Abstract
Thousands of people each year suffer from peripheral nerve injury. Treatment options are limited, and recovery is often incomplete. Treadmill exercise can enhance nerve regeneration; however, this appears to occur in a sex-dependent manner. Females respond best to short duration, high speed interval training; whereas, males respond best to slower, continuous training. Previous studies have shown a role for testosterone in this process, but the role of estrogen is unknown. To evaluate the role of estrogen signaling in treadmill exercise, we blocked estrogen receptor (ER) signaling during treadmill exercise in males and female wild type mice. The right common fibular (CF) branch of the sciatic nerve was cut and repaired with fibrin glue that contained the ER antagonist ICI 182,780. Estradiol-filled or blank Silastic capsules were implanted subcutaneously at the time of nerve transection. Starting three days post-transection, exercised mice received treadmill training using the paradigm appropriate to their sex 5 days a week for 2 weeks. Fourteen days after the initial nerve transection, motoneurons whose axons had regenerated at least 1.5 mm distal to the original cut sites were labeled with a retrograde tracer. Regeneration was quantified by counting the number of fluorescent labeled motoneurons in the lumbar region of the spinal cord. Both treadmill training and estradiol administration increased the number of motoneurons participating in axon regeneration, but these effects were blocked by ER antagonist treatment. Estrogen signaling is important for the enhancing effects of treadmill exercise on motoneuron participation after peripheral nerve cut.
Keywords: Estrogen, Exercise, Regeneration, Peripheral Nervous System, Estrogen Receptor
INTRODUCTION
Injured peripheral nerves have the ability to regenerate, but the process is slow and often functional recovery is very poor (Frostick et al., 1998; Scholz et al., 2009). Modest exercise in the form of treadmill training has been successful in promoting peripheral nerve regeneration and functional recovery by increasing the length of regenerating axons (Sabatier et al., 2008; Wilhelm et al., 2012; Wood et al., 2012), reducing misdirection of axons (Sabatier et al., 2011), and increasing the number of motoneurons whose axons successfully participate in regeneration (English et al., 2009). The pattern of exercise needed to produce these effects is sex-specific (Wood et al., 2012). One hour of low speed (10 m/min) continuous treadmill exercise daily for two weeks produces significant enhancement of axon profile lengths in males but not females; whereas, 4 repetitions of running at a faster speed (20 m/min) for 2 minutes with 5 min breaks interspersed (interval training) significantly enhances the lengths of axon profiles of females but not males (Wood et al., 2012). The mechanisms underlying these sex differences are not completely understood, but sex steroid signaling appears to be important (Wood et al., 2012; English et al., 2014; Thompson et al., 2014). Elucidating the mechanisms by which treadmill exercise enhances axon regeneration is critical for the development of strategies to enhance peripheral nerve regeneration and provide more positive functional outcomes in patients where exercise may not be possible immediately after injury.
Androgen signaling may mediate some of the effects of treadmill exercise on axon regeneration. Males trained using the continuous paradigm have significantly increased serum testosterone levels after two weeks of treadmill exercise (Wood et al., 2012). Blocking this increase by castration (Wood et al., 2012) or by treating male mice systemically with the androgen receptor antagonist flutamide (Thompson et al., 2014) blocks the enhancing effects of continuous treadmill exercise on axon elongation. No change in serum testosterone levels is found in males or females after interval training (Wood et al., 2012), suggesting a less important role for systemic androgen signaling in the mechanisms underlying interval exercise-mediated enhancements. Androgen signaling could still be a part of the mechanisms engaged by exercise in females that receive interval training as blocking androgen receptor signaling via flutamide treatment reduced the exercise-mediated enhancement of axon elongation in these animals (Thompson et al., 2014). In addition, treating unexercised females with anastrozole, a cytochrome CP450 aromatase inhibitor that reduces the conversion of androgens to estrogens, augmented axon elongation (Wood et al., 2012). Although these results suggest that androgen signaling is sufficient to mediate the effects of treadmill exercise on axon regeneration, it is possible that other factors such as estrogens are also involved in the signaling cascade.
Several previous studies have found that treatment with a non-aromatizable form of testosterone produces less enhancement of axon elongation than treatment with the aromatizable form of testosterone, suggesting that androgens can work synergistically with estrogen signaling to enhance axon regeneration (Tanzer and Jones, 1997; Sharma et al., 2009). The biosynthetic pathway for androgens and estrogens is found in motoneurons (Coirini et al., 2002; Rakotoarivelo et al., 2004) and glial cells in the spinal cord (Patte-Mensah et al., 2004; Garcia-Ovejero et al., 2005). Testosterone in these cells can be converted to estradiol via the cytochrome P450 enzyme aromatase. Aromatase is expressed in neurons under basal conditions and has been shown to be upregulated in some cells after injury (Garcia-Ovejero et al., 2005; Schaeffer et al., 2010). Thus, increases in testosterone could produce increases in estradiol. Exogenous estradiol administration has been shown to enhance axon regeneration after peripheral nerve crush and transection injuries (Tanzer and Jones, 1997; Jones et al., 2000; Islamov et al., 2002; McMurray et al., 2003; Murashov et al., 2004; Huppenbauer et al., 2005; Sharma et al., 2009; Sekiguchi et al., 2012). Both isoforms (α, β) of estrogen receptors (ER) have been found in the sciatic nerve and motoneurons in the lumbar spinal cord of adult female mice (Islamov et al., 2003) and the expression of ER mRNA increases after peripheral injury (Islamov et al., 2003). Therefore, we hypothesized that ER signaling is a mechanism by which treadmill exercise enhances axon regeneration after sciatic nerve transection injuries. A preliminary report of some of this work has been presented in the abstract form (Wilhelm et al., 2015).
METHODS
Subjects
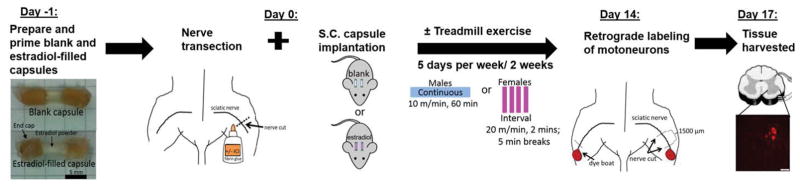
All experimental methods have been approved by Institutional Animal Care and Use Committee of College of Charleston and conform to the Guidelines for the Use of Animals in Research of the Society of Neuroscience. Motoneuron participation in axon regeneration was studied in 63 gonadally intact male (n = 33) and female (n = 30) wild type C57BI/6J mice aged from 2–5 months old (Jackson Labs, Bar Harbor, ME). Mice were separated by sex and then randomly assigned to one of six experimental groups (see Table 1): (1) untreated without treadmill exercise, (2) untreated with treadmill exercise, (3) estradiol treatment without treadmill exercise, (4) ER antagonist treatment with treadmill exercise, (5) ER antagonist treatment without treadmill exercise, or (6) ER antagonist treatment subcutaneously administered away from transection site without treadmill exercise. An additional group of male mice were treated with systemic exogenous estradiol and ICI 182,780 at the site of nerve transection. The numbers of animals studied in each group were selected based on the results of an a priori power sample size estimate (Lenth), using data from previously published reports (English et al., 2009) and alpha= 0.05 and power = 0.8. See Figure 1 for an overview of methods.
Table 1.
Summary of Experimental Groups
| Group # | Continuous Treadmill Exercise | Interval Treadmill Exercise | Estrogen Receptor Antagonist Treatment | Estradiol Treatment | Sex | N |
|---|---|---|---|---|---|---|
| 1 | No | No | No | No | M | 4 |
| 1 | No | No | No | No | F | 5 |
| 2 | Yes | No | No | No | M | 6 |
| 2 | No | Yes | No | No | F | 4 |
| 3 | No | No | No | Yes | M | 7 |
| 3 | No | No | No | Yes | F | 7 |
| 4 | Yes | No | Yes | No | M | 4 |
| 4 | No | Yes | Yes | No | F | 4 |
| 5 | No | No | Yes | No | M | 4 |
| 5 | No | No | Yes | No | F | 5 |
| 6 | No | No | Yes; s.c. at nape of neck | No | M | 4 |
| 6 | No | No | Yes, s.c. at nape of neck | No | F | 5 |
| 7 | No | No | Yes | Yes | M | 4 |
| Total | 63 | |||||
Figure 1.
Diagram of the experimental procedure used to label motoneurons. Mice were treated with blank or estradiol filled capsules immediately prior to nerve transection and repair surgery. Mice then were treated with no exercise or were treadmill trained using a continuous (males) or interval (females) paradigm for 5 days per week for 2 weeks. Two weeks after the nerve transection and repair surgery, neurons were labeled using retrograde tracers applied 1.5mm distal to the original lesion site. Three days later, mice were euthanized and tissue was harvested for histologic analysis. Motoneurons labeled with dye were counted to measure the number of motoneurons participating in axon regeneration.
Nerve Transection and Repair Surgery
Under isoflurane (2 – 5 % in oxygen) or ketamine (80–100 mg/kg)/xylazine (5–10 mg/kg) anesthesia, the CF nerve was cut unilaterally just distal to its branch point from the sciatic nerve in all mice. Nerves were repaired by end to end anastomosis and secured with 3–5 μL fibrin glue (de Vries et al., 2002; MacGillivray, 2003). To assess the importance of ER signaling in the effects of exercise on the participation of motoneurons in regeneration after axotomy, mice in groups 4 & 5 were treated with an ER antagonist, ICI 182,780 (Tocris, Bristol, UK). ICI 182,780 (1 μM) was mixed into the fibrin glue solution immediately prior to application to the cut CF nerve. Mice in group 6 also received ICI 182,780 treatment; however, in these mice the ICI 182,780 was mixed into fibrin glue and applied to the subcutaneous space at the nape of the mouse’s neck. In these mice the fibrin glue applied at the time of nerve transection and repair did not contain the ER antagonist. This group was included to assess whether the ER antagonist’s effects on motoneuron participation were due to local effects at the cut nerve or due to more systemic effects. For all mice, the contralateral CF nerve was surgically exposed but not transected to serve as an unoperated (intact) control. The surgical incisions were closed using sutures. All mice were given meloxicam (1–2 mg/kg PO; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) for up to 3 days post operation to alleviate pain.
Exogenous Estradiol Administration
To assess the effects of estradiol administration on motoneuron participation in regeneration, mice in groups 3 & 7 were treated with systemically administered exogenous estradiol. Estradiol capsules were made of 15 mm of Silastic tubing (Dow Corning, Midland, MI; inner diameter: 1.67 mm; outer diameter 3.18 mm; length: 6 mm) filled with 5mm of estradiol powder (approximately 20–30 micrograms; Letco, Decatur, AL) and flanked on each side by 5mm wooden stick segments. The ends of the capsules were covered with Medical Adhesive A (Dow Corning). Capsules were soaked in 0.9% saline for 24 hours prior to implantation to prime the estradiol powder for release. Two estradiol-filled capsules were implanted subcutaneously at the nape of the neck immediately prior to the nerve transection surgery. This approach allows estradiol to be released gradually with a rate of release proportional to the capsule surface area (Smith, 1977) and has been described previously (Islamov et al., 2002; McMurray et al., 2003; Sharma et al., 2009). Mice in experimental groups that did not receive estradiol treatment received two similarly made capsules that were not filled with estradiol (blank capsules).
Treadmill Exercise
Three days after initial nerve transection surgery a subset of mice (n = 18) received exercise on a treadmill for 5 days a week for 2 weeks using a continuous or interval training paradigm (Sabatier et al., 2008). Previous studies have shown a sex difference in the effectiveness of treadmill exercise to enhance the elongation of regenerating axons (Wood et al., 2012), therefore, mice received exercise in a paradigm dependent on their sex. Female mice received exercise using an interval paradigm in which each mouse ran 4 repetitions at a speed of 20 m/min for 2 minutes with 5 minutes of rest interspersed. Male mice received exercise for 1 hour at a slower speed of 10 m/min (continuous training paradigm).
Retrograde Labeling of Motoneurons
Although axon counting is the most commonly used parameter to measure regenerative success after peripheral nerve injuries, we used neuron cell body counts rather than axon counts to compare conditions of axon regeneration to avoid the confounding variable of axon sprouting (Wood et al., 2011). Axon counts do not necessarily reflect the number of neurons that regenerate axons as sensory neurons generate almost twice as many axon branches as motoneurons (Redett et al., 2005). Using a method of labeling cell bodies whose axons regenerated provides a reliable measure of regenerative success independent of the number of axon branches that are emitted from each axon proximal to the injury site (Brushart, 1993; Boyd and Gordon, 2003; Wood et al., 2011) and will allow for us to independently assess the effects of treatment on motoneuron populations.
Fourteen days after the initial nerve transection surgery, mice sustained a second survival surgery in which the axons and cell bodies of neurons were labeled using a retrograde tracer dye. Under isoflurane anesthesia, the previously cut and repaired CF was re-exposed and cut approximately 1.5 mm distal to the original cut site. This distance from the original transection site was chosen because in previous experiments the average growth of untreated regenerating axons in C57Bl/6J mice at 2 weeks post-transection was 1 mm. Treadmill exercise enhanced the average length of regenerating axon profiles to approximately 3 mm. Therefore, we applying the retrograde tracer at 1.5 mm allows labeling of some motoneurons in untreated, unexercised mice and allows the assessment of any enhancement of regeneration due to treatment or exercise. The intact CF, which was not cut in the initial surgery, was also exposed and cut at approximately the same location distal to the branch point from the sciatic nerve as the right CF. Both nerves were draped over small pieces of Parafilm prior to transection. A small well of petroleum jelly was created around the nerve. The nerve was not cut until the well was created to prevent excessive damage to the epineurium. Cut nerves were soaked in sterile water for 5 minutes. The water was then removed, and the nerve was soaked in crystals of dextran amine (MW 10,000 fixable) conjugated to a fluorophore (AlexaFluor594 or AlexaFluor488; Molecular Probes, Waltham, MA) hydrated with water for up to 1 hour. Nerves were checked every 5 – 10 minutes, and tracer solutions were replenished as necessary. At the end of the application period, the proximal stumps of the nerves contained visible color, indicating that the dye was taken up by the axons. Although previous studies have shown comparable labeling of motoneurons by both tracers (Boyd and Gordon, 2001; Boyd and Gordon, 2002; English, 2005), the same tracer was used on both nerves in each individual animal. The tracer solution, Parafilm, and petroleum jelly were removed, and the entire surgical field was irrigated with normal saline solution prior to surgical closure.
Tissue Harvesting and Analysis of Motoneuron Participation
Three days following retrograde labeling, all mice were euthanized with an overdose of pentobarbital euthanasia solution. The uteri of females were removed and weighed to determine the effectiveness of estradiol administration. The mice were then perfused with 0.9% saline followed by 4% paraformaldehyde. Spinal cord segments L3 – L5 were harvested, cryoprotected for 3 days in 20% sucrose, and stored at 4° C. Serial transverse sections were cut at 20 μm thick on a cryostat (Leica, Buffalo Grove, IL), mounted directly onto subbed slides, and then cover slipped. Sections were imaged a low magnification (10 – 20x) using an epifluorescence light microscope (Olympus BX43F, Center Valley, PA) with Texas Red and FITC filters. Researchers blinded to the experimental conditions applied to the tissues captured the images and counted the number of labeled neurons in the ventral horn of L3-L5 segments of the spinal cord. Only neurons with detectable cell borders containing retrograde fluorescent label that filled the cell soma and a visible nuclear area devoid of label were included in this study. Double counting was corrected using the method of Abercrombie (Abercrombie, 1946).
Data Analysis
The average number of labeled motoneurons in each group were compared using a two-way (sex and treatment) analysis of variance (ANOVA). The average uterine weights from treated and untreated females were compared using t-tests for independent samples. Probabilities of less than 0.05 were considered statistically significant.
RESULTS
Application of retrograde tracer dyes to cut CF nerves in mice resulted in the labeling of motoneuron cell bodies in the lumbar spinal cord. To identify the number of motoneurons in the intact CF motoneuron pool, we labelled the contralateral intact CF nerve in all mice. A two-way ANOVA was used to compare the number of labeled motoneurons counted in each treatment condition for both sexes. There was no significant difference between the mean numbers of motoneurons labeled with either AlexaFluor 488 or AlexaFluor 594; therefore, the results using each dye were combined within each treatment condition. The main effect of sex yielded a F ratio of F(1, 102) =2.04, p= 0.16. The main effect of treatment condition yielded an F ratio of F(13, 102) = 20.1, p ≤ 0.0001; therefore, final significance of differences in means was studied using post hoc paired (Fisher’s least significant difference, LSD) testing where appropriate. The interaction effect was not statistically significant, F (11, 102) = 1.46, p = 0.16. There was no significant difference in the mean number of motoneurons labeled from the intact side for any treatment condition for either sex (Figure 2).
Figure 2.
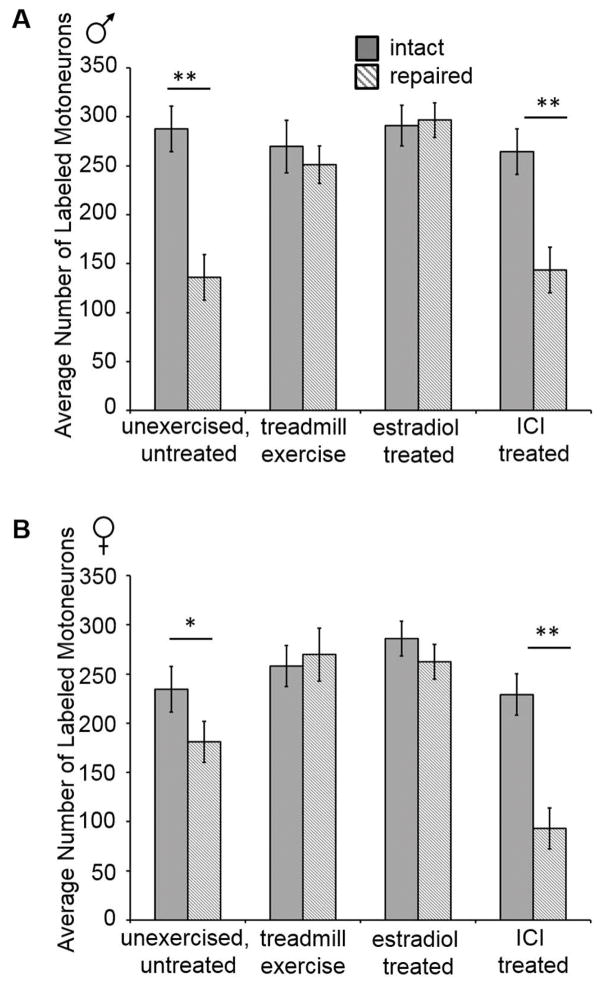
Comparisons of the effects of estradiol treatment, treadmill exercise, and ER antagonist ICI 182,780 (ICI) treatment on the number of labeled motoneurons in males (A) and females (B). The mean (± standard deviations) number of motoneurons labeled by tracer application to the transected and repaired CF nerve 1.5 mm distal to the original cut site (hashed bars) and to the intact CF nerve (solid bars) for males (A) and females (B) are shown. No treatment altered the number of labeled motoneurons in the intact side in either sex. No differences were found in the number of labeled motoneurons between the intact side and the transected and repaired side in mice treated with estradiol or with treadmill exercise using the continuous paradigm (A) or interval paradigm (B). Significantly fewer motoneurons were labeled in the transected and repaired side in mice treated with ICI compared to the intact side of the same animals. * p ≤ 0.04 ; ** p ≤0.0001
Nerve transection and surgical repair reduced the number of labeled motoneurons by an average of 38%. The mean number of back-labelled CF motoneurons which regenerated axons 1.5mm from the site of nerve repair was approximately 47% of the total number of labelled intact motoneurons without any treatments applied for males (intact: 287.8 ± 76.1, repaired: 136 ± 11.4, p ≤ 0.0001; Figure 2A) and approximately 77% of the total of labeled intact motoneurons for females (intact: 234.2 ± 38.2, repaired: 181.2 ± 32.7, p ≤ 0.04; Figure 2B). The total number of untreated, unexercised labelled CF motoneurons from the cut side counted in these experiments was larger than in some previous reports (English, 2005; English et al., 2009), but those studies applied the retrograde tracer much farther distal to the original cut and repair site (5 – 12 mm), thus reducing the likelihood that as many neurons would have had axons that regenerated to that distance compared to the relatively shorter distance of 1.5 mm. Examples of sections from the spinal cord ipsilateral to the cut CF nerve of control, treadmill exercised, and estradiol treated male and female mice are shown in Figure 3.
Figure 3.
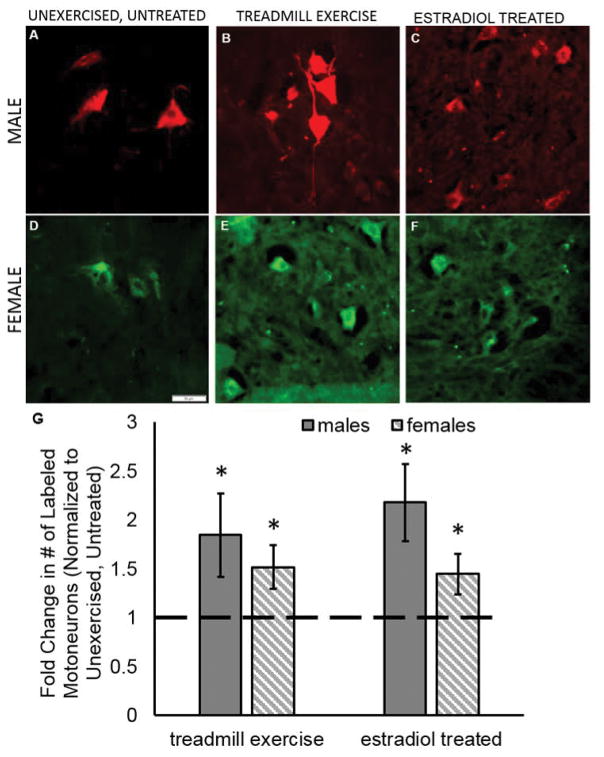
Exercise and estradiol treatments enhance the number of motoneurons that participate in regeneration in males and females. Sample pictographs from unexercised, untreated male (A) and female (D), treadmill exercise treated male (B) and female (E), and estradiol treated male (C) and female (F). Motoneurons were labeled 1.5 mm distal to the original cut site using either AlexaFluor 594 (red) or AlexaFluor 488 (green) conjugated tracers. Both retrograde tracers were used in both sexes, and no differences were found in the number of cells counted within a treatment group when separated by tracer. Scale bar 50 μm. (G) Treatment with estradiol or exercise using either the continuous paradigm (males; sold bar) or interval paradigm (females; hashed bar) significantly increased the number of labeled motoneurons compared to unexercised, untreated controls (dashed line). Data are normalized to unexercised, untreated control motoneuron counts. Each sex was normalized to the controls of the same sex. Errors bars, ± standard deviations. * p ≤ 0.001
Treadmill Exercise Enhances Participation Of Motoneurons In Regeneration In Both Continuous And Interval Training Paradigms
A previous study demonstrated that two weeks of treadmill exercise enhanced the number of tibial and CF motoneurons participating in regeneration and resulted in an increase in the number of motoneuron cell bodies containing retrograde tracer (English et al., 2009). However, the study found that CF neurons only show enhancement if the mice receive treadmill exercise using the interval paradigm and not the continuous paradigm. More recently it has been shown that the effectiveness in treadmill exercise is sex dependent (Wood et al., 2012). Previous studies did not differentiate between the sexes when administering the treadmill exercise paradigms. Therefore, we hypothesized that treadmill exercise would increase the number of CF motoneurons containing retrograde labeling if the treadmill exercise paradigm was administered appropriate to the sex of the mouse. We found that two weeks of treadmill exercise using the continuous paradigm for males increased the number of labeled motoneurons in the transection and surgical repair side by more than 1.8 times compared to untreated, unexercised controls (exercised: 251 ± 58.1; p ≤ 0.0001; Figure 3G). For females exercised using the interval paradigm, more than 1.5 times as many motoneurons were labeled compared to untreated, unexercised controls (exercised: 274.8 ± 40.4; p = 0.0003; Figure 3G). Consistent with previous reports (English et al., 2009), we found that treadmill exercise itself did not result in increased numbers of labeled motoneurons in the uncut side (Figure 2).
Estradiol Treatment Enhances Axon Regeneration Similar To Treadmill Exercise
Because sex differences in motoneuron axon elongation after peripheral nerve injury have been shown in response to treadmill exercise, we hypothesized that sex steroid hormones such as estradiol may be part of the signaling mechanism that produces the exercise-mediated enhancement in regeneration. The effectiveness of sustained release estradiol treatments was evaluated by comparing the weights of the uterine tissue in treated and untreated female mice. Uterine weights from estradiol treated females (223.2 ± 30.5 mg) were significantly heavier than those from untreated females (156.7 ± 10.3 mg; t(6.14) = −5.063, p ≤ 0.002). The increase in uterine weight is evidence that the estradiol treatment resulted in a systemic effect on ERs. We assume that the estradiol has similar effects on ERs in all tissues.
To test the effectiveness of estradiol to increase the number of motoneurons participating in axon regeneration, we compared the number of labeled motoneurons from mice treated with estradiol to untreated, unexercised controls. Estradiol treatment significantly increased the number of motoneurons containing retrograde tracer in both males (estradiol treatment: 296.7 ± 53.6; p ≤ 0.0001; Figure 3G) and females (estradiol treatment: 262.3 ± 37.1; p ≤ 0.001; Figure 3G). The number of motoneurons containing retrograde label in mice treated with estradiol was similar to the number found in mice treated with treadmill exercise (males, p = 0.06; females, p = 0.62). Both estradiol and exercise treatments increased the number of motoneurons containing retrograde label to approximately the same number as was counted in the intact motoneuron pools (Figure 2), suggesting that these treatments may be able to elicit the participation of a very large portion of the motoneurons able to participate in regeneration after peripheral axotomy. Estradiol treatment did not result in increased numbers of labeled motoneurons in the uncut side (males, p = 0.95; female, p = 0.06; Figure 2).
ER Antagonist Treatments at the Nerve Repair Site Block the Enhancing Effects of Exercise
To test the hypothesis that estradiol signaling may be important for the treadmill exercise-mediated enhancement in the number of motoneurons participating in regeneration, we treated mice with the ER antagonist ICI 181,780 (ICI) for two weeks and treated the mice with treadmill exercise using a sex appropriate paradigm. Inhibition of ER signaling via ICI treatment at the site of nerve repair prevented the increase in labelled motoneurons found in mice receiving treadmill exercise in males (ICI + exercise: 143.5 ± 27.4, p = 0.0003; Figure 4A & B) and females (ICI + exercise: 93.2 ± 16.8, p ≤ 0.0001; Figure 4C & D). Blocking ER signaling during continuous exercise in males reduced the number of motoneurons containing by approximately 56% compared to untreated males that were trained using the continuous paradigm. This number was comparable to the number of motoneurons counted in the untreated, unexercised group of males (Figure 4B). Blocking ER signaling during interval exercise in females produced an even greater reduction in the number of labeled motoneurons compared to untreated, exercised females. For the females, fewer motoneurons were counted in mice receiving interval exercise and ER antagonist than in untreated, unexercised mice (p ≤ 0.03; Figure 4D).
Figure 4.
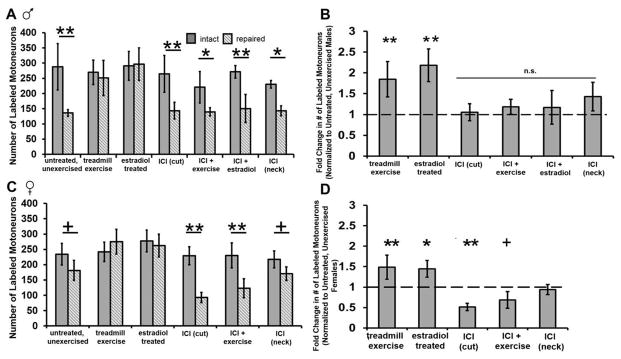
Blocking ER signaling using the ER antagonist ICI 182,780 (ICI) prevents the exercise-mediated increase in motoneuron participation in axon regeneration. (A) The mean number of motoneurons labeled by tracer application to the transected and repaired CF nerve 1.5 mm distal to the original cut site (hashed bars) and to the intact CF nerve (solid bars) for males (A) and females (B) are shown. Mean numbers of labeled motoneurons from the cut and repaired side normalized to untreated, unexercised male (C) and female (D) mice are shown. Values from untreated, unexercised mice are represented by the dashed line. Error bars, ± standard deviations. ** p ≤ 0.0001; * p ≤ 0.003; + p ≤ 0.05
To test the hypothesis that blocking ER signaling in unexercised mice would alter the number of motoneurons participating in regeneration, we treated a subset of mice with ICI 182,780 at the nerve transection site during surgical repair and did not treat them with exercise. For males, blocking ER signaling at the transection site did not alter the number of motoneurons participating in regeneration compared to untreated, unexercised males (p = 0.81) suggesting that ER signaling may not be important for regeneration in the absence of exercise (Figure 4). However, for females, ER antagonist treatment in female significantly reduced the number of motoneurons containing retrograde label compared to untreated, unexercised females (p = 0.002) suggesting that ER signaling in females is important for motoneuron regeneration (Figure 4).
To confirm the site of action of the ER antagonist, ICI 182,780 was mixed into fibrin glue and applied to the subcutaneous tissue at the nape of the neck in a subset of male and female mice during the initial nerve transection surgery. When applied to away from the site of nerve transection and repair, the ER antagonist had no effect on the number of motoneurons containing retrograde label (males: 162 ± 21.9; females: 186 ± 14.2; Figure 4). This suggests that the effects of the ER antagonist likely are specific to the site of nerve repair.
To test the location at which estradiol may be having its effects on motoneuron participation in regeneration, a subset of male mice were treated systemically with estradiol capsules and received an application of ICI 182,780 mixed with the fibrin glue at the site of nerve transection at the time of surgical repair. Blocking ER signaling at the site of the surgical repair blocked the enhancing effects of estradiol treatment (150.5 ± 21.9, p ≤ 0.0001; Figure 4A & B). This suggests that the ER signaling at the may be acting at the site of the nerve repair to exert its effects.
DISCUSSION
Modest exercise in the form of treadmill training results in a significant enhancement of axon regeneration in mice (Sabatier et al., 2008; English et al., 2009; Wilhelm et al., 2012). Sex steroids such as androgens and estrogens have been suspected to be involved in the exercise-mediated enhancement because the effects of the exercise are dependent on the sex of the participant (Wood et al., 2012). In this study we explored the involvement of ER signaling in the treadmill exercise-mediated enhancement in participation of motoneurons in axon regeneration after peripheral nerve transection injury using ICI 182,780, an ER antagonist. The main finding of this study is that in both sexes, the effects of exercise was eliminated completely with ER antagonist treatment indicating that ER signaling is required. In addition, ER signaling is important for regeneration, in the absence of exercise, for females.
Exogenous estradiol treatment increased the number of motoneurons participating in axon regeneration in males and females. This finding is consistent with previous studies that have shown that the estrogens can enhance axon regeneration after peripheral nerve crush and transection injuries (Tanzer and Jones, 1997; Jones et al., 2000; Islamov et al., 2002; McMurray et al., 2003; Murashov et al., 2004; Huppenbauer et al., 2005; Sharma et al., 2009; Sekiguchi et al., 2012). Application of exogenous estradiol enhances the number of axon profiles found in the distal stump (Islamov et al., 2002), the number of motoneurons whose axons begin to regenerate (Murashov et al., 2004), the length of axon profiles (Tanzer and Jones, 1997; Sharma et al., 2009) and as shown in the current study, the number of motoneurons whose axons begin to regenerate. Blocking ER signaling at the site of nerve repair prevented the enhancing effects of systemic exogenous estradiol administration on motoneuron participation. Thus we conclude that the effects of estradiol on regenerating axons is dependent on ER signaling local to the site of the nerve cut and surgical repair.
Estrogen signaling at the site axon regeneration is important for motoneuron axon regeneration in the absence of exercise for females. Blocking ERs at the surgical repair site significantly reduced the number of motoneurons whose axons regenerated at least 1.5 mm two weeks after transection in unexercised females. Interestingly, males did not have the same reduction in motoneuron participation suggesting that estradiol may not be as important for regeneration of those axons in the absence of exercise.
The involvement of ER signaling in axon regeneration at the site of nerve repair is not surprising as previous studies have reported ERs in the sciatic nerve (Jung-Testas et al., 1994; Fex Svenningsen and Kanje, 1999; Islamov et al., 2003; Altun and Kurutas, 2015). After sciatic nerve crush injury, mRNA for ERs α and β have been shown to increase in expression and accumulate in the somas as well as the peripheral axons of axotomized motoneurons in the regenerating sciatic nerve (Islamov et al., 2003). Although ERs can be found in motoneurons, the identity of the cells in which this critical ER signaling takes place and the relationship between the signaling and exercise are not completely understood. The ability of the ER antagonist to block the estradiol- and exercise-mediated enhancements of motoneuron participation in axon regeneration when applied to the site of nerve cut and repair but not when applied to other regions of the body away from the site of nerve injury suggests that estradiol is acting at the site of transection to produce its effects. It is possible for ERs on other cells at the nerve repair site, such as Schwann cells, to effect axon regeneration as these peripheral glial cells have been shown to have ERs as well (Jung-Testas et al., 1994; Fex Svenningsen and Kanje, 1999; Altun and Kurutas, 2015). The promotion of axon regeneration mediated by ER signaling could occur at the site of nerve transection via a non-genomic ERK-activated signaling pathway (Islamov et al., 2003; Murashov et al., 2004) and by partially downregulating PTEN, a negative regulator of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Smith et al., 2009). Inhibiting PTEN has been shown to promote robust sprouting of neurons (Christie and Zochodne, 2013).
English and colleagues (Wood et al., 2012; Thompson et al., 2014) have shown that exercise-mediated elongation in axons after sciatic nerve transection and surgical repair in mice is dependent on androgen signaling. Male mice trained using the continuous exercise paradigm have significantly increased serum testosterone levels after two weeks of exercise (Wood et al., 2012). Blocking this increase by castration (Wood et al., 2012) or by treating mice systemically with the androgen receptor antagonist flutamide (Thompson et al., 2014) blocks the enhancing effects of treadmill exercise, suggesting a role for androgen signaling in exercise-mediated effects on axon regeneration. The current study demonstrates that blocking estrogens but not androgens prevents the enhancing effects of treadmill exercise. Taken together, these findings suggest that both androgens and estrogens are involved in the process of axon regeneration. Several studies have found that treating mice with a non-aromatizable form of testosterone produces less enhancement of axon elongation than treating mice with the aromatizable form of testosterone, suggesting that androgens work synergistically with estrogen signaling to enhance axon regeneration (Tanzer and Jones, 1997; Sharma et al., 2009). It is possible that with treadmill exercise, both androgens and estrogens are working synergistically to mediate the effects of exercise on axon regeneration. These sex steroids could be effecting different cell types or have different locations of action. Alternatively, they could both share similar mechanisms of action as both have been shown to alter brain derived neurotrophic factor (BDNF), a protein known to mediate axon regeneration.
Blocking ER signaling during treadmill exercise prevents the exercise-mediated enhancement in motoneuron participation in axon regeneration. The mechanism by which ER signaling may be promoting axon regeneration is unclear and likely involves several different molecules. BDNF may have a role in this process as BDNF production is thought to be regulated by increases in neuronal activity during exercise (Berchtold et al., 2001) and is downstream of neuronal androgen (Sharma et al., 2010) and estrogen signaling (Berchtold et al., 2001). Estrogen and exercise have been shown to interact to regulate BDNF mRNA and protein expression in the hippocampus (Berchtold et al., 2001; Berchtold et al., 2002). It is possible that similar interactions between estrogens and BDNF exist in the spinal cord and may mediate the effects of exercise after peripheral nerve transection. BDNF is required for the enhancing effects of treadmill exercise on axon regeneration after transection (Wilhelm et al., 2012). Both continuous and interval treadmill exercise paradigms increase BDNF mRNA expression in the motoneurons (Wilhelm et al., 2012; Wood et al., 2012). Reducing BDNF expression in regenerating neurons or in Schwann cells in the distal stump prevents the enhancement of axon regeneration by exercise (Wilhelm et al., 2012). Future studies should examine the link between BDNF and estrogen signaling in peripheral nerve regeneration.
It is likely that complex interactions exit between regenerating axons and the regenerative environment. Based on the results of the present studies as well as previously published (Wilhelm et al., 2012; Wood et al., 2012; Thompson et al., 2014), we conclude that estrogens and androgens likely interact with BDNF to mediate the enhancing effects of treadmill exercise on motoneuron regeneration. By improving our understanding of the mechanisms that underlie the effects of treadmill exercise on axon regeneration, we can begin to develop and improve pharmacological treatments for patients that are not able to engage in exercise after traumatic nerve injury.
Acknowledgments
Thanks to Lindsay Wright, Sandy Pang, Carly Phillips, and Travis Green for technical assistance. This work was supported financially in part by the College of Charleston Undergraduate Research and Creative Activities, a grant to the College of Charleston from the Howard Hughes Medical Institute through the Pre-College and Undergraduate Science Education Program, and a grant from the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R15NS099983. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations. The authors have no conflicts of interest to report.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Altun I, Kurutas EB. G Protein-Coupled Estrogen Receptor Levels After Peripheral Nerve Injury in an Experimental Rat Model. World Neurosurg. 2015;84:1903–1906. doi: 10.1016/j.wneu.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Cotman CW. Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J Neurosci Res. 2002;68:511–521. doi: 10.1002/jnr.10256. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J Neurobiol. 2001;49:314–325. doi: 10.1002/neu.10013. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie KJ, Zochodne D. Peripheral axon regrowth: new molecular approaches. Neuroscience. 2013;240:310–324. doi: 10.1016/j.neuroscience.2013.02.059. [DOI] [PubMed] [Google Scholar]
- Coirini H, Gouezou M, Liere P, Delespierre B, Pianos A, Eychenne B, Schumacher M, Guennoun R. 3 Beta-hydroxysteroid dehydrogenase expression in rat spinal cord. Neuroscience. 2002;113:883–891. doi: 10.1016/s0306-4522(02)00224-5. [DOI] [PubMed] [Google Scholar]
- de Vries J, Menovsky T, van Gulik S, Wesseling P. Histological effects of fibrin glue on nervous tissue: a safety study in rats. Surg Neurol. 2002;57:415–422. doi: 10.1016/s0090-3019(02)00736-x. discussion 422. [DOI] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Sabatier M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J Comp Neurol. 2009;517:245–255. doi: 10.1002/cne.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Ward PJ. Exercise, neurotrophins, and axon regeneration in the PNS. Physiology (Bethesda) 2014;29:437–445. doi: 10.1152/physiol.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex Svenningsen A, Kanje M. Estrogen and progesterone stimulate Schwann cell proliferation in a sex- and age-dependent manner. J Neurosci Res. 1999;57:124–130. doi: 10.1002/(SICI)1097-4547(19990701)57:1<124::AID-JNR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J Neurosci. 2005;25:4004–4013. doi: 10.1523/JNEUROSCI.5279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islamov RR, Hendricks WA, Jones RJ, Lyall GJ, Spanier NS, Murashov AK. 17Beta-estradiol stimulates regeneration of sciatic nerve in female mice. Brain Res. 2002;943:283–286. doi: 10.1016/s0006-8993(02)02827-5. [DOI] [PubMed] [Google Scholar]
- Islamov RR, Hendricks WA, Katwa LC, McMurray RJ, Pak ES, Spanier NS, Murashov AK. Effect of 17 beta-estradiol on gene expression in lumbar spinal cord following sciatic nerve crush injury in ovariectomized mice. Brain Res. 2003;966:65–75. doi: 10.1016/s0006-8993(02)04191-4. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Alexander TD, Brown TJ, Tanzer L. Gonadal steroid enhancement of facial nerve regeneration: role of heat shock protein 70. J Neurocytol. 2000;29:341–349. doi: 10.1023/a:1007157105835. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Actions of steroid hormones- and growth factors on glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1994;48:145–154. doi: 10.1016/0960-0760(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Lenth RV. Java Applets for Power and Sample Size (2006–9) [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg. 2003;18:480–485. doi: 10.1046/j.0886-0440.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- McMurray R, Islamov R, Murashov AK. Raloxifene analog LY117018 enhances the regeneration of sciatic nerve in ovariectomized female mice. Brain Res. 2003;980:140–145. doi: 10.1016/s0006-8993(03)02984-6. [DOI] [PubMed] [Google Scholar]
- Murashov AK, Islamov RR, McMurray RJ, Pak ES, Weidner DA. Estrogen increases retrograde labeling of motoneurons: evidence of a nongenomic mechanism. Am J Physiol Cell Physiol. 2004;287:C320–326. doi: 10.1152/ajpcell.00542.2003. [DOI] [PubMed] [Google Scholar]
- Patte-Mensah C, Penning TM, Mensah-Nyagan AG. Anatomical and cellular localization of neuroactive 5 alpha/3 alpha-reduced steroid-synthesizing enzymes in the spinal cord. J Comp Neurol. 2004;477:286–299. doi: 10.1002/cne.20251. [DOI] [PubMed] [Google Scholar]
- Rakotoarivelo C, Petite D, Lambard S, Fabre C, Rouleau C, Lumbroso S, de Weille J, Privat A, Carreau S, Mersel M. Receptors to steroid hormones and aromatase are expressed by cultured motoneurons but not by glial cells derived from rat embryo spinal cord. Neuroendocrinology. 2004;80:284–297. doi: 10.1159/000083611. [DOI] [PubMed] [Google Scholar]
- Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. J Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, To BN, Nicolini J, English AW. Effect of axon misdirection on recovery of electromyographic activity and kinematics after peripheral nerve injury. Cells Tissues Organs. 2011;193:298–309. doi: 10.1159/000323677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer V, Meyer L, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Sciatic nerve injury induces apoptosis of dorsal root ganglion satellite glial cells and selectively modifies neurosteroidogenesis in sensory neurons. Glia. 2010;58:169–180. doi: 10.1002/glia.20910. [DOI] [PubMed] [Google Scholar]
- Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009;25:339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- Sekiguchi H, Ii M, Jujo K, Renault MA, Thorne T, Clarke T, Ito A, Tanaka T, Klyachko E, Tabata Y, Hagiwara N, Losordo D. Estradiol triggers sonic-hedgehog-induced angiogenesis during peripheral nerve regeneration by downregulating hedgehog-interacting protein. Lab Invest. 2012;92:532–542. doi: 10.1038/labinvest.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Coughlin L, Porter RG, Tanzer L, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Effects of electrical stimulation and gonadal steroids on rat facial nerve regenerative properties. Restor Neurol Neurosci. 2009;27:633–644. doi: 10.3233/RNN-2009-0489. [DOI] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2010;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Smith EDD, Davidson J. Hormone Administration: Peripheral and Intracranial Implants. New York: Academic Press; 1977. [Google Scholar]
- Smith JA, Zhang R, Varma AK, Das A, Ray SK, Banik NL. Estrogen partially down-regulates PTEN to prevent apoptosis in VSC4.1 motoneurons following exposure to IFN-gamma. Brain Res. 2009;1301:163–170. doi: 10.1016/j.brainres.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer L, Jones KJ. Gonadal steroid regulation of hamster facial nerve regeneration: effects of dihydrotestosterone and estradiol. Exp Neurol. 1997;146:258–264. doi: 10.1006/exnr.1997.6529. [DOI] [PubMed] [Google Scholar]
- Thompson NJ, Sengelaub DR, English AW. Enhancement of peripheral nerve regeneration due to treadmill training and electrical stimulation is dependent on androgen receptor signaling. Dev Neurobiol. 2014;74:531–540. doi: 10.1002/dneu.22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JC, Copley TA, Acosta MC, Harrell JL. Estrogen signaling is necessary for the exercise-mediated increase in motoneuron participation in axon regeneration after peripheral nerve injury in mice. Society for Neuroscience; Chicago, IL: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32:5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K, Wilhelm JC, Sabatier MJ, Liu K, Gu J, English AW. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev Neurobiol. 2012;72:688–698. doi: 10.1002/dneu.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MD, Kemp SW, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Ann Anat. 2011;193:321–333. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]