MT1-MMP is required for efficient tumor dissemination in experimental metastatic disease. Oncogene. 2007;27:3274–3281. doi: 10.1038/sj.onc.1210982. [DOI] [PubMed] [Google Scholar]
- 177.Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, Hu Y, Sampson NS, Zucker S, Cao J. Inhibition of matrix metalloproteinase-14 (MMP-14)-mediated cancer cell migration. J Biol Chem. 2011;286:33167–33177. doi: 10.1074/jbc.M111.256644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, Davis MJ, Davis GE. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J Cell Sci. 2009;122:4558–4569. doi: 10.1242/jcs.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 180.Koike T, Vernon RB, Hamner MA, Sadoun E, Reed MJ. MT1-MMP, but not secreted MMPs, influences the migration of human microvascular endothelial cells in 3-dimensional collagen gels. J Cell Biochem. 2002;86:748–758. doi: 10.1002/jcb.10257. [DOI] [PubMed] [Google Scholar]
- 181.Zhang W, Matrisian LM, Holmbeck K, Vick CC, Rosenthal EL. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: Three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Takino T, Tsuge H, Ozawa T, Sato H. MT1-MMP promotes cell growth and ERK activation through c-Src and paxillin in three-dimensional collagen matrix. Biochem Biophys Res Commun. 2010;396:1042–1047. doi: 10.1016/j.bbrc.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 185.Lauer JL, Fields GB. Collagen in Cancer. In: Bagley RG, editor. The Tumor Microenvironment. Springer Science + Business Media LLC; 2010. pp. 477–507. [Google Scholar]
- 186.Foley CJ, Luo C, O’Callaghan K, Hinds PW, Covic L, Kuliopulos A. Matrix metalloproteinase-1a promotes tumorigenesis and metastasis. J Biol Chem. 2012;287:24330–24338. doi: 10.1074/jbc.M112.356303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Makareeva E, Han S, Vera JC, Sackett DL, Holmbeck K, Phillips CL, Visse R, Nagase H, Leikin S. Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res. 2010;70:4366–4374. doi: 10.1158/0008-5472.CAN-09-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Narayanan AS, Meyers DF, Page RC, Welgus HG. Action of Mammalian Collagenases on Type-I Trimer Collagen. Collagen Rel Res. 1984;4:289–296. doi: 10.1016/s0174-173x(84)80036-9. [DOI] [PubMed] [Google Scholar]
- 189.Ng MR, Brugge JS. A stiff blow from the stroma: Collagen crosslinking drives tumor progression. Cancer Cell. 2009;16:455–457. doi: 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 190.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol. 2015;7:1120–1134. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krantz SB, Shields MA, Dangi-Garimella S, Cheon EC, Barron MR, Hwang RF, Rao MS, Grippo PJ, Bentrem DJ, Munshi HG. MT1-MMP cooperates with Kras(G12D) to promote pancreatic fibrosis through increased TGF-β signaling. Mol Cancer Res. 2011;9:1294–1304. doi: 10.1158/1541-7786.MCR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-b and promotes tumor invasion and angiogenesis. Genes Develop. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 193.Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaissé JM, Foged NT. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem. 2002;277:44061–44067. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 194.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nguyen HL, Kadam P, Helkin A, Cao K, Wu S, Samara GJ, Zhang Q, Zucker S, Cao J. MT1-MMP Activation of TGF-β Signaling Enables Intercellular Activation of an Epithelial-mesenchymal Transition Program in Cancer. Curr Cancer Drug Targets. 2016;16:618–630. doi: 10.2174/1568009616666160216125634. [DOI] [PubMed] [Google Scholar]
- 196.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 198.Knudson CB, Knudson W. Cartilage proteoglycans. Seminars Cell Dev Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 199.Pratta M, Yao W, Decicco C, Tortorella MD, Liu R-W, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 200.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Smith GN., Jr The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Front Biosci. 2006;11:3081–3095. doi: 10.2741/2034. [DOI] [PubMed] [Google Scholar]
- 203.Walling HW, Raggatt LJ, Irvine DW, Barmina OY, Toledano JE, Goldring MB, Hruska KA, Adkisson HD, Burdge RE, Gatt CJ, Jr, Harwood DA, Partridge NC. Impairment of the collagenase-3 endocytotic receptor system in cells from patients with osteoarthritis. Osteoarthritis Cartilage. 2003;11:854–863. doi: 10.1016/s1063-4584(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 204.Yamamoto K, Okano H, Miyagawa W, Visse R, Shitomi Y, Santamaria S, Dudhia J, Troeberg L, Strickland DK, Hirohata S, Nagase H. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016;56:57–73. doi: 10.1016/j.matbio.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Özler K, Aktaş E, Atay Ç, Yılmaz B, Arıkan M, Güngör Ş. Serum and knee synovial fluid matrix metalloproteinase-13 and tumor necrosis factor-alpha levels in patients with late-stage osteoarthritis. Acta Orthop Traumatol Turc. 2016;50:356–361. doi: 10.3944/AOTT.2015.15.0115. [DOI] [PubMed] [Google Scholar]
- 206.Baragi VM, Becher G, Bendele AM, Biesinger R, Bluhm H, Boer J, Deng H, Dodd R, Essers M, Feuerstein T, Gallagher BM, Jr, Gege C, Hochgürtel M, Hofmann M, Jaworski A, Jin L, Kiely A, Korniski B, Kroth H, Nix D, Nolte B, Piecha D, Powers T, Richter F, Schneider M, Steeneck C, Sucholeiki I, Taveras A, Timmermann A, Van Veldhuizen J, Weik J, Wu X, Xia B. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: Evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 2009;60:2008–2018. doi: 10.1002/art.24629. [DOI] [PubMed] [Google Scholar]
- 207.Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man C-F, Bornemeier DA, Banotai CA, Mueller WT, McConnell P, Yan C, Baragi V, Lesch C, Roark WH, Wilson M, Datta K, Guzman R, Han H-K, Dyer RD. Discovery and Characterization of a Novel Inhibitor of Matrix Metalloprotease-13 That Reduces Cartilage Damage in Vivo without Joint Fibroplasia Side Effects. J Biol Chem. 2007;282:27781–27791. doi: 10.1074/jbc.M703286200. [DOI] [PubMed] [Google Scholar]
- 208.Novinec M, Lenarčič B. Cathepsin K: a unique collagenolytic cysteine peptidase. Biol Chem. 2013;394:1163–1179. doi: 10.1515/hsz-2013-0134. [DOI] [PubMed] [Google Scholar]
- 209.Boonen S, Singer AJ. Osteoporosis management: impact of fracture type on cost and quality of life in patients at risk for fracture I. Curr Med Res Opin. 2008;24:1781–1788. doi: 10.1185/03007990802115796. [DOI] [PubMed] [Google Scholar]
- 210.Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci. 2012;9:825–832. doi: 10.7150/ijms.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.D’Amelio P, Sassi F. Osteoimmunology: from mice to humans. Bonekey Rep. 2016;5:802. doi: 10.1038/bonekey.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nakamura H, Sato G, Hirata A, Yamamoto T. Immunolocalization of matrix metalloproteinase-13 on bone surface under osteoclasts in rat tibia. Bone. 2004;34:48–56. doi: 10.1016/j.bone.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 213.McClelland P, Onyia JE, Miles RR, Tu Y, Liang J, Harvey AK, Chandrasekhar S, Hock JM, Bidwell JP. Intermittent administration of parathyroid hormone (1–34) stimulates matrix metalloproteinase-9 (MMP-9) expression in rat long bone. J Cell Biochem. 1998;70:391–401. [PubMed] [Google Scholar]
- 214.Li J, Liao EY, Dai RC, Wei QY, Luo XH. Effects of 17 beta-estradiol on the expression of interstitial collagenases-8 and -13 (MMP-8 and MMP-13) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in ovariectomized rat osteoblastic cells. J Mol Histol. 2004;35:723–731. doi: 10.1007/s10735-004-6206-3. [DOI] [PubMed] [Google Scholar]
- 215.Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, Sondergaard BC. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10:R63. doi: 10.1186/ar2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Panwar P, Lamour G, Mackenzie NCW, Yang H, Ko F, Li H, Brömme D. Changes in structural-mechanical properties and degradability of collagen during aging-associated modifications. J Biol Chem. 2015;290:23291–23306. doi: 10.1074/jbc.M115.644310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, Roberts JD, Fay WP, Birkedal-Hansen H, Holmbeck K, Sabeh F, Allen ED, Weiss SJ. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202:663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ruddy JM, Ikonomidis JS, Jones JA. Multidimensional Contribution of Matrix Metalloproteinases to Atherosclerotic Plaque Vulnerability: Multiple Mechanisms of Inhibition to Promote Stability. J Vasc Res. 2016;53:1–16. doi: 10.1159/000446703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.López B, González A, Díez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–1654. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 220.Zavadzkas JA, Mukherjee R, Rivers WT, Patel RK, Meyer EC, Black LE, McKinney RA, Oelsen JM, Stroud RE, Spinale FG. Direct Regulation of Membrane Type-1 Matrix Metalloproteinase Following Myocardial Infarction Causes Changes in Survival, Cardiac Function and Remodeling. Am J Physiol Heart Circ Physiol. 2011;301:H1656–H1666. doi: 10.1152/ajpheart.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Koenig GC, Rowe RG, Day SM, Sabeh F, Atkinson JJ, Cooke KR, Weiss SJ. MT1-MMP-Dependent Remodeling of Cardiac Extracellular Matrix Structure and Function Following Myocardial Infarction. Am J Pathol. 2012;180:1863–1878. doi: 10.1016/j.ajpath.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Brás LE. A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis. J Am Coll Cardiol. 2015;66:1364–1374. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Iyer RP, de Castro Brás LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields GB, Lindsey ML. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. J Mol Cell Cardiol. 2016;100:109–117. doi: 10.1016/j.yjmcc.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shiryaev SA, Cieplak P, Aleshin AE, Sun Q, Zhu W, Motamedchaboki K, Sloutsky A, Strongin AY. Matrix metalloproteinase proteolysis of the mycobacterial HSP65 protein as a potential source of immunogenic peptides in human tuberculosis. FEBS J. 2011;278:3277–3286. doi: 10.1111/j.1742-4658.2011.08244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sathyamoorthy T, Tezera LB, Walker NF, Brilha S, Saraiva L, Mauri FA, Wilkinson RJ, Friedland JS, Elkington PT. Membrane Type 1 Matrix Metalloproteinase Regulates Monocyte Migration and Collagen Destruction in Tuberculosis. J Immunol. 2015;195:882–891. doi: 10.4049/jimmunol.1403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, Robertson BD, D’Armiento J, Friedland JS. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Talmi-Frank D, Altboum Z, Solomonov I, Udi Y, Jaitin DA, Klepfish M, David E, Zhuravlev A, Keren-Shaul H, Winter DR, Gat-Viks I, Mandelboim M, Ziv T, Amit I, Sagi I. Extracellular Matrix Proteolysis by MT1-MMP Contributes to Influenza-Related Tissue Damage and Mortality. Cell Host Microbe. 2016;20:458–470. doi: 10.1016/j.chom.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 231.Solomonov I, Talmi-Frank D, Milstein Y, Addadi S, Aloshin A, Sagi I. Introduction of correlative light and airSEM™ microscopy imaging for tissue research under ambient conditions. Sci Rep. 2014;4:5987. doi: 10.1038/srep05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Udi Y, Grossman M, Solomonov I, Dym O, Rozenberg H, Moreno V, Cuniasse P, Dive V, Arroyo AG, Sagi I. Inhibition mechanism of membrane metalloprotease by an exosite-swiveling conformational antibody. Structure. 2015;23:104–115. doi: 10.1016/j.str.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 233.Olsen BR, Ninomiya Y. Collagens: Overview of the family. In: Kreis T, Vale R, editors. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins. 2. Oxford University Press; 1999. pp. 380–383. [Google Scholar]
- 234.Di Lullo GA, Sweeney SM, Körkkö J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 235.Beck K, Chan VC, Shenoy N, Kirkpatrick A, Ramshaw JAM, Brodsky B. Destabilization of osteogenesis imperfecta collagen-like model peptides correlates with the identity of the residue replacing glycine. Proc Natl Acad Sci USA. 2000;97:4273–4278. doi: 10.1073/pnas.070050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lauer JL, Bhowmick M, Tokmina-Roszyk D, Lin Y, Van Doren SR, Fields GB. The Role of Collagen Charge Clusters in the Regulation of Matrix Metalloproteinase Activity. J Biol Chem. 2014;289:1981–1992. doi: 10.1074/jbc.M113.513408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Leeming DJ, He Y, Veidal S, Nguyen Q, Larsen D, Koizumi M, Segovia-Silvestre T, Zhang C, Zheng Q, Sun S, Cao Y, Barkholt V, Hägglund P, Bay-Jensen A, Qvist P, Karsdal M. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011;16:616–628. doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- 238.Dragsbæk K, Neergaard JS, Hansen HB, Byrjalsen I, Alexandersen P, Kehlet SN, Bay-Jensen AC, Christiansen C, Karsdal MA. Matrix Metalloproteinase Mediated Type I Collagen Degradation - An Independent Risk Factor for Mortality in Women. EBioMedicine. 2015;2:723–729. doi: 10.1016/j.ebiom.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hu J, Van den Steen PE, Sang Q-XA, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 240.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.O’Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, Galin FS, Blalock JE. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol. 2009;217:51–54. doi: 10.1016/j.jneuroim.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Weathington NM, van Houwelinge AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 243.Welgus HG, Jeffrey JJ, Stricklin GP, Eisen AZ. The gelatinolytic activity of human skin fibroblast collagenase. J Biol Chem. 1982;257:11534–11539. [PubMed] [Google Scholar]
- 244.O’Farrell TJ, Guo R, Hasegawa H, Pourmotabbed T. Matrix Metalloproteinase-1 Takes Advantage of the Induced Fit Mechanism To Cleave the Triple-Helical Type I Collagen Molecule. Biochemistry. 2006;45:15411–15418. doi: 10.1021/bi060849d. [DOI] [PubMed] [Google Scholar]
- 245.Marini S, Fasciglione GF, de Sanctis G, D’Alessio S, Politi V, Coletta M. Cleavage of bovine collagen I by neutrophil collagenase MMP-8. Effect of pH on the catalytic properties as compared to synthetic substrates. J Biol Chem. 2000;275:18657–18663. doi: 10.1074/jbc.M000283200. [DOI] [PubMed] [Google Scholar]
- 246.Welgus HG, Jeffrey JJ, Eisen AZ. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981;256:9511–9515. [PubMed] [Google Scholar]
- 247.Yu Z, Visse R, Inouye M, Nagase H, Brodsky B. Defining Requirements for Collagenase Cleavage in Collagen Type III Using a Bacterial Collagen System. J Biol Chem. 2012;287:22988–22997. doi: 10.1074/jbc.M112.348979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.