Abstract
Objective
We sought to describe and evaluate longitudinal use of intra-articular injections after treatment initiation among adults with radiographically confirmed knee osteoarthritis (OA).
Method
Using data from the Osteoarthritis Initiative, we included participants with radiographically confirmed OA (Kellgren-Lawrence grade (K-L) ≥ 2) in ≥ 1 knee at baseline. With 9 years of data, 412 participants newly initiating hyaluronic acid or corticosteroid injections with their index visit were identified. For each type of injection initiated, socio-demographic and clinical characteristics were described by patterns of treatments (one-time use, switched, or continued injections). Multinomial logistic models estimated the extent to which patient-reported symptoms (post-initial injection and changes over time) were associated with patterns of injection use.
Results
Of those initiating injections, ~19% switched, ~21% continued injection type, and ~60% did not report any additional injections. For participants initiating corticosteroid injections, greater symptoms post-initial injection were associated with lower odds of continued use compared to one-time users (adjusted odds ratio (aOR) for WOMAC pain: 0.91; 95%, confidence interval (CI): 0.83 to 0.99; aORstiffness: 0.77; CI: 0.63 to 0.94; aORphysical function: 0.97; CI: 0.94 to 1.00). Symptom changes over time (e.g. worsened or improved) were not associated with patterns of injections use.
Conclusion
After treatment initiation, the proportion of patients switching injection use and one-time users was substantial. Symptoms post-initial injection appear to be associated with patterns of injection use. The extent to which these patterns are an indication of lack of impact on patient-reported symptoms should be explored.
Keywords: Intra-articular injections, Switching, Discontinuation, Longitudinal studies, Knee osteoarthritis
Introduction
Osteoarthritis (OA) is the most commonly seen arthritis among U.S. adults1–3. Among OA-affected joints, knee OA is one of the leading causes of disability among adults living in the community3. OA is a slowly progressive joint disease and currently has no cure. Generally, the treatment goals for non-pharmacologic or pharmacologic treatment of OA include pain relief, improved mobility, delayed disease progression, and improved quality of life. For those whose non-pharmacologic interventions or symptom-relieving medications are ineffective, intra-articular injections may be recommended to attempt to more directly target underlying pathophysiological processes 4–7.
Although several guidelines for the treatment of knee OA have been issued, the recommendations for use of intra-articular injections such as corticosteroid or hyaluronic acid injections are inconsistent4,8,9. Costs contributing to the long-term use of injections due to the chronic symptoms of knee OA could be substantial10. Intra-articular injections are increasingly common and particularly among patients newly diagnosed with knee OA 11. Given the concern regarding increase in use and the associated economic burden for patients with knee OA, evaluating how patients use these modalities over time is important. Examining patterns of injections use can help understand the switching and/or augmentation of treatment related to clinical outcomes12,13. However, no previous studies have evaluated treatment patterns for intra-articular injection use among patients with knee OA living in the community.
This study sought 1) to describe and evaluate longitudinal use of intra-articular injections after treatment initiation; and 2) to identify factors associated with patterns of treatment use among adults with radiographically confirmed knee OA. With the data derived from yearly visits from the Osteoarthritis Initiative (OAI), we were able to identify newly initiated injection users and examine factors associated with treatment patterns. We hypothesized that the patterns of injection use among participants initiating injections would be associated with more severe OA symptoms and/or changes in symptoms over time compared to those received one-time injections.
Method
Study sample
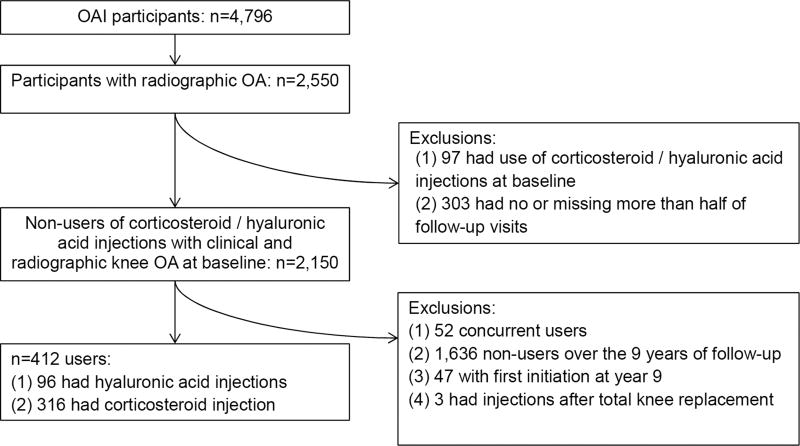
Publicly available data from the OAI were used14. The OAI study was originally a prospective cohort which enrolled participants from Baltimore, MD; Columbus, OH; Pittsburgh, PA; and Pawtucket, RI from 2004 through 2006. Using these four study sites, 4,796 patients with established knee OA or at high risk for developing knee OA were enrolled. For this present retrospective cohort study, we used information from annual assessments from baseline through year 9. Figure 1 shows the study sample for the current study. We first included patients with radiographically confirmed OA (defined as having a Kellgren-Lawrence grade (K-L) >2) at baseline (n=2,550). From this group, participants who reported already receiving injections (corticosteroid or hyaluronic) at baseline (n=97) were excluded. In addition, participants with no follow-up assessments or missing > half of these assessments were also excluded (n=303). From the remaining 2,150, we further excluded those reporting use of both injections (concurrent users, n=52), not reporting any injection use over the 9 years of follow-up (n=1,636), reporting first initiation at year 9 (n=47), and reporting injections after total knee replacement (n=3). The final analytic sample consisted 412 participants initiating injection use.
Figure 1.
Flowchart of study participants.
Index Knee for Analysis
In OAI, symptoms and x-rays for both knees were collected separately. We selected an index knee for use in the analysis based on the presence of symptoms (knee pain) and radiographic evidence of OA. For participants with only one radiographically confirmed OA knee at baseline, that knee was used as the index knee. If both knees were radiographically confirmed with OA, then the knee with greater pain at baseline measured by Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scale was used. If both pain scores were equal, then the knee with worse K-L grade was used as the index knee.
Injections use, index visit, and patterns defined
Injection use was assessed separately for both knees in OAI. Participants were first asked “During the past 6 months, have you had any injections in either of your knees for treatment of arthritis?” For those who answered yes, participants were then asked questions “During the past 6 months, have you had an injection of hyaluronic acid (Synvisc or Hyalgan) in either of your knees for treatment of your arthritis? These injections are given as a series of 3 to 5 weekly injections.” To assess corticosteroid injections, participants were asked: “During the past 6 months, have you had an injection of steroids (cortisone, corticosteroids) in either of your knees for treatment of your arthritis?” The visit that participants reported their initial injection was used as the index visit.
After identifying the initial injection and index visit, we used the first reporting of injection use during the follow-up to determine if participants continued or switched their injection use. Switching injection users were then defined as reporting at least one injection other than their initial injection during follow-up. For example, for participants who initiated corticosteroid injection use, reporting a hyaluronic acid injection constituted a switch. Continued users were defined as reporting more than one injection of the same type during follow-up. Those who did not report either a switching or continuing use during follow-up were considered as one-time injection users.
Symptoms
In the OAI, knee-related symptoms such as pain, stiffness, and physical function were examined annually using WOMAC, with a 5-point Likert scale. The range of scores was 0 to 20 for pain, 0 to 8 for stiffness, and 0 to 68 for physical function15. For each subscale, higher numbers indicated worse symptoms. We were interested in evaluating patient-reported symptoms in two ways: 1) symptoms post-initial injection; and 2) longitudinal change in symptoms. We believed that these two metrics offered complementary (but distinct) information. The first one is symptoms measured at the post-initial injection. Conceptually, we believe this captures patents’ response to the initial treatment. If participants have higher symptoms post-initial injection, this may represent a surrogate for chronic and persistent symptoms of the knee16–18. In addition, we also evaluated longitudinal change in symptoms over the disease course which conceptually could also be associated with treatment use.
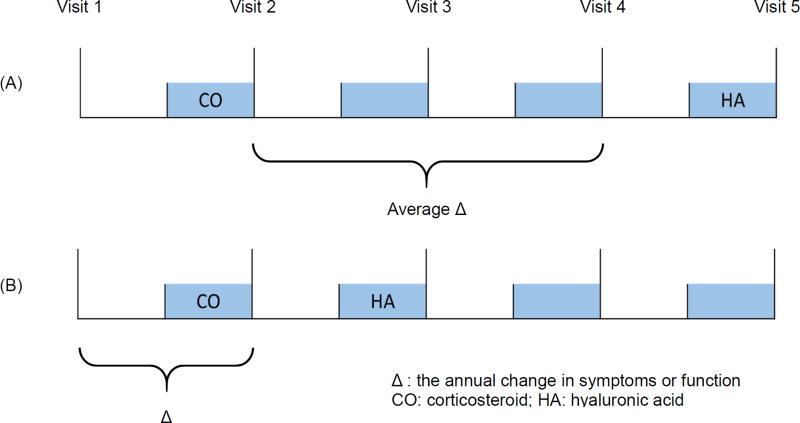
To determine the intervals of the first switching/continued injections use among one-time users, we used frequency matching to match the distribution of follow-up time intervals of the first reported switching/continuation among those switched and continued users to one-time users. Symptoms post-initial injection were measured at the index visit in which the initial injection was reported. In general, change in symptoms were assessed between the index visit (where the initial injection was reported) to the visit before the switching/continued injection use. To evaluate average changes of symptoms, we had to account for the varied number of years between visits. To do so, the difference of symptoms between index visit of injections use and one visit before the visit reporting switched/continued injection use was first calculated and then divided by the appropriate time intervals (Figure 2A). For example, if the participants reported an initial injection at visit 2 and reported switching/continued injection use at visit 5, the average change of symptoms (e.g. WOMAC pain) between visits was calculated as: (pain score (visit 4) – pain score (visit 2)) / 2. However, participants could initiate injections use at visit 2 and report switching/continued injection use at one year after initiation (i.e. visit 3). In this scenario, the difference between the index visit and one year before was used to represent the change in symptoms between the initial injection and switched/continued injection use given the OAI’s data structure (Figure 2B). Using these change scores adjusted for time intervals, we created 3 categories to define minimal clinically important changes for each symptom: 1) improved, 2) no change, and 3) worsened. A negative change in WOMAC scores indicated improvement ranging from −4.6 to −1.2 for pain, −1.5 to −0.5 for stiffness, and −9.9 to −4.1 for physical function19–22. The minimal threshold was used for creating the categories (e.g. improved pain was defined as < −1.2; worsened pain was defined as > 1.2; and no change was defined as −1.2 to 1.2).
Figure 2.
Scheme of the study design to define changes between visits among injection users.
Covariates
Sociodemographic characteristics including age, sex, race/ethnicity, income, and education were evaluated. Clinical characteristics such disease severity, knee-related symptoms, insurance coverage, general health status, and physical activity were also assessed.23 Sociodemographic variables (e.g. sex, race/ethnicity, and household income) were considered time-invariant variables and thus using information from the enrollment. Information from the index visit were used for other variables.
Sociodemographic variables were self-reported. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, and other. Educational levels were collapsed and categorized as high school or less, some college or college graduate, and at least some graduate school. Annual household income was categorized into three levels: < $25,000, $25,000–50,000, and > $50,000.
Clinical characteristics such as disease severity was measured based on K-L grade and joint space width (JSW). A detailed protocol regarding the measurement of JSW has been documented elsewhere14,24. If JSW measures were implausible (e.g. the distance between plateau and rim was > 6.5mm), we treated these as missing as these measures could be due to poorly positioned knees14. Multi-joint symptoms were present if participants had frequent pain, aching, or stiffness in ≥ two joints other than knee. Cumulative measures were used to assess the history of knee injury and surgery. Information was collected on prior knee injuries if participants had limited ability to walk for ≥ two days reported at any previous visit. A history of having knee surgery included arthroscopy, ligament repair or meniscectomy at any previous visit.
General health status was evaluated using the 12-item Short-Form Health Survey (SF-12)25, with summary scores for physical and mental health ranging from 0 to 100 and higher scores indicating better health status. Body mass index (BMI) was calculated using measured height and weight [weight (kg)/height (m)2]. Participants were categorized into: < 25, normal weight; 25 to < 30, overweight; and ≥ 30, obese26. Information on depressive symptoms were collected using Centers for Epidemiologic Studies Depression Scale (CES-D). A CES-D score >16 indicated elevated depressive symptoms27. Comorbidity status was evaluated using the Charlson index and then categorized into 0, 1, and ≥ 228. Health coverage status and physical activity were self-reported. The Physical Activity Scale for the Elderly (PASE) consisting 26 items was used to assess activities including occupational, household, and leisure work over the past week, with higher scores indicating greater activities29.
Statistical analyses
Descriptive statistics including means and standard deviations (SD) for continuous variables and percentages for categorical variables were first calculated to describe socio-demographic and clinical characteristics by patterns of injections use for types of injections initiated at the index visit. Average yearly changes in symptoms between treatment initiation and switching/continued treatment use were also examined. Multicollinearity was evaluated and ruled out before the model building process by evaluating the correlations between the covariates of interest. Six multinomial logistic models were then built to evaluate the association between two operational expressions (symptoms post-initial injection; and change in symptoms) for three symptoms (pain, stiffness, and physical function) with patterns of injection use. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) for each group compared to a common reference group (one-time users) were estimated after adjusting for socio-demographics and clinical/functional factors.
Due to the concern of missing data on some of the potential confounders (e.g. ~13% of K-L score were missing among participants initiating corticosteroid injections) with selected sample for the current investigation and the OAI protocol, multiple imputation was used to impute the missing values30,31. We applied the Fully Conditional Specification method for imputation of missing data using SAS PROC MI FCS32. We used all available information from the covariates including socio-demographics and clinical/functional factors (e.g. multi-joint symptoms, history of knee surgery or injury, CES-D, and comorbidity status) as variables in the imputation model to impute the missing values. Twenty imputed datasets were created. We then incorporated the imputed values to reanalyze each completed dataset. We finally combined estimates and generated valid inferences using SAS PROC MIANALYZE to compare results. Due to the small sample size, however, we were not able to examine such relationships among participants initiating hyaluronic acid use.
Results
Overall, 96 initiated of hyaluronic acid injections and 316 initiated corticosteroid injections. Regardless of types of initial injection, nearly 1 in 5 participants switched or continued the initial injection use and approximately 60% of participants did not receive any additional injections. Socio-demographic and clinical characteristics of participants initiating hyaluronic acid injections are presented in Table 1. The average age of those who switched from hyaluronic injections to corticosteroids was 62.0 years whereas the average age for those continuing with hyaluronic injections or who had only one injection was 65.6 years and 67.0 years, respectively. Fifty-two percent of those who switched injections were women, while 40.0% of those who continued and 46.6% of one-time users were women. Nearly two thirds of continued users and one-time users had at least some graduate school education whereas half of those switching injections use had graduate level education. Half of participants who switched injections use had K-L grade 4 and reports of multi-joint symptoms and history of knee injuries were common. WOMAC pain at the index visit was 5.8 (SD: 3.7) for switching users whereas those who continued use and one-time users were 4.0 (SD: 2.9) and 6.3 (SD: 3.7), respectively.
Table 1.
Clinical characteristics by patterns of hyaluronic acid injection use (N=96*).
| Characteristics | Switching users (n=23) |
Continued users (n=15) |
One-time users (n=58) |
|
|---|---|---|---|---|
| Mean (SD) age in years | 62.0 (8.0) | 65.6 (7.4) | 67.0 (8.6) | |
| Mean intervals | 2.4 (1.9) | 1.5 (0.9) | NA | |
| Women (%)a | 52.2 | 40.0 | 46.6 | |
| Race/ethnicity (%)a | ||||
| Non-Hispanic White | 82.6 | 93.3 | 89.7 | |
| Non-Hispanic Black | 8.7 | 0 | 6.9 | |
| Other | 8.7 | 6.7 | 3.5 | |
| Education (%)a | ||||
| High school or less | 8.7 | 20.0 | 12.3 | |
| Some college | 39.1 | 20.0 | 22.8 | |
| College graduate | 8.7 | 33.3 | 19.3 | |
| Some graduate school or above | 43.5 | 26.7 | 45.6 | |
| Income (%)a | ||||
| <$25,000 | 0 | 13.3 | 3.5 | |
| $25,000 - $50,000 | 34.8 | 6.7 | 22.8 | |
| >$50,000 | 65.2 | 80.0 | 73.7 | |
| Body mass index (%) | ||||
| Normal | 13.0 | 20.0 | 15.5 | |
| Overweight | 34.8 | 20.0 | 31.0 | |
| Obese | 52.2 | 60.0 | 53.5 | |
| Health care coverage (%) | 100.0 | 100.0 | 100.0 | |
| Kellgren-Lawrence grade (%) | ||||
| 2 | 15.0 | 15.4 | 18.4 | |
| 3 | 35.0 | 46.2 | 46.9 | |
| 4 | 50.0 | 38.5 | 34.7 | |
| Multi-joint symptoms (%) | 78.3 | 46.7 | 60.3 | |
| History of knee injury (%) | 69.6 | 46.7 | 56.9 | |
| History of knee surgery (%) | 56.5 | 46.7 | 53.5 | |
| Depressive symptoms (CES-D >16) (%) | 13.0 | 20.0 | 9.3 | |
| Comorbidity status | ||||
| 0 | 73.9 | 64.3 | 72.4 | |
| 1 | 17.4 | 28.6 | 19.0 | |
| ≥2 | 8.7 | 7.1 | 8.6 | |
| WOMAC scores, mean (SD) | ||||
| Pain | 5.8 (3.7) | 4.0 (2.9) | 6.3 (3.7) | |
| Stiffness | 2.7 (1.7) | 2.3 (1.5) | 3.0 (1.6) | |
| Physical function | 17.1 (11.5) | 14.2 (9.9) | 18.7 (12.1) | |
| Joint space width, mean (SD) | 3.4 (1.9) | 4.6 (1.7) | 3.9 (2.2) | |
| SF-12 PCS, mean (SD) | 40.2 (9.9) | 41.5 (9.1) | 41.2 (10.4) | |
| SF-12 MCS, mean (SD) | 54.0 (7.6) | 53.5 (11.5) | 56.2 (7.6) | |
| PACE, mean (SD) | 154.2 (70.4) | 177.4 (64.2) | 145.1 (82.3) | |
Abbreviation: CES-D, Centers for Epidemiologic Studies Depression Scale; MCS, SF-12 Mental Component Summary scores; PACE, Physical activity scale for the elderly; PCS, SF-12 Physical Component Summary scores; SD, standard deviation; WOMAC, The Western Ontario and McMaster Universities Arthritis Index.
Information at enrollment was used.
Number of participants with missing information: education (1), annual household income (1), health care coverage (5), Kellgren-Lawrence grade (14), CES-D (4), comorbidity status (1), WOMAC Physical function (2), joint space width (24), SF-12 PCS (5), SF-12 MCS (5), PACE (2).
For participants initiating corticosteroid injections (Table 2), the average age of one-time users was 68.7 years (SD: 9.5 years) whereas those switched or continued were 67.2 years and 67 years of age, respectively. The majority of participants initiating corticosteroid injections had annual household income >$50,000. For clinical characteristics, nearly half of participants switched injection use had K-L grade 2 relative to the other groups (e.g. continued: 38.6%, one-time users: 30.2%). Overall, the majority of participants initiating corticosteroid injections had multi-joint symptoms. Knee-specific symptoms such WOMAC pain at the index visit was 7.0 (SD: 4.4) for switching users whereas those who continued and one-time users were 5.2 (SD: 4.0) and 6.3 (SD: 4.1), respectively.
Table 2.
Clinical characteristics by patterns of corticosteroid injection use (N=316*).
| Characteristics | Switching users (n=56) |
Continued users (n=75) |
One-time users (n=185) |
|
|---|---|---|---|---|
| Mean (SD) age in years | 67.2 (9.5) | 67.0 (7.9) | 68.7 (9.5) | |
| Mean intervals | 2.3 (1.7) | 1.9 (1.5) | NA | |
| Women (%)a | 66.1 | 60.0 | 64.3 | |
| Race/ethnicity (%)a | ||||
| Non-Hispanic White | 76.8 | 74.7 | 78.4 | |
| Non-Hispanic Black | 14.3 | 20.0 | 18.4 | |
| Other | 8.9 | 5.3 | 3.2 | |
| Education (%)a | ||||
| High school or less | 14.3 | 14.7 | 21.7 | |
| Some college | 21.4 | 26.7 | 27.7 | |
| College graduate | 28.6 | 24.0 | 20.1 | |
| Some graduate school or above | 35.7 | 34.7 | 30.4 | |
| Income (%)a | ||||
| <$25,000 | 14.3 | 12.0 | 15.8 | |
| $25,000 - $50,000 | 28.6 | 28.0 | 33.7 | |
| >$50,000 | 57.1 | 60.0 | 50.5 | |
| Body mass index (%) | ||||
| Normal | 14.3 | 12.0 | 13.0 | |
| Overweight | 33.9 | 45.3 | 41.1 | |
| Obese | 51.8 | 42.7 | 46.0 | |
| Health care coverage (%) | 100.0 | 100.0 | 98.9 | |
| Kellgren-Lawrence grade (%) | ||||
| 2 | 48.9 | 38.6 | 30.2 | |
| 3 | 28.9 | 38.6 | 39.0 | |
| 4 | 22.2 | 22.9 | 30.8 | |
| Multi-joint symptoms (%) | 66.1 | 58.7 | 51.9 | |
| History of knee injury (%) | 42.9 | 49.3 | 51.4 | |
| History of knee surgery (%) | 23.2 | 32.0 | 37.3 | |
| Depressive symptoms (CES-D >16) (%) | 18.2 | 13.3 | 14.0 | |
| Comorbidity status | ||||
| 0 | 52.7 | 58.7 | 65.8 | |
| 1 | 32.7 | 24.0 | 15.2 | |
| ≥2 | 14.6 | 17.3 | 19.0 | |
| WOMAC scores, mean (SD) | ||||
| Pain | 7.0 (4.4) | 5.2 (4.0) | 6.3 (4.1) | |
| Stiffness | 3.0 (1.8) | 2.5 (1.8) | 2.9 (1.7) | |
| Physical function | 20.2 (12.7) | 17.2 (13.6) | 19.6 (13.2) | |
| Joint space width, mean (SD) | 4.7 (1.9) | 4.4 (2.0) | 4.2 (1.9) | |
| SF-12 PCS, mean (SD) | 39.4 (8.7) | 41.1 (10.3) | 41.3 (9.9) | |
| SF-12 MCS, mean (SD) | 54.8 (10.4) | 55.1 (9.5) | 53.8 (9.3) | |
| PACE, mean (SD) | 141.0 (81.0) | 137.9 (71.8) | 137.3 (73.8) | |
Abbreviation: CES-D, Centers for Epidemiologic Studies Depression Scale; MCS, SF-12 Mental Component Summary scores; PACE, Physical activity scale for the elderly; PCS, SF-12 Physical Component Summary scores; SD, standard deviation; WOMAC, The Western Ontario and McMaster Universities Arthritis Index.
Information at enrollment was used.
Number of participants with missing information: education (1), annual household income (1), health care coverage (10), Kellgren-Lawrence grade (42), CES-D (7), comorbidity status (2), WOMAC Physical function (7), joint space width (94), SF-12 PCS (16), SF-12 MCS (16), PACE (20).
Table 3 shows the association between symptoms post-initial injection and switching or continuing corticosteroid injections compared to one-time users. In relation to one-time users, greater knee-specific symptoms post-initial injection were associated with lower odds of continued injections use (adjusted odds ratio (aOR) for WOMAC pain: 0.91; 95%, confidence interval (CI): 0.83 to 0.99; aORstiffness: 0.77; CI: 0.63 to 0.94; aORphysical function: 0.97; CI: 0.94 to 1.00). Compared with one-time users, symptoms post-initial injection did not appear to be associated with switching injections use among initiators of corticosteroid injection.
Table 3.
Association* between symptoms post-initial injection and first switching/continuation of corticosteroid injections among participants with radiographic confirmed knee osteoarthritis.
| Switching users | Continued users | ||
|---|---|---|---|
| Pain | Odds Ratio (95% Confidence Interval) | ||
| Crude | 1.04 (0.97 to 1.12) | 0.93 (0.87 to 1.00) | |
| Adjusteda | 0.97 (0.88 to 1.07) | 0.91 (0.83 to 0.99) | |
| Stiffness | |||
| Crude | 1.02 (0.86 to 1.21) | 0.88 (0.75 to 1.03) | |
| Adjusteda | 0.83 (0.66 to 1.05) | 0.77 (0.63 to 0.94) | |
| Physical function | |||
| Crude | 1.00 (0.98 to 1.03) | 0.99 (0.97 to 1.01) | |
| Adjusteda | 0.97 (0.94 to 1.00) | 0.97 (0.94 to 1.00) | |
Odds ratios (95% Confidence Intervals) were estimated using participants with one-time use of injections as the reference group.
Adjusted for age at the index visit, sex, K-L grade, comorbidity status, and SF-12 physical component scores.
Table 4 shows the association between the clinically relevant change in symptoms with first switching/continuation compared to one-time users among participants initiating corticosteroid injections use. For symptoms in pain and physical function, approximately half of participants experienced no clinically meaningful difference regardless of patterns of injection use. Reaching a priori defined minimally clinical important differences in worsened or improved pain was not associated with patterns of switching or continued injections use compared to one-time users. No association was observed for stiffness and physical function. The findings derived from analyses which used multiple imputation methods remained similar (data not shown).
Table 4.
Association* between average change in symptoms and first switching/continuation of corticosteroid injections among participants with radiographic confirmed knee osteoarthritis.
| Switching (n=79) | Continued (n=90) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pain | Improved Pain¶ | No changes | Worsened Pain | Improved Pain¶ | No changes | Worsened Pain | |
| (n=16) | (n=41) | (n=22) | (n=14) | (n=49) | (n=27) | ||
|
| |||||||
| Crude | 0.69 | Reference | 1.11 | 0.48 | Reference | 1.00 | |
| (0.32 to 1.49) | (0.54 to 2.31) | (0.24 to 0.99) | (0.52 to 1.89) | ||||
|
| |||||||
| Adjusteda | 0.61 | Reference | 0.66 | 0.56 | Reference | 0.99 | |
| (0.25 to 1.47) | (0.27 to 1.61) | (0.26 to 1.22) | (0.49 to 2.01) | ||||
|
| |||||||
| Stiffness | Improved Stiffness¶ | No changes | Worsened Stiffness | Improved Stiffness¶ | No changes | Worsened Stiffness | |
| (n=27) | (n=31) | (n=21) | (n=34) | (n=24) | (n=32) | ||
|
| |||||||
| Crude | 0.96 | Reference | 0.66 | 1.39 | Reference | 1.19 | |
| (0.46 to 2.00) | (0.31 to 1.43) | (0.70 to 2.78) | (0.59 to 2.38) | ||||
|
| |||||||
| Adjusteda | 1.02 | Reference | 0.47 | 1.53 | Reference | 1.34 | |
| (0.44 to 2.33) | (0.18 to 1.19) | (0.72 to 3.24) | (0.64 to 2.82) | ||||
|
| |||||||
| Physical function | Improved Function¶ | No changes | Worsened Function | Improved Function¶ | No changes | Worsened Function | |
| (n=17) | (n=40) | (n=22) | (n=18) | (n=45) | (n=27) | ||
|
| |||||||
| Crude | 0.57 | Reference | 0.63 | 0.58 | Reference | 0.58 | |
| (0.26 to 1.23) | (0.30 to 1.31) | (0.29 to 1.15) | (0.30 to 1.12) | ||||
|
| |||||||
| Adjusteda | 0.55 | Reference | 0.42 | 0.71 | Reference | 0.68 | |
| (0.23 to 1.33) | (0.17 to 1.05) | (0.34 to 1.49) | (0.33 to 1.41) | ||||
Odds ratios (95% Confidence Intervals) were estimated using participants with one-time use of injections as the reference group (n=243).
Adjusted for age at the index visit, sex, K-L grade, comorbidity status, and SF-12 physical component scores
A negative change in WOMAC scores indicated improvement ranging from −4.6 to −1.2 for pain, −1.5 to −0.5 for stiffness, and −9.9 to −4.1 for physical function. The minimal threshold was used for creating the categories (e.g. improved pain was defined as < −1.2; worsened pain was defined as > 1.2; and no change was defined as −1.2 to 1.2).
Discussion
To our knowledge, this present study is the first study to describe the patterns of intra-articular injection use in patients with knee OA. Our data suggest that a substantial proportion of knee OA patients initiating intra-articular injections use switched their treatment or used it for one time, regardless of the initial therapy or actual symptoms change. We found that approximately 1 in 5 participants initiating injections had switched injection type, but that channeling into the hyaluronic injections was not apparent. While 24.0% of hyaluronic acid initiators switched to corticosteroid injections, 17.8% of corticosteroid initiators switched to hyaluronic acid injections. We also observed that it was reported symptoms post-initial injection rather than clinically meaningful changes in symptoms (e.g. improved or worsened) over time that appeared to be associated with patterns of use among corticosteroid users.
We hypothesized that the patterns of injection use among persons with knee OA would be associated with more severe symptoms and/or changes in symptoms over time compared to those who only received one-time injections. Although we were not able to examine such a relationship among participants initiating hyaluronic acid injections owing to a limited sample size available for analysis, our findings did support the hypothesis that greater symptoms post-initial injection were associated with lower odds of continued treatment use among participants initiating corticosteroid injections. Further, we observed that clinically meaningful changes in knee symptoms over time were not associated with either switching or continued injections compared to one-time users. Several explanations could be responsible for this observation. It might be the channeling effects of injection use rather than the actual symptoms changes since current clinical guidelines are still conflicting4,8,9,33,34. A similar phenomenon was found in a study of celecoxib compared to other NSAIDs in OA patients35. Another potential explanation is that the relation between change in symptoms and actual treatments received may not be a linear. As such, it may affect the treatment decisions, particularly in persons with OA16,17. Since the sensitization of symptoms could be both influenced by both physical and psychological factors, it could be that sustained chronic symptoms rather than the success or failure of treatment affect treatment decisions.
In addition to symptoms of the knee, the decision to continue or switch intra-articular injections among patients might be driven by several factors including patient choice, physician specialty, insurance reimbursement, and cost reasons36,37. Indeed, the cost to manage OA could be substantial38–40. Typically, patients switch from less extensive and cheaper treatments (e.g. corticosteroid injections) to newer and more expensive therapies that may target more directly the underlying pathophysiological effects (e.g. hyaluronic acid injections). However, we observed that approximately 1 in 4 participants initiating hyaluronic acid injection use switched to corticosteroid injections. One potential explanation could be that “clinical equipoise” still exists and physicians and patients frequently switch between available options41,42. The end result may be additional economic and human burden of OA. In addition, although general practitioners can administer corticosteroid injections, hyaluronic acid injections are typically provided by specialists such as an orthopaedic surgeons or rheumatologists. Therefore, the extent of switching from hyaluronic injections to corticosteroid injections observed in this study may be a reflection of ease of convenience, rather than preference of injection type37.
Treatment switching, continuation, and discontinuation in populations with chronic disease might be due to suboptimal efficacy, safety, and tolerability of treatment modalities12,13. We did observe that there were high percentages of switching in both groups and this could be due to patients’ attempts to manage chronic pain with potentially suboptimal treatments. Indeed, recent evidence shows that viscosupplements can last through 26 weeks but no similar evidence exists for corticosteroid injections among persons with knee OA43,44. More studies regarding the long-term efficacy using multiple treatments might be needed. We observed a high proportion of one-time injection use in this study. While in some clinical scenarios this may indicate potentially intolerable side effects of the modalities, adverse events and side effects of intra-articular injections are rare and more often due to localized reactions (e.g. swelling or redness on the injection site) rather than systematic effects from the agent45. In this present study, we were not able to evaluate this relationship since information related to adverse effects is limited in the OAI. The high proportion of one-time use may also be reflective of lack of perceived efficacy of the treatment46.
Strengths and limitations of this study are acknowledged. This is the first study examining patterns of intra-articular injections use for patients with knee OA. Although participants were not newly diagnosed with OA and thus may have injection use before entering into the study, a new user design was used to minimize the bias47. Using data from the OAI, we were able to evaluate the associations between clinical outcomes and patterns of injections use longitudinally. The low number of participants initiating hyaluronic acid injections limited our ability to develop models of patterns of injection use in this group. Despite the comprehensive assessment in OAI, physicians’ prescribing notes or chart information are lacking. Therefore, the extent to which treatment switching and continued observed in this study related to safety or tolerability of injections use is unknown. Although the indication of injections was self-reported and assessed for the 6 months before annual visits in the study, the proportion of participants receiving either injections is comparable to previous study48. However, we may still miss the identification of the initiation, continuation or switching during the intervals of assessments given the questionnaires used in OAI (i.e., 6 months window before the annual visit to assess treatment use). If this misclassification was non-differential, the observed association could be toward the null. In addition, there is a potential for mismatch between the timing of actual injections use and assessments of symptoms. We may not have the optimal window to examine the associations of injection use and symptoms for all participants. However, we examined symptoms using two operational definitions which provided complementary but distinct information. Last, confounding by indication may arise when individuals have an "indication" for use of the drug even if the study population consists of subjects with the same disease. Traditionally, multivariable regression is used to account for differences in measured covariates between subjects49. Despite that we were able to adjust the indices to deal with the potential confounding by indication given the comprehensive measurement and information on the disease severity that might affect patients in seeking treatment in OAI, we could not rule out the possibility that our findings may still be biased by factors such as the patients’ preference of physicians or specialists that is not measured in the OAI.
In conclusion, we found that approximately 19% of patients with radiographically confirmed knee OA who initiated injections switched injection type and ~60% did not receive any injections after their initial injection. Furthermore, among those initiating corticosteroid injection use, we also observed that symptoms post-initial injection might be associated with patterns of injections use and approximately half of participants experienced no clinically meaningful change on symptoms regardless of patterns of injection use. Despite that the proportion of patients switching injection use and onetime users was substantial in both types of commonly used treatment agents, there is currently consistent approach for intra-articular injections use in treatment guidelines for patients with knee OA. Further, these phenomena may suggest that longer-term efficacy regarding symptom relief and/or slowing disease progression of these agents maybe suboptimal among patients with OA in the real-world setting.
Acknowledgments
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Role of funding source
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Disease (Project number 268201000020C-1-0-1 entitled TAS::75 0888::TAS to Charles Eaton).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Shao-Hsien Liu conceived the study, conducted the analyses, and wrote substantial portions of this article. This research was conducted under the guidance of Kate Lapane with substantial inputs from the remaining authors. All authors contributed to the interpretation of the data, preparation of the final manuscript, and revising it critically for important scientific content. The final version of this manuscript was approved by all authors.
Competing interests
Authors have no competing interests directly related to this study. Dr. McAlindon reported grants from Sanofi Aventis, Abbvie, Fidia, Samumed, and Pfizer and served as a paid consultant to Flexion Therapeutics, Samumed, Plexxikon Inc, Federal Trade Commission, Regeneron Orthogen, McNeil Consumer HC on work unrelated to this research.
References
- 1.Brault M, Hootman J, Helmick CG, Thesis KA, Armour BS. Prevalence and common causes of disability among adults - United States, 2005. Morb. Mortal. Wkly. Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Annals of Internal Medicine. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am. J. Public Health. 1994;84:351–358. doi: 10.2105/AJPH.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Kon E, Filardo G, Drobnic M, et al. Non-surgical management of early knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012;20(3):436–49. doi: 10.1007/s00167-011-1713-8. [DOI] [PubMed] [Google Scholar]
- 6.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J. Orthop. 2014;5(3):351–61. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehling P, Moser C, Maixner W. How does surgery compare with advanced intra-articular therapies in knee osteoarthritis: current thoughts. Ther. Adv. Musculoskelet. Dis. 2016;8(3):72–85. doi: 10.1177/1759720X16642405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 9.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J. Am. Acad. Orthop. Surg. 2013;21:571–6. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 10.Arnold W, Fullerton DSP, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J. Manag. Care Pharm. 2007;13(4 Suppl):S3–19. doi: 10.18553/jmcp.2007.13.s4.3. quiz S20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenig KM, Ong KL, Lau EC, et al. The Use of Hyaluronic Acid and Corticosteroid Injections Among Medicare Patients With Knee Osteoarthritis. J. Arthroplasty. 2016;31(2):351–355. doi: 10.1016/j.arth.2015.08.024. doi: http://dx.doi.org/10.1016/j.arth.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Zhao SZ, Wentworth C, Burke TA, Makuch RW. Drug switching patterns among patients with rheumatoid arthritis and osteoarthritis using COX-2 specific inhibitors and non-specific NSAIDs. Pharmacoepidemiol. Drug Saf. 2004;13(5):277–287. doi: 10.1002/pds.909. [DOI] [PubMed] [Google Scholar]
- 13.MK, KHK, SXK, et al. Drug switching patterns among patients taking non-steroidal anti-inflammatory drugs: A retrospective cohort study of a general practitioners database in the United Kingdom. Pharmacoepidemiol. Drug Saf. 2001;10(6):517–524. doi: 10.1002/pds.653. [DOI] [PubMed] [Google Scholar]
- 14.Nevitt MC, Felson DTLG. [Accessed October6, 2016];The Osteoarthritis Initiative Protocol for the Cohort Study. 2006 Available at: http://oai.epiucsf.org/datarelease/docs/StudyDesignProtocol.pdf.
- 15.Roos EM, Klässbo M, Lohmander LS. WOMAC osteoarthritis index Reliability, validity, responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand. J. Rheumatol. 1999;28(4):210–215. doi: 10.1016/S0161-4754(00)90101-5. [DOI] [PubMed] [Google Scholar]
- 16.Dieppe PA. Relationship between symptoms and structural change in osteoarthritis: What are the important targets for therapy? Journal of Rheumatology. 2005;32:1147–1149. [PubMed] [Google Scholar]
- 17.Sofat N, Kuttapitiya A. Future directions for the management of pain in osteoarthritis. Int. J. Clin. Rheumtol. 2014;9(2):197–276. doi: 10.2217/ijr.14.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modi A, Sen S, Adachi JD, et al. Gastrointestinal symptoms and association with medication use patterns, adherence, treatment satisfaction, quality of life, and resource use in osteoporosis: baseline results of the MUSIC-OS study. Osteoporos. Int. 2016;27:1227–1238. doi: 10.1007/s00198-015-3388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vignon E, Piperno M, LeGraverand MPH, et al. Measurement of radiographic joint space width in the tibiofemoral compartment of the osteoarthritic knee: Comparison of standing anteroposterior and Lyon schuss views. Arthritis Rheum. 2003;48(2):378–384. doi: 10.1002/art.10773. [DOI] [PubMed] [Google Scholar]
- 20.Dougados M, Hawker G, Lohmander S, et al. OARSI/OMERACT criteria of being considered a candidate for total joint replacement in knee/hip osteoarthritis as an endpoint in clinical trials evaluating potential disease modifying osteoarthritic drugs. J. Rheumatol. 2009;36(9):2097–9. doi: 10.3899/jrheum.090365. [DOI] [PubMed] [Google Scholar]
- 21.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower ex. Arthritis Rheum. 2001;45(4):384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal art. Am. J. Sports Med. 2010;38(5):891–902. doi: 10.1177/0363546509354163. [DOI] [PubMed] [Google Scholar]
- 23.Lapane KL, Liu S-H, Dubé CE, Driban JB, McAlindon TE, Eaton CB. Factors Associated with the Use of Hyaluronic Acid and Corticosteroid Injections among Patients with Radiographically Confirmed Knee Osteoarthritis: A Retrospective Data Analysis. Clin. Ther. 2017;39(2):347–358. doi: 10.1016/j.clinthera.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Duryea J, Li J, Peterfy CG, Gordon C, Genant HK. Trainable rule-based algorithm for the measurement of joint space width in digital radiographic images of the knee. Med. Phys. 2000;27(3):580–591. doi: 10.1118/1.598897. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.2307/3766749. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013:1–70. [Google Scholar]
- 27.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- 28.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med. Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J. Sports Med. Phys. Fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 30.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayati Rezvan P, Lee KJ, Simpson JA. The rise of multiple imputation: a review of the reporting and implementation of the method in medical research. BMC Med. Res. Methodol. 2015;15(1):30. doi: 10.1186/s12874-015-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 33.Kaspar J, Kaspar S, Orme C, DeBeer JDV. Intra-articular steroid hip injection for osteoarthritis: A survey of orthopedic surgeons in Ontario. Can. J. Surg. 2005;48(6):461–469. [PMC free article] [PubMed] [Google Scholar]
- 34.AJS SMB-Z, Dekker J, et al. Agreement of general practitioners with the guideline-based stepped-care strategy for patients with osteoarthritis of the hip or knee: a cross-sectional study. BMC Fam. Pract. 2013;14:33. doi: 10.1186/1471-2296-14-33. doi: http://dx.doi.org/10.1186/1471-2296-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosis G, Stijnen T, Castellsague J, et al. Channeling and prevalence of cardiovascular contraindications in users of cyclooxygenase 2 selective nonsteroidal antiinflammatory drugs. Arthritis Care Res. 2006;55(4):537–542. doi: 10.1002/art.22096. [DOI] [PubMed] [Google Scholar]
- 36.Berchi C, Degieux P, Halhol H, Danel B, Bennani M, Philippe C. Impact of falling reimbursement rates on physician preferences regarding drug therapy for osteoarthritis using a discrete choice experiment. Int. J. Pharm. Pract. 2016 Apr 24;(2):114–22. doi: 10.1111/ijpp.12220. [DOI] [PubMed] [Google Scholar]
- 37.Law A, Ray MD, Knapp K, Balesh J. Unmet Needs in the Medication Use Process: Perceptions of Physicians, Pharmacists, and Patients. J. Am. Pharm. Assoc. 2003;43(3):394. doi: 10.1331/154434503321831111. [DOI] [PubMed] [Google Scholar]
- 38.Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J. Rheumatol. 2006;33(6):1152–1158. [PubMed] [Google Scholar]
- 39.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Osteoarthritis and absenteeism costs: evidence from US National Survey Data. J. Occup. Environ. Med. 2010;52(3):263–8. doi: 10.1097/JOM.0b013e3181cf00aa. [DOI] [PubMed] [Google Scholar]
- 40.White AG, Birnbaum HG, Janagap C, Buteau S, Schein J. Direct and indirect costs of pain therapy for osteoarthritis in an insured population in the United States. J. Occup. Environ. Med. 2008;50(9):998–1005. doi: 10.1097/JOM.0b013e3181715111. [DOI] [PubMed] [Google Scholar]
- 41.Freedman B. Equipoise and the ethics of clinical research. N. Engl. J. Med. 1987;317(3):141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 42.Perry DC, Griffin XL, Parsons N, Costa ML. Designing clinical trials in trauma surgery: overcoming research barriers. Bone Joint Res. 2014;3(4):123–9. doi: 10.1302/2046-3758.34.2000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jüni P, Hari R, Rutjes AWS, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015 Oct 22;(10):CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J. Pain Res. 2015;8:217–228. doi: 10.2147/JPR.S83076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014;10(1):11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas E, Croft PR, Paterson SM, Dziedzic K, Hay EM. What influences participants’ treatment preference and can it influence outcome? Results from a primary care-based randomised trial for shoulder pain. Br. J. Gen. Pract. 2004;54(499):93–96. [PMC free article] [PubMed] [Google Scholar]
- 47.Ray WA. Evaluating Medication Effects Outside of Clinical Trials: New-User Designs. Am. J. Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 48.Dhawan A, Mather RC, III, Karas V, et al. An epidemiologic analysis of clinical practice guidelines for non-arthroplasty treatment of osteoarthritis of the knee. Arthrosc. - J. Arthrosc. Relat. Surg. 2014;30(1):65–71. doi: 10.1016/j.arthro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J. Am. Geriatr. Soc. 1999;47(6):749–54. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]