Abstract
Strong tolerance to HBV surface antigens limits the therapeutic effect of the conventional HBsAg vaccination in both preclinical animal models and patients with chronic hepatitis B (CHB) infection. In contrast, we observed that clinical CHB patients presented less immune tolerance to the preS1 domain of HBV large surface antigen. To study whether targeting the weak tolerance of preS1 region could improve therapy gain, we explored vaccination with the long peptide of preS1 domain for HBV virions clearance. Our study showed that this preS1-polypeptide rather than HBsAg vaccination induced robust immune responses in the HBV carrier mice. The anti-preS1 rapidly cleared HBV virions in vivo and blocked HBV infection to hepatocytes in vitro. Intriguingly, vaccination of preS1-polypeptide even reduced the tolerized status of HBsAg, opening a therapeutic window for the host to respond to the HBsAg vaccine. A sequential administration of antigenically distinct preS1-polypeptide and HBsAg vaccines in HBV carrier mice could finally induce HBsAg-HBsAb serological conversion and clear chronic HBV infection in the carrier mice. These results suggest that preS1 can function as a therapeutic vaccination for the control of CHB.
Keywords: Vaccine, PreS1 domain, HBV surface antigens (HBsAg), Immune tolerance, HBV carrier mice
Introduction
Persistent HBV infection still represents a substantial threat to the public health, despite the existence of effective prophylactic vaccines. More than 2 billion people are infected with hepatitis B virus (HBV), and 350 million become chronic HBV carriers worldwide. Nearly one million people die from hepatitis B-related diseases every year (1). Thus, there remains an urgent need for effective treatment strategies to limit the enormous burden of viral hepatitis on global health. The HBV genome encodes three overlapping viral surface antigens, named small (S), middle (M), and large (L) proteins respectively. “S protein”, known as HBsAg, is the common C-terminal domain of these three proteins, which is the most abundant surface antigen. M protein is derived from a transcript initiating at the upstream start codon of HBsAg. L protein is composed of the N-terminal polypeptide preS1domain and the adjacent M protein (2). HBsAg is the most active component of conventional HBV vaccines. Although it elicits strong immunogenicity as a preventive vaccine in healthy people, the current HBsAg vaccines cannot induce anti-HBs for viral-clearance in CHB patients (3). High levels of viral antigens in circulation have been shown to induce host immune tolerance with impaired DC, NK or T cell, B cell functions, and thus contribute to HBV persistence (4-7). How to break or bypass the immune tolerance and induce anti-HBV immune responses is still a major challenge in the development of HBV therapeutic vaccines.
The correlation between high viral antigen load and the dysfunction of the immune system in chronic infections has been documented (7, 8). A recent study further showed that the threshold of antigen expression is the dominant factor in determining the fate of T-cell in liver (9). However, in certain mouse models, studies also demonstrated that some antigens of HBV could induce effective immune responses because of the trace expression quantity or high immunogenicity, like the HBV non-structure protein polymerase (10) and the “non-tolerogen” HBcAg (11-13). These implied the potential alternative HBV vaccine candidates other than the tolerized HBsAg and HBeAg antigens. In HBV infection, the defective viral particles containing HBsAg generally outnumber infectious HBV virions by up to 1,000:1 (2). Unlike HBsAg, preS1 region exists primarily in mature infectious HBV virions and thus its level is much lower than that of HBsAg (14). Besides, preS1 domain mediates the viral interaction with the cellular receptor for hepatocyte entry (15, 16) and plays essential roles in the assembly and release of HBV virions (17), making it a potential target for HBV therapy.
Though the B-cell and T-cell epitopes of preS1 sequence are well-characterized (18-20) and preS1 peptides have been shown to protect from HBV infection to chimpanzee (21), the tolerized state of preS1 and its unique contribution for vaccination in chronic HBV infection has not been well defined. Since preS1 domain contains HBV binding epitopes for cell entry and exists at much lower levels than HBsAg, we sought to determine whether it presented a much weaker tolerized status than that of HBsAg in CHB hosts, and thus may be feasible to efficiently induce immune responses to preS1 domain for protective anti-HBV immunity. Indeed, we observed both antibody and specific T cell responses to preS1 region other than HBsAg in clinical CHB patients. The anti-preS1 antibody correlated well with the reduction of preS1 and HBV-DNA. In the further study, a murine CHB model with persistent viremia and immune tolerance to the viral antigens was used (22, 23). We observed that preS1- polypeptide was a potential immunogen in HBV carrier mice and its vaccination could clear HBV virions. Unexpectedly, we observed that preS1-polypeptide vaccination even reduced the tolerized status of HBsAg, providing a potential therapeutic strategy for treating CHB patients.
Results
PreS1 domain presents more immunogenicity than HBsAg in clinical CHB patients
To dissect the tolerized status towards preS1 domain and HBsAg in HBV infection, we compared the levels of antigens and antibodies in clinical HBV patients. We observed that preS1 antigen presented in much less quantity (∼10 fold) than HBsAg in serum (Fig. 1A). Anti-preS1, but not anti-HBs, was detectable in both AHB and CHB patients (Fig. 1B), corresponding to the specific T cell response to preS1 domain but not HBsAg in CHB patients (Fig. 1C). All these suggest that CHB hosts have less tolerance towards preS1 domain than HBsAg.
Fig. 1. PreS1 domain is less immune tolerized than HBsAg in CHB patients.
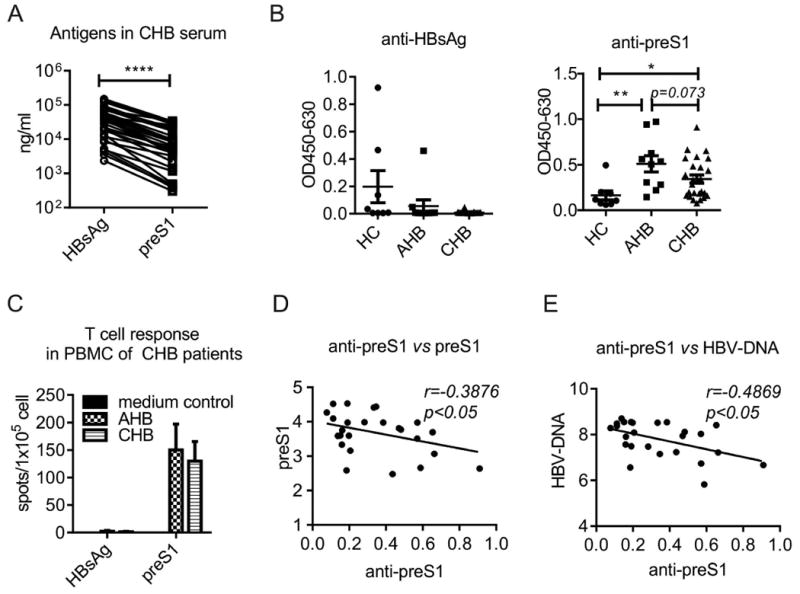
(A) HBsAg and preS1 antigen levels in CHB patients (n=25) were tested by ELISA. The correlation between HBsAg and preS1 was analyzed using two-tailed correlation test. (B) Anti-HBs and anti-preS1 in HC (n=8), AHB (n=10) and CHB (n=25) were tested by ELISA (Dilution 1:10). (C) PBMCs from patients (AHB, n=2; CHB, n=14) were collected and then stimulated with 5ug/ml HBsAg or preS1. Specific T cell response to HBsAg and preS1 were tested by IFN-γ secreting ELISPOT. (D) and (E) In CHB patients, the correlation between anti-preS1 (OD450-630) and preS1 (log10 ng/mL) and the correlation between anti-preS1 (OD450-630) and HBV-DNA (log10 IU/mL) were analyzed. Error bars in data represent mean ± SEM. *p < 0.05, **p < 0.01, ****p < 0.0001 by two-tailed correlation test (A,D and E) or unpaired t test (B).
In clinic, the appearance of anti-preS1 in patients implies a better recovery from acute hepatitis B (AHB) (24-26). So we investigated whether there were relationships between anti-preS1 and reduction of HBV infection. We observed that anti-preS1 was negatively correlated with preS1 antigen (Fig. 1D), as well as HBV-DNA (Fig. 1E). The results implied that the immune responses to preS1 domain might be associated with a potential recovery from CHB infection. All these raise the possibility that preS1, presenting in less quantity, together with its essential role in covering HBV-DNA, might serve as an immunogenic antigen and thus be an effective therapeutic vaccine to clear HBV virions in CHB infection.
The level of preS1 antigen is significant lower than that of HBsAg in the murine HBV model
We have reported a murine model with i.v infection of AAV-HBV1.3, which partially mimics immunological characteristics of chronic HBV infection (22, 23). The tolerance to HBsAg was induced by its level in serum and could be reversed by reducing its titer with a neutralizing monoclonal antibody (mAb), leading to immune responses to the conventional HBsAg vaccine (27). We compared the antigen levels of preS1 and HBsAg in the same model. PreS1 antigen was 10-fold lower than HBsAg in the peripheral (Fig. 2A), regardless of the HBV infection doses (Fig. 2B), similar to that in the clinical patients. We also observed that preS1 antigen presented in much less quantity than HBsAg in liver (Fig. 2C).
Fig. 2. PreS1 antigen is much lower than HBsAg in HBV carrier mice.
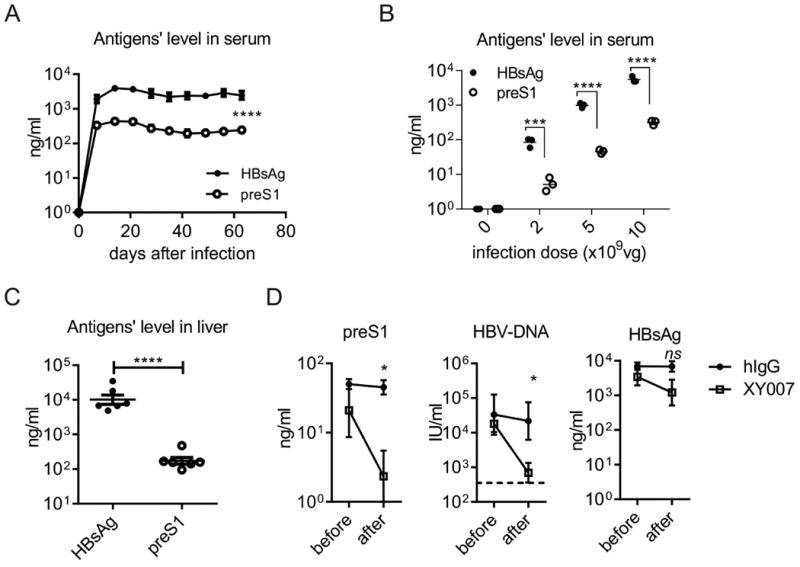
(A) C57BL/6 mice (n=4/group, 6-8 weeks old, male) were i.v. infected with 1×1010 viral genome equivalents(vg) of AAV-HBV1.3 viruses in 200 μl saline. Blood samples were collected every week after infection. The antigen levels of HBsAg and preS1 were detected by ELISA. (B) Three doses of virus ranging from 2×109 to 1×1010vg were delivered to C57BL/6 mice (n=3/group, 6-8 weeks old, male). Four weeks later, the antigen level in the serum was measured by ELISA. (C) C57BL/6 mice were infected with 1×1010 vg of viruses. The mice were sacrificed to collect liver tissue 8 weeks later. The protein levels of preS1 antigen and HBsAg in grinded tissue were detected by ELISA. (D) The carrier mice were i.p injected with 200ug monoclonal antibody XY007 specific to preS1 domain, and one day later, preS1 and HBsAg antigen levels in serum were tested by ELISA, HBV-DNA in serum was extracted and was tested by qPCR as the manufacture mentioned. One representative result out of three independent experiments for panel A (N=12/group), or four for panel B (N=16/group), or three for panel D (N=15/group) is shown. Error bars in data represent mean ± SEM. “ns” means “no significant difference”. *p < 0.05, ***p < 0.001, ****p < 0.0001 by two-way ANOVA (A) or unpaired t test (B, C, D).
PreS1 domain has been reported to be contained mainly on HBV virions in clinical samples (14). To confirm this in the murine model, HBV carrier mice were i.p. injected with the mAb XY007 specific to preS1 sequence. We observed that XY007 efficiently clear both serum preS1 antigen and HBV-DNA to an undetectable level, but there was no significant change for the level of HBsAg (Fig. 2D). Thus, preS1 antigen presents in low quantity in the HBV murine model, similar to that in CHB patients. All these raises the possibility that preS1 might function as a potential breakthrough point for breaking HBV immune tolerance.
PreS1 domain is not tolerized in HBV carrier mice
We first determined the immunogenicity of preS1 domain by vaccinating C57BL/6 mice with preS1-polypeptide formulated in the indicated adjuvants (fig. S1). Then the immune responses of preS1-polypeptide vaccine and the conventional HBsAg vaccine were compared in HBV carrier mice. As mentioned, though HBsAg vaccine elicits strong immune response in WT mice, it did not induce HBsAb seroconversion in carrier mice (Fig. 3A). And there was no specific T cell response to HBsAg induced in contrast to its robust immune response observed in WT mice (Fig. 3B). Thus the induction of HBsAb is a challenging clinical goal for the conventional HBsAg vaccine to achieve because of the immune tolerance induced the circulating HBsAg.
Fig. 3. Unlike HBsAg, preS1 domain is not tolerized in HBV carrier mice.
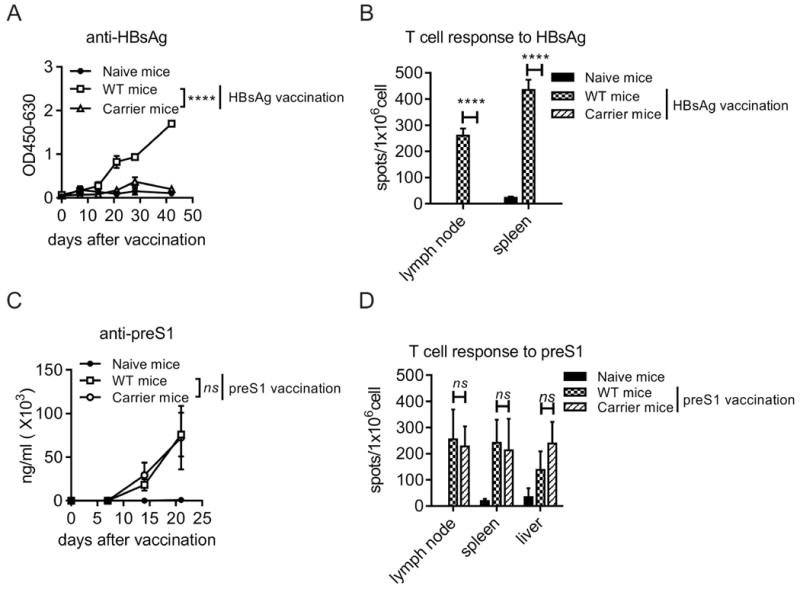
C57BL/6 (n=4/group, 6-8 weeks old, male) WT and stable HBV carrier mice were vaccinated with preS1-polypeptide or HBsAg formulated in IFA adjuvant, respectively. (A) Anti-HBs in serum after HBsAg vaccination in WT and carrier mice were determined at the indicated time points by ELISA. (B) In HBsAg vaccinated WT and carrier mice, 2×105 lymphocytes from lymph node and spleen were collected 28 days after the 2nd vaccination. Then the cells were stimulated with 5ug full-length HBsAg or BSA, and the specific T cell responses to HBsAg were tested by IFN-γ ELISPOT. (C) Anti-preS1 in serum after preS1-polypeptide vaccination in WT and carrier mice were determined at the indicated time points by ELISA. (D) In preS1-polypeptide vaccinated WT and carrier mice, 2×105 lymphocytes from lymph node, spleen and liver were collected 28 days after the 2nd vaccination. Then the cells were stimulated with 5ug preS1 polypeptide or BSA. The specific T cell responses to preS1 domain were tested by IFN-γ ELISPOT. One representative result out of three (WT mice, N=14) or four (Carrier mice, N=14) independent experiments is shown. Error bars in data represent mean ± SEM. “ns” means “no significant difference”. ****p < 0.0001 by two-way ANOVA (A, C) or unpaired t test (B, D).
However, unlike the tolerized HBsAg antigen, HBV carriers that were vaccinated with preS1-polypeptide generated specific antibodies to preS1 domain, the level of which is comparable to that in WT mice (Fig. 3C). We also determined the specific T cell responses to preS1 region in dLN, spleen and liver after preS1-polypeptide vaccination, all of which were similar to the responses developed in WT mice (Fig. 3D). These results suggest that, contrary to HBsAg, preS1 domain is not a viral tolerogen in the HBV carrier model and could be used as a therapeutic vaccine for CHB infection.
PreS1-polypeptide vaccination effectively prevents HBV infection
In clinical setting, preS1 domain alone had never been used as a preventive vaccine for HBV infection. To determine whether preS1-polypeptide vaccination could be used for HBV prevention, mice were inoculated s.c. with preS1-polypeptide as the indicated time schedule in Fig. 4A. This vaccination induced preS1-specific antibody (Fig. 4B) and cleared the preS1 antigens in serum after AAV-HBV infection, similar to the clinical HBsAg vaccination (Fig. 4C). Most importantly, the HBV DNA in the serum was also undetectable at the end of the experiment (Fig. 4D), indicating the viral clearance by endogenous antibody to preS1 domain. These results demonstrate that preS1-polypeptide vaccination can effectively prevent HBV infection in vivo.
Fig. 4. PreS1-polypeptide functions as an effective preventive vaccine for HBV infection.
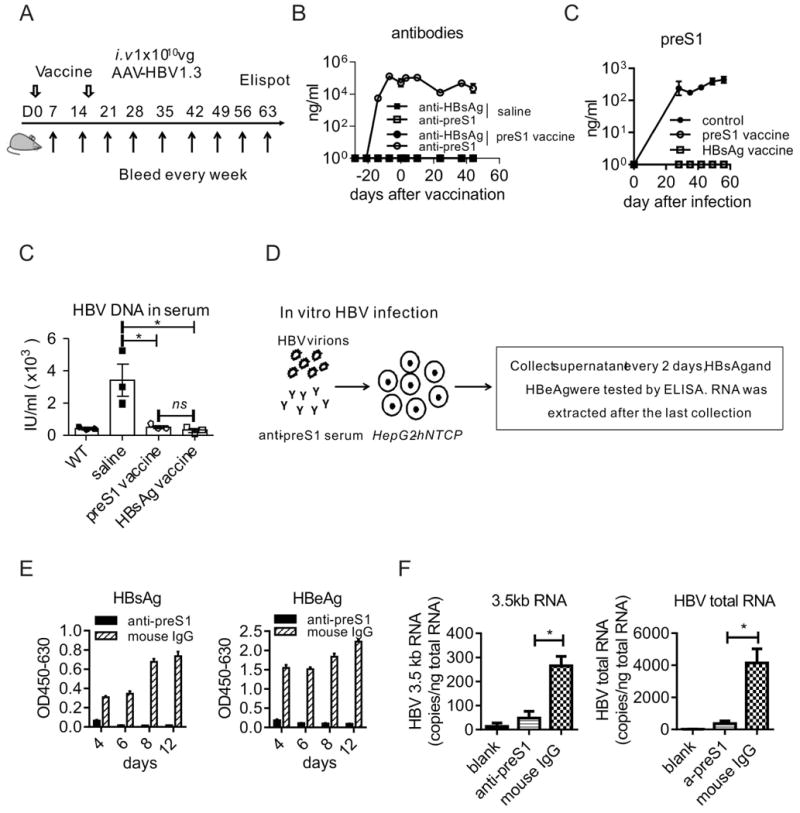
(A) Time schedule for preS1-polypeptide vaccination to prevent HBV infection. (B) Only anti-preS1 but no anti-HBs, was detected after preS1 vaccination and HBV infection in preS1-polypeptide vaccinated mice. (C) The preS1 antigen level in serum after HBV infection were tested by ELISA. (D) The HBV-DNA in serum was extracted per the manufacturer's instructions and tested by qPCR. (E) A schematic diagram of blocking HBV infection to hepatocytes in vitro. Briefly, HepG2-hNTCP cells were inoculated with 1×107 genome equivalents of HBV in the presence of anti-preS1 serum or control mouse IgG, and incubated for 24 hours. Then, the cells were washed with medium three times and maintained in PMM medium. The supernatant of the culture was collected and medium was changed every 2 days. The levels of HBsAg (F) and HBeAg (G) in the supernatant were measured by ELISA. HBV viral RNAs in infected cells were extracted at the indicated time points, the HBV specific 3.5kb RNA(H) and HBV total RNA(I) were quantified by RT-qPCR. One representative result out of three independent experiments is shown for panel A, B, C, and D (N=12/group) and for panel E, F, G, H, I (N=18 wells/group). Error bars in data represent mean ± SEM. “ns” means “no significant difference”. *p < 0.05 by unpaired t test (D, H, I).
As HBV is a hepadnavirus that infects only humans and a few primates (28), mouse models cannot be used to test the direct HBV infection/re-infection to the host liver in vivo. To investigate whether the preS1 antisera could block HBV entry/re-entry to hepatocytes, we infected HepG2-hNTCP cells with HBV viruses in the presence of anti-preS1 serum and incubated for 24h (Fig. 4E), as reported previously (16). The production of both HBsAg and HBeAg in the cell culture media was significantly reduced when anti-preS1 was added, but not the control antibody (Fig. 4F and 4G). It indicated the HBV infection to hepatocytes was blocked by the antisera to preS1 domain. This was further demonstrated by the reduction of HBV viral replicative intermediates, including the 3.5 kb HBV RNA and the total HBV RNA (Fig. 4H and 4I). To further confirm that anti-preS1 alone could inhibit the infection and subsequent replication of HBV in hepatocytes, we repeated the experiment by mAb XY007 (fig. S2). Thus, using the murine HBV model and HepG2-hNTCP infection system, we demonstrated that preS1-polypeptide was potentially an effective vaccine for preventing HBV infection.
PreS1-polypeptide serves as a therapeutic vaccine in HBV carrier mice
To determine the therapeutic effects of preS1 polypeptide vaccine, we challenged the HBV carrier mice with preS1 vaccine, and boosted with the same dose 14 days later, antigen and antibody responses were tested at the indicated time points (Fig. 5A). As mentioned, HBsAg vaccine can't induce immune response in HBV carrier mice. Even on 35 days post-vaccination with HBsAg, none of the carrier mice showed a significant reduction in preS1 antigen (Fig. 5B), and HBV-DNA in serum (Fig. 5C). On the contrary, preS1-polypeptide vaccination induced anti-preS1 immune response, and then preS1 antigen decreased sharply after the induction of anti-preS1. It was cleared completely on day 14 post vaccine boost (Fig. 5B). Moreover, the anti-preS1 resulted in HBV-DNA clearance in the serum (Fig. 5C). The in vitro HBV inhibition assay showed that the anti-preS1 in HBV carrier mice resulted in a significant reduction in the secretion of HBsAg and HBeAg in the HepG2-hNTCP cells in vitro system (Fig. 5D). The RNA levels of HBV in HepG2-hNTCP also indicated that anti-preS1 induced in carrier mice blocked HBV infection/re-infection to hepatocytes efficiently (Fig. 5E).
Fig. 5. PreS1-polypeptide functions as therapeutic vaccine in HBV in carrier mice.
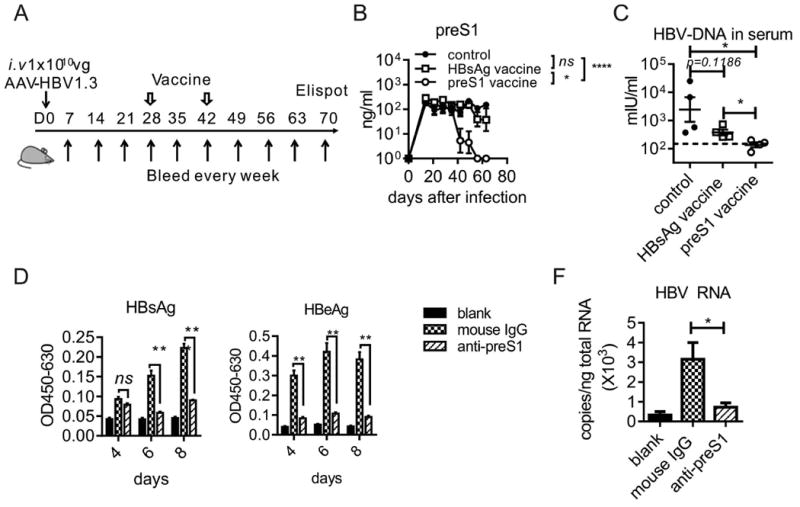
(A) Time schedule for testing preS1 antigen tolerance in HBV carrier mice. C57BL/6 (n=4/group, 6-8 weeks old, male) WT and stable HBV carrier mice were vaccinated with preS1-polypeptide or HBsAg formulated in IFA adjuvant, respectively. (B) After preS1-polypeptide or HBsAg vaccination in carrier mice, preS1 antigen level in serum were determined by ELISA at the indicated time points. (C) HBV-DNA in serum of each group was extracted and determined by qPCR. (D) Anti-sera induced in HBV carrier mice with preS1 vaccination blocked HBV infection to HepG2-hNTCP in vitro. The levels of HBsAg and HBeAg in the supernatants were measured by ELISA at the indicated time points. (E) The total RNAs of the infected cells were extracted on day 10 of HBV infection in vitro, and the HBV specific RNAs were measured with RT-qPCR. One representative result out of four independent experiments is shown for panel A and B (N=21/group), three for panel C (N=12/group), and three for panel D and E (N=12 wells/group). Error bars in data represent mean ± SEM. “ns” means “no significant difference”. *p < 0.05, **p<0.01, ***p < 0.001, ****p < 0.0001 by two-way ANOVA (B) or unpaired t test (C, D, E).
We repeated the experiment in the HLA-A2.1/ transgenic HBV carrier mice model (23). The same as in C57BL/6 carrier model, vaccination with preS1 polypeptide rather than HBsAg induced the clearance of the preS1 antigen. The ELISPOT results for T cell response corresponded well to the antibody response (fig. S3). Together, these results indicated that preS1 antigen is not tolerized in HBV carrier mice. The viral-specific immune response to preS1 domain can clear HBV viral particle and potentially block the HBV infection/ re-infection to hepatocytes.
Administration of preS1-polypeptide partially restores host immune responses to HBsAg
In the study of applying preS1-polypeptide as preventive vaccination for HBV infection, we unexpectedly observed that the level of HBsAg significantly decreased (Fig. 6A). However, we could not detected significant anti-HBsAg in the serum (Fig.4B). The decrease but not clearance of HBsAg may result from the weak anti-HBsAg response induced by preS1-polypeptide vaccination, which couldn't completely neutralize the large scale HBsAg circulating in the serum. To test this hypothesis, we detected the specific B cell response to HBsAg by ELISPOT and observed that preS1-polypeptide vaccination indeed induced HBsAg-specific antibody response (Fig. 6B). As preS1 domain is always coexist with HBsAg in HBV L-HBsAg during infection, the non-tolerized preS1 specific T cells could cross-reactivate the tolerized B cells to HBsAg (20), thus might partially restore the host immune response toward HBsAg. To test the hypothesis, we detected the serum HBsAg in carrier mice after preS1 vaccination. Intriguingly, the preS1-polypeptide vaccination definitely reduced HBsAg (Fig. 6C) and induced the specific B cell response to HBsAg even in HBV carrier mice (Fig. 6D).
Fig. 6. PreS1-polypeptide vaccination induce the B-cell response to HBsAg in HBV mice.
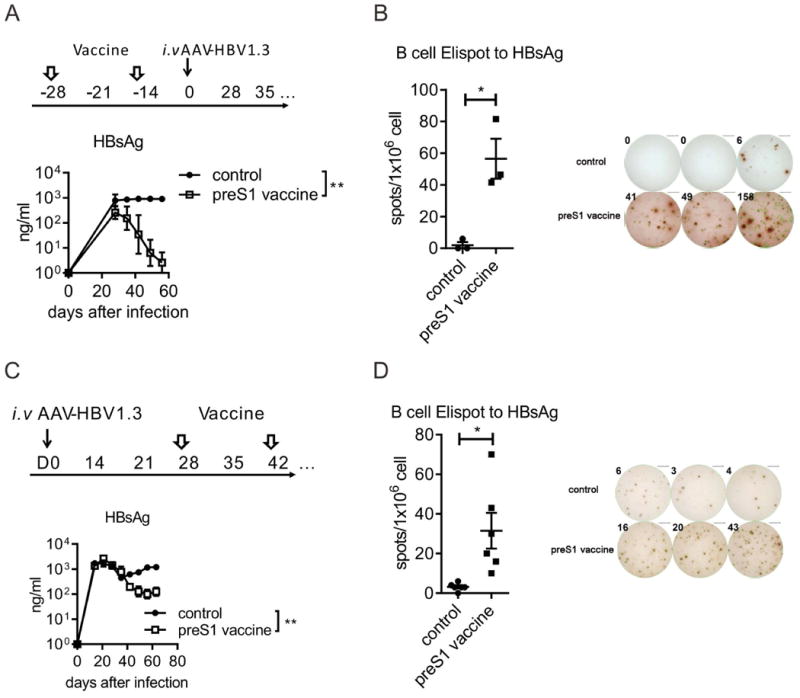
(A) After preS1-polypeptide vaccination, mice were infected with HBV, the level of HBsAg in serum was tested by ELISA, which indicated that preS1 pre-vaccination diminished HBsAg level in serum. (B) In the preS1-prevaccinated mice, 2×105 splenocytes were collected 28 days after HBV infection. The specific B cell response to HBsAg was tested by B cell-ELISPOT assay. (C) In HBV carrier mice, preS1-polypeptide was vaccinated at day 28 and 42 after AAV-HBV inoculation. The HBsAg variation in carrier mice were tested by ELISA. (D) In preS1-polypeptide vaccinated HBV carrier mice, 2×105 splenocytes were collected. The specific B cell response to HBsAg was tested by B cell-ELISPOT assay. One (A, B, C) or two (D) representative results out of three (N=11/group) independent experiments is shown. Error bars in data represent mean ± SEM. *p < 0.05, **p<0.01 by two-way ANOVA (A,C) or one tailed t test (B, D).
Thus, we proposed that the preS1 vaccination may open a therapeutic window for the hosts who are deeply tolerized to the HBsAg vaccine. We designed a sequential vaccine of preS1-polypeptide prior to HBsAg in HBV carrier mice to test this hypothesis. The schedule was as shown in Fig. 7A. Compared to HBsAg vaccination alone, the priming with preS1-polypeptide prior to HBsAg vaccine cleared both preS1 and HBsAg antigens in HBV carrier mice (Fig. 7B). We determined the antibody in serum and observed that such combination induced specific B cell response to HBsAg and made anti-HBs seroconversion (Fig. 7C), a marker for clinical cure to CHB infection. And most importantly, specific T cell response to HBsAg could also be detected both in spleen and liver (Fig. 7D). The specific T cell response induced the reduction both of the HBV RNAs and DNA in hepatocytes (Fig. 7E, 7F and 7G). These response to HBsAg was also accompanied by the diminished IHC staining of HBV in the liver (Fig. 7H). Thus, the strategy of priming with preS1 prior to HBsAg vaccination might serve as a effective treatment for clinical chronic hepatitis B virus infection in the future.
Fig. 7. HBsAg boost immunization amplifies preS1-polypeptide induced anti-HBV response for viral clearance.
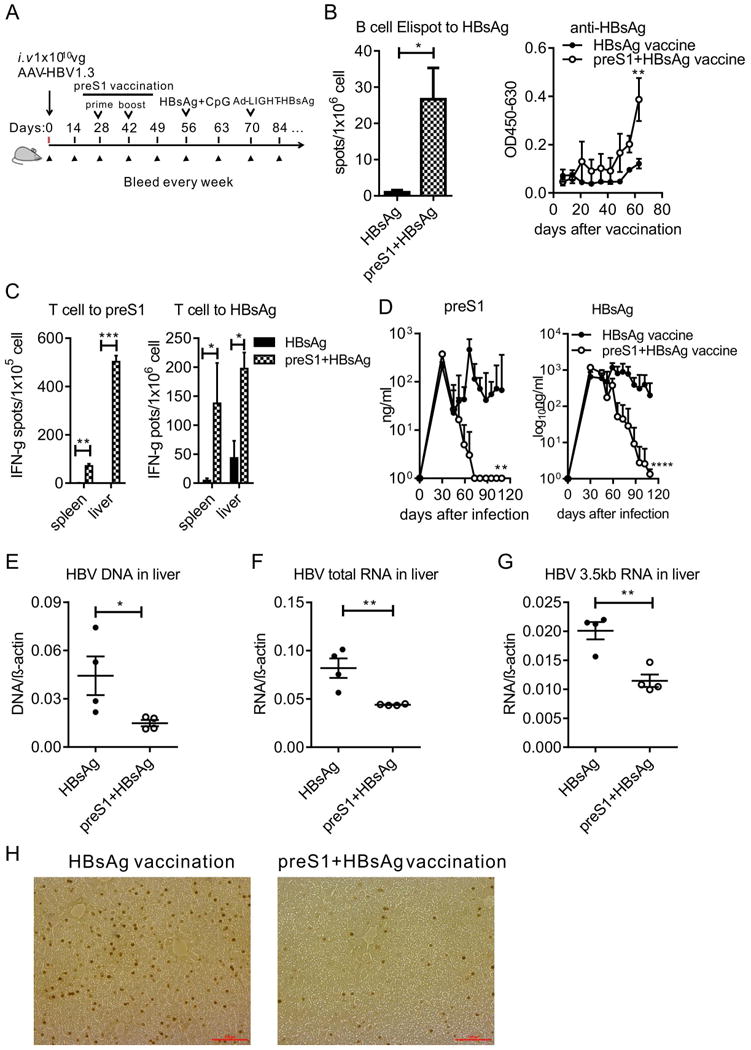
(A) Time schedule for preS1-polypeptide combination with HBsAg as vaccine to treat HBV carrier mice. (B) After carrier mice were vaccinated with preS1-polypeptide + HBsAg, the preS1 and HBsAg antigens in serum were tested by ELISA. (C) The B cell response to HBsAg was tested by B cell ELISPOT, and anti-HBs in serum was tested by ELISA. (D) Mice were sacrificed on the last day, 2×105 lymphocytes from lymph node, spleen and liver were collected. Then the cells were stimulated with 5ug of preS1 polypeptide, HBsAg or BSA, and the specific T cell response was measured by IFN-γ secretion T cell ELISPOT assay. (E) The HBV total RNAs and (F) intermediate products 3.5kb RNA in the liver tissue were determined with RT-qPCR. (G) HBV DNA in the liver was determined by qPCR. (H) IHC staining for HBcAg in hepatocytes. One representative result of three (N=11/group; B, C, D) or two (N=7; E, F, G and H) independent experiments is shown. Error bars in data represent mean ± SEM. *p < 0.05, **p<0.01, ***p < 0.001, ****p < 0.0001 by two-way ANOVA (B, C) or unpaired t test (D, E, F and G).
Discussion
In this study, we have explored whether the preS1-polypeptide vaccination is a potential treatment for CHB infection. We first analyzed the levels of preS1 antigen and HBsAg in clinical HBV patients, and compared the immunogenicity of these two viral antigens in HBV carrier mice. PreS1 domain of the L-HBsAg presents strong immunogenicity for both B-cell and T-cell responses in contrast to HBsAg, the major toleragen in CHB patients. Actually, the appearance of anti-preS1 indicated a potential recovery from HBV infection. By using the HBV carrier model, we confirmed the immunogenicity of preS1 polypeptide. The anti-preS1 induced by preS1-polypeptide cleared HBV-DNA in carrier mice and blocked HBV infection/re-infection to hepatocytes. Furthermore, preS1-polypeptide vaccination even weakened the HBsAg tolerized status and the subsequent vaccination with HBsAg could induce anti-HBs seroconversion in HBV carrier mice.
Unlike preS1 polypeptide, HBsAg has been widely used as a prophylactic vaccine against HBV, but it cannot induce immune responses to clear HBsAg in CHB patients and even in some healthy recipients (4, 29-31). To further enhance the vaccine immunogenicity and the immune protection from HBV infection, the L-HBsAg containing preS1 domain has been included in the third generation HBV vaccines (32-34). However, by using the HBV carrier mouse model, we observed that, same as the conventional HBsAg vaccine, the L-HBsAg containing protein vaccination showed no therapeutic effects in the tolerized model either (fig. S4). Failing in induction of immune response to preS1 domain by the L-HBsAg containing protein vaccination might due to an overwhelming immune tolerance to HBsAg, a physical link of preS1 domain to HBsAg, or too little amount of preS1 region containing in the vaccine (35, 36). It is also possible that alum, the currently clinically used adjuvant, might not be potent enough to induce detectable responses. Whether preS1 region itself alone can be applied as a vaccine has not been directly tested. Here, we found the antigenicity of preS1 was weak than that of HBsAg, it can only induce robust with stronger adjuvants, like incomplete Freund Adjuvant (IFA). However, this adjuvant limits the preS1-polypeptide vaccination usage in clinical. We are exploring several other formulation for preS1 polypeptide vaccination, including a nano-vaccine that contains preS1 region to enhance its immunogenicity.
In the clinic, anti-preS1 appears early in the course of AHB (37). Even the occasional appearance of anti-preS1 in a few patients with chronic aggressive hepatitis or treated with antiviral agents indeed correlates well with better health improvement (38). However, the random clinical analyses of the appearance of anti-preS1 in CHB have not been conclusive for the correlation between the changes in immune status and viral persistence. In CHB infection, the soluble circulating HBsAg induces lymphocytes anergy that fail to receive the secondary danger signals for sustaining their activation. However, preS1 domain is contained on HBV virions, which viral DNA can significantly enhance the immunogenicity of preS1. By comparing the immune status between preS1 and HBsAg in clinical patients, we observed that preS1 was much less immune tolerance. And the appearance of antibody and T cell responses to preS1 domain in clinical patients correlated with a better prognosis of the significant decrease of serum HBV-DNA. Recently, an entry inhibitor peptide derived from the preS1 region, Myrcludex-B, has been investigated as a treatment for HBV infection. This peptide has been shown to inhibit HBV infection in vivo and hinder the amplification of the cccDNA pool in initially infected hepatocytes (39). Here, we demonstrated preS1-polypeptide vaccination can induce a strong antibody response in HBV carrier mice, thus made it a unique candidate vaccine for treating HBV infection. Compared with this Myrcludex-B, such vaccination would be much more potent in inducing intrinsic anti-preS1 and would provide long-term protection from HBV infection. Further clinical trials using preS1 region are warranted.
In fact, CD4+ but not CD8+ T cell responses were induced by preS1-polypeptide vaccination (fig. S5A and 5B), with no liver damage indicated by normal ALT and AST levels in serum (fig. S5C and 5D). Consistently, the only CTL epitope in the preS1 region identified is for human HLA-A11-restricted (40, 41). Lack of CTLs after preS1-polypeptide vaccination may have liver escape from unexpected injuries, meanwhile the preS1-vaccine induced anti-preS1 antibodies in the peripheral circulation can clear HBV virions, block HBV re-infection to healthy hepatocytes in patients, and thus dilute and even diminish the HBV-infected hepatocytes gradually. Unexpectedly, we observed preS1-polypeptide vaccination partially restored immune response to HBsAg during HBV infection, and thus open a therapeutic window for the host to fully respond to the HBsAg vaccine. To amplify the response to HBsAg induce by preS1-polypeptide vaccination and induce stronger and multi-specific T cell responses for completely viral clearance in HBV carrier mice, the sequential combination of preS1-polypeptide prior to the HBsAg vaccination was administered. We showed that preS1-polypeptide priming before vaccination with HBsAg restored both the B cell and T cell response to HBsAg in the periphery and liver, ultimately resulting in the HBsAb seroconversion and the gradual clearance of HBV in the liver. IHC staining also showed a dramatic decrease in the number of core-positive hepatocytes in the livers of HBV carrier mice. This strategy indeed makes HBsAg, the preventive vaccine, to achieve an unexpected therapeutic effect in HBV carrier mice. However, it is not yet clear how preS1 region vaccination helped to restore the immune response to HBsAg. In HBV infection, preS1 domain is always fused with HBsAg within L-HBsAg, thus T cells to preS1 region may help immune response to HBsAg (20, 42). Moreover, the immune-complex of endogenous anti-preS1 with HBV virions may further enhance anti-HBV immune response. The function of the B cell and T cell responses to preS1 domain in the restoration of anti-HBV immunity is under investigation. In summary, preS1-polypeptide vaccine can reverse HBV-tolerance in HBV carrier mice and may serve a potentially effective therapeutic strategy for treating chronic hepatitis B infection.
Materials and methods
Patients
In this study, the patient clinical information was summarized as in Table 1. Sera and PBMCs isolated from blood samples of healthy people (HC, n=8), acute HBV patients (AHB, n=10) and chronic hepatitis B patients (CHB, n=25) were collected and stored by the Beijing 302 Hospital (Beijing, China). The serum levels of anti-HBs and anti-preS1 were measured by ELISA. HBsAg, HBeAg and preS1 antigen levels were measured by ELISA. The HBV-DNA in serum was measured by qPCR. Consent for the collection of serum and PBMC samples was given by each patient in writing and authorized by the Hospital Ethics Review Committee.
Table 1. The clinical characteristics of study cohort.
| Patients | Age (yr ±SD) | Gender (n=male, %) | Viremia (IU/ml, ± SD) | HBeAg (n=positive, %) | anti-HBeAg (n=positive, %) | ALT (IU/ml ±SD) | AST (IU/ml ±SD) |
|---|---|---|---|---|---|---|---|
| Health control (n=8) | 44.43 ± 13.66 | n=4, 50% | —* | n=0, 0 | —* | —* | —* |
| AHB (n=10) | 34.78 ± 11.44 | n=6, 60% | 1.46E+06± 2.99E+06 | n=2, 20% | n=8, 80% | 757.00 ± 437.67 | 337.78 ± 302.92 |
| CHB (n=25) | 25.2 ± 8.89 | n=19, 76% | 1.55E+08 ± 1.5E+08 | n=25, 100% | n=0, 0 | 225.28 ± 337.88 | 110.24 ± 126.34 |
Data not tested.
Mice and reagents
C57BL/6 mice were purchased from Vital River Laboratories Animal Technology Co. (Beijing, China). HLA-A2.1 transgenic mice (C57BL/6-Tg(HLA-A2.1)1Enge/JNju) were purchased from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Mice were maintained under specific pathogen-free condition in BSL-2+ animal facility, and animal experiments were followed with protocol no. DWSWAQ (ABSL-2) 2012205 at the Institute of Biophysics, Chinese Academy of Sciences. Six- to eight-week-old male mice were used in all experiments. All animal experiments were performed in compliance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Biomedical Research Ethics Committee of the Institute of Biophysics of the Chinese Academy of Sciences.
Recombinant proteins used in this study include PreS1 standard-polypeptide (PrimeGene Biotechnology Co., Ltd, Shanghai, China), recombinant HBsAg (Key-Biotechnology Co., Ltd, Beijing, China) for vaccination and HBsAg (ayw serotype) (PrimeGene Biotechnology Co., Ltd, Shanghai, China) for ELISPOT testing. The Large-HBsAg containing protein for vaccination was a commercial products that was purified from patient sera (Key- Biotechnology Co., Ltd, Beijing, China). Both the commercial standard preS1 and the lab-produced preS1 polypeptides are the proteins of 12 Kdalton and contain only the sequence of preS1 domain of HBV large-HBsAg, which is comprised of preS1, preS2, and S regions. The ELISA kits were used for testing HBsAg, HBeAg (Shanghai Kehua Bio-engineering Co., Ltd, Shanghai, China), and preS1 (Shanghai Alpha Biotechnology Co., Ltd, Shanghai, China). For the ELISA to test anti-preS1, the plates was coated with the commercial standard preS1 protein at 2ug/ml in PBS, the samples was added at 1:10, 1;100 and 1:1000 dilutions. Then a secondary antibody of goat-anti-mouse IgG (HRP conjugated) (Cwbiotech, Beijing, China) was added for chromogenic reaction. The purchased anti-preS1 antibody was an IgG2a monoclonal antibody, which was raised against HBV preS1 antigen (Santa Cruz, CA, USA). The anti-preS1 XY007 monoclonal antibody was screened and produced in our lab via yeast display techniques. The peptides for anti-HBs (ayw) testing 111-140 aa (PGSSTTSTGPCRT CMTTAQGTSMYPSCCCT) were synthesized by China Peptides Co., Ltd (Shanghai, China).
Virus and AAV-HBV1.3 infection
The HepG2-hNTCP cell line and HBV-D (subtype, ayw) virus were kindly provided by Professor Wenhui Li (National Institute of Biological Sciences, Beijing, China). The AAV-HBV1.3 virus was purchased from the Beijing FivePlus Molecular Medicine Institute (Beijing, China). This recombinant virus carries 1.3 copies of the HBV genome (genotype D, serotype ayw) and is packaged in AAV serotype 8 (AAV8) capsids. Adult C57BL/6 and C57BL/6-Tg (HLA-A2.1) mice were injected with the experimentally indicated amounts of recombinant virus (diluted to 200 μl with saline) by tail vein injection. After four weeks or more, the stable HBV carrier mice were used for vaccination (22). The mice were bled through the ophthalmic vein at the indicated time points in the respective experiments to monitor HBV surface antigen (HBsAg), HBV preS1 antigen (preS1), HBs antibody (HBsAb), preS1 antibodies and HBV genomic DNA in serum.
PreS1-polypeptide vaccine preparation
The coding sequence of preS1-polypeptide was synthesized by PCR from cDNA extracted from the livers of AAV-HBV1.3 infected mice. We used the following primers: 5′-CGGGATCCatgggg cagaatctttccacca and 3′-CCGCTCGAGctaggcctgaggatgagtgtttct (43). Then, the sequence was subcloned into plasmid SUMO-pET-28a to yield the expression plasmid. The plasmid was transformed into E.coli BL21(DE3) cells. The expression of fusion protein was induced by 1 mmol/L isopropyl-beta-D-thiogalactopyranoside (IPTG) at 37°C and left overnight. Then, the induced cells were harvested and sonicated. The supernatant of the cell lysates was purified by Ni-NTA (GE Healthcare, PA, USA) column chromatography. Finally, the 6× His-SUMO tag was cut by SUMO-protease, and preS1 was separated by molecular size exclusion and ion-exchange chromatography (Superdex-75, and HiTrap QFF, GE Healthcare).
Vaccination
Alum adjuvant (Alhydrogel® 2%) and MPLA Synthetic VacciGrade were purchased from InvivoGen (Toulouse, France). CpG-1826 (TCCATGACGTTCCTGACGTT) was synthesized by Life Technologies Corporation (Carlsbad, USA). Incomplete Freund's Adjuvant was purchased from Sigma-Aldrich (St. Louis, MO, USA) (Supplementary Fig. 1). The adjuvants were mixed with 5 μg of rHBsAg or 10 μg of preS1 for use in vaccination. All vaccines were injected subcutaneously.
HBV DNA and RNA detection
Serum HBV DNA was extracted from 200 μl of serum and measured following the manufacturer's instructions (careHBV, Qiagen, Hilden, Germany). Liver HBV DNA was extracted from 50 mg of liver tissue using a genomic DNA kit (Tiagen Biotech, Beijing, China). The total RNA was extracted from the livers of AAV-HBV1.3 infected mice with TRIzol reagent (Invitrogen, United States). The RNA was reverse-transcribed (RT-PCR) using the RevertAid First Strand cDNA synthesis Kit (Thermo Scientific, United States). The samples were analyzed by using quantitative PCR (qPCR) with the following HBV-specific primers: HBV-HBsAg–real–F:5′-CACATCAGGATTCCTAGGACC-3′; HBV-HBsAg-real-R: 5′-GGTGAGTGATTGGAGGTTG-3′. HBV-3.5kb-real-F:5′-GAGTGTGGATTCGCACTCC- 3′; HBV-3.5kb-real–R:5′-GAGGCGAGGGAGTTCTTCT-3′. HBV-total-real-F:5′-TCACCA GCACCATGCAAC-3′; and HBV-total-real-R:5′-AAGCCACCCAAGGCACAG-3′. Real- time PCR was performed using the SYBR Premix Ex Taq kit (Takara, Japan) with an ABI Fast 7500 Real-Time PCR System.
HBV infection and inhibition assays in vitro
The in vitro HBV infection and inhibition assays were performed as previously reported (16). Briefly, 1×107 copies of genome-equivalent HBV were inoculated into the culture medium of 1×105 HepG2-hNTCP cells in 48-well plates in the presence of the preS1 antibody or anti-preS1 serum and incubated for 24 hours. Cells were then washed with medium three times and maintained in PMM medium (William's E medium (Gibco, United States) with 5 μg/ml transferrin, 5 ng/ml sodium selenite, 3 μg/ml insulin (ITS) (Corning, United States), 2 mM L-glutamine, 10 ng/ml EGF (Sigma, United States), 2% DMSO, 100 U/ml penicillin, and 100 μg/ml streptomycin). The medium was changed every 2 days. Viral infection at different time points was analyzed by measuring viral antigen and viral RNAs in the culture medium.
ELISPOT Assay
The lymph nodes, spleen and liver were harvested at the indicated time points after immunization with rHBsAg or preS1-polypeptide vaccines. The intrahepatic lymphocytes were isolated by enzymatic digestion. Briefly, the liver tissues were digested by collagenase IV (Roche, Basel, Switzerland) at 37°C for 15 min. The suspension was centrifuged at 30g for 1 min to remove hepatocytes. The lymphocytes were then pelleted by centrifugation at 400g for 10 min and further purified with 40% and 70% Percoll solutions by centrifugation at 800g for 20 min at room temperature. The cells were collected from the interface, and the red blood cells were removed with ACK buffer to make a single-cell suspension.
CD4-deleted and CD8-deleted lymphocytes were collected through magnetic separation with anti-APC Microbeads (Miltenyi Biotec Inc. Bergisch Gladbach, Germany) following the manufacturer's protocol. Briefly, the splenocytes were first stained with the APC-conjugated primary antibody, and the cells were then magnetically labeled with anti-APC microbeads. Then, the cell suspension was loaded onto a MACS column, which was placed in the magnetic field of a MACS separator. The magnetically labeled CD4+ or CD8+ cells were retained within the column. The unlabeled cells were collected as CD4-deleted or CD8-deleted cells.
The PBMCs from HBV patients were stored in liquid nitrogen. And after thawed at 37°C, lymphocytes were pelleted by centrifugation at 400g for 5 min in 10ml complete medium. Then cells were put into medium to make a single-cell suspension. For detecting antigen-specific immune response, lymphocytes were incubated for 48 h at 37°C in complete medium containing full-preS1 protein or HBsAg peptide-pools that covers all identified HBsAg T cell epitopes in an IFNγ ELISPOT plate (Merck Millipore, Billerica, Massachusetts, USA). After incubation, the IFN-γ secretion was analyzed using a biotinylated anti-IFN-γ antibody and streptavidin-HRP (BD Biosciences, Franklin Lakes, New Jersey, USA). Finally, the spots were visualized with AEC substrate and quantified with an auto-analyzing system.
Statistics
Error bars in data represent mean ± SEM. Data were analyzed using an unpaired two-tailed t test or two-way ANOVA by the GraphPad Prism statistical software (GraphPad Software Inc., San Diego, CA, USA). A value of P<0.05 was considered statistically significant (*P<0.05; **P<0.01; ***P<0.001; and ****P<0.0001). The correlation between HBsAg and preS1, anti-preS1 and preS1, anti-preS1 and HBV-DNA in clinical HBV patients were analyzed using two-tailed correlation test. A value of P<0.05 was considered statistically significant correlation.
Supplementary Material
Acknowledgments
This work was supported by the National Key Basic Research Program of China (No. 2012CB910203 and No. 2012CB519000) and the National Grand Program on Key Infectious Diseases (No. 2012ZX10002006) to Y-XF and HP; National Nature and Science Foundation of China (No. 81471579 and 81641063) to HP; and NIH funding (R01AI095097) to LS and Y-XF. YB, Y-XF and HP designed the experiments. We thank Dr. Wenhui Li for providing the HepG2-hNTCP cell line and HBV virus (ayw subtype) for HBV infection experiment in vitro. We thank Daryl Harmon for editorial assistance.
References
- 1.Organization WH, Hepatitis B. Fact sheet. 2015 [Google Scholar]
- 2.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 3.Dienstag JL, Stevens CE, Bhan AK, Szmuness W. Hepatitis B vaccine administered to chronic carriers of hepatitis b surface antigen. Ann Intern Med. 1982;96:575–579. doi: 10.7326/0003-4819-96-5-575. [DOI] [PubMed] [Google Scholar]
- 4.Shi B, Ren G, Hu Y, Wang S, Zhang Z, Yuan Z. HBsAg Inhibits IFN-α Production in Plasmacytoid Dendritic Cells through TNF-α and IL-10 Induction in Monocytes. PLoS ONE. 2012;7:e44900. doi: 10.1371/journal.pone.0044900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan XZ, Wang M, Li Hw, Zhuang H, Xu D, Wang FS. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. doi: 10.1007/s10875-004-6249-y. [DOI] [PubMed] [Google Scholar]
- 6.Han Q, Lan P, Zhang J, Zhang C, Tian Z. Reversal of hepatitis B virus-induced systemic immune tolerance by intrinsic innate immune stimulation. Journal of Gastroenterology and Hepatology. 2013;28:132–137. doi: 10.1111/jgh.12034. [DOI] [PubMed] [Google Scholar]
- 7.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of Functional CD8+ T-Cell Responses to the Gag Protein of Human Immunodeficiency Virus Type 1 Correlates Inversely with Viral Load in Plasma. Journal of Virology. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay SS, Wong YC, McDonald DM, Wood NA, Roediger B, Sierro F, McGuffog C, et al. Antigen expression level threshold tunes the fate of CD8 T cells during primary hepatic immune responses. Proc Natl Acad Sci U S A. 2014;111:E2540–2549. doi: 10.1073/pnas.1406674111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609–8620. doi: 10.1128/JVI.76.17.8609-8620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Sallberg M, Hughes J, Jones J, Guidotti LG, Chisari FV, Billaud JN, et al. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol. 2005;79:3016–3027. doi: 10.1128/JVI.79.5.3016-3027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMattia MA, Watts NR, Stahl SJ, Grimes JM, Steven AC, Stuart DI, Wingfield PT. Antigenic switching of hepatitis B virus by alternative dimerization of the capsid protein. Structure. 2013;21:133–142. doi: 10.1016/j.str.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milich DR, Schodel F, Hughes JL, Jones JE, Peterson DL. The hepatitis B virus core and e antigens elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J Virol. 1997;71:2192–2201. doi: 10.1128/jvi.71.3.2192-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petit MA, Zoulim F, Caipel F, Dubanchet S, Dauguet C, Trepo C. Variable expression of preS1 antigen in serum during chronic hepatitis B virus infection: An accurate marker for the level of hepatitis B virus replication. Hepatology. 1990;11:809–814. doi: 10.1002/hep.1840110515. [DOI] [PubMed] [Google Scholar]
- 15.Klingmuller U, Schaller H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol. 1993;67:7414–7422. doi: 10.1128/jvi.67.12.7414-7422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruss V, Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari C, Penna A, Bertoletti A, Cavalli A, Valli A, Schianchi C, Fiaccadori F. The preS1 antigen of hepatitis B virus is highly immunogenic at the T cell level in man. J Clin Invest. 1989;84:1314–1319. doi: 10.1172/JCI114299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu WG, Wei J, Xia HC, Yang XX, Li F, Li GD, Wang Y, et al. Identification of the immunogenic domains in HBsAg preS1 region using overlapping preS1 fragment fusion proteins. World J Gastroenterol. 2005;11:2088–2094. doi: 10.3748/wjg.v11.i14.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milich DR, McLachlan A, Moriarty A, Thornton GB. A single 10-residue pre-S(1) peptide can prime T cell help for antibody production to multiple epitopes within the pre-S(1), pre-S(2), and S regions of HBsAg. J Immunol. 1987;138:4457–4465. [PubMed] [Google Scholar]
- 21.Neurath AR, Seto B, Strick N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine. 1989;7:234–236. doi: 10.1016/0264-410x(89)90235-1. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Liu L, Zhu D, Peng H, Su L, Fu YX, Zhang L. A mouse model for HBV immunotolerance and immunotherapy. Cell Mol Immunol. 2014;11:71–78. doi: 10.1038/cmi.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dion S, Bourgine M, Godon O, Levillayer F, Michel ML. Adeno-Associated Virus-Mediated Gene Transfer Leads to Persistent Hepatitis B Virus Replication in Mice Expressing HLA-A2 and HLA-DR1 Molecules. Journal of Virology. 2013;87:5554–5563. doi: 10.1128/JVI.03134-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei J, Wang YQ, Lu ZM, Li GD, Wang Y, Zhang ZC. Detection of anti-preS1 antibodies for recovery of hepatitis B patients by immunoassay. World J Gastroenterol. 2002;8:276–281. doi: 10.3748/wjg.v8.i2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Ditzhuijsen TJ, Kuijpers LP, Koens MJ, Rijntjes PJ, van Loon AM, Yap SH. Hepatitis B pre-S1 and pre-S2 proteins: clinical significance and relation to hepatitis B virus DNA. J Med Virol. 1990;32:87–91. doi: 10.1002/jmv.1890320204. [DOI] [PubMed] [Google Scholar]
- 26.Whalley SA, Murray JM, Brown D, Webster GJM, Emery VC, Dusheiko GM, Perelson AS. Kinetics of Acute Hepatitis B Virus Infection in Humans. The Journal of Experimental Medicine. 2001;193:847–854. doi: 10.1084/jem.193.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu D, Liu L, Yang D, Fu S, Bian Y, Sun Z, He J, et al. Clearing Persistent Extracellular Antigen of Hepatitis B Virus: An Immunomodulatory Strategy To Reverse Tolerance for an Effective Therapeutic Vaccination. J Immunol. 2016;196:3079–3087. doi: 10.4049/jimmunol.1502061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganem D, Varmus HE. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 29.Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HLA, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280–289. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang Z, Shen F, et al. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol Immunol. 2009;46:2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HLA. Hepatitis B Virus Lacks Immune Activating Capacity, but Actively Inhibits Plasmacytoid Dendritic Cell Function. PLoS ONE. 2011;6:e15324. doi: 10.1371/journal.pone.0015324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDermott AB, Cohen SB, Zuckerman JN, Madrigal JA. Hepatitis B third-generation vaccines: improved response and conventional vaccine non-response--evidence for genetic basis in humans. J Viral Hepat. 1998;5(Suppl 2):9–11. doi: 10.1046/j.1365-2893.1998.0050s2009.x. [DOI] [PubMed] [Google Scholar]
- 33.Yap I, Chan SH. A new pre-S containing recombinant hepatitis B vaccine and its effect on non-responders: a preliminary observation. Ann Acad Med Singapore. 1996;25:120–122. [PubMed] [Google Scholar]
- 34.Shouval D, Roggendorf H, Roggendorf M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Med Microbiol Immunol. 2015;204:57–68. doi: 10.1007/s00430-014-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prange R, Clemen A, Streeck RE. Myristylation is involved in intracellular retention of hepatitis B virus envelope proteins. J Virol. 1991;65:3919–3923. doi: 10.1128/jvi.65.7.3919-3923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerlich WH. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Med Microbiol Immunol. 2015;204:39–55. doi: 10.1007/s00430-014-0373-y. [DOI] [PubMed] [Google Scholar]
- 37.Klinkert MQ, Theilmann L, Pfaff E, Schaller H. Pre-S1 antigens and antibodies early in the course of acute hepatitis B virus infection. J Virol. 1986;58:522–525. doi: 10.1128/jvi.58.2.522-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellstrom U, Lindh M, Krogsgaard K, Sylvan S. Demonstration of an association between detection of IgG antibody reactivity towards the C-terminal region of the preS1 protein of hepatitis B virus and the capacity to respond to interferon therapy in chronic hepatitis B. J Gastroenterol Hepatol. 2008;23:804–810. doi: 10.1111/j.1440-1746.2007.05174.x. [DOI] [PubMed] [Google Scholar]
- 39.Volz T, Allweiss L, Ben MM, Warlich M, Lohse AW, Pollok JM, Alexandrov A, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Jin Y, Shih WK, Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988;168:293–306. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desmond CP, Bartholomeusz A, Gaudieri S, Revill PA, Lewin SR. A systematic review of T-cell epitopes in hepatitis B virus: identification, genotypic variation and relevance to antiviral therapeutics. Antivir Ther. 2008;13:161–175. [PubMed] [Google Scholar]
- 42.Milich DR. Immune response to hepatitis B virus proteins: relevance of the murine model. Semin Liver Dis. 1991;11:93–112. doi: 10.1055/s-2008-1040428. [DOI] [PubMed] [Google Scholar]
- 43.Lu YY, L K, Cheng J, Wang L, Liu Y, Zhang LX. Cloning and expression of the preS1 gene of hepatitis B virus in yeast cells. Hepatobiliary Pancreat Dis Int. 2002;1:238–242. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


