Abstract
Hepatic cystogenesis in Polycystic Liver Disease (PLD) is associated with increased levels of cAMP in cholangiocytes lining liver cysts. TGR5, a G protein-coupled bile acid receptor, is linked to cAMP and expressed in cholangiocytes. Therefore, we hypothesized that TGR5 might contribute to disease progression. We examined expression of TGR5 and Gα proteins in cultured cholangiocytes and in livers of animal models and humans with PLD. In vitro, we assessed cholangiocyte proliferation, cAMP levels, and cyst growth in response to: (i) TGR5 agonists [taurolithocholic acid (TLCA), oleanolic acid (OA) and two synthetic compounds]; (ii) a novel TGR5 antagonist (SBI-115); and (iii) a combination of SBI-115 and pasireotide, a somatostatin receptor (SSTR) analog. In vivo, we examined hepatic cystogenesis in OA-treated PCK rats and after genetic elimination of TGR5 in double mutant TGR5−/−;Pkhd1del2/del2 mice. Compared to control, expression of TGR5 and Gαs (but not Gαi and Gαq) proteins was increased 2–3-fold in cystic cholangiocytes in vitro and in vivo. In vitro, TGR5 stimulation enhanced cAMP production, cell proliferation and cyst growth by ~40%; these effects were abolished after TGR5 reduction by shRNA. OA increased cystogenesis in PCK rats by 35%; in contrast, hepatic cystic areas were decreased by 45% in TGR5-deficient TGR5−/−;Pkhd1del2/del2 mice. TGR5 expression and its co-localization with Gαs were increased ~2-fold upon OA treatment. Levels of cAMP, cell proliferation and cyst growth in vitro were decreased by ~30% in cystic cholangiocytes after treatment with SBI-115 alone and by ~50% when SBI-115 was combined with pasireotide. Conclusion: TGR5 contributes to hepatic cystogenesis by increasing cAMP and enhancing cholangiocyte proliferation. Our data suggest that a TGR5 antagonist alone or concurrently with SSTR agonists represents novel therapeutic approaches in PLD.
Keywords: TGR5 antagonist, cholangiocytes, PCK rat, 3-D culture, PLD treatment
INTRODUCTION
The polycystic liver disease (PLD) is a group of genetic disorders characterized by cholangiocyte-derived liver cysts. PLD co-exists with autosomal dominant (AD-) or autosomal recessive (AR-) polycystic kidney disease (PKD). ADPKD results from mutations in PKD1 and PKD2 genes while mutations in the PKHD1 gene are responsible for renal and hepatic cystogenesis in ARPKD. Isolated autosomal dominant PLD (ADPLD) is a rare condition due to mutations in SEC63, PRKCSH or LRP5 genes.1–3 Hepatic cystogenesis in all the PLDs is sex-dependent; females often have a more severe disease and higher total liver volume.2
Mutations in PLD-related genes initiate the formation of hepatic cysts which continue to grow as a result of multiple downstream phenomena including cholangiocyte hyper-proliferation, dysregulated cell cycle, enhanced fluid secretion, decreased intracellular calcium and global changes in messenger RNA (mRNA), microRNA (miRNA) and protein expression.2, 3 Intracellular cAMP (which levels are markedly increased in PLD)4 is a well-characterized regulator of cellular pathways associated with hepatic cystogenesis. For example: (i) cholangiocyte proliferation involves multiple cAMP down-stream effectors, including EPAC1, EPAC2, PKA and ERK1/2;5 (ii) expression of the cell cycle proteins is controlled by components of cAMP signaling;3, 6 (iii) fluid secretion into the cystic lumen requires the water channel, AQP1, and the ion transporters, CFTR and AE2, all influenced by cAMP;7 and (iv) cilia in cystic cells lack components of the cAMP machinery (e.g., adenylyl cyclase 5/6, A-kinase anchoring protein 150, PKA and phosphodiesterase 4C) subsequently interrupting cross-talk between cAMP and intracellular calcium; as a result, cholangiocyte proliferation is enhanced contributing to cyst growth.3, 8 Taken together, these observations implicate cAMP as is an important central component in the network of dysregulated signaling pathways in PLD and provide the rationale for cAMP-targeted strategies in disease treatment. Indeed, we have shown that suppression of cAMP in cystic cholangiocytes by octreotide and pasireotide, synthetic octapeptide agonists of somatostatin receptors (SSTRs), inhibited hepatic cystogenesis in animal models of PLD.4, 9 Moreover, octreotide decreased liver volume (by ~5%) in patients with PLD and improved quality of life.10–12 While somatostatin analogs are considered to be the only available drug option, treatment is costly ($7,000–$11,000 per month), changes in liver volume are modest, up to 15% of patients do not respond to treatment and after drug withdrawal the liver volume returns back to pre-treatment levels.11 Thus, a search for new therapeutic options remains of a great importance.
TGR5, a G protein-coupled bile acid receptor linked to cAMP signaling, is expressed in a variety of tissues and known to influence energy homeostasis, inflammation, immune responses, insulin secretion and gallbladder relaxation.13–15 Recently, TGR5 has emerged as a promising therapeutic target.13, 14, 16, 17
In the liver, TGR5 is expressed in sinusoidal cells, Kupffer cells, gallbladder epithelia and cholangiocytes but not in hepatocytes.13, 16, 18 TGR5 is stimulated by bile acids (e.g., lithocholic, chenodeoxycholic, deoxycholic, and cholic acids), xenobiotic ligands (i.e., oleanolic acid) and semi-synthetic derivatives (e.g., INT-777 and obeticholic acid).13, 14 Importantly, we previously reported that the concentration of lithocholic, chenodeoxycholic and cholic acids is significantly higher in livers of the PCK rats (an animal model of PLD) compared to WT counterparts.19 TGR5 agonists are known to affect downstream signaling events via coupling to Gαs, Gαi and Gαq proteins.13, 18, 20 Moreover, stimulation of TGR5 in different cell types, including cholangiocytes, has been shown to increase cAMP and affect cell proliferation.18, 20–23
Thus, given that: (i) hyper-proliferation is one of the major mechanisms driving cyst expansion in PLD;2, 3 (ii) cAMP is increased in cholangiocytes of animal models and humans with PLD,3, 4, 9 (iii) levels of TGR5 agonists are increased in cystic livers; and (iv) TGR5 is linked to cAMP signaling,13, 18 we investigated the effects of pharmacological activation and genetic and pharmacological inactivation of TGR5 on hepatic cystogenesis in PLD and the potential mechanisms involved.
We found that TGR5 and Gαs proteins are overexpressed in PLD. As anticipated, stimulation of TGR5 in cystic cholangiocytes increased cAMP levels, cell proliferation and cyst growth in vitro; these effects were eliminated after TGR5 reduction by shRNA. The TGR5 agonist, OA, worsened hepatic cystogenesis in vivo in PCK rats while genetic depletion of TGR5 by generating TGR5−/−;Pkhd1del2/del2 double mutant mice reduced cyst growth. Finally, cAMP levels, cell proliferation and cyst expansion in vitro were decreased in cystic cholangiocytes after treatment with a novel small molecule TGR5 antagonist, SBI-115. Concurrent targeting of cAMP by SBI-115 and pasireotide, a synthetic agonist of SSTRs, suppressed cAMP, cell proliferation and cyst expansion in vitro more effectively than each drug alone. Together these data suggest that TGR5 activation facilitates cyst growth while its inhibition attenuates hepatic cystogenesis via modulation of cAMP signaling. Thus, TGR5 antagonists alone or concurrently with agonists of SSTRs represent a novel therapeutic approach for PLD.
MATERIALS AND METHODS
Cell cultures, reagents and animals
Control and PCK rat cystic cholangiocytes (derived from wild type [WT] and PCK rats, respectively); and control and ADPKD human cystic cholangiocytes (derived from healthy humans and ADPKD patients, respectively) were used and maintained as described.9 TGR5 was activated by taurolithocholic acid (TLCA), oleanolic acid (OA; both from Sigma-Aldrich, St. Louis, MO); and two synthetic compounds - compound "1” (C1) - (2-(ethylamino)-6-(3-(4-(trifluoromethoxy)phenyl)-propanoyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine-4carbox-amide) and compound “2” (C2) – 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethyl-isoxazole-4-carboxamide. The small molecule TGR5 antagonist (SBI-115, m-tolyl 5-chloro-2-(ethylsulfonyl) pyrimidine-4-carboxylate) was identified by throughput screening of a 50,000 compound chemical library in TGR5-expressing CHO-K1 cells. C1, C2 and SBI-115 were synthesized in the Sanford Burnham Prebys Medical Discovery Institute at >95% purity by HPLC following the outlined procedure.24, 25 Cystic cholangiocytes were stably transfected with TGR5 or control shRNAs (Santa Cruz Biotechnology, Santa Cruz, CA). Rodents were housed in a 12-hour light-dark facility on a standard diet and water ad lib. The number of animals used is indicated for each experiment. The protocol was approved by the Mayo Institutional Animal Care and Use Committee.
RNA preparation, sequencing and data analysis
of rat and human control and cystic cholangiocytes (n=3 for each cell line) is described in Supporting Methods.
Western blot
Proteins were separated by 4–15% SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and incubated first with primary anti-TGR5 (Santa Crus H-90; which was found to work best in our experimental systems), anti-p-Erk1/2 (Santa Cruz), anti-total ERK1/2 (Cell Signaling, Danvers, MA), anti-Gα (Abcam, Cambridge, MA) and anti-β-actin antibodies and then with corresponding secondary horseradish peroxidase-conjugated (Invitrogen Carlsbad, CA) or IRdye 680 or 800 (Odyssey) antibodies. Bands were visualized with ECL Plus Western Blotting Detection kit (BD Biosciences, San Jose, CA) or Odyssey Li-Cor Scanner (Li-Cor, Lincoln, NE). Integrated density of protein band was assessed by ImageJ (NIH, Bethesda, MD) and data presented as arbitrary units compared to β-actin.
Immunofluorescence confocal microscopy
Livers from WT (Harlan Sprague Dawley) and PCK (our colony) rats; WT, Pkd2WS25/−, Pkhd1del2/del2 and TGR5−/− mice (all of C57BL/6 background and our colonies); healthy humans and patients with ADPKD and ADPLD (provided by the Mayo Clinical Core) were incubated with primary antibodies to TGR5, Gα and PCNA (Santa Cruz) and then with respective secondary fluorescent antibodies (Molecular Probes, Eugene, OR). Nuclei were stained with DAPI (4-,6-diamidino-2-phenylindole; Invitrogen). Livers were analyzed by ZeissLSM-510 microscope (Carl Zeiss, Thornwood, NY). Fluorescence intensity (arbitrary units) of protein staining was assessed by Adobe Photoshop Elements 10 and expressed as percent change in cystic cholangiocytes compared to respective controls in which fluorescence intensity was considered to be equal 100%. Quantitation of TGR5 co-localization with Gαs was performed using Image J.
Immunogold transmission electron microscopy (IG-TEM)
Livers from WT and PCK rats were incubated with anti-TGR5 antibody (1:20) and then with a gold-conjugated secondary antibody (1:100, Electron Microscopy Sciences), post-fixed with 2.5% glutaraldehyde, enhanced with silver mixture (R-Gent SE-EM), and incubated again with 1% osmium tetroxide. Specimens were observed using a Joel 12 electron microscope (Joel USA, Peabody, MA). Number of immuno-gold particles was assessed by ImageJ.
cAMP
was detected by the Bridge-It cAMP designer cAMP assay (Mediomics, St. Louis, MO). Cholangiocytes (10,000/well) were incubated with TLCA, OA, C1 and C2 (all, 25 µM), SBI-115 (100 and 200 µM) and pasireotide (20 µM, MedChemexpress, Monmouth Junction, NJ) for 15–30 min. Doses of drugs were chosen based on published data9, 18 or dose ranging (SBI-115).
Cell proliferation
was determined by CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) and by cell counting using the Cellometer Auto4 (Nexcelom Bioscience) cell counter. Cholangiocytes (2500 cells/well) were grown for 24–48 hours and then treated with TGR5 agonists (all, 25 µM), SBI-115 (100 and 200 µM) and pasireotide (20 µM) for additional 24 hours. Alterations in cell proliferation after treatment were expressed as percent change compared to un-treated cholangiocytes in which cell proliferation was considered to be equal 100%.
Three-Dimensional (3-D) cultures
Microdissected from PCK rat livers cystic bile ducts or cultured cholangiocytes were grown in 3-D matrices as described4, 5, 9 and treated with TLCA and OA (both, 25 µM) daily. Images were taken at days 1 (24 hours after seeding) and 3. The circumference of cystic structures was measured by ImageJ as described.4
3-D Cholangiocyte spheroids
Cholangiocyte spheroids were generated by a hanging drop approach26, 27 using Perfect 96-well hanging drop plate (3-D Biomatrix, Ann Arbor, MI). Each hanging drop (40 µL of media) contains 10,000 cholangiocytes in suspension. Spheroids were formed within 24 hour and grown for additional 96 hour before treatment. Microphotographs were taking before (day1) and after treatment (day 3). Circumferences of spheroids were assessed by Image J.
Treatment protocol
PCK rats (4–6 weeks old, n=5 females, n=5 males) were injected intraperitoneally with OA (25 mg/kg bw) daily for 6 weeks. Dose of OA and route of administration were chosen based on published studies.28 Drug doses were adjusted to the animal weight weekly. Control PCK rats (4–6 weeks old, n=4 females, n=4 males) received equal doses of DMSO. Serum biochemistry, gross anatomy and cystic and fibrotic areas were analyzed as described.9 Both male and female rats were used in this study because of gender differences in human PLD patients.2
Statistical analysis
The data are expressed as the MEAN±SD. Statistical analysis was performed by Student’s t-test; results were considered statistically significant at p<0.05.
Details on other experimental procedures are described in the Supporting Methods.
RESULTS
TGR5 is overexpressed in rat and human cystic cholangiocytes
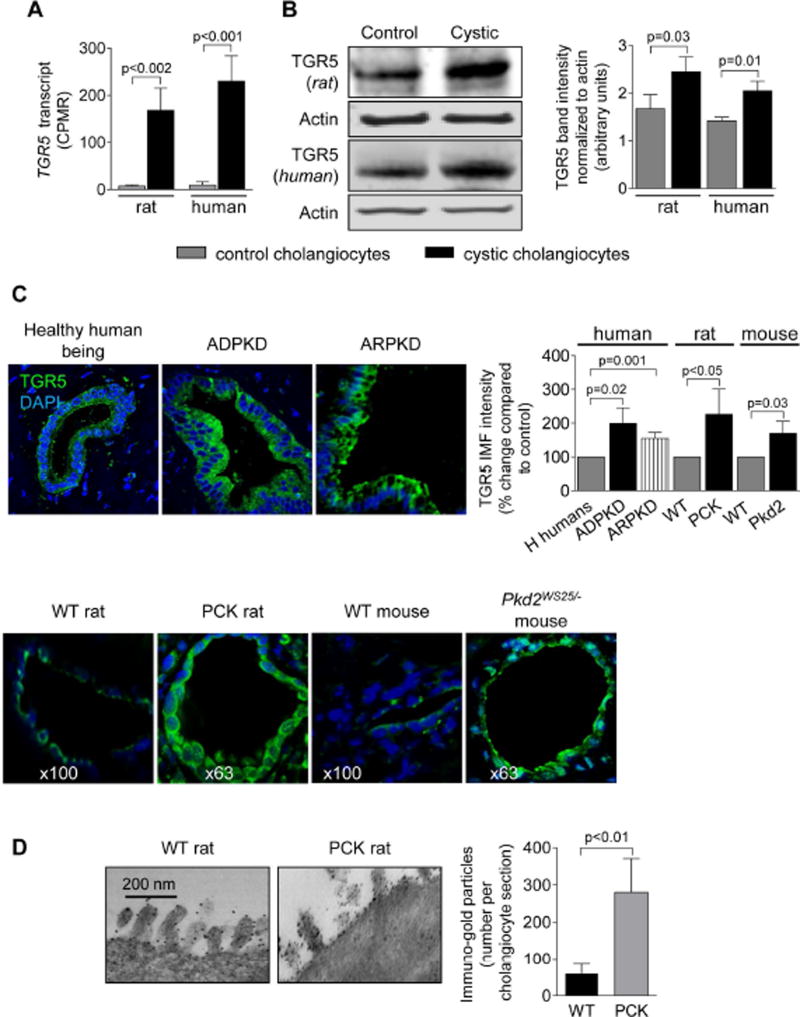
Copy numbers of TGR5 transcript were increased ~20-fold (Fig. 1A) and expression of TGR5 protein was higher by 40–45% (Fig. 1B) in cultured cystic cholangiocytes compared to control. Increased immuno-reactivity of TGR5 (up to 2-fold) was seen in vivo in cholangiocytes of patients and animal models with PLD (Fig. 1C). IG-TEM demonstrated that the number of TGR5-positive immuno-gold particles was ~4.5-fold greater in cholangiocytes of PCK rats compared to WT confirming over-expression of TGR5 in PLD (Fig. 1D).
Figure 1.
TGR5 is over-expressed in PLD. (A) Higher copy numbers of TGR5 transcript per million reads (CPMR) were observed in cultured cystic cholangiocytes. n=3 for each cell line. (B) Representative western blotting and quantitation of TGR5 band density show increased levels of TGR5 protein in cystic cholangiocytes. n=3 for each cell line. (C) Representative images and quantitation of relative TGR5 IMF intensity confirm up-regulation of TGR5 protein in humans and rodents with PLD. n=5 livers for each condition. Nuclei stained with DAPI. (D) IG-EM images and quantitation analysis show the increased numbers of TGR5-positive immuno-gold particles along apical membrane and microvilli of cholangiocytes in PCK rats. n=10 cholangiocyte sections per group. Data are presented as MEAN±SD. Abbreviation: IMF – immunofluorescence, H humans – healthy humans, WT – wild type.
TGR5 agonists increase cAMP levels in cultured cystic cholangiocytes
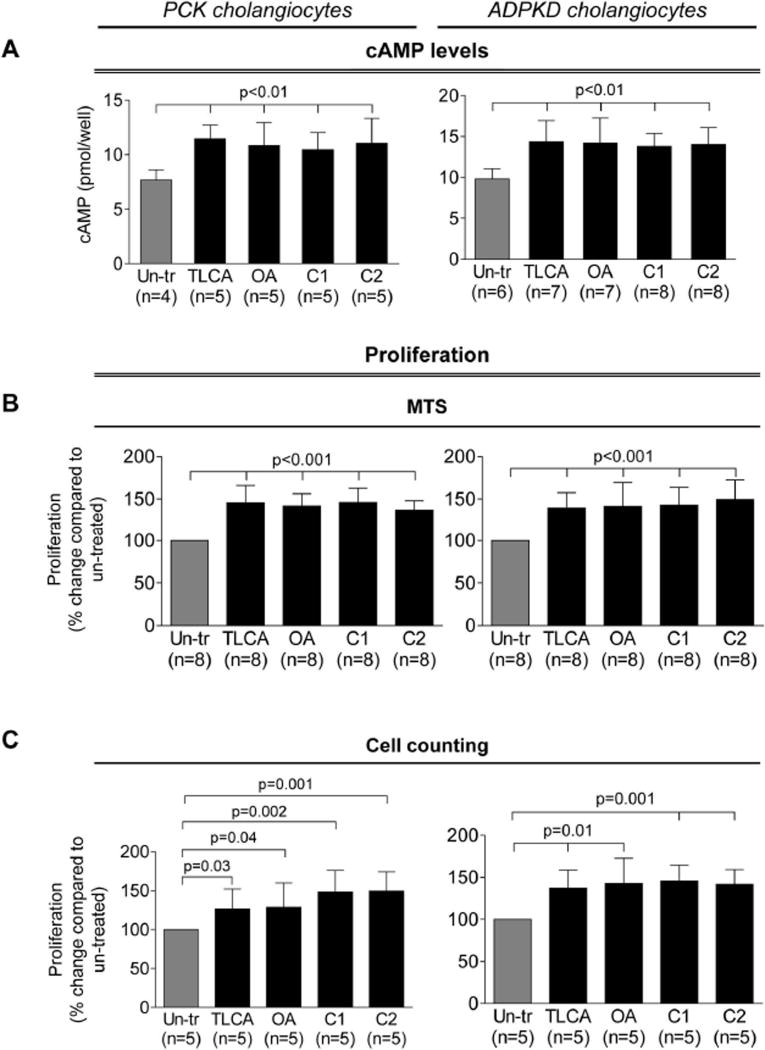
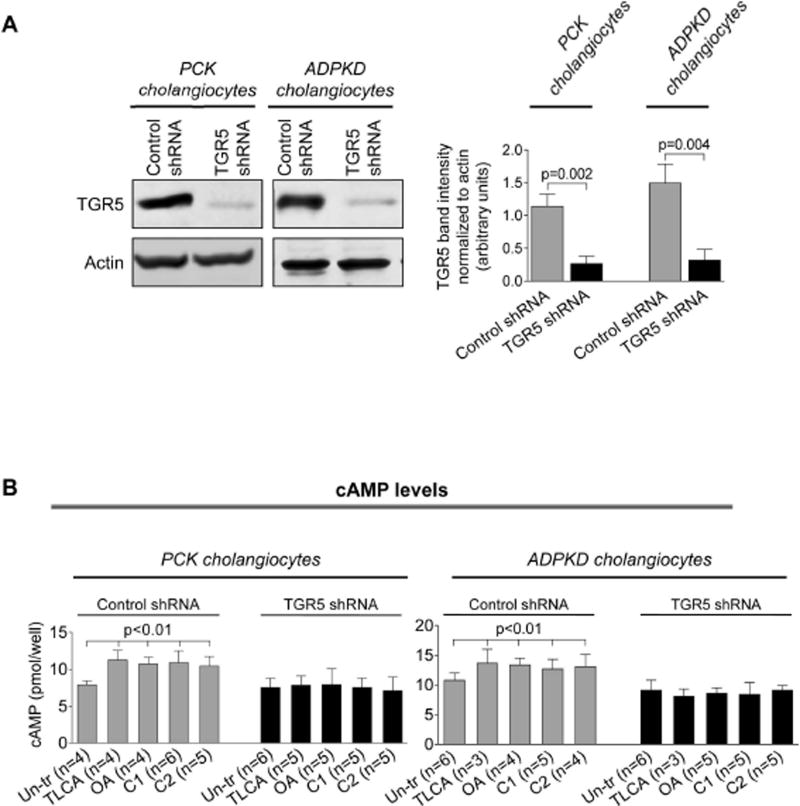
Levels of cAMP upon TLCA, OA, C1 and C2 treatment were increased by 39–50% in PCK cholangiocytes and by 41–46% in ADPKD cholangiocytes (Fig. 2A). TGR5 agonists also increased cAMP (by 27–33%) in cholangiocytes derived from WT rats and healthy humans (Supporting Fig. 1).
Figure 2.
TGR5 agonists increases cAMP levels, cell proliferation and cyst growth in 3-D cultures. (A) TLCA, OA, C1 and C2 (all, 25 µM) enhanced cAMP production in rat and human cystic cholangiocytes compared to un-treated cholangiocytes. (B) All four TGR5 agonists increase cholangiocyte proliferation assessed by MTS absorbance and (C) cell counting. (D) Representative images and scatter plots demonstrate accelerated expansion of freshly dissected PCK bile ducts upon treatment with TLCA and OA. All data are presented as MEAN±SD. Abbreviation: Un-tr – un-treated.
TGR5 agonists increase proliferation of cystic cholangiocytes
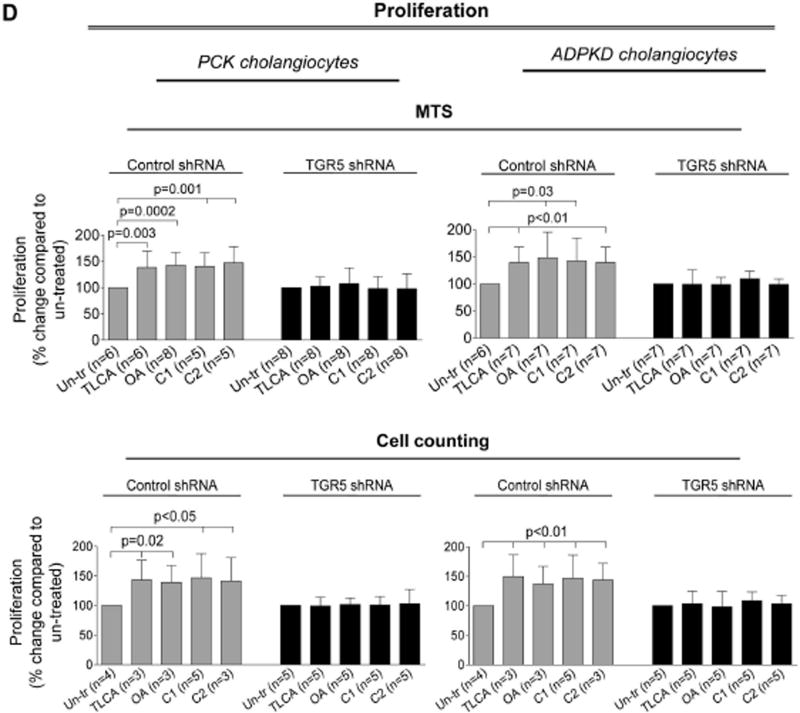
All TGR5 agonists increased proliferation of PCK cholangiocytes by 37–51% and ADPKD cholangiocytes by 39–49% as assessed by MTS absorbance (Fig. 2B) and cell counting (Fig. 2C). TGR5 agonists also increased (by 23–30%) proliferation of cholangiocytes derived from WT rats and healthy humans (Supporting Fig. 2).
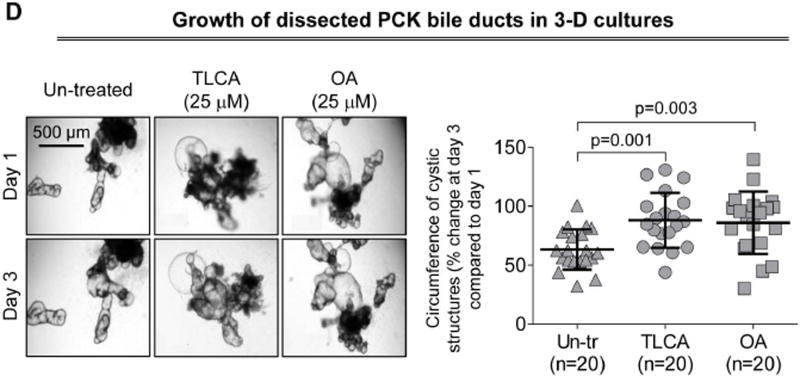
TGR5 agonists accelerate growth of isolated PCK bile ducts in 3-D culture
PCK bile ducts progressively grow under basal conditions (Fig. 2D). At day 3, they were ~60% larger compared to day 1. TLCA and OA accelerated cyst growth increasing circumferences of bile ducts, respectively, by 88% and 92%.
Oleanolic acid increases hepato-renal cystogenesis in PCK rats
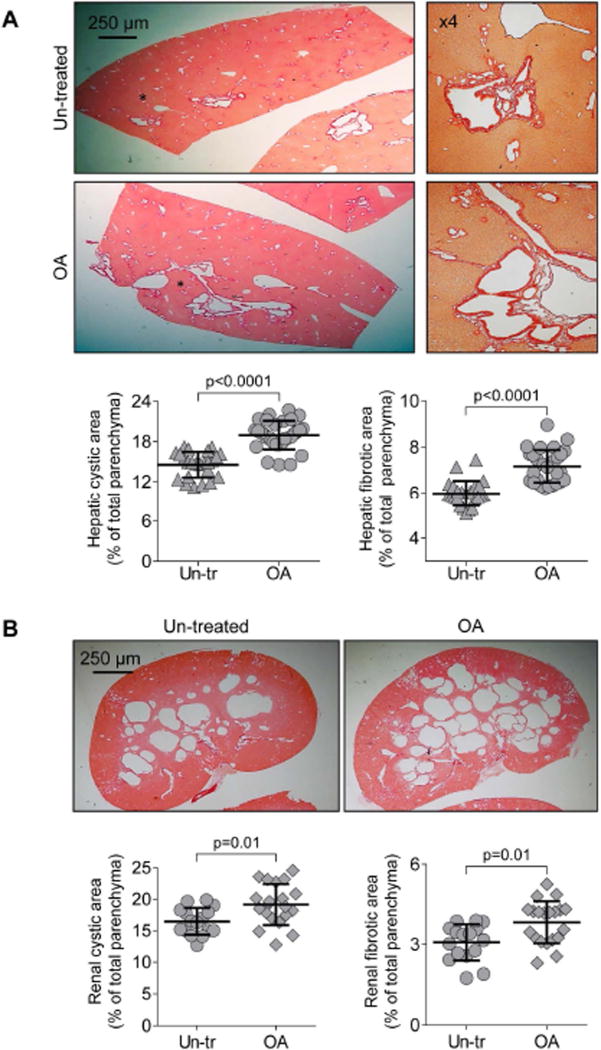
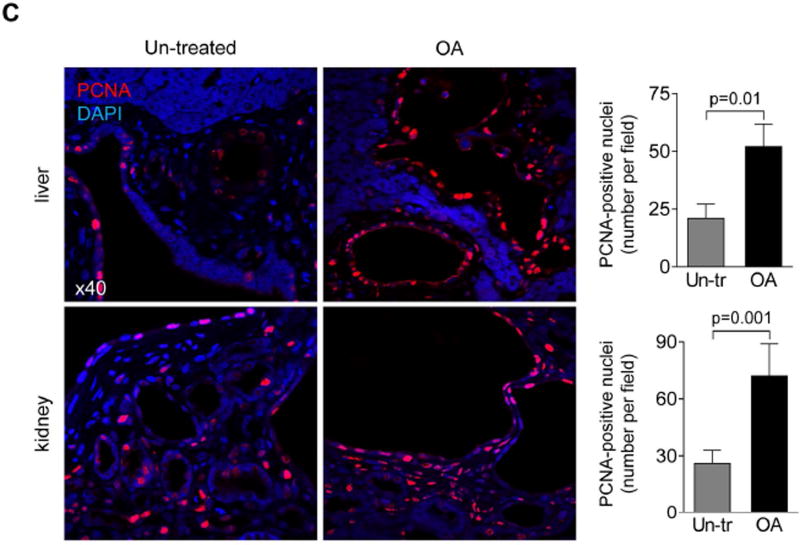
OA was well tolerated, without mortality or toxicity (i.e., no hair or weight loss). Serum biochemistries were comparable in un-treated and OA-treated PCK rats (Supporting Table 1). OA worsened hepato-renal cystogenesis as evidenced by increased: (i) liver weights (~25%); (ii) kidney weights (~22%); (iii) hepatic cystic areas (~32%); (iv) hepatic fibrotic areas (~19%); (v) renal cystic areas (~26%); and (vi) renal fibrotic areas (~22%; Supporting Table 2 and Fig. 3A–B). After OA treatment, the numbers of PCNA-positive nuclei were higher 2.5-fold in cystic cholangiocytes and 2.8-fold in cystic renal epithelia compared to respective controls (Fig. 3C).
Figure 3.
Oleanolic acid (OA) increases hepatic and renal cystogenesis in PCK rats. Drug-treated rats (n=5 females; n=5 males) received daily injection of OA (25 mg/kg) and control group (n=4 females, n=4 males) were injected with equal doses of DMSO for 6 weeks. (A) Representative images of picrosirius red stained liver and (B) kidney sections of un-treated and OA-treated PCK rats. Area depicted by asterisk (A, left panels) is shown at higher magnification on right. Scatter plots depict cystic and fibrotic areas of individual liver lobes (three liver lobes from each rat) and kidneys (two kidneys from each rat) analyzed. (C) Representative images of liver and kidney section from un-treated and OA-treated PCK rats stained with PCNA antibody and quantitative analysis show a greater number of PCNA-positive nuclei after OA treatment. n=15 microscopic fields for each organ and group. Abbreviation: Un-tr – un-treated. All data are presented as MEAN±SD.
TGR5 reduction in cystic cholangiocytes abolishes effects of TGR5 agonists on cAMP levels, Erk1/2 phosphorylation, cell proliferation and cyst growth in vitro
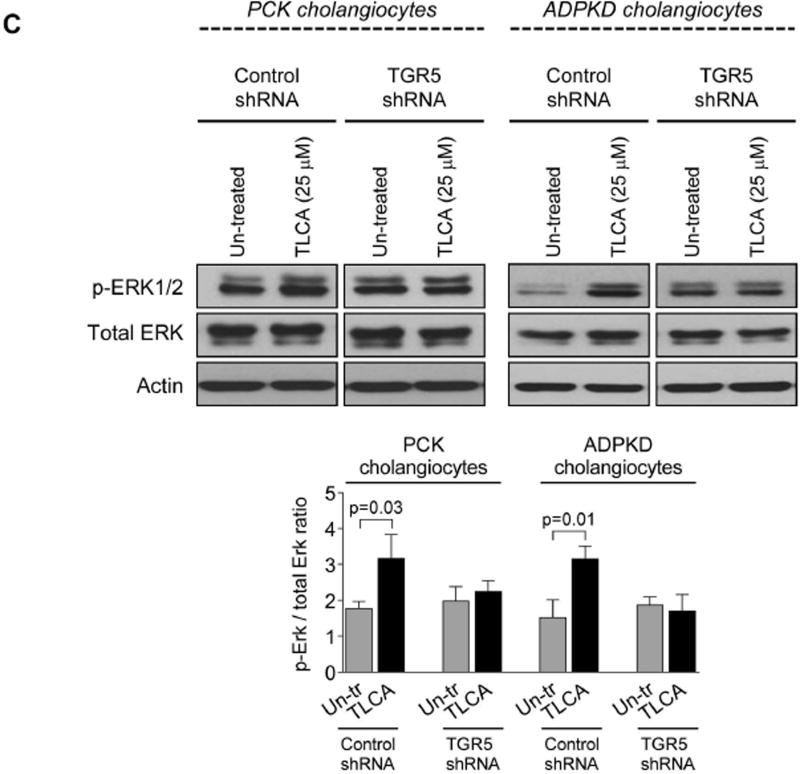
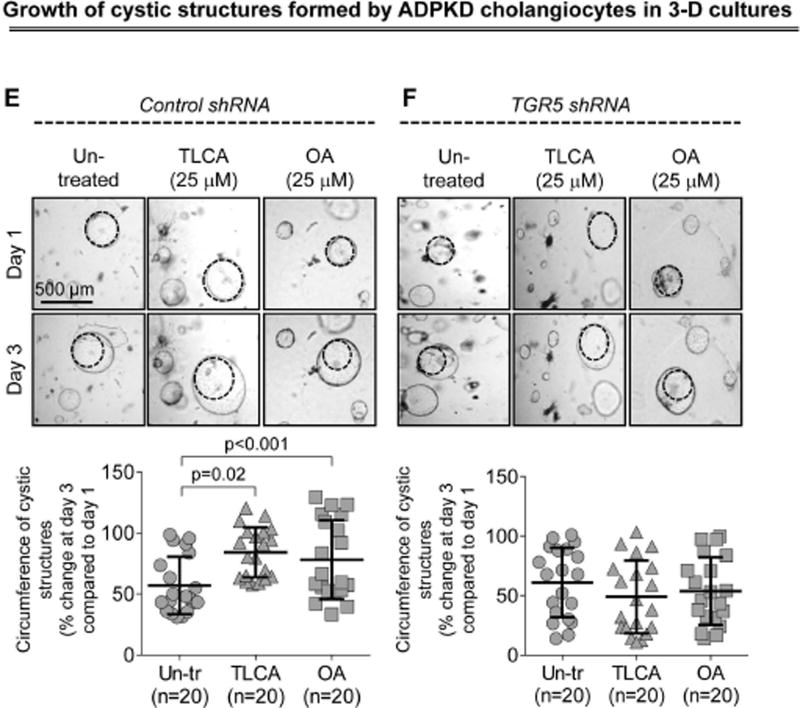
Western blots demonstrate that TGR5 levels were decreased by ~80% in TGR5-shRNA-transfected cholangiocytes compared to cholangiocytes transfected with control shRNA (Fig. 4A). Compered to un-transfected cholangiocytes, control-shRNA-transfected cholangiocytes have comparable levels of cAMP, cell proliferation and rate of cyst growth after treatment with TGR5 agonists (Supporting Fig. 3). Control-shRNA-transfected cholangiocytes respond to TGR5 ligands by increasing cAMP levels, Erk1/2 phosphorylation and cell proliferation; these effects were eliminated in cholangiocytes with reduced levels of TGR5 (Fig. 4B–D). Similarly to freshly isolated PCK bile ducts, growth of cystic structures formed by cultured control-shRNA-transfected ADPKD cholangiocytes was enhanced by 47% and 36% in the presence of TLCA and OA, respectively (Fig. 4E). No changes in circumference of cystic structures were noticed in TGR5-shRNA-transfected cholangiocytes (Fig. 4F).
Figure 4.
Depletion of TGR5 abolishes effects of its agonists on cAMP levels, Erk1/2 phosphorylation, cell proliferation and cyst growth. (A) Representative western blots and quantitation of TGR5 band density demonstrate reduced expression of TGR5 in cystic cholangiocytes stably transfected with TGR5 shRNA compared to control-shRNA-transfected cholangiocytes. n=3 for each cell line. (B) Levels of cAMP were increased in response to treatment with TGR5 agonists (all, 25 µM) in cholangiocytes transfected with control shRNA but not TGR5 shRNA. (C) Representative western blots and quantitation analysis demonstrate that TLCA increased Erk phosphorylation in control-shRNA-transfected rat and human cystic cholangiocytes but not in TGR5-shRNA depleted cells. n=3 for each cell line. (D) Stimulation of control-shRNA-transfected cholangiocytes with TGR5 agonists (all, 25 µM) accelerated their proliferation; these effects were abolished in TGR5-depleted cells. (E) Representative images and scatter plots show that growth of cysts formed by control-shRNA-transfected ADPKD cholangiocytes is increased in response to TGR5 agonists compared to un-treated controls. (F) No differences in circumference of cystic structures formed by cholangiocytes transfected with TGR5 shRNA were observed in the absence or presence of TGR5 agonists. All data are presented as MEAN±SD. Abbreviation: Un-tr – un-treated.
Genetic elimination of TGR5 in mice with PLD decreases hepatic cystogenesis
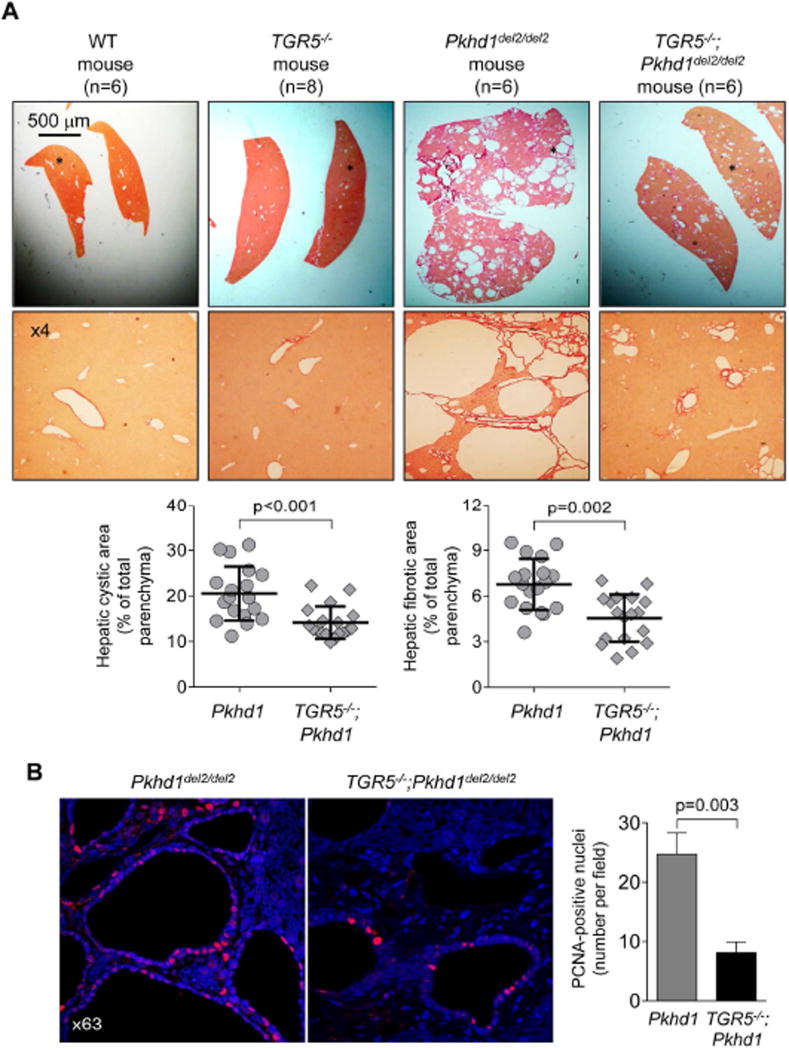
To further examine the involvement of TGR5 in PLD, we generated double-mutant mice yielding TGR5−/−;Pkhd1del2/del2 phenotype. Consistent with a previous report, TGR5−/− mice were healthy and fertile.29 Serum biochemistries (Supporting Table 3), gross anatomy (Supporting Table 4) and morphology of liver, cholangiocyte and primary cilium (Supporting Fig. 4) were comparable in WT and TGR5−/− mice. Pkhd1del2/del2 rodents have multiple hepatic cysts.30 Consistent with our observations in other animal models of PLD, TGR5 was over-expressed in Pkhd1del2/del2 mice compared to WT but was not detected in TGR5−/− mice and TGR5−/−;Pkhd1del2/del2 mice (Supporting Fig. 5). In contrast to Pkhd1del2/del2 littermates, in double mutant TGR5−/−;Pkhd1del2/del2 mice (both males and females), we observed reductions in: (i) liver weight by 30%; (ii) hepatic cystic areas by 31%; and (iii) hepatic fibrotic areas by 33% (Fig. 5A and Supporting Table 4). Serum biochemistries were similar in all groups of mice (Supporting Table 3). Attenuated hepatic cystogenesis in double mutant TGR5−/−;Pkhd1del2/del2 mice was associated with 3-fold decreased quantity of PCNA-positive nuclei (Fig. 5B).
Figure 5.
Hepatic cystogenesis and cholangiocyte proliferation is decreased in double mutant TGR5−/−;Pkhd1del2/del2 mice. (A) Representatives liver section stained with picrosirius red show that cysts were absent in age- (5–7 month old) and sex-matched wild type (WT; n=3 males, n=3 females) and TGR5−/− (n=3 males, n=5 females) rodents but present in Pkhd1del2/del2 mice (n=3 males, n=3 females); TGR5−/−;Pkhd1del2/del2 mice (n=3 males, n=3 females) have reduced hepatic cystogenesis. Asterisks in upper panel depict areas shown underneath with higher power. Scatter plots demonstrate that cystic and fibrotic areas are decreased in TGR5−/−;Pkhd1del2/del2 mice compared to Pkhd1del2/del2 littermates. Three liver lobes from each mouse were analyzed. (B) Number of PCNA-positive nuclei were greater in cholangiocytes of TGR5−/−;Pkhd1del2/del2 mice compared to Pkhd1del2/del2 rodents. n=20 microscopic cholangiocyte fields per group. All data are presented as MEAN±SD.
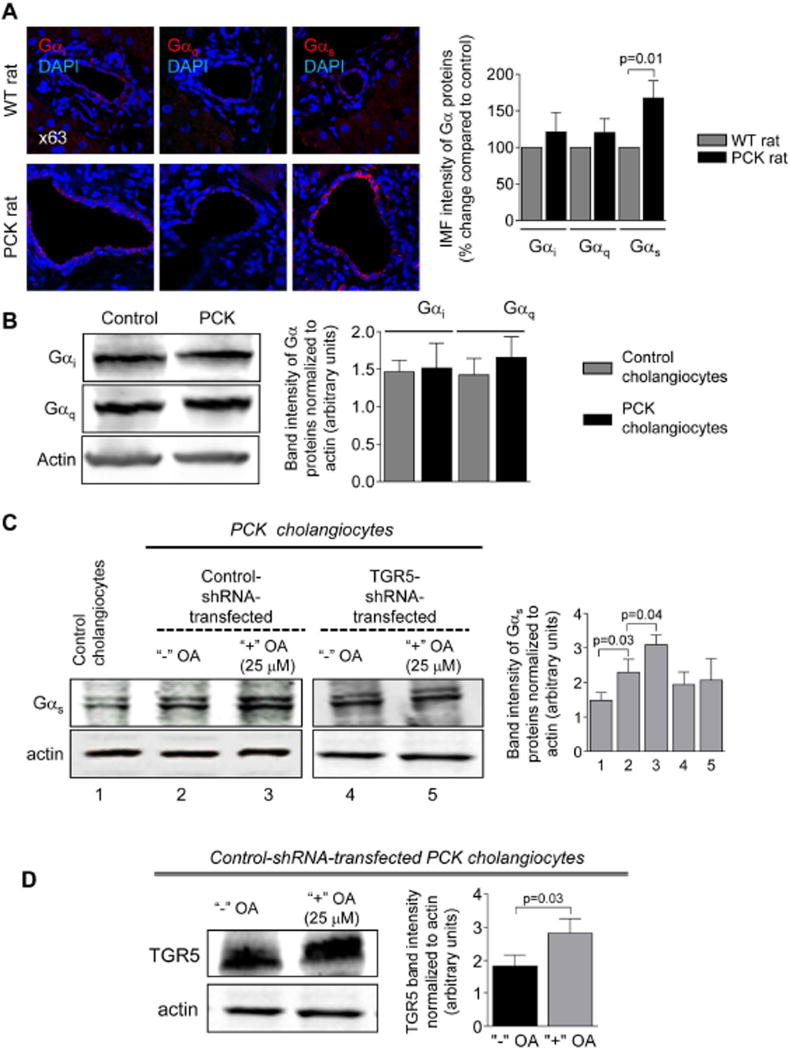
Expression of Gαs proteins is increased in PLD
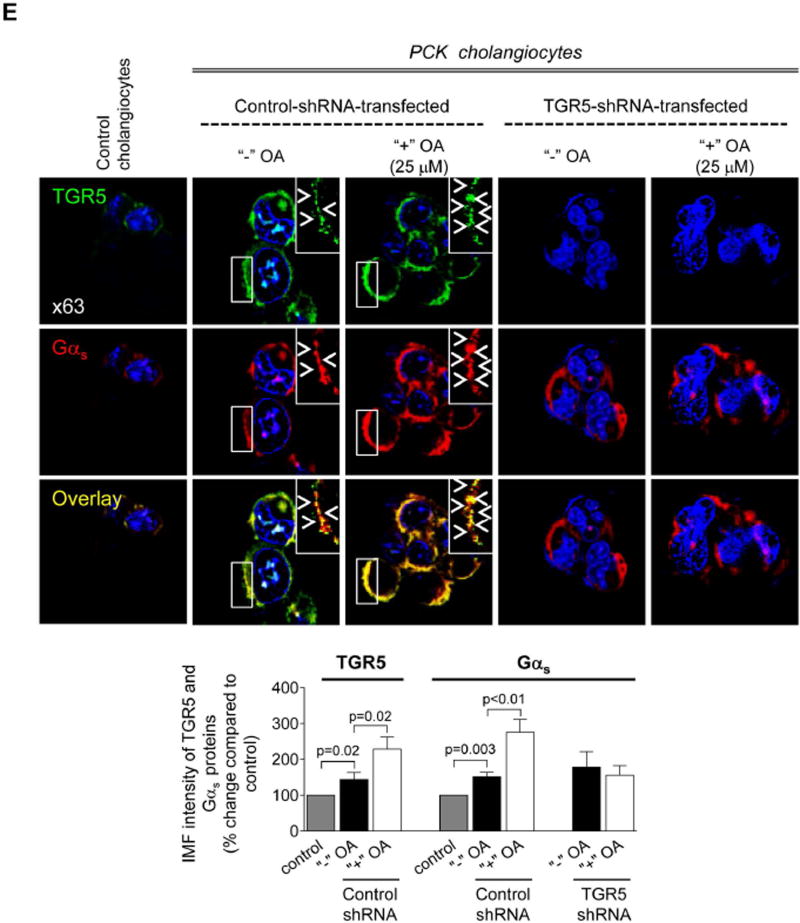
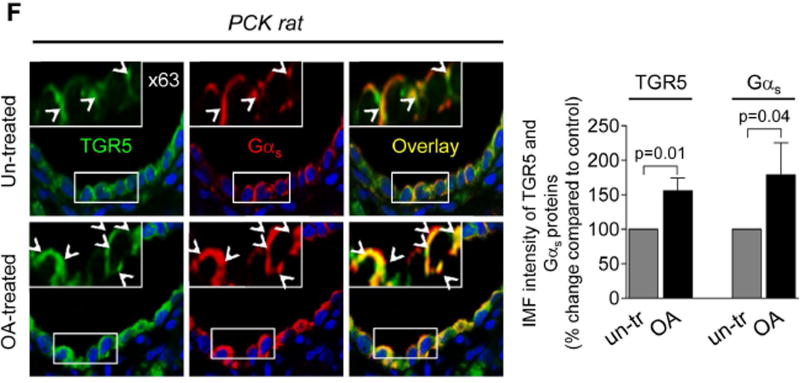
We investigated the expression of Gα proteins known to be coupled with TGR5.20 Levels of Gαi and Gαq were identical in control and cystic cholangiocytes (Fig. 6A–B). In contrast, immunoreactivity of Gαs in cholangiocytes of PCK rats was increased by ~67% compared to WT rats (Fig. 6A). Similarly, in cultured cystic cholangiocytes expression of Gαs was ~50% higher compared to control (i.e., cholangiocytes derived from wild type rats; Fig. 6C and E). OA treatment increased levels of Gαs by ~30% in control-shRNA-transfected cholangiocytes but no changes were observed in TGR5-depleted cholangiocytes (Fig. 6C and E). Up-regulation of TGR5 (by ~50%) was also seen in response to OA (Fig. 6D and E). Consistent with an in vitro observation, 59% and 48% greater immunoreactivity of Gαs and TGR5, respectively, was observed in cholangiocytes of OA-treated PCK rats (Fig. 6F). Finally, we found by confocal microscopy that co-localization of TGR5 with Gαs proteins in cystic cholangiocytes was increased ~2-fold after TGR5 stimulation (Costes’ test, p<0.001; Fig. 6 E–F).
Figure 6.
Expression of Gαs is increased in cystic cholangiocytes. (A) Representative images and quantitation of relative IMF intensity of Gα proteins demonstrate that levels of Gαi and Gαq were comparable in cholangiocytes of wild type (WT) and PCK rats while Gαs expression was increased in cystic cholangiocytes of PCK rats. n=5 livers for each group. (B) Representative western blots and quantitation of band intensity of Gα proteins further confirmed that Gαi and Gαq are equally expressed in cultured control (i.e., cholangiocytes derived from wild type rat) and cystic cholangiocytes. n=3 for each cell line. (C) Expression of Gαs protein was higher in PCK cholangiocytes compared to control as demonstrated by representative western blots (protein bands were numbered from 1 to 5) and quantitation of Gαs band intensity in the absence of TGR5 agonists (line 1 versus line 2). OA treatment increased Gαs levels in cholangiocytes transfected with control shRNA (line 2 versus line 3) but not in TGR5-shRNA-transfected cholangiocytes (line 4 versus line 5). No differences in Gαs expression was noticed between cholangiocytes transfected with control shRNA or TGR5 shRNA in the absence of treatment (line 2 versus line 4 and line 5). n=3 for each condition. (D) Representative western blots and quantitation of TGR5 band intensity show that OA treatment increases expression of TGR5 in cholangiocytes. n=3 for each condition. (E) Representative confocal images and quantitation of IMF intensity of Gαs and TGR5 confirmed that both proteins are over-expressed in cystic cholangiocytes and their immunoreactivity is further increases upon stimulation with OA. Co-localization of TGR5 with Gαs also increases after OA application. The arrows highlight some examples of co-localization. The upper right insets show higher power of the areas outlined in the main panel. n=5 for each condition. (F) OA treatment also increased immunoreactivity of TGR5 and Gαs and their co-localization in cholangiocytes of PCK rats. The arrows highlight some examples of co-localization. The upper left insets show higher power of the areas outlined in the main panel. Representative of 4–5 rats per group. All data are presented as MEAN±SD.
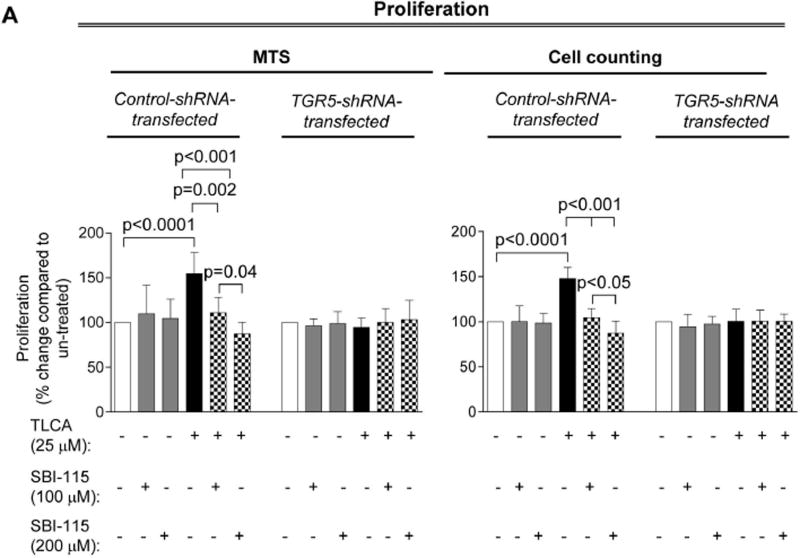
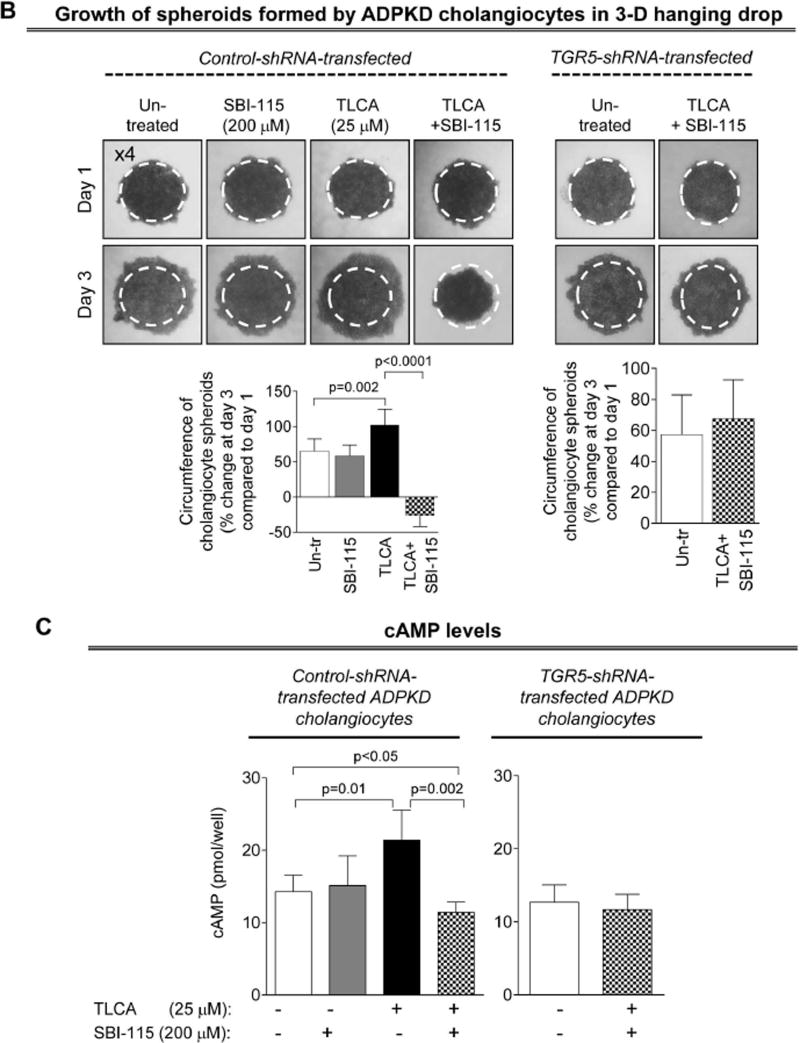
A TGR5 antagonist, SBI-115, decreases cell proliferation, cholangiocyte spheroid growth and cAMP levels in cystic cholangiocytes
SBI-115 alone had no effects on cell proliferation, spheroid growth and cAMP levels in shRNA-transfected ADPKD cholangiocytes. In contract, stimulation of cholangiocytes with TLCA following by SBI-115 treatment resulted in dose-dependent (by 32–48%) inhibition of cell proliferation (Fig. 7A). Under similar conditions, SBI-115 reduced growth of cholangiocyte spheroids (Fig. 7B) and levels of cAMP (Fig. 7C) by ~30%. These processes were not affected in TGR5-depleted ADPKD cholangiocytes pre-treated with TLCA (Fig. 7A–C). Moreover, to provide additional evidence that TGR5 antagonism decreases cAMP in a TGR5-dependent fashion, we increased cAMP levels in cystic cholangiocytes by forskolin (i.e., independently of TGR5) and found that SBI-115 had no effect on forskolin-induced cAMP production (Supporting Figure 6).
Figure 7.
SBI-115 decreases proliferation, growth of cholangiocyte spheroids in vitro and cAMP levels in ADPKD cholangiocytes. (A) SBI-115 alone had no effect on cholangiocyte proliferation. In contrast, proliferation triggered by pre-treatment of cystic cholangiocytes with TLCA was inhibited in response to SBI-115 in a dose-dependent fashion. n=15 for each condition. (B) Representative images of cholangiocyte spheroids and quantitative assessment show that SBI-115 alone did not affect their growth while TLCA-induced spheroid expansion was decreased upon treatment with TGR5 antagonist. n=8 for each condition. (C) Levels of cAMP in ADPKD cholangiocytes stimulated with TLCA were inhibited by SBI-115. n=8 for each condition. The effects of SBI-115 were absent in TGR5-depleted ADPKD cholangiocytes. All data are presented as MEAN±SD.
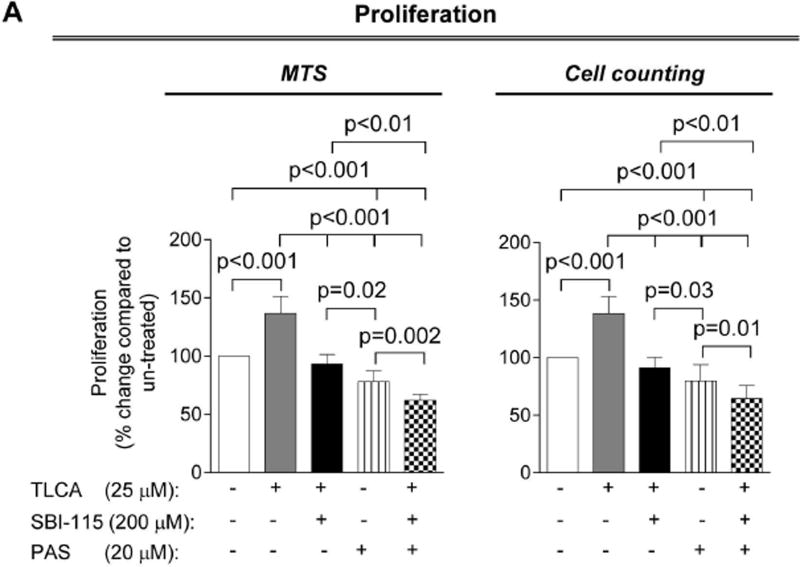
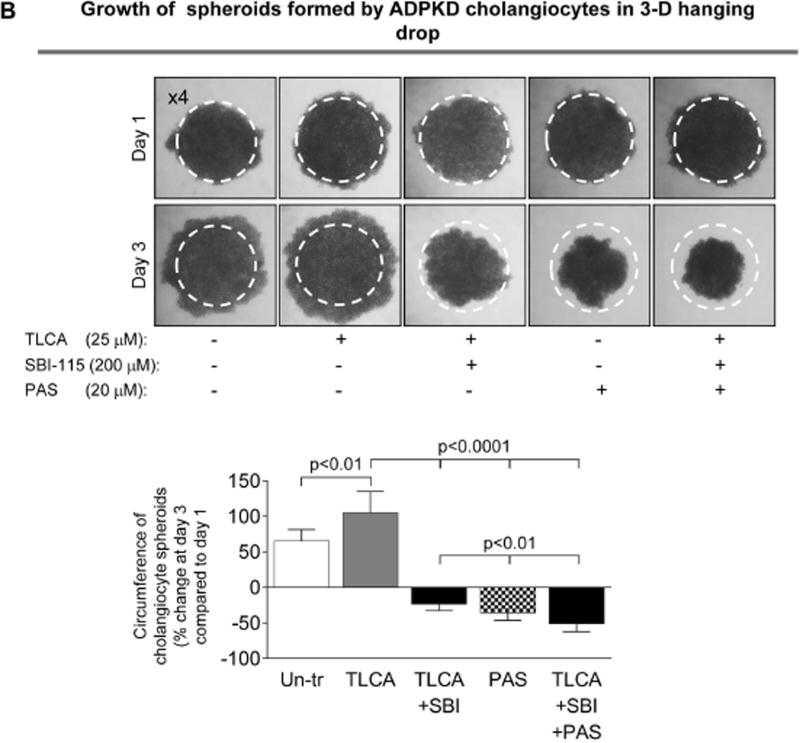
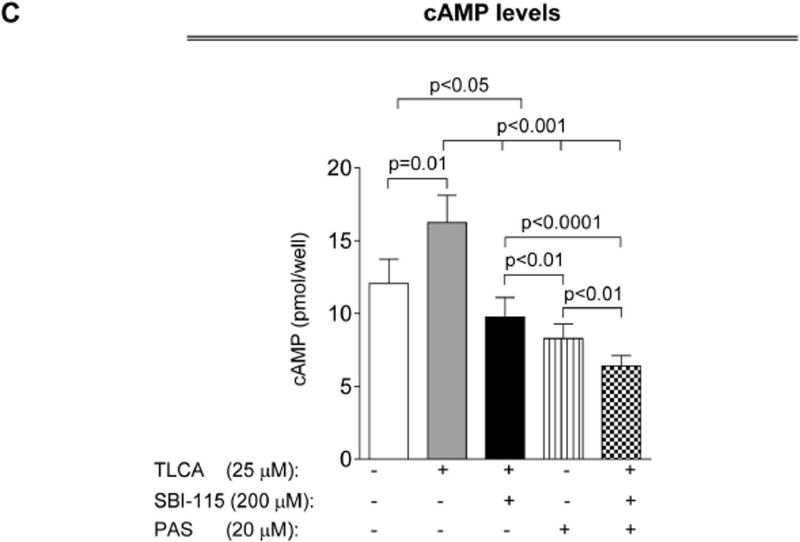
Concurrently, SBI-115 and pasireotide decrease cell proliferation, cholangiocyte spheroid growth and cAMP levels in vitro more effectively than each drug alone
Finally, we tested how the simultaneous targeting of elevated cAMP in cystic cholangiocytes by SBI-115 and pasireotide will affect the cellular processes known to play an important role in hepatic cystogenesis. We observed that cell proliferation, spheroid growth and cAMP levels in control-shRNA-transfected ADPKD cholangiocytes were decreased after treatment with SBI-115 by 20–33% and by 35–40% in response to pasireotide. However, a greater reduction (by 45–55%) in cell proliferation, spheroid growth and cAMP was detected in response to drug combination (Fig. 8A–C). In addition, cAMP levels, cell proliferation and spheroid growth was decreased by ~30–35% in TGR5-shRNA-depleted cystic cholangiocytes in response to pasireotide alone (Supporting Fig. 7) suggesting that pasireotide affects these processes independently of TGR5.
Figure 8.
Concurrent treatment of ADPKD cholangiocytes by SBI-115 and pasireotide decreased cell proliferation, cholangiocyte spheroid growth and cAMP levels. Bar graphs, representative images of cholangiocyte spheroids and quantitative assessment demonstrate that SBI-115 alone and pasireotide alone or in combination decreased (A) proliferation of ADPKD cholangiocytes, (B) spheroid growth in 3-D and (C) cAMP levels. SBI-115 and pasireotide combination affected these processes to a higher extend than each drug alone. n=8 for each experimental condition. All data are presented as MEAN±SD.
DISCUSSION
The key findings of our study are: (i) TGR5 is overexpressed in PLD and its agonists accelerates disease progression; (ii) pharmacologic inhibition of TGR5 in cystic cholangiocytes in vitro by a novel small molecule antagonist or in vivo by genetic elimination of TGR5 in an animal model of PLD attenuates disease progression; (iii) TGR5 contributes to hepatic cystogenesis by enhancing cAMP production and Gαs expression; and (iv) concurrent targeting of cAMP machinery in cystic cholangiocytes by TGR5 antagonists and SSTR agonists inhibits cell proliferation and cyst growth in vitro more substantially than each drug alone. Together, these data suggest that TGR5 contributes to PLD and its inhibition represents a potential therapeutic approach. Furthermore, targeting of elevated cAMP in cystic cholangiocytes via two different pathways [i.e., TGR5 antagonists and SSTR agonists (currently the only available pharmacologic option for PLD patients)] may be an approach worth evaluating.
Our data show that the hyper-proliferative effects of TGR5 agonists in PLD are TGR5-dependent. All tested TGR5 agonists modestly but significantly accelerated proliferation (i.e., one of the major mechanisms involved in cyst growth) of cultured rat and human cystic cholangiocytes, and increased the number of PCNA-positive cholangiocytes in PCK rats treated with OA. In TGR5-depleted cholangiocytes in vitro or in cholangiocytes of double mutant TGR5−/−;Pkhd1del2/del2 mice with genetically eliminated TGR5, the effects of TGR5 agonists on cell proliferation was absent. In line with our observations, cholangiocyte hyper-proliferation in response to TGR5 agonists has been reported previously.21, 31 For example, TLCA feeding increased the number of PCNA-positive cholangiocytes in rats while cholic acid triggered cholangiocyte proliferation in WT but not in TGR5−/− mice.31,21 Moreover, TGR5 agonists have been shown to enhance growth of human cells derived from endometrial cancer, gastric adenocarcinoma and cholangiocarcinoma (CCA).21,22, 32
Using different approaches, we demonstrated that TGR5 is up-regulated in PLD at the message and protein levels and its activation worsens hepatic cystogenesis. Accumulating data suggest that TGR5 levels are increased in a number of different pathological conditions and might influence disease outcome. Indeed, over-expression of TGR5 in gastric adenocarcinoma was associated with decreased rate of patient survival22 and a high copy number of TGR5 gene was found to be an indicator of worse prognosis in gastric and breast cancer patients.33 Up-regulation of TGR5 was also reported in patients with CCA and pancreatic cancers.21, 33 Thus, there appears to be a growing number of diseases17, 21, 23, 32 in addition to PLD in which an effective antagonist to TGR5 might be of value.
Why expression of TGR5 is increased in PLD (or in other pathological conditions) is unclear. Our preliminary data suggest that over-expression of TGR5 in cystic cholangiocytes is associated with decreased levels of miR-204 and miR-708 which target TGR5 mRNA. This potential mechanism of TGR5 up-regulation in PLD is now under investigation in our laboratory.
To further reveal the contribution of TGR5 to PLD, we genetically eliminated this bile acid receptor in cystic cholangiocytes by crossing TGR5−/− and Pkhd1del2/del2 mice. It has been reported previously that TGR5−/− mice which we used for cross-breeding had body weight and bile acid pool size comparable to WT littermates.29 We also observed no differences in morphology of the liver (e.g., no cysts), of cholangiocytes and of cholangiocyte primary cilia in TGR5−/− mice compared to WT. In contrast, Pkhd1del2/del2 mice, a well-characterized model of PLD, develop multiple liver cysts by the age of 7–9 months.30 Consistent with our observations in other animal models of PLD (i.e., PCK rat and Pkd2WS25/− mice), TGR5 was significantly overexpressed in cholangiocytes lining liver cysts in Pkhd1del2/del2 rodents. In contrast, no TGR5 expression was observed in TGR5−/−;Pkhd1del2/del2 double-mutant mice and depletion of TGR5 significantly reduced cystic and fibrotic areas in TGR5−/−;Pkhd1del2/del2 mice, providing perhaps the best evidence that TGR5 plays an important role in hepatic cystogenesis.
Several reports suggest that TGR5 could be coupled to Gαs, Gαi and Gαq proteins.18, 20, 34 In order to understand the mechanisms by which stimulation of TGR5 enhances hepatic cystogenesis, we studied the presence of Gα proteins in PLD. Expression of Gαi and Gαq was similar between control and diseased cholangiocytes but levels of Gαs (the release of which accelerates cAMP production) were up-regulated in PCK and ADPKD cystic cholangiocytes. In line with this, all four tested TGR5 agonists increased cAMP levels in cystic cholangiocytes to a higher extend (by ~12–17%) than in control cholangiocytes. Effects of TGR5 stimulation were abolished after TGR5 reduction by shRNA. We noticed that levels of cAMP were comparable in control-shRNA-transfected and TGR5-shRNA-transfected cholangiocytes in the absence of treatment. TGR5 enhances cAMP production only in response to its stimulation. In cultured cholangiocytes under basal conditions, TGR5 is silent due to the absence of TGR5 agonists in culture media. However, control-shRNA-transfected cholangiocytes (but not TGR5-depleted cholangiocytes) respond to TGR5 stimulation by increased cAMP levels.
We also demonstrated that expression of both, Gαs and TGR5 proteins and their co-localization was increased in response to OA in vitro and in vivo. These data suggest that exposure of cystic cholangiocytes to TGR5 agonists might account for overexpression of both TGR5 and Gαs in PLD leading to cAMP elevation. An increased expression of TGR5 upon exposure to its ligands has also been observed in oesophageal epithelium, gastric myenteric plexus and macrophages.35–37
To our knowledge, no reports describe the identification, synthesis or pharmacologic activity of TGR5 antagonists. SBI-115 (further details of which will be published elsewhere), a novel small molecule, was identified from a high-throughput screen in CHO-K1 cells expressing TGR5. We confirmed and validated the effects SBI-115 in PLD and found that this TGR5 antagonist reduced cell proliferation and growth of cholangiocyte spheroids in vitro by decreasing cAMP levels in cystic cholangiocytes in a TGR5-dependent manner. Moreover, concurrent targeting of cAMP machinery by SBI-115 and an SSTR analog, pasireotide, inhibited these processes more effectively compared to each drug alone. While in vivo pre-clinical studies are needed to further demonstrate the potential beneficial effects of TGR5 targeting in PLD, our extensive experience using different activators and inhibitors of hepatic cystogenesis4–7, 9, 38, 39 suggest that results of two in vitro experimental systems (i.e., cell proliferation and cyst growth) reliably predicts outcomes of in vivo studies in different animal models of PLD.
Finally, while our in vitro and in vivo data strongly suggest that TGR5 agonists/antagonists affect hepatic cystogenesis in PLD by direct activation/inhibition of cholangiocyte TGR5, we acknowledge that other mechanisms might also be contributing factors. One such indirect mechanism might involve modulation of cholangiocyte proliferation by the hormone GLP-1 which is released by intestinal L-cells in response to TGR5 stimulation.40 Although experimental evidence suggests that cholangiocytes express the receptor for GLP-1 (i.e., GLP-1R) and its activation increases cell proliferation,41 further studies are needed to assess the role of the GLP-1 in TGR5-mediated cyst growth. However, we found that levels of GLP-1R are decreased in human and rat cystic cholangiocytes, respectively, 16-fold and 37-fold (Supporting Fig. 8) suggesting that a potential contribution of GLP-1 to hepatic cystogenesis might not be significant.
In conclusion, we demonstrated that TGR5 agonists contribute to hepatic cystogenesis by increasing cAMP production and expression of Gαs proteins. We also show that TGR5 antagonist alone or in combination with other drugs that lower cAMP in cystic cholangiocytes is a promising therapeutic target in PLD.
Supplementary Material
Acknowledgments
Grant support: This work was supported by DK24031 grant from the NIH, the Mayo Clinic, the Clinical Core and Optical Microscopy Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), the Mayo Translational PKD Center (NIDDK P30DK090728), the Mayo Translational PKD Center Pilot and Feasibility Award, and by the Eileen Creamer O'Neill Award from the PKD Foundation
Abbreviations
- PKD
polycystic kidney disease
- PLD
polycystic liver disease
- ADPKD
autosomal dominant PKD
- ARPKD
autosomal recessive PKD
- PKD1
polycystic kidney 1
- PKD2
polycystic kidney 2
- PKHD1
polycystic kidney and hepatic disease 1
- PRKCSH
protein kinase C substrate 80K-H
- PCK
polycystic kidney
- TLCA
taurolithocholic acid
- OA
oleanolic acid
- cAMP
cyclic adenosine monophosphate
- ERK
extracellular signal-regulated protein kinase
- EPAC
exchange protein activated by cAMP
- PKA
protein kinase A
- AQP1
aquaporin 1
- CFTR
cystic fibrosis transmembrane conductance regulator
- AE2
anion exchanger
- AKAP150
A-kinase anchoring protein
- PDE4C
phosphodiesterase 4C
Footnotes
Conflict of interest: The authors have declared no conflicts of interest exist.
TM, AM & NL supervised the project & wrote the manuscript. TM, AM, MLP, BR, BH, PYL and XF performed experiments and contributed to discussion. AP, ES and RA identified and synthesized the TGR5 compounds and contributed to discussion. TC contributed to design of SBI-115 experiments and discussion.
References
- 1.Wills ES, Roepman R, Drenth JP. Polycystic liver disease: ductal plate malformation and the primary cilium. Trends in molecular medicine. 2014 doi: 10.1016/j.molmed.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Masyuk TV, Masyuk AI, La Russo NF. Therapeutic Targets In Polycystic Liver Disease. Current drug targets. 2015 doi: 10.2174/1389450116666150427161743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perugorria MJ, Masyuk TV, Marin JJ, et al. Polycystic liver diseases: advanced insights into the molecular mechanisms. Nature reviews. Gastroenterology & hepatology. 2014 doi: 10.1038/nrgastro.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masyuk TV, Masyuk AI, Torres VE, et al. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–16. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Banales JM, Masyuk TV, Gradilone SA, et al. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–74. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masyuk TV, Radtke BN, Stroope AJ, et al. Inhibition of Cdc25A suppresses hepato-renal cystogenesis in rodent models of polycystic kidney and liver disease. Gastroenterology. 2012;142:622–633. e4. doi: 10.1053/j.gastro.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banales JM, Masyuk TV, Bogert PS, et al. Hepatic cystogenesis is associated with abnormal expression and location of ion transporters and water channels in an animal model of autosomal recessive polycystic kidney disease. The American journal of pathology. 2008;173:1637–46. doi: 10.2353/ajpath.2008.080125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi YH, Suzuki A, Hajarnis S, et al. Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10679–84. doi: 10.1073/pnas.1016214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masyuk TV, Radtke BN, Stroope AJ, et al. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58:409–21. doi: 10.1002/hep.26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–61. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larusso NF, Masyuk TV, Hogan MC. Polycystic Liver Disease: The Benefits of Targeting cAMP. Clin Gastroenterol Hepatol. 2016;14:1031–4. doi: 10.1016/j.cgh.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neijenhuis MK, Gevers TJ, Nevens F, et al. Somatostatin analogues improve health-related quality of life in polycystic liver disease: a pooled analysis of two randomised, placebo-controlled trials. Alimentary pharmacology & therapeutics. 2015;42:591–8. doi: 10.1111/apt.13301. [DOI] [PubMed] [Google Scholar]
- 13.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46:302–12. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nature reviews. Gastroenterology & hepatology. 2014;11:55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 15.Keitel V, Reich M, Haussinger D. TGR5: Pathogenetic Role and/or Therapeutic Target in Fibrosing Cholangitis? Clinical reviews in allergy & immunology. 2014 doi: 10.1007/s12016-014-8443-x. [DOI] [PubMed] [Google Scholar]
- 16.Keitel V, Haussinger D. TGR5 in cholangiocytes. Current opinion in gastroenterology. 2013;29:299–304. doi: 10.1097/MOG.0b013e32835f3f14. [DOI] [PubMed] [Google Scholar]
- 17.Hodge RJ, Nunez DJ. Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype? Diabetes Obes Metab. 2016;18:439–43. doi: 10.1111/dom.12636. [DOI] [PubMed] [Google Scholar]
- 18.Masyuk AI, Huang BQ, Radtke BN, et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. American journal of physiology. Gastrointestinal and liver physiology. 2013;304:G1013–24. doi: 10.1152/ajpgi.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz-Garrido P, Marin JJ, Perugorria MJ, et al. Ursodeoxycholic acid inhibits hepatic cystogenesis in experimental models of polycystic liver disease. Journal of hepatology. 2015;63:952–61. doi: 10.1016/j.jhep.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong J, Behar J, Wands J, et al. Role of a novel bile acid receptor TGR5 in the development of oesophageal adenocarcinoma. Gut. 2010;59:170–80. doi: 10.1136/gut.2009.188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich M, Deutschmann K, Sommerfeld A, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65:487–501. doi: 10.1136/gutjnl-2015-309458. [DOI] [PubMed] [Google Scholar]
- 22.Cao W, Tian W, Hong J, et al. Expression of bile acid receptor TGR5 in gastric adenocarcinoma. American journal of physiology. Gastrointestinal and liver physiology. 2013;304:G322–7. doi: 10.1152/ajpgi.00263.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carino A, Graziosi L, D'Amore C, et al. The bile acid receptor GPBAR1 (TGR5) is expressed in human gastric cancers and promotes epithelial-mesenchymal transition in gastric cancer cell lines. Oncotarget. 2016 doi: 10.18632/oncotarget.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piotrowski DW, Futatsugi K, Warmus JS, et al. Identification of Tetrahydropyrido[4,3-d]pyrimidine Amides as a New Class of Orally Bioavailable TGR5 Agonists. ACS medicinal chemistry letters. 2013;4:63–8. doi: 10.1021/ml300277t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans KA, Budzik BW, Ross SA, et al. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. Journal of medicinal chemistry. 2009;52:7962–5. doi: 10.1021/jm901434t. [DOI] [PubMed] [Google Scholar]
- 26.Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp. 2011 doi: 10.3791/2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware MJ, Colbert K, Keshishian V, et al. Generation of Homogenous Three-Dimensional Pancreatic Cancer Cell Spheroids Using an Improved Hanging Drop Technique. Tissue Eng Part C Methods. 2016;22:312–21. doi: 10.1089/ten.tec.2015.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong DW, Kim YH, Kim HH, et al. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharmaceutics & drug disposition. 2007;28:51–7. doi: 10.1002/bdd.530. [DOI] [PubMed] [Google Scholar]
- 29.Vassileva G, Golovko A, Markowitz L, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. The Biochemical journal. 2006;398:423–30. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woollard JR, Punyashtiti R, Richardson S, et al. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney international. 2007;72:328–36. doi: 10.1038/sj.ki.5002294. [DOI] [PubMed] [Google Scholar]
- 31.Alpini G, Glaser SS, Ueno Y, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–86. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 32.Casaburi I, Avena P, Lanzino M, et al. Chenodeoxycholic acid through a TGR5-dependent CREB signaling activation enhances cyclin D1 expression and promotes human endometrial cancer cell proliferation. Cell Cycle. 2012;11:2699–710. doi: 10.4161/cc.21029. [DOI] [PubMed] [Google Scholar]
- 33.Chen MC, Chen YL, Wang TW, et al. Membrane bile acid receptor TGR5 predicts good prognosis in ampullary adenocarcinoma patients with hyperbilirubinemia. Oncol Rep. 2016;36:1997–2008. doi: 10.3892/or.2016.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen DD, Godfrey CB, Niklas C, et al. The bile acid receptor TGR5 does not interact with beta-arrestins or traffic to endosomes but transmits sustained signals from plasma membrane rafts. The Journal of biological chemistry. 2013;288:22942–60. doi: 10.1074/jbc.M113.455774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang B, Zhao S, Tao Z, et al. Controlled bile acid exposure to oesophageal mucosa causes up-regulation of nuclear gamma-H2AX possibly via iNOS induction. Biosci Rep. 2016;36 doi: 10.1042/BSR20160124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou H, Zhou S, Gao J, et al. Upregulation of bile acid receptor TGR5 and nNOS in gastric myenteric plexus is responsible for delayed gastric emptying after chronic high-fat feeding in rats. Am J Physiol Gastrointest Liver Physiol. 2015;308:G863–73. doi: 10.1152/ajpgi.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Garcia S, Castaneda-Sanchez JI, Jimenez-Arellanes A, et al. Macrophage Activation by Ursolic and Oleanolic Acids during Mycobacterial Infection. Molecules. 2015;20:14348–64. doi: 10.3390/molecules200814348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gradilone SA, Habringer S, Masyuk TV, et al. HDAC6 is overexpressed in cystic cholangiocytes and its inhibition reduces cystogenesis. The American journal of pathology. 2014;184:600–8. doi: 10.1016/j.ajpath.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masyuk TV, Huang BQ, Ward CJ, et al. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303–10. doi: 10.1016/j.gastro.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Brighton CA, Rievaj J, Kuhre RE, et al. Bile Acids Trigger GLP-1 Release Predominantly by Accessing Basolaterally Located G Protein-Coupled Bile Acid Receptors. Endocrinology. 2015;156:3961–70. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marzioni M, Alpini G, Saccomanno S, et al. Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology. 2007;133:244–55. doi: 10.1053/j.gastro.2007.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.