Introduction
While direct-acting antivirals (DAAs) for HCV have substantially improved sustained virologic response (SVR) rates, the risk of developing hepatocellular carcinoma (HCC) persists over a decade.(1) HCC incidence will keep rising over the next two decades even with DAAs unless HCV diagnosis and treatment uptake rates are drastically improved.(1) HCC risk prediction with clinical risk factors such as older age, liver fibrosis, and metabolic disorders is not accurate enough, and there is still no clinically established strategy of HCC screening in the post-SVR population.(1) Applying biannual HCC screening recommended in the guideline to the whole post-SVR population will not be a practically feasible option given the already overburdened HCC screening capability as evidenced by the low utilization rate below 15%.(1) Post-SVR HCC risk prediction by means of a transcriptome signature-based HCC risk score may address the challenge as suggested in our recent study.(2)
Case Presentation
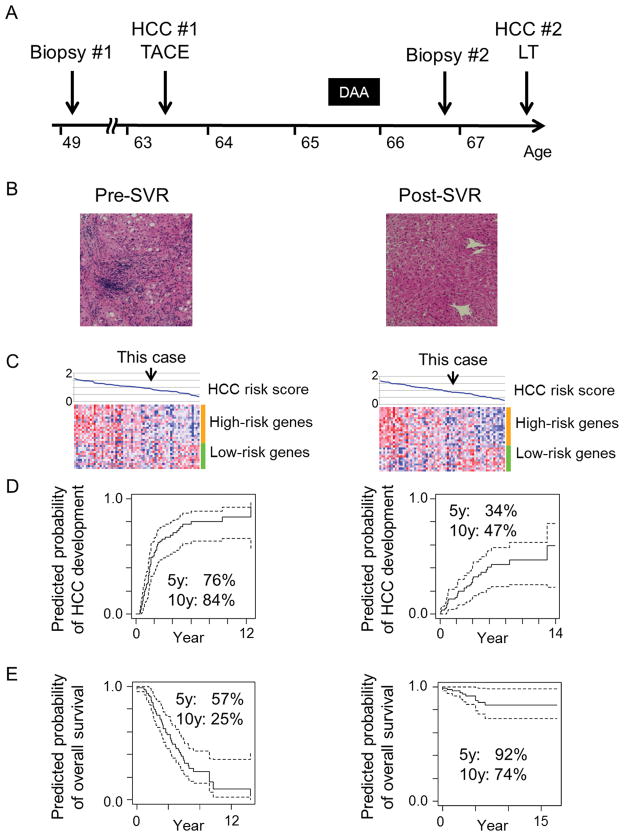
A 63-year-old Caucasian man with chronic HCV genotype 1 cirrhosis (MELD score: 8), type 2 diabetes, and hypertension developed three small HCC tumors (7-mm and 11-mm tumors in segment 7 and 19-mm tumor in segment 8). He underwent transcatheter arterial chemoembolization (TACE), resulting in a complete radiologic response, and was subsequently listed for liver transplantation (LT) based on MELD exception for HCC (Fig. 1A). Serum alpha-fetoprotein (AFP) level was decreased from 9.8 ng/mL at baseline to 4 ng/mL. He was then regularly followed up with contrast-enhanced CT or MRI and AFP every 3 months. While on the transplant waiting list, he underwent HCV treatment with ledipasvir 90 mg and sofosbuvir 400 mg daily for 24 weeks, and achieved an SVR at the age of 66.
Figure 1.
(A) Timeline of clinical events. (B) H & E staining of liver biopsy tissue pre- and post-SVR. (C) Expression pattern of the HCC risk gene signature in the reference series of pre- (n=63) and post- (n=64) SVR patients. Samples (columns) were ordered according to the HCC risk score calculated from the gene signature as a relative measure within the normalized range between 0 and 2 in the respective reference patient series as previously described.(2) Red and blue colors in the heatmap indicate high and low relative gene expression after sample-wise standardization by subtracting mean and dividing by standard deviation, respectively. (D) Predicted probability of HCC development using a Cox regression model based on the HCC risk score. Dashed lines indicate 95% confidence interval of the predicted probability. (E) Predicted probability of overall survival.
The patient had been hesitant to receive LT due to anticipated complications and well-preserved liver function without any sign of HCC for 3 years, and sought to assess his HCC recurrence risk and future need for regular HCC screening and LT by our HCC risk gene signature.(2) At the time, there was no evidence of HCC, and AFP level was consistently below 10 ng/mL since the TACE. Liver biopsy tissue was obtained, and compared with pre-SVR liver tissue. His transaminase levels improved and synthetic function remained stable after SVR (Table 1). H&E staining revealed almost complete regression of liver fibrosis and inflammation (Fig. 1B). The HCC risk signature in an FDA-approved platform was analyzed as previously described.(2, 3) The profiles were projected onto two reference series of HCC patients with active HCV infection (n=63) and after SVR (n=64) treated with curative surgery and followed for up to 15 years (Fig. 1C).(2, 3) His predicted long-term HCC risk was improved after SVR, but still remained substantial (Fig. 1D, E). Therefore, HCC screening was continued at 3-month intervals.
Table 1.
Change of clinical and biochemical variables after achieving an SVR.
| Variable | Pre-SVR | Post-SVR |
|---|---|---|
| White blood cell (/mm3) | 3,500 | 6,900 |
| Hemoglobin (g/dL) | 16.1 | 15.2 |
| Platelet count (× 103/mm3) | 125 | 161 |
| Total bilirubin (mg/dL) | 0.5 | 0.5 |
| Albumin (g/dL) | 4.1 | 4.5 |
| AST (IU/L) | 61 | 22 |
| ALT (IU/L) | 69 | 16 |
| Prothrombin time (INR) | 1.1 | 1.1 |
| AFP (ng/ml) | 10.6 | 4 |
| MELD score | 8 | 9 |
AST: aspartate transaminase, ALT: alanine transaminase, INR: international normalized ratio, AFP: alpha-fetoprotein, MELD: model for end-stage liver disease.
Despite the significant improvement of liver histology and biochemistry after SVR, a 22 mm lesion in segment 7 with typical contrast enhancement pattern for HCC was determined by MRI (OPTN class 5B) at the age of 67. AFP level remained low. This early stage HCC tumor (AJCC stage I) was successfully treated by LT. The explanted liver demonstrated this tumor to be a poorly differentiated HCC but with no sign of microvascular invasion, i.e., local disease progression, supporting that the tumor was detected at earlier stage by the close follow-up as recommended from the gene signature assessment.
Discussion
HCV will remain a major cause of HCC in the coming decades, and HCC incidence may be higher in DAA-based SVR compared to interferon-based SVR.(1) Given the anticipated exponential growth of a post DAA-based SVR patient population, identification of a subset of SVR patients at risk of HCC is an urgent unmet need. Allocating HCC screening resources to high-risk patients will enable more cost-effective and practically feasible management of the patients. This case report suggests that molecular risk biomarker-based cancer risk prediction is a viable option, which is readily applicable for HCC risk prediction after surgical resection without changing clinical practice to obtain the sample, and will also serve as the basis to explore serum surrogate biomarkers for non-surgery post-SVR patients.
Acknowledgments
Grant support:
NIH/NIDDK DK099558, European Union ERC-2014-AdG-671231HEPCIR, Irma T. Hirschl Trust, US Department of Defense (W81XWH-16-1-0363) to Y.H.
NIH Loan Repayment Program to G.Y.I.
We thank Dr. Laurence Bennett for providing invaluable clinical information and critical reading of the manuscript.
References
- 1.Baumert TF, Jühling F, Ono A, Hoshida Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med. 2017 doi: 10.1186/s12916-017-0815-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS, et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30:879–890. doi: 10.1016/j.ccell.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King LY, Canasto-Chibuque C, Johnson KB, Yip S, Chen X, Kojima K, et al. A genomic and clinical prognostic index for hepatitis C-related early-stage cirrhosis that predicts clinical deterioration. Gut. 2015;64:1296–1302. doi: 10.1136/gutjnl-2014-307862. [DOI] [PMC free article] [PubMed] [Google Scholar]