Abstract
Background & Aims
Statins have been variably shown to decrease risk and complications of chronic liver diseases (CLDs). We performed a systematic review and meta-analysis to evaluate the association between statins and risk of cirrhosis and related complications in patients with CLDs.
Methods
Through a systematic literature search up to March 2017, we identified 13 studies (3 randomized trials, 10 cohort studies) in adults with CLDs, reporting the association between statin use and risk of development of cirrhosis, decompensated cirrhosis, improvements in portal hypertension, or mortality. Pooled relative risk (RR) estimates with 95% CIs were calculated using random effects model. GRADE criteria were used to assess quality of evidence.
Results
Among 121,058 patients with CLDs (84.5% with hepatitis C), 46% were exposed to statins. In patients with cirrhosis, statin use was associated with 46% lower risk of hepatic decompensation (4 studies; RR, 0.54; 95% CI, 0.46–0.62; I2=0%; moderate quality evidence), and 46% lower mortality (5 studies; RR, 0.54; 95% CI, 0.47–0.61; I2=10%; moderate quality evidence). In patients with CLD without cirrhosis, statin use was associated with a non-significant (58% lower) risk of development of cirrhosis or fibrosis progression (5 studies; RR, 0.42; 95% CI, 0.16–1.11; I2=99%; very low quality evidence). In 3 randomized controlled trials, statin use was associated with 27% lower risk of variceal bleeding or progression of portal hypertension (hazard ratio, 0.73; 95% CI, 0.59–0.91; I2=0%; moderate quality evidence).
Conclusion
Based on a systematic review and meta-analysis, statin use is probably associated with lower risk of hepatic decompensation and mortality, and might reduce portal hypertension, in patients with CLDs. Prospective observational studies and randomized controlled trials are needed to confirm this observation.
Keywords: cholesterol-lowering drug, RCT, liver fibrosis, cirrhosis, meta-analysis
INTRODUCTION
Chronic liver diseases (CLDs) are a significant cause of morbidity and mortality worldwide. According to the Centers for Disease Control, in 2013, CLDs and cirrhosis were among the top 15 causes of death in the United States, and the mortality rate continues to increase each year leading to over 35,000 deaths in 20131. Currently, there are no medications to prevent or reverse hepatic decompensation, though treatment of underlying etiology may slow progression of fibrosis.
Statins are one class of medications being studied to determine their effect on progression and decompensation of CLDs. Statins are lipid-lowering agents, which act by inhibiting the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase, leading to inhibition of cholesterogenesis and decreased serum cholesterol levels2–10. Besides their lipid-lowering effects, statins also decrease oxidative stress and inflammation by decreasing activation of inflammatory cells11–14, and improve endothelial function by increasing synthesis of nitric oxide, restoring the function of endothelial cells, and increasing the number of endothelial progenitor cells15–23. In vitro and preclinical studies have also suggested a favorable impact of statins on hepatic inflammation, fibrosis, and hepatic vascular tone24–29. Epidemiological studies have observed a protective association between statin use and hepatocellular cancer30–32. Recent studies have also suggested an association between statin use and risk of fibrosis progression and hepatic decompensation in patients with CLDs, although the effects have been variable33–43.
Hence, to better understand the association between statin use and outcomes in patients with CLDs, we performed a systematic review with meta-analysis of randomized controlled trials (RCTs) and cohort studies to evaluate the association between statin exposure and (a) risk of fibrosis progression and development of cirrhosis (in patients with non-cirrhotic CLDs), (b) risk of hepatic decompensation and mortality (in all patients with cirrhotic or non-cirrhotic CLDs), and (c) risk of progression of portal hypertension (in patients with cirrhosis). We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria to appraise quality of evidence.
METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines44, using an a priori established protocol.
Selection Criteria
We included RCTs or cohort studies that met the following inclusion criteria: (1) conducted in adults with CLDs due to any cause, and reported baseline degree of CLDs – fibrosis without cirrhosis, compensated cirrhosis, or decompensated cirrhosis, (2) evaluated and clearly defined exposure to statins, (3) reported association between statin exposure and cirrhosis-related outcomes in patients, (4) with an acceptable measure of association such as hazard ratios (HRs), relative risk (RR) or odds ratios (ORs), or provided data for their calculations. Inclusion was not restricted by study size, language, or publication type. We excluded: (a) case-control studies (high risk of recall bias) and cross-sectional studies (confounding by disease severity, and unable to infer exposure-outcome association), (b) studies focusing only on association between statin use and risk of HCC and (c) studies which provided insufficient information to calculate measure of association. When the same population was used in multiple publications, only data from the most recent all-inclusive report were included.
Search Strategy
To identify all relevant articles on the effect of statins in CLD, we first conducted a systematic literature search of MEDLINE (1946 through August 8, 2016), Embase (1988 through August 8, 2016), Cochrane Central Register of Controlled Trials (1991 through July, 2016), Cochrane Database and Systematic Reviews (2005 through August 3, 2016), Scopus (1823 through August 8, 2016), and Web of Science (1975 through August 8, 2016) databases, with no language restrictions; after peer review, an updated literature search of PubMed was performed on March 25, 2017 to identify any additional studies. The search strategy was designed and implemented by an experienced medical librarian with input from the study’s investigators, using controlled vocabulary supplemented with keywords, for studies on statin use in CLDs. The details of the search strategy are reported in the Supplementary Appendix. Two reviewers (RGK, SS) independently assessed the title and abstract of studies identified in the primary search for inclusion, and the full text of remaining articles were examined to determine whether they met inclusion criteria. In addition, conference abstracts from major gastroenterology and hepatology conferences (American Association for the Study of Liver Diseases, European Association for the Study of the Liver, and Digestive Disease Week) from 2012–2016, as well as bibliography of the selected articles and review articles on the topic were manually searched for additional studies.
Data Abstraction
Two reviewers (RGK, SS) independently abstracted data onto a standardized form. Data collected from each study included the following: time period of study/year of publication, country of the population studied, study design, inclusion and exclusion criteria, type of medication, dose and duration of statin use (if reported), primary outcome reported, secondary outcomes, information source for exposure measurement, total number of participants in each group (statin exposure vs no exposure), HRs, RRs or ORs, and 95% confidence intervals (CI) with and without adjustment for confounding factors. We extracted data on the following confounding risk factors for progression of fibrosis and/or decompensation of cirrhosis: age, sex, etiology of chronic liver disease, Child-Pugh score, diabetes mellitus, coronary artery disease, alcoholic liver disease/alcohol use, LDL, use of ACE-Is and aspirin, and use of nonstatin lipid-lowering medications.
Risk of bias was assessed using the Cochrane risk of bias tool for RCTs45, and using the Newcastle Ottawa Scale for cohort studies46.
Outcomes Assessed
Our primary outcomes of interest were the association between statin use and (a) risk of fibrosis progression and/or development of cirrhosis (in patients with baseline non-cirrhotic CLDs), (b) risk of developing decompensated cirrhosis (in patients with baseline compensated cirrhosis), (c) all-cause mortality (in all patients with CLDs) and (d) progression of portal hypertension (variceal bleeding) (in patients with cirrhosis with established portal hypertension). RCTs and observational studies were analyzed separately. Subgroup analyses based on underlying etiology of CLDs were performed for outcomes if there were ≥2 studies for each subgroup.
Statistical Analysis
We used the random-effects model described by DerSimonian and Laird to calculate pooled RR and 95% CI47. Adjusted RR reported in studies was used for analysis to account for confounding variables. We assessed heterogeneity using the I2 statistic; values of <30%, 30%–59%, 60%–75%, and >75% were classified as low, moderate, substantial, and considerable heterogeneity, respectively48. Due to paucity of number of studies, a formal assessment of publication bias using funnel plots was not performed. All p-values were two-tailed, and a probability level <0.05 was considered statistically significant.
Quality of Evidence
We utilized the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework to evaluate the quality of the evidence (or confidence in effect estimates)49. In determining quality of evidence, the following factors were considered: risk of bias in the body of the evidence, imprecision, indirectness (addressing a different population, intervention, or outcome, from the one under consideration), inconsistency of findings, publication bias, magnitude of effect, dose-response relationship and other biases. The GRADE approach accounts for the inherent limitations in observational studies (selection bias, unmeasured confounding, etc.) by starting at low quality, and then potentially upgrading or downgrading based on the aforementioned factors.
RESULTS
Search Results
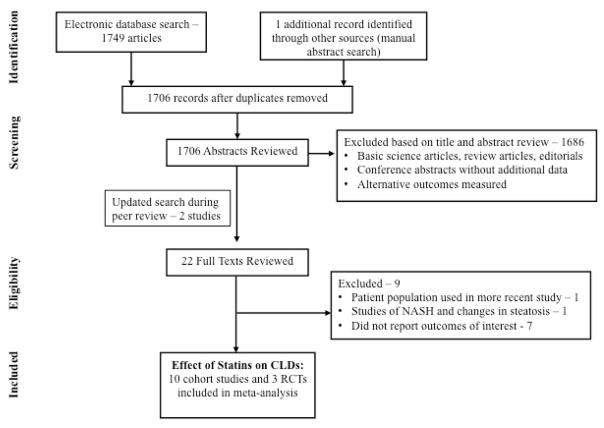
Of the 1706 unique studies identified using our search criteria, 13 studies met our inclusion criteria and were included in our meta-analysis (3 RCTs and 10 observational studies)33–43, 50, 51 Six studies included participants with non-cirrhotic CLDs35, 39–43, six studies included participants with compensated cirrhosis36, 37, 42, 43, 50, 51, and three studies included individuals with decompensated cirrhosis33, 34, 38. One study was excluded as it represented a population already included in another, more recent study;52 one small RCT of statins in NASH was excluded due to insufficient information53. Figure 1 shows the flow diagram summarizing study identification and selection.
Figure 1.
Study selection flowsheet
Characteristics of Included Studies
The characteristics of these studies are shown in Table 1. Overall, these studies included 121,058 patients with CLDs, of whom 46% were exposed to statins. Four cohort studies were conducted in patients with HCV mono-infection, and one was conducted in patients with HIV-HCV co-infection; two studies were conducted exclusively in patients with HBV. One study was conducted exclusively in patients with alcoholic liver disease, and one study reported outcomes stratified in patients with HBV, HCV and alcoholic liver disease. Four observational studies were conducted in Asia, and five were conducted in the United States; among the five conducted in the US, three were conducted in the veteran population. The three included RCTs were conducted primarily to evaluate the efficacy of statins in patients with portal hypertension.
Table 1.
Characteristics of Included Studies Assessing the Risk of Cirrhosis and Related Complications with Statin Use
| Study; Location |
Design | Time period; Follow-up (y) |
Total no. of patients (statin users) |
Etiology of CLD; baseline patient population |
Outcomes of interest |
Outcome | Variables adjusted for* |
|
|---|---|---|---|---|---|---|---|---|
| Statins users | Non-users | |||||||
| Chang, 2017; Taiwan50 | Propensity-score matched cohort | 2000–2013; Statin users vs. non-users: 5.5 vs. 5.4 | 1350 (675, 50%) | HBV, HCV, EtOH; compensated cirrhosis | Decompensated cirrhosis; Mortality | Decompensated cirrhosis: 14% Mortality: 9% |
28% 18% |
1,2,3,5,14,16,18,19 |
| Bang, 2016; Denmark51 | Propensity-score matched cohort | 1995–2010; NR | 3530 (706, 20%) | EtOH; compensated and decompensated cirrhosis | Mortality | In patients with compensated cirrhosis: 88 per 1000p-y; In patients with decompensated cirrhosis: 142 per 1000p-y |
200 per 1000p-y 240 per 1000p-y |
1,2,19,21 |
| Huang, 2016; Taiwan35 | Propensity-score matched cohort | 1997–2009; Statin users vs. non-users: 4.7 vs. 4.6 | 13086, (6543, 50%) | HBV; non-cirrhotic CLD | Cirrhosis; Decompensated cirrhosis | Cirrhosis: 2.6%; Decompensated cirrhosis: 0.9% |
6.1%; 1.9% |
1–5,8,11,12,16 |
| Oliver, 2016; United States43 | Retrospective cohort | 1999–2010; 6.2 | 5985 (949, 16%) | HIV-HCV co-infection; non-cirrhotic CLD | Progression to cirrhosis | 37.8% developed cirrhosis | 1,3–5,12,15,16,17 | |
| Simon, 2016; United States39 | Retrospective cohort | 2001–2014; statin users vs. non-users: 6.8 vs. 8.2 | 9135 (4165, 46%) | HCV; non-cirrhotic CLD | Progression to cirrhosis | Cirrhosis: 14.0% | 21.4% | NR |
| Hsiang, 2015; Hong Kong42 | Propensity-score matched cohort | 2000–2012; statin users vs. non-users: 1.6 vs. 2.6 | 53513 (2%) | HBV; non-cirrhotic CLD, compensated cirrhosis | Mortality | Mortality within 1y: 6.5% | 14.3% | 1,3,6,7,9,13,14,18,19 |
| Mohanty, 2015; United States37 | Propensity-score matched cohort | 1996–2009; statin users vs. non-users: 2.3 vs. 1.7 | 2747 (685, 25%) | HCV; compensated cirrhosis | Decompensated cirrhosis, variceal hemorrhage, death | Decompensated cirrhosis: 2.1%; Variceal hemorrhage: 0.5%; Death: 6.3% |
3.9%; 1.3%; 11.1% |
1–7,10,12–15,20 |
| Simon, 2015; United States40 | Retrospective cohort | 2000–2004; 3.5 | 543 (29, 5%) | HCV; non-cirrhotic CLD | Progression of fibrosis | Fibrosis progression: 10% | 28% | 4,6,8 |
| Yang, 2015; Taiwan41 | Propensity-score matched cohort | 1999–2010; statin users vs. non-users: 378843 p-y vs. 687559 p-y | 84213 (28071, 33%) | HCV; non-cirrhotic CLD | Cirrhosis | Cirrhosis: 6.0% | 21.9% | 1–3,21 |
| Kumar, 2014; United States36 | Retrospective cohort | 1988–2011; statin users vs. non-users: 3 vs. 2.5 | 243 (81, 33%) | Mixed; compensated cirrhosis | Decompensated cirrhosis; mortality | Decompensated cirrhosis: 30.0%; Survival (median, in y): 10.8y |
40.5%; 6.3y |
7–11 |
| Abraldes, 2016; Spain34 | RCT | 2010–2013; 1 | 147 (69, 47%) | Mixed; decompensated cirrhosis with recent variceal bleeding | Recurrent variceal hemorrhage; mortality | Recurrent variceal hemorrhage: 25%; Death: 9% |
28%; 22% |
NA |
| Pollo-Flores, 2015; Brazil38 | RCT | 2011–2013; 0.25 | 24 (13, 54%) | Mixed; decompensated cirrhosis | Reduction in portal hypertension | Reduction in portal hypertension: 55% | 0% | NA |
| Abraldes, 2009; Spain33 | RCT | 2004–2007; 0.1 | 55 (28, 50%) | Mixed; decompensated cirrhosis | Reduction in portal hypertension | Reduction in portal hypertension: 32% | 11% | NA |
1, age; 2, gender; 3, diabetes mellitus; 4, body mass index/obesity; 5, hypertension; 6, platelet count; 7, albumin; 8 NAFLD/steatosis; 9, beta-blocker; 10, MELD; 11, HCC; 12, lipid panel; 13, total bilirubin; 14, cardiovascular disease; 15, race; 16, treatment of underlying CLD (HBV, HCV, HIV); 17, disease-specific prognostic factors (CD4 count for HIV; HCV genotype); 18, cirrhosis complications; 19, other medications (warfarin, antiplatelet/NSAID, fibrate, loop diuretic, other diuretic, ACE-I/ARB, nucleos(t)ide analogue, metformin, sulfonylurea, thiazolidinedione, insulin); 20, other comorbidities (CKD, PAD, etc.); 21, socioeconomic status
Patient-level characteristics are summarized in eTable 1. Patients exposed to statins were generally more likely to have associated diabetes, metabolic syndrome, and be exposed to metformin, aspirin and angiotensin-converting enzyme inhibitors, as compared to patients non-exposed to statins. Propensity-score matching was performed in 6 cohorts, and matched cohorts were comparable with regard to metabolic syndrome and exposure to concomitant therapies.
The median Newcastle-Ottawa quality score for the observational studies was 8 (range, 8–9); 8 of 8 studies were considered high quality. eTable 2 shows the methodological quality of observational studies, and eFigure 1 shows the methodological quality of RCTs.
Risk of Development of Cirrhosis or Progression of Fibrosis
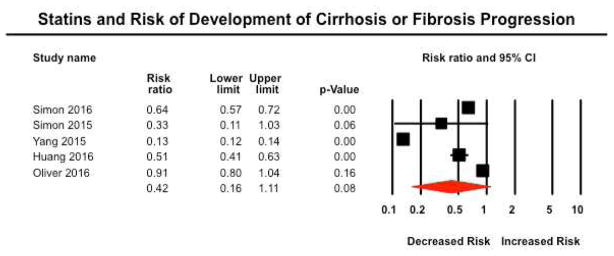
Five studies reported risk of development of cirrhosis (or progression of fibrosis) in patients with baseline non-cirrhotic CLDs35, 39–41, 43. In three studies, diagnosis of cirrhosis (or fibrosis progression) was ascertained based on a combination of administrative claims codes and calculated serum fibrosis markers (FIB-4 or APRI); one study was a post-hoc analysis of the HALT-C trial included patients with paired liver biopsies, with fibrosis progression defined based on increase in Ishak fibrosis stage by ≥ 2. One Taiwanese study in patients with HBV relied exclusively on administrative claims codes. Overall, on random-effects meta-analysis, risk of development of cirrhosis (or progression of fibrosis) was 58% lower in patients exposed to statins, as compared to non-exposed patients (RR, 0.42; 95% CI, 0.16–1.11), with considerable heterogeneity (I2=99%) (Figure 2). On exclusion of one study, which relied exclusively on administrative claims codes with very large protective effect size, a 35% lower risk of development of cirrhosis (or progression of fibrosis) in statin-exposed patients was a more conservative estimate (RR, 0.65; 95% CI, 0.48–0.87), with considerable heterogeneity (I2=89%). Three studies specifically reported the outcome of fibrosis progression (based on either paired liver biopsies or serum fibrosis markers)39, 40, 43. On meta-analysis of these studies, statin exposure was associated with a 27% lower risk of fibrosis progression (RR, 0.73; 95% CI, 0.54–1.00, p=0.049).
Figure 2.
Forest plot showing the association between statin exposure and risk of progression of fibrosis or development of cirrhosis
Overall confidence in these estimates was very low, primarily because of the observational nature of these studies with inherent biases due to absence of randomization, and imprecision due to wide CIs.
Risk of Decompensation of Cirrhosis
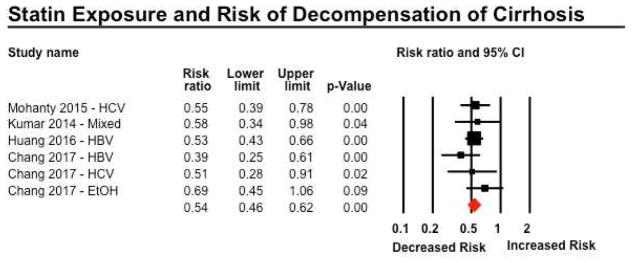
Four studies reported on risk of progression to decompensated cirrhosis, in patients baseline compensated cirrhosis36, 37, 50 or non-cirrhotic CLDs35. Three studies relied on validated administrative claims codes for development of decompensated cirrhosis, and one used individual medical record review. On meta-analysis, statin use was associated with a 46% lower risk of progression to decompensated cirrhosis (RR 0.54; 95% CI, 0.46–0.62), with minimal heterogeneity (I2=0%) (Figure 3). On restricting analysis only to patients with baseline compensated cirrhosis (excluding patients with non-cirrhotic CLDs), a similar protective association of between statin use and risk of hepatic decompensation was observed (RR, 0.54; 95% CI, 0.44–0.66). On subgroup analyses by etiology of underlying CLD, no considerable differences were observed in patients with CLD due to HBV, HCV, alcoholic liver disease or mixed etiology (Table 2).
Figure 3.
Forest plot showing the association between statin exposure and risk of decompensation of cirrhosis. Of note, on restricting analysis only to patients with baseline compensated cirrhosis (excluding patients with non-cirrhotic CLDs in Huang et al), a similar protective association of between statin use and risk of hepatic decompensation was observed (RR, 0.54; 95% CI, 0.44–0.66)
Table 2.
Subgroup analysis based on etiology of chronic liver diseases
| Etiology of CLD | No. of studies | Risk Ratio (95% CI) | p-interaction |
|---|---|---|---|
| Outcome: Mortality | |||
| HBV | 2 | 0.63 (0.27–1.45) | 0.84 |
| HCV | 2 | 0.67 (0.40–1.13) | |
| Alcoholic liver disease | 2 | 0.56 (0.40–0.79) | |
| Mixed | 2 | 0.50 (0.32–0.76) | |
| Outcome: Decompensated Cirrhosis | |||
| HBV | 2 | 0.49 (0.37–0.64) | 0.62 |
| HCV | 2 | 0.54 (0.40–0.73) | |
| Alcoholic liver disease | 1 | 0.69 (0.45–1.06) | |
| Mixed | 1 | 0.58 (0.34–0.98) | |
Overall confidence in these estimates was moderate, primarily because of the observational nature of these studies with inherent biases due to absence of randomization; due to large effect size, evidence was rated up.
Risk of Mortality
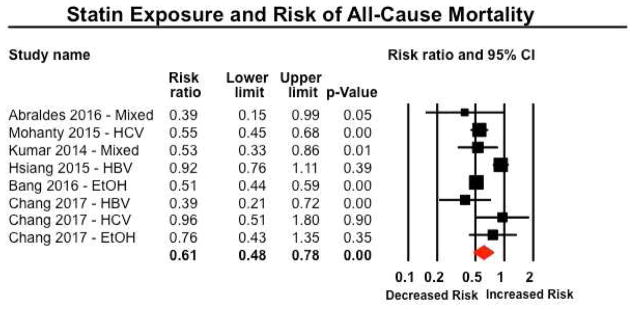
Six studies reported on risk of mortality in patients with CLDs (five studies in patients with cirrhosis, one study in patients with non-cirrhotic CLDs)34, 36, 37, 42, 50, 51. Five studies were observational studies, and one was an RCT primarily focusing on addition of simvastatin or placebo to standard of care in patients with decompensated cirrhosis with prior variceal bleeding. On meta-analysis, statin use was associated with a 39% lower risk of mortality (RR 0.61; 95% CI, 0.48–0.78), with considerable heterogeneity (I2=77%) (Figure 4). On restricting analysis only to patients with baseline cirrhosis (excluding patients with non-cirrhotic CLDs),42 a similar protective association was observed (RR, 0.54; 95% CI, 0.47–0.61), with minimal heterogeneity (I2=10%). On subgroup analyses by etiology of underlying CLD, no considerable differences were observed in patients with CLD due to HBV, HCV, alcoholic liver disease or mixed etiology (Table 2).
Figure 4.
Forest plot showing the association between statin exposure and risk of all-cause mortality. On restricting analysis only to patients with baseline cirrhosis (excluding patients with non-cirrhotic CLDs),42 a similar protective association was observed (RR, 0.54; 95% CI, 0.47–0.61), with minimal heterogeneity (I2=10%).
When focusing on use of statins only in patients with baseline cirrhosis, overall confidence in estimates was moderate, primarily because of the observational nature of these studies with inherent biases due to absence of randomization, and large effect size; in evaluating all patients with CLDs, evidence was rated as low quality due to serious inconsistency.
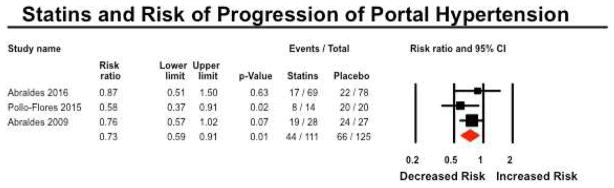
Improvement in Portal Hypertension
Four studies reported on risk of re-bleeding or clinically significant improvement in portal hypertension in patients with baseline cirrhosis.33, 34, 37, 38 On meta-analysis of 3 RCTs, statin use was associated with a 27% lower risk of re-bleeding (or failure to improve portal hypertension) (RR 0.73; 95% CI, 0.59–0.91), with minimal heterogeneity (I2=0%) (Figure 5). In the single observational study in patients with compensated HCV-related cirrhosis, statin use was associated with a 61% lower risk of portal hypertension-related bleeding (RR, 0.39; 95% CI, 0.19–0.79).
Figure 5.
Forest plot showing the association between statin exposure and risk of progression of portal hypertension
Based on RCTs, there was moderate confidence in estimates supporting the use of statins for decreasing risk of variceal bleeding in patients with established portal hypertension. Evidence was rated down for imprecision due to small number of events (110 events in 236 patients), and short duration trials.
DISCUSSION
In this systematic review and meta-analysis of 13 studies on the association between statins and risk of clinically relevant outcomes in 121,058 patients with CLDs, we made several key observations. First, moderate quality evidence supports the use of statins for decreasing the risk of hepatic decompensation by 46% especially in patients with baseline compensated cirrhosis, though there is very low confidence in estimates supporting the use of statins to decrease risk of fibrosis progression or development of cirrhosis in patients with non-cirrhotic CLDs. Second, low quality evidence suggests that statins may lower mortality risk by 39% in all patients with CLDs; in a subset of patients with baseline cirrhosis, moderate quality evidence suggests a 46% lower risk of mortality with statin use. Third, moderate quality evidence also suggests a moderate benefit of statins in decreasing the risk of variceal bleeding or progression of portal hypertension by 27%, especially in patients with known portal hypertension. This study, along with previous evidence on the potential protective association between statin use and risk of HCC in patients with CLDs, provides further impetus to perform larger RCTs to evaluate the role of statins in high-risk patients, such as those with compensated cirrhosis.
In vitro and preclinical studies have shown various effects of statins on hepatic cells and vasculature. In cirrhosis, one of the leading causes of decompensation is increasing hepatic vascular resistance which causes increased portal pressure, potentially mediated by insufficient vasodilator nitric oxide54–56. In a randomized trial of 30 patients with cirrhosis with clinically significant portal hypertension (HVPG ≥12mmHg), Zafra et al found a significant increase in nitric oxide levels in hepatic venous blood in patients who received simvastatin, as compared to patients who received placebo24. Abraldes et al made a similar observation in CCl4 cirrhotic rats treated with simvastatin, with a selective increase in hepatic nitric oxide production, which was associated with improvement in liver sinusoidal endothelial dysfunction and decreased vasoconstriction25. Other studies have shown statins may also have anti-inflammatory effects and anti-fibrotic properties on hepatic cells26. Trebicka and colleagues observed early treatment with atorvastatin in bile duct ligated rats results in inhibition of hepatic stellate cells leading to significantly reduced fibrosis as measured by hepatic hydroxyproline content27.
We were unable to perform a meta-analysis of dose and duration effects of statins due to insufficient information. However, four included observational studies suggested that the effects of statins were dose-dependent, with strong effect estimates in patients with >1 year of statin use as compared to patients with shorter duration of use. Similarly, several different types of statins were studied, and observed effects were assumed to be class-specific effects. However, it is possible that lipophilic and lipophobic statins may have differential efficacy in decreasing fibrosis progression.
The strengths of this systematic review include: (a) comprehensive and systematic literature search with well-defined inclusion criteria; (b) quantitative and qualitative assessment of several clinically relevant outcomes in pre-defined subgroups of patients with CLDs; and (c) GRADE-based quality assessment to allow effective translation of findings into clinical guidelines.
Our study has several limitations. First, evidence was based on observational studies for several outcomes. Observational studies lack the experimental random allocation of the intervention necessary to test exposure-outcome hypotheses optimally. Despite adjusting for numerous covariates, it is not possible to eliminate the potential of residual confounding, in particular, confounding by indication, and for potential immortal time bias. Immortal time bias refers to failure to account for time between disease diagnosis (for example, chronic liver diseases) and exposure ascertainment (for example to statins) in survival analyses which may overestimate the beneficial effects of exposure.57 We rated down the quality of evidence accordingly to low quality for all estimates derived from observational studies. Second, considerable heterogeneity was observed for some outcomes, including risk of progression of fibrosis or cirrhosis, and mortality. Subgroup analyses based on etiology of underlying CLD could not explain observed heterogeneity; however, heterogeneity in effect estimates for mortality were explained by excluding one study which included patients with non-cirrhotic CLD, whereas the effects were homogenous in patients with cirrhosis. Other potential sources of heterogeneity, which could not be adequately assessed, include methodological differences in identifying patients with CLDs, exposure ascertainment (definition of statin exposure), and outcome assessment (definition of fibrosis progression/cirrhosis, decompensated cirrhosis, etc.). It is important to note, however, that heterogeneity was primarily due to differences in effect size and not in effect direction, and summary estimates in all included studies suggested a favorable effect of statins on outcomes of interest. Third, studies did not consistently adjust for the same confounding variables; only 6 studies performed propensity score matching. Moreover, depending on outcome, specific confounding factors may be more relevant. For example, for the outcome “development of cirrhosis or progression of fibrosis”, previous etiological treatment for liver disease, whether definitive cure of the etiological factor was achieved, baseline platelet count and albumin concentration, fibrosis scores and histology whenever available, are relevant. However, it is important to note that statin use is more likely to be observed in patients with metabolic syndrome, which is an independent factor associated with fibrosis progression and adverse outcomes in patients with CLDs; hence, any potential bias due to failure to adjust for confounders, may possibly accentuate the observed protective effects of statins. Fourth, some studies relied on administrative codes, and their performance for CLDs, cirrhosis- and related complications may be unreliable. Finally, due to the small number of studies, statistical tests for small study effects were not performed; given the nascency of the field, reporting bias is a valid concern. We did not contact investigators of original studies for unpublished data.
In conclusion, through a systematic review and meta-analysis, moderate quality evidence suggests beneficial effect of statins on risk of hepatic decompensation and mortality, and variceal bleeding, especially in patients with known compensated cirrhosis, and low quality evidence suggests a mortality benefit in patients with CLDs. Together with previous evidence on the potential protective association between statin exposure and risk of HCC, and their safety in this patient population, this suggests that statins may potentially improve patient-relevant outcomes in patients with CLDs and improve survival without significant additional costs. Large, pragmatic RCTs in patients with compensated cirrhosis, are required to confirm these observations.
Supplementary Material
Acknowledgments
Funding support: Dr. Singh is supported by the NIH/NLM training grant T15LM011271. Dr. Loomba is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award and grant K23-DK090303 and R01-DK106419-03. Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES010337
Footnotes
Conflicts of Interest: None
Author contributions:
Rebecca G. Kim: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission
Siddharth Singh: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, study supervision, approved final submission
Larry J. Prokop: literature review, critical revision of the manuscript, approved final submission
Rohit Loomba: study concept and design, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu J, Murphy SL, Kochanek KD, et al. Deaths: Final Data for 2013. National Vital Statistics Reports. 2016;64:1–119. [PubMed] [Google Scholar]
- 2.Grundy SM. HMG-CoA Reductase Inhibitors for Treatment of Hypercholesterolemia. N Engl J Med. 1988;319:24–33. doi: 10.1056/NEJM198807073190105. [DOI] [PubMed] [Google Scholar]
- 3.Endo A, MK, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;1976:323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- 4.Tsujita Y, Kuroda M, Tanzawa K, et al. Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Atherosclerosis. 1979;32:307–313. doi: 10.1016/0021-9150(79)90174-6. [DOI] [PubMed] [Google Scholar]
- 5.Shigematsu H, Hata Y, Yamamoto M, et al. Treatment of hypercholesterolemia with a HMG CoA reductase inhibitor (CS-500). I. Phase I study in normal subjects. Geriatr Med. 1979;17:1564–1570. [Google Scholar]
- 6.Yamamoto A, Sudo H, Endo A. Therapeutic effects of ML-236B in primary hypercholesterolemia. Atherosclerosis. 1980;35:259–266. doi: 10.1016/0021-9150(80)90124-0. [DOI] [PubMed] [Google Scholar]
- 7.Mabuchi H, Haba T, Tatami R, et al. Effects of an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10 levels in patients with familial hypercholesterolemia. N Engl J Med. 1981;305:478–482. doi: 10.1056/NEJM198108273050902. [DOI] [PubMed] [Google Scholar]
- 8.Tobert JA, Bell GD, Birtwell J, et al. Cholesterol-lowering effect of mevinolin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, in healthy volunteers. J Clin Invest. 1982;69:913–919. doi: 10.1172/JCI110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Lovastatin Study Group II. Therapeutic response to lovastatin (mevinolin) in nonfamilial hypercholesterolemia: a multicenter study. JAMA. 1986;256:2829–2834. doi: 10.1001/jama.1986.03380200067023. [DOI] [PubMed] [Google Scholar]
- 10.Havel RJ, Hunninghake DB, Illingworth DR, et al. Lovastatin (mevinolin) in the treatment of heterozygous familial hypercholesterolemia: a multicenter study. Ann Intern Med. 1987;107:609–615. doi: 10.7326/0003-4819-107-5-609. [DOI] [PubMed] [Google Scholar]
- 11.Musial J, Undas A, Gajewski P, et al. Anti-inflammatory effects of simvastatin in subjects with hypercholesterolemia. Int J Cardiol. 2001:77. doi: 10.1016/s0167-5273(00)00439-3. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Rifai N, Lowenthal SP. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation. 2001;103:1191–1193. doi: 10.1161/01.cir.103.9.1191. [DOI] [PubMed] [Google Scholar]
- 13.Albert MA, Staggers J, Chew P, et al. The pravastatin inflammation CRP evaluation (PRINCE): rationale and design. Am Heart J. 2001;141:893–898. doi: 10.1067/mhj.2001.115297. [DOI] [PubMed] [Google Scholar]
- 14.Lefer DJ. Statins as potent antiinflammatory drugs. Circulation. 2002;106:2041–2042. doi: 10.1161/01.cir.0000033635.42612.88. [DOI] [PubMed] [Google Scholar]
- 15.McGirt MJ, Lynch JR, Parra A, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 16.Kalinowski L, Dobrucki LW, Brovkovych V, et al. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation. 2002;105:933–938. doi: 10.1161/hc0802.104283. [DOI] [PubMed] [Google Scholar]
- 17.Laufs U, Fata VL, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 18.Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llevadot J, Murasawa S, Kureishi Y, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson TJ, Meredith IT, Yeung AC, et al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 22.Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 23.O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 24.Zafra C, Abraldes JG, Turnes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–55. doi: 10.1053/j.gastro.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Moreno M, Ramalho LN, Sancho-Bru P, et al. Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am J Physiol Gastrointest Liver Physiol. 2009;296:G147–G156. doi: 10.1152/ajpgi.00462.2007. [DOI] [PubMed] [Google Scholar]
- 27.Trebicka J, Hennenberg M, Odenthal M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702–712. doi: 10.1016/j.jhep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Mura VL, Pasarin M, Meireles CZ, et al. Effects of Simvastatin Administration on Rodents with Lipopolysaccharide-Induced Liver Microvascular Dysfunction. Hepatology. 2013;57:1172–1181. doi: 10.1002/hep.26127. [DOI] [PubMed] [Google Scholar]
- 29.Marrone G, Maeso-Diaz R, Garcia-Cardena G, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015;64:1434–1443. doi: 10.1136/gutjnl-2014-308338. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–32. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Tsan Y, Lee C, Wang J, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. Value Health. 2012;15:A602–A603. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 32.El-Serag HB, Johnson ML, Hachem C, et al. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136:1601–8. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraldes JG, Albillos A, Banares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–8. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 34.Abraldes JG, Villanueva C, Aracil C, et al. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150:1160–1170. e3. doi: 10.1053/j.gastro.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y-W, Lee C-L, Yang S-S, et al. Statins Reduce the Risk of Cirrhosis and Its Decompensation in Chronic Hepatitis B Patients: A Nationwide Cohort Study. Am J Gastroenterol. 2016;111:976–85. doi: 10.1038/ajg.2016.179. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59:1958–65. doi: 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- 37.Mohanty A, Tate JP, Garcia-Tsao G. Statins Are Associated With a Decreased Risk of Decompensation and Death in Veterans With Hepatitis C-Related Compensated Cirrhosis. Gastroenterology. 2016;150:430–40. doi: 10.1053/j.gastro.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollo-Flores P, Soldan M, Santos UC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: A randomized controlled trial. Dig Liver Dis. 2015;47:957–63. doi: 10.1016/j.dld.2015.07.156. [DOI] [PubMed] [Google Scholar]
- 39.Simon TG, Bonilla H, Yan P, et al. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47–57. doi: 10.1002/hep.28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon TG, King LY, Zheng H, et al. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18–23. doi: 10.1016/j.jhep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y-H, Chen W-C, Tsan Y-T, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63:1111–7. doi: 10.1016/j.jhep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Hsiang JC, Wong GL-H, Tse Y-K, et al. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J Hepatol. 2015;63:1190–7. doi: 10.1016/j.jhep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Oliver NT, Hartman CM, Kramer JR, et al. Statin drugs decrease progression to cirrhosis in HIV/hepatitis C virus coinfected individuals. AIDS. 2016;30:2469–2476. doi: 10.1097/QAD.0000000000001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. [Google Scholar]
- 46.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Google Scholar]
- 47.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 48.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–7. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Chang FM, Wang YP, Lang HC, et al. Statins decrease the risk of decompensation in HBV- and HCV-related cirrhosis: A population-based study. Hepatology. 2017 doi: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- 51.Bang UCBT, Bendtsen F. The impact of statins on mortality in patients with compensated or decompensated alcoholic cirrhosis in a nationwide retrospective study. Hepatology. 2016;54:131A–132A. [Google Scholar]
- 52.Butt AA, Yan P, Bonilla H, et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: Results from ERCHIVES. Hepatology. 2015;62:365–74. doi: 10.1002/hep.27835. [DOI] [PubMed] [Google Scholar]
- 53.Dongiovanni P, Petta S, Mannisto V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–12. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Gupta TK, Toruner M, Chung MK, et al. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926–931. doi: 10.1002/hep.510280405. [DOI] [PubMed] [Google Scholar]
- 55.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 56.Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 57.Targownik LE, Suissa S. Understanding and Avoiding Immortal-Time Bias in Gastrointestinal Observational Research. Am J Gastroenterol. 2015;110:1647–50. doi: 10.1038/ajg.2015.210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.