Abstract
Background
Epithelial ovarian cancer (EOC) remains the most lethal gynecologic malignancy. The objective of this study was to compare the anti-tumor activity of HER2/neu-targeting monoclonal antibodies, trastuzumab (T), pertuzumab (P), combination of trastuzumab and pertuzumab (T+P) and trastuzumab-emtansine (T-DM1) in EOC with high HER2/neu expression.
Methods
Primary EOC cell lines were established and cell blocks were analyzed for HER2/neu expression. Cytostatic, apoptotic and antibody-dependent cell-mediated cytotoxicity (ADCC) activities of T, P, T+P and T-DM1 were evaluated in vitro. The in vivo antitumor activity was tested in xenograft models with 3+ HER2/neu expression.
Results
High (3+) HER2/neu expression was detected in 40% of the primary EOC cell lines. T, P, T+P, and T-DM1 were similarly effective in inducing strong ADCC against primary EOC cell lines expressing 3+ HER2/neu. The combination of T and P was more cytostatic when compared with that of T or P used alone (p<0.0001 and p<0.0001, respectively). T-DM1 induced significantly more apoptosis when compared with T+P (p<0.0001). Finally, T-DM1 was significantly more effective in tumor growth inhibition in vivo in EOC xenografts overexpressing HER2/neu when compared to T alone, P alone and T+P (p=0.04).
Conclusion
In vitro and in vivo experiments with 3+ HER2/neu expressing EOC revealed limited anti-tumor activity of T or P. T-DM1 showed superior anti-tumor activity to T and P as single agents and as a combination. Our preclinical data support the design of clinical studies with T-DM1 for the treatment of chemotherapy-resistant EOC overexpressing HER2/neu.
Keywords: trastuzumab, pertuzumab, T-DM1, HER2/neu, epithelial ovarian carcinoma
INTRODUCTION
Epithelial ovarian cancer (EOC) remains the most lethal gynecologic malignancy and the fifth most frequent cause of cancer-related deaths in women in the United States [1]. Approximately 75% of EOC patients are diagnosed with advanced disease, which results in a poor 5-year overall survival rate of 20% to 30% [2]. The standard-of-care management of EOC consists of either primary surgical cytoreduction followed by platinum-based chemotherapy or neoadjuvant chemotherapy followed by interval cytoreduction [3]. The dismal prognosis of EOC patients underscores the need to identify novel effective treatment options for patients with advanced or recurrent disease resistant to conventional chemotherapy.
The human epidermal growth factor receptor 2 (HER2, ErbB-2 or HER-2/neu) gene, which encodes the HER2/neu receptor tyrosine kinase, plays an important role in coordinating the complex ErbB signaling network that is responsible for regulating cell growth and differentiation [4]. C-erbB2 gene amplifications and mutations have been identified in a variety of cancers, and it is predictive of response to adjuvant therapy and prognosis [5, 6]. However, observed rates of HER2/neu overexpression/amplification in EOC show considerable variation ranging from 8% to 66% with considerable intra-tumoral heterogeneity in receptor expression found in about 50% of the HER2/neu 2+ and 3+ EOC samples [7]. The low incidence of c-erbB2 gene amplification and/or HER2/neu overexpression and the high HER2/neu intratumoral heterogeneity in EOC have so far significantly limited the impact of HER2-targeted therapies in the treatment of recurrent chemotherapy-resistant EOC [7].
The introduction of trastuzumab (T) in the treatment of HER2/neu-positive metastatic breast cancer patients favorably changed the natural history of this disease. T has multiple mechanisms of action to inhibit tumor growth, which includes inhibition of downstream signaling by blocking either HER2/neu homodimerization [8] or ligand independent HER2/HER3 heterodimerization [9]. T also inhibits HER2 activation by inhibiting the cleavage of its extracellular domain, thus preventing the formation of the truncated and very active form of HER2/neu, namely p95HER2 [10]. Finally, T induces antibody dependent cell-mediated cytotoxicity (ADCC) [11]. Despite its effectiveness and multiple mechanisms of action, resistance to T remains an important clinical issue. Approximately, 15% of patients who receive T experience recurrence as a result of acquired resistance [12]. Nevertheless, tumor cells continue to rely on the HER2 oncogene as gene amplification and protein overexpression are still present in T-resistant HER2/neu+ clones [13].
Pertuzumab (P) is a recombinant, humanized, monoclonal antibody that binds to the extracellular dimerization domain II of HER2/neu, which is located on the opposite side of the domain IV recognized by trastuzumab. P inhibits heterodimerization of HER2/neu with EGFR, HER3 and HER4 [14], whereas T is preferentially active against HER2/neu homodimers [8]. More specifically, P inhibits HER2/neu heterodimerization [8] and the activation of downstream cell signaling pathways which are critical for tumor growth. However, P, unlike T, is not capable of preventing the formation of the p95HER2 truncated form [10]. Hence, T and P mechanisms of action are likely to be at least in part complementary, and provide, when combined, a more complete blockade of HER2/neu downstream signaling than either antibody alone.
A sophisticated addition to the HER2/neu-targeted therapies armamentarium is trastuzumab emtansine (T-DM1, Kadcyla, Genentech/Roche). T-DM1 is an antibody-drug conjugate (ADC) endowed with superior clinical activity when compared to T naked antibody and accordingly, TDM-1 is currently FDA-approved for patients with HER2/neu positive breast cancer harboring T-resistant disease [15]. In T-DM1, the antibody and the cytotoxic agent are conjugated by means of a non-cleavable linker. This linker combines the HER2/neu targeting ability of T with the cytotoxic effects of maytansine (ie, DM1, a potent anti-microtubule agent). Once the ADC is internalized into tumor cells by receptor-mediated endocytosis and processed, DM1 binds to tubulin and inhibits microtubule assembly, leading to cessation of mitosis and eventual apoptosis in dividing tumor cells [15].
The objective of this study was to evaluate and compare the anti-tumor activity of HER2/neu-targeting monoclonal antibodies, T, P, combination of trastuzumab and pertuzumab (T+P) and T-DM1 in epithelial ovarian carcinoma with high HER2/neu expression status. We report the first evidence that combination of T and P has additive anti-tumor activity in EOC with 3+ HER2/neu expression. More importantly, we demonstrate that T-DM1 is significantly more potent than single agent T, P and their combination in comparative in vitro and in vivo experiments. These preclinical results strongly support the use of T-DM1 as novel agent against patients harboring chemotherapy-resistant EOC with high HER2/neu expression.
METHODS
Establishment of EOC Cell Lines
Study approval was obtained from the Institutional Review Board (IRB), and all patients signed consent prior to tissue collection according to the institutional guidelines. Ten primary EOC cell lines (Table 1) were established from fresh tumor biopsy samples. Briefly, fresh autologous tumor cells were obtained from ovarian tumor biopsies from all patients. Fresh tumor cell lines were maintained in RPMI 1640 (Invitrogen, Grand Island, NY), supplemented with 10% fetal bovine serum (FBS, Invitrogen) at 37 °C, 5% CO2. Briefly, single cell suspensions were obtained by processing solid tumor samples under sterile conditions at room temperature. Viable tumor tissue was mechanically minced in RPMI 1640 to portions no larger than 1–3 mm3 and washed twice with RPMI 1640. The portions of minced tumor were then placed into 250 ml trypsinizing flasks containing 30 ml of enzyme solution [0.14% Collagenase Type I (Sigma St. Louis, MO) and 0.01% DNAse (Sigma, 2000 KU/mg)] in RPMI 1640, and incubated on a magnetic stirring apparatus overnight at 4°C. Enzymatically dissociated tumor was then filtered through 150 μm nylon mesh to generate a single cell suspension. The resultant cell suspension was then washed twice in RPMI 1640 plus 10% FBS. All experiments were performed with fresh or cryopreserved tumor cultures which had at least 90% viability and contained >99% tumor cells. The epithelial nature and the purity of tumor cultures was verified by immunohistochemical staining and flow cytometric analysis with antibodies against cytokeratin and vimentin as previously described [16]. Tumors were staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system. Patient characteristics are noted in Table 1. Primary EOC cell lines with limited passages (i.e. <50) were used in the experiments listed below and corresponding cell blocks were analyzed for HER2/neu surface expression by immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH).
Table 1.
Characteristics and demographic data of ovarian cancer cell lines
| Cell line | Age | Race | FIGO* stage | Primary site | Histology | Flow cytometry (MFI)** | FISH*** | Flow cytometry IHC score cell block | IHC Tissue block |
|---|---|---|---|---|---|---|---|---|---|
| KRCH31**** | 69 | W | IV | Ovary | Serous | 119.68 | Amplified | 3+ | 3+ |
| OVA11 | 79 | W | IC | Ovary | Clear cell | 290.76 | Amplified | 3+ | 1+ |
| OVA10 | 51 | W | IIC | Ovary | Clear cell | 299.61 | Amplified | 3+ | 2+ |
| OVA14 | 58 | W | IIIC | Ovary | Serous | 138.87 | Amplified | 3+ | 3+ |
| OVA1 | 64 | W | IA | Ovary | Serous | 63.79 | Not amplified | 2+ | 1+ |
| OVA5 | 60 | W | IIIC | Ovary | Serous | 85.31 | Not amplified | 2+ | 2+ |
| OVA13 | 42 | W | IIIC | Ovary | Clear cell | 54.20 | Not amplified | 2+ | NA***** |
| OVA12 | 32 | W | IC | Ovary | Clear cell | 27.10 | Not amplified | 1+/0 | 0 |
| OVA3 | 53 | W | IIIA | Ovary | Serous | 13.92 | Not amplified | 1+/0 | 0 |
| OVA4 | 64 | W | IIIC | Ovary | Serous | 20.65 | Not amplified | 1+/0 | 1+ |
FIGO: International Federation of Gynecology and Obstetrics;
MFI: mean fluorescence intensity;
FISH: fluorescent in situ hybridization.
Primary cell line established from a patient in progression after multiple chemotherapy regimens and confirmed to be highly resistant in vitro by extreme drug resistant (EDR) assay to multiple chemotherapy drugs including paclitaxel (Supplementary Figure 2).
Data Not Available
Immunostaining of Cell Blocks of Primary EOC
Cell blocks were obtained from all ten EOC cell lines and reviewed by a gynecologic surgical pathologist to confirm the presence of epithelial ovarian cancer cells. Briefly, HER2/neu immunohistochemical staining was performed on paraffin-embedded 5 μm sections of cell blocks after deparaffinisation and rehydration, using the c-erbB-2 antibody (Thermo Fisher Scientific, Fremont, CA) at 1:800 dilution. HER2/neu staining intensity was graded per the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) 2013 breast scoring criteria [17]. Using similar IHC methods, HER2/neu staining was performed on paraffin-embedded 5 μm sections of available tissue blocks obtained from the patients from which the primary ovarian cancer cell lines were established. Appropriate positive and negative controls were used with each case.
Fluorescent In Situ Hybridization (FISH) of Cell Blocks From Primary EOC
Fluorescent in situ hybridization (FISH) analysis was performed using the PathVysion HER2 DNA FISH Kit (Abbott Molecular Inc., Abbott Park, IL, USA) according to the manufacturer’s instructions. Cell block sections of 5 μm were deparaffinised and rehydrated, followed by acid pretreatment and proteinase K digestion. A probe mix containing an orange probe directed against the HER2 gene (Vysis, Inc., Downers Grove, IL, USA, LSI HER2) and a green probe directed against the pericentromeric region of chromosome 17 (Vysis CEP 17) were added and specimens were denatured for 5 minutes at 73°C. Slides were then incubated overnight in a humidity chamber at 37°C and washed the day after when a fluorescence mounting medium, containing 4, 6-diamidino-2-phenylindole (DAPI), was applied. Fluorescent signals in at least 30 non-overlapping interphase nuclei with intact morphology were scored using a Zeiss Axioplan 2 microscope (Carl Zeiss Meditec, Inc., Dublin, CA, USA) with a 100x planar objective, using a triple band-pass filter that permits simultaneous blue, green, and red colors. Tumor cells were scored for the number of orange (HER2) and green (chromosome 17) signals. A case was scored as amplified when the ratio of the number of fluorescent signals of HER2/neu to chromosome 17 was ≥2.
Tests for ADCC
Standard 4-hour chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Hypaque-separated PBLs from several healthy donors in combination with rituximab (R), T, P, T+P and T-DM1 against primary EOC target cell lines at effector to target ratios (E:T) of 20:1 and 40:1. The release of 51Cr from target cells was measured as evidence of tumor cell lysis after exposure of the tumor cells to 2.0 μg/ml of T, P, T+P or 2.0 μg/ml of T-DM1. Dose-response experiments were performed in order to determine the optimal antibody dosing for ADCC experiments. Controls included the incubation of target cells alone or with PBLs or mAb separately. As a positive control condition, 1% sodium dodecyl sulfate (SDS) was used to achieve complete lysis of target cells. Chimeric anti-CD20 mAb rituximab 2.0 μg/ml was used as the negative control in all bioassays. The percentage cytotoxicity of T, P, T+P and T-DM1 was calculated by the following formula: % cytotoxicity = 100 × (E−S)/(T−S), where E is the experimental release, S is the spontaneous release by target cells, T is the maximum release by target cells lysed with 0.1% SDS.
Cell Viability Assay
EOC cell lines were plated at log phase of growth in 6-well tissue culture plates at a density of 20,000–40,000 cells in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Mediatech, Manassas, VA), and 1% fungizone (Life Technologies, Carlsbad, CA). Cells were incubated at 37°C, 5% CO2. After 24 hours of incubation, cells were treated with R, T, P, T+P and T-DM1. R, T and P were used at a concentration of 20μg/ml. T+P was used at a concentration of 10 μg/ml of each drug and T-DM1 was used at a concentration of 2μg/ml [18]. Three days after drug treatment, cells were harvested in their entirety, centrifuged and stained with propidium iodide (2μl of 500 μg/ml stock solution in PBS). Analysis was performed using a flow cytometry based assay to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. A minimum of three independent experiments per cell line was performed.
In vivo treatment
The in vivo antitumor activity of T, P, T+P and T-DM1 was tested in xenograft models with 3+ HER2/neu expression. Briefly, four to six week old CB-17/SCID mice were given a single subcutaneous injection of 7 × 106 OVA10 cells (HER2/neu 3+) in approximately 300 μL of a 1:1 solution of sterile PBS containing cells and Matrigel (BD Biosciences). Once the tumor volume was approximately 0.2 cm3, the mice were randomized into treatment groups (ie, 5 to 6 per group); those treated with R (15mg/kg), T (15 mg/kg), P (15 mg/kg), T+P (7.5 mg/kg+ 7.5 mg/kg) and T-DM1 (15 mg/kg). Drug dosages were chosen according to previous studies conducted on different xenograft models [18, 19]. All treatment drugs were given as intravenous (IV) weekly injections for three to five times to different groups of mice based on prior literature [18]. In some experiments mice were euthanized one week after the last injection (i.e., day 22) for necropsy studies while in other experiments mice were observed for overall survival as the primary outcome measure. Tumor measurements were recorded once weekly. Mice were sacrificed if tumor volume reached 1 cm3 using the formula (width2 x height)/2. Animal care and euthanasia were carried out according to the rules and regulations as set forth by the Institutional Animal Care and Use Committee (IACUC).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7 (GraphPad Software, Inc. San Diego, CA). The differences in ADCC levels by 4-hr chromium release assays as well as the inhibition of proliferation in the EOC cell lines after exposure to drugs were evaluated by the two-tailed unpaired student t-test. Unpaired t-test was used to evaluate significant differences in the tumor volumes at specific time points in the in vivo experiments. Overall survival data were analyzed and plotted using the Kaplan-Meier method. Survival curves were compared using the log-rank test. Differences in all comparisons were considered statistically significant at p-values < 0.05.
RESULTS
HER2/neu expression in primary EOC cell lines
We evaluated erbB-2 gene amplification by FISH and HER2/neu protein expression by IHC in ten primary EOC culture cell lines (Table 1). Gene amplification and high levels (ie, 3+ staining) of HER2 protein expression by IHC were detected in 40% of the EOC cell lines (i.e. 4 out of 10). Three cell lines had low (2+) HER2 expression on IHC, while the remaining three cell lines had negligible expression (ie, 1+/0, Table 1). We utilized 4 primary EOC cell lines with similar growth rate [ie, KRCH31 (21 hrs doubling time), OVA4 (24 hrs doubling time), OVA11 (22 hrs doubling time), and OVA10 (21 hrs doubling time)] a clear cell ovarian carcinoma, for the additional in vitro and in vivo experiments described below. Supplementary Figure 1 shows representative images of KRCH31 (HER2/neu 3+) and OVA4 (HER2/neu 1+/0).
T, P, T+P and T-DM1 mediated ADCC against primary EOC
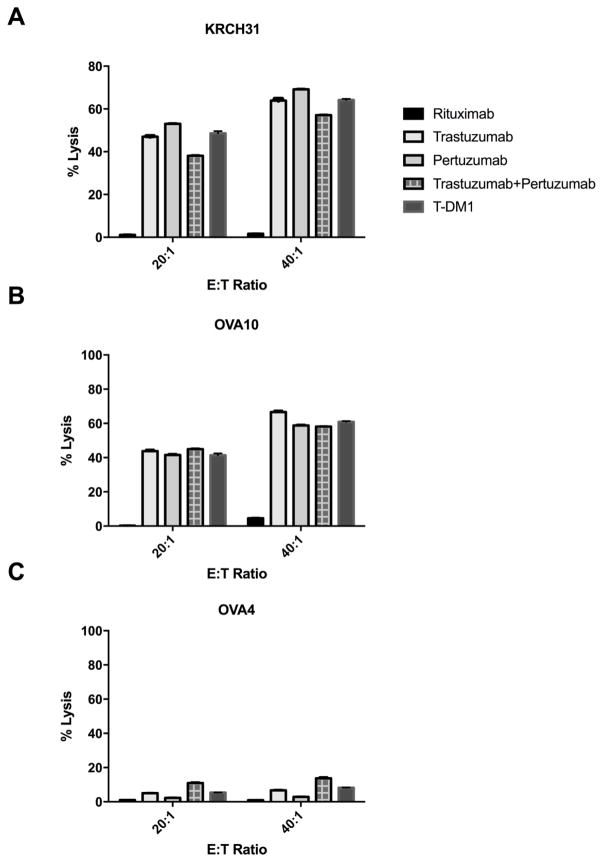
Three representative primary EOC cell lines [two HER2/neu 3+ (ie, KRCH31 and OVA10) and one HER2/neu 1+/0 (ie, OVA4)] were tested for their sensitivity to PBL-mediated cytotoxicity when challenged with heterologous PBLs collected from several healthy donors in standard 4-h 51Cr release assays. EOC cell lines were consistently found to be resistant to PBL-mediated cytotoxicity when combined with PBLs and R (2.5 μg/mL) at E:T ratios of 20:1 and 40:1 (data not shown). We then investigated the sensitivity of EOC cell lines to heterologous PBLs in the presence of T, P, T+P and T-DM1 at 2.5 μg/mL (Figure 1). T, P, T+P and T-DM1 were similarly effective in inducing strong ADCC against primary EOC cell lines expressing 3+ HER2/neu [i.e., KRCH31, OVA10] and did not induce significant cytotoxicity against the HER2/neu 1+/0 cell line [i.e., OVA4, Figure 1]. No significant difference in the level of cytotoxic activity was detected when comparing T, P, T+P and T-DM1 against any of the primary cell lines tested (p not significant).
Figure 1.
ADCC results (mean ± SD) of rituximab (R), trastuzumab (T), pertuzumab (P), trastuzumab plus pertuzumab (T+P) and T-DM1 in two representative HER2/neu 3+ expressing cell lines (KRCH31, panel A; OVA10, panel B), and one representative 1+/0 HER2 expressing cell line (OVA4, panel C).
In vitro cytotoxicity in the absence of effector cells
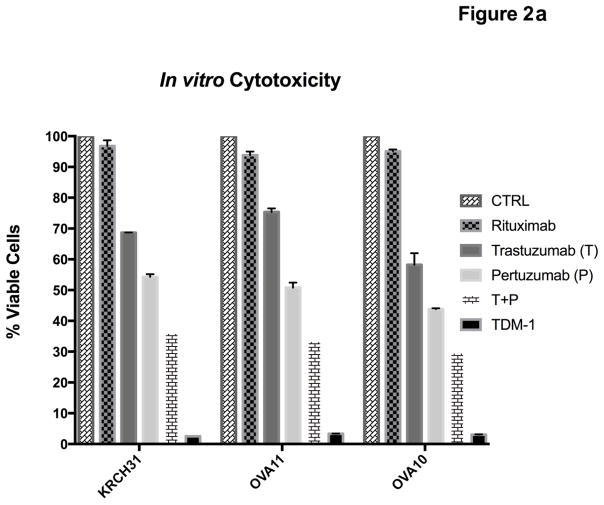
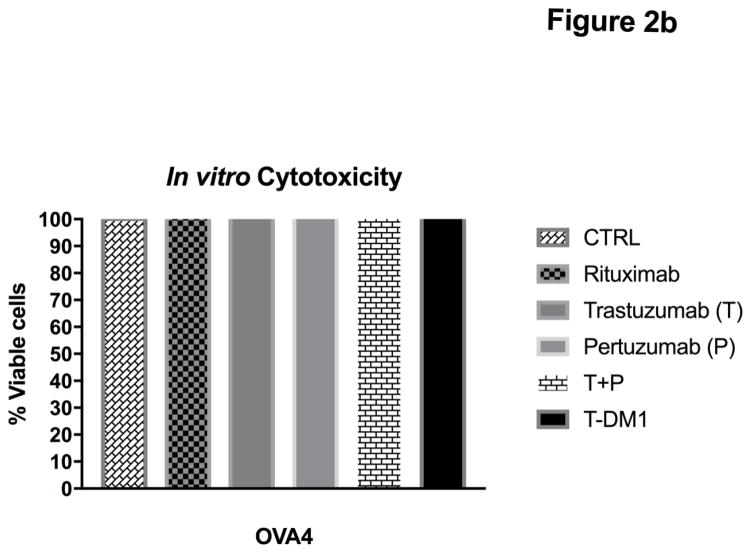
Inhibition of proliferation by T, P, T+P and T-DM1 was tested in vitro in a total of four primary EOC cell lines available [3 endowed with high HER2/neu expression (ie, KRCH31, OVA11, OVA10) and 1 with low to negligible HER2/neu expression (ie, OVA4)](Table 1). The effect of P alone (20μg/ml) was compared with that of T alone (20μg/ml), with that of a combination of both antibodies (total concentration 20μg/ml, ratio 1:1) and with that of T-DM1 (2 μg/ml). Mean percentage of viable cells + SD for T alone was 68.54±7.82; P alone was 49.80±4.51; T+P was 33.24±3.34; and T-DM1 was 3.01±0.38 (Figure 2). P was significantly more effective than T in inhibiting the proliferation of 3+ HER2/neu expressing EOC cell lines (p=0.0003). T+P was more cytostatic when compared with that of T or P used alone against all three cell lines (Figure 2, p<0.0001 and p<0.0001 respectively). Finally, T-DM1 was dramatically more potent in inducing apoptosis against the high Her2/neu expressor cell lines when compared with T+P (Figure 2, p<0.0001).
Figure 2.
Proliferation assay results demonstrating % viable cells for three HER2/neu 3+ overexpressing cell lines and one HER2/neu 1+/0 expressing control cell line (i.e., OVA4). T-DM1 significantly inhibited in vitro EOC proliferation compared to other groups (p<0.0001).
In vivo antitumor activity
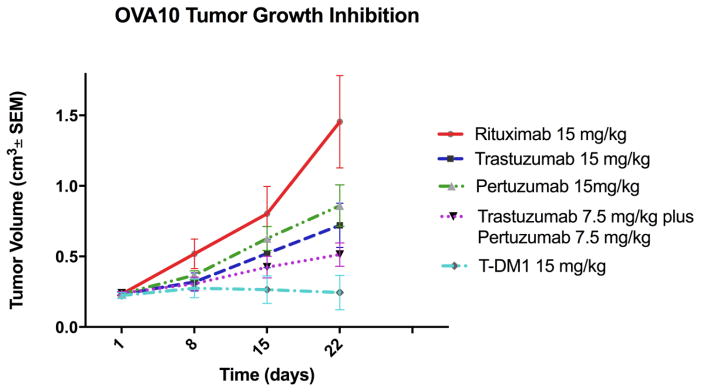
In vivo experiments comparing the antitumoral action of T, P, T+P and T-DM1 were performed using mice injected with OVA10 cells (Figure 3). The OVA10 tumor model was selected for the in vivo experiments because of the high expression of HER2/neu receptors, the in vitro sensitivity to anti-HER2/neu treatment and its ability to consistently grow as xenografts in vivo in SCID mice. As described in the Methods section, all drug treatments were given weekly for 3 to 5 weeks [18] after which mice were either immediately sacrificed for necropsy evaluation or placed into observation until death or meeting criteria for euthanasia according to the IACUC guidelines.
Figure 3.
Antitumor activity of T-DM1 compared to R, T, P and T+P in EOC xenograft tumor models with OVA10 (HER2/neu 3+). Mice were treated with three to five doses administered once a week intravenously as described in Methods. A significant difference in tumor growth inhibition was detected in T-DM1-treated group when compared to the other treatment groups.
Injections of T-DM1 showed remarkable inhibition of tumor growth in mice xenografts with 3+ HER2/neu expression (ie, OVA10, Figure 3). Accordingly, we detected a significant difference in growth inhibition emerging at 22 days (p=0.0008) for mice treated with T, at 15 days for mice treated with P (p=0.005), and at 22 days (p=0.02) for mice treated with T+P when compared to T-DM1-treated mice (Figure 3). T-DM1 was the only drug capable of consistently preventing in vivo tumor growth during treatment in most of the animals (Figure 3). Accordingly, a significant survival advantage was also seen in T-DM1-treated mice when compared to those treated with T alone (p=0.02), P alone (p=0.03) and T+P (p=0.004) (supplementary Figure 3). Importantly, four of the six T-DM1-treated mice were alive and disease free at 36 days after the completion of T-DM1 injections. In contrast, no statistically significant difference in mean overall survival were observed when comparing T+P treated mice to P-only or T-only treated mice (p not significant). Together, these data demonstrate that T-DM1 is endowed with a significantly more potent anti-tumoral effect on ovarian cancer cells when compared to T, P as well as T+P.
DISCUSSION
The dismal prognosis of advanced ovarian cancer patients has driven much research with the aim of developing novel agents to improve disease outcome. Overexpression of HER2/neu in EOC has been reported to have a considerable variation ranging from 8% to 66% [7], while the level of HER2/neu tyrosine phosphorylation has been reported to be much higher (>90%) [20]. Consistent with this view, recent studies from our group performing objective, domain-specific HER2/neu measurement in serous EOC report that the intracellular (ICD) and extracellular (ECD) HER2/neu domains are differentially expressed in a large number of HER2/neu positive ovarian carcinoma [21]. Accordingly, in this work, we demonstrated for the first time that combination of P and T has additive therapeutic effect and that T-DM1 is dramatically more potent when compared to T, P or the combination in EOC with high level of HER2/neu expression in preclinical models of chemotherapy-naïve and chemotherapy-resistant ovarian cancer.
The introduction of T in the treatment of HER2-positive metastatic breast cancer favorably changed the natural history of this disease. Although breast and ovarian cancer have notable distinctions, they may share some pathways such as HER2/neu that can be exploited for therapeutic gain. Consistent with this view, a phase II trial on the efficacy of T as single agent reported that 7% of heavily pretreated ovarian cancer patients responded with a partial response (PR), with 39% of the patients demonstrating prolonged stabilization of the disease [22].
P shows mechanistic advantages that distinguish it from T, particularly in regards to HER2/neu heterodimerization. A single-arm phase II study in which P was evaluated in patients who had received up to three T containing regimens, found a 24% response rate (RR) with 50% of patients demonstrating disease stabilization [23]. This study suggested a role of P in treating T-resistant HER2/neu positive breast cancer [23]. In EOC patients, P has been the most extensively studied HER2/neu inhibitor in ovarian cancer, with over 600 patients enrolled in three large Phase II and one Phase III trials [24–27]. Makhija et al., performed a randomized, placebo-controlled, Phase II trial of gemcitabine plus P in 130 women with platinum-resistant, recurrent ovarian carcinoma [25]. The objective RR was 13.8% among women treated with gemcitabine plus P compared with 4.6% for those treated with gemcitabine alone, favoring the P arm [72].
Another Phase III trial, PENELOPE, evaluated the addition of P to chemotherapy in platinum-resistant ovarian carcinoma patients with low HER3 expression. After investigators’ selection of the chemotherapy backbone (single-agent topotecan, weekly paclitaxel, or gemcitabine), 156 patients were randomly assigned to receive either placebo or P as an add-on. Although adding P to chemotherapy did not significantly improve PFS, subgroup analyses showed trends in PFS favoring P in the gemcitabine and paclitaxel cohorts [27].
The benefit of dual treatment with HER2/neu-targeted antibodies with complementary mechanism of growth inhibition has been explored in HER2/neu positive metastatic breast cancer. The efficacy of adding P to T plus taxanes in the front-line treatment of HER2/neu-positive metastatic breast cancer was studied in a randomized, multinational, phase III trial, CLEOPATRA (Clinical Evaluation of Pertuzumab and Trastuzumab) trial [28]. Patients were randomized to receive either P or matched placebo as an add-on to the standard-of-care T and docetaxel. The authors reported an approximate 6-month prolongation of PFS (18.5 vs 12.4 months, p<0.001) and improved ORR (80.2% vs 69.3%) in the P arm. Improvement in OS was then reported with P, T, and docetaxel after a follow-up of 50 months [29]. Consistent with these results, in our current study, we were able to demonstrate the complementary anti-tumor activity of T and P in preclinical experiments in EOC. Accordingly, after an expert panel discussion in 2014, ASCO recommended that the combination of P, T and a taxane should constitute front-line treatment for patients with HER2-positive metastatic breast cancer and T-DMl should be reserved for second-line treatment [30].
To our knowledge, this is the first report investigating the anti-tumor activity of T-DM1 in HER2/neu overexpressing EOC. Our experiments consistently demonstrated that T-DM1 has a strong growth inhibitory effect on HER2/neu-positive EOC cell lines in vitro as well as in HER2/neu-positive EOC xenografts in vivo. While both T-DM1 and T and P evoked similar ADCC against HER2/neu overexpressing EOC cell lines, T-DM1 was found to be dramatically more effective than T, P and T+P in inhibiting cell proliferation and similarly, improving survival of HER2/neu-positive xenograft-bearing mice. Future studies using primary high grade serous ovarian carcinoma xenografts overexpressing HER2/neu will be necessary to validate further the in vivo data obtained with OVA10 (ie, a clear cell ovarian cancer cell line). Our results showing strong anti-tumor activity of T-DM1 in EOC are in line with previously reported data from HER2/neu-positive metastatic breast cancer. EMILIA, the Phase III, randomized, international trial enrolled over 900 women with HER2/neu-positive locally advanced or metastatic breast cancer who had previously been treated with T and a taxane [31]. Patients were randomized to T-DMl or lapatinib plus capecitabine in a 1:1 ratio and the primary end points of EMILIA were independently assessed PFS and OS. T-DM1 demonstrated superior activity to lapatinib-capecitabine with significantly improving PFS by 3.2 months and OS by 5.8 months with less toxicity in a population of T failures [31]. Based on these promising results demonstrated in EMILIA, the US FDA approved T-DM1 as the first ADC available for women with advanced HER2/neu-positive breast cancer in 2013. These data set the stage for the MARIANNE trial which was designed to answer an important clinical question of whether T-DM1-containing regimens are better than or non-inferior to T plus a taxane in the front-line setting [32]. In this phase III trial, over 1000 patients were randomized to receive T plus a taxane (control arm), T-DM1 plus placebo (T-DM1 arm), or T-DM1 plus P. T-DM1 and T-DM1 plus P showed non-inferior PFS compared with the control arm, but failed to show superiority (median PFS was 13.7 months with T plus a taxane, 14.1 months with T-DM1, and 15.2 months with T-DM1 plus P). Of interest, fewer patients discontinued treatment because of adverse events, and health-related quality of life was maintained for longer in the T-DM1 arm [32]. Based on the results from EMILIA and MARIANNE trials, the National Comprehensive Cancer Network (NCCN) has recently added T-DM1 as one of the first-line treatment option for patients with HER2/neu-positive metastatic breast cancer who are not considered candidates with the preferred regimen of P, T, and taxane [33]. Because of the superior anti-tumor activity of T-DM1 in comparison to other clinically available HER2/neu-targeted drugs and its favorable adverse effect profile, the preclinical data presented here strongly support the clinical evaluation of T-DM1 for the treatment of chemotherapy-resistant EOC.
In conclusion, our preclinical in vitro and in vivo experiments with 3+ HER2/neu expressing EOC cell lines and xenografts revealed limited anti-tumor activity of T or P, in line with previously published studies [34, 35]. In contrast, T-DM1 showed superior anti-tumor activity to single agent T or P as well as to the combination of both. T-DM1 may represent a valid treatment option for HER2/neu-positive EOC patients with recurrent disease resistant to chemotherapy.
Supplementary Material
Highlights.
T-DM1 is a novel ADC with remarkable activity against HER2 amplified EOC.
T-DM1 is more potent than T+P against EOC with HER2/neu expression.
Clinical studies with T-DM1 in HER2 overexpressing EOC are encouraged.
Acknowledgments
Financial and grant support: This work was supported in part by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, and grants from the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to A.D. Santin. This investigation was also supported by NIH Research Grant CA-16359 from the NCI to A.D. Santin.
Footnotes
Disclosure of Potential Conflicts of Interest. The authors declare no conflict of interest or previous publication. All of the authors fulfill the conditions required for authorship.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Herzog TJ. The current treatment of recurrent ovarian cancer. Current oncology reports. 2006;8:448–54. doi: 10.1007/s11912-006-0074-9. [DOI] [PubMed] [Google Scholar]
- 3.Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Noske A, Schwabe M, Weichert W, Darb-Esfahani S, Buckendahl AC, Sehouli J, et al. An intracellular targeted antibody detects EGFR as an independent prognostic factor in ovarian carcinomas. BMC Cancer. 2011;11:294. doi: 10.1186/1471-2407-11-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steffensen KD, Waldstrom M, Jeppesen U, Jakobsen E, Brandslund I, Jakobsen A. The prognostic importance of cyclooxygenase 2 and HER2 expression in epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17:798–807. doi: 10.1111/j.1525-1438.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 7.Wen W, Chen WS, Xiao N, Bender R, Ghazalpour A, Tan Z, et al. Mutations in the Kinase Domain of the HER2/ERBB2 Gene Identified in a Wide Variety of Human Cancers. The Journal of molecular diagnostics: JMD. 2015;17:487–95. doi: 10.1016/j.jmoldx.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh R, Narasanna A, Wang SE, Liu S, Chakrabarty A, Balko JM, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71:1871–82. doi: 10.1158/0008-5472.CAN-10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- 11.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 12.Chung A, Cui X, Audeh W, Giuliano A. Current status of anti-human epidermal growth factor receptor 2 therapies: predicting and overcoming herceptin resistance. Clinical breast cancer. 2013;13:223–32. doi: 10.1016/j.clbc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 14.Metzger-Filho O, Winer EP, Krop I. Pertuzumab: optimizing HER2 blockade. Clin Cancer Res. 2013;19:5552–6. doi: 10.1158/1078-0432.CCR-13-0518. [DOI] [PubMed] [Google Scholar]
- 15.Black J, Menderes G, Bellone S, Schwab CL, Bonazzoli E, Ferrari F, et al. SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine Serous Carcinoma with HER2/Neu Expression. Mol Cancer Ther. 2016;15:1900–9. doi: 10.1158/1535-7163.MCT-16-0163. [DOI] [PubMed] [Google Scholar]
- 16.Varughese J, Cocco E, Bellone S, Bellone M, Todeschini P, Carrara L, et al. High-grade, chemotherapy-resistant primary ovarian carcinoma cell lines overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Gynecol Oncol. 2011 Mar 30; doi: 10.1016/j.ygyno.2011.03.002. 2011 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skirnisdottir I, Sorbe B, Seidal T. The growth factor receptors HER-2/neu and EGFR, their relationship, and their effects on the prognosis in early stage (FIGO I–II) epithelial ovarian carcinoma. Int J Gynecol Cancer. 2001;11:119–29. doi: 10.1046/j.1525-1438.2001.011002119.x. [DOI] [PubMed] [Google Scholar]
- 18.English DP, Bellone S, Schwab CL, Bortolomai I, Bonazzoli E, Cocco E, et al. T-DM1, a novel antibody-drug conjugate, is highly effective against primary HER2 overexpressing uterine serous carcinoma in vitro and in vivo. Cancer Med. 2014;3:1256–65. doi: 10.1002/cam4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black JD, Lopez S, Cocco E, Bellone S, Altwerger G, Schwab CL, et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br J Cancer. 2015;113:1020–6. doi: 10.1038/bjc.2015.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–81. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 21.Carvajal-Hausdorf DE, Schalper KA, Bai Y, Black J, Santin AD, Rimm DL. Objective, domain-specific HER2 measurement in uterine and ovarian serous carcinomas and its clinical significance. Gynecol Oncol. 2017 doi: 10.1016/j.ygyno.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283–90. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–44. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon MS, Matei D, Aghajanian C, Matulonis UA, Brewer M, Fleming GF, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. J Clin Oncol. 2006;24:4324–32. doi: 10.1200/JCO.2005.05.4221. [DOI] [PubMed] [Google Scholar]
- 25.Makhija S, Amler LC, Glenn D, Ueland FR, Gold MA, Dizon DS, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol. 2010;28:1215–23. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 26.Kaye SB, Poole CJ, Danska-Bidzinska A, Gianni L, Del Conte G, Gorbunova V, et al. A randomized phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. Ann Oncol. 2013;24:145–52. doi: 10.1093/annonc/mds282. [DOI] [PubMed] [Google Scholar]
- 27.Kurzeder C, Bover I, Marme F, Rau J, Pautier P, Colombo N, et al. Double-Blind, Placebo-Controlled, Randomized Phase III Trial Evaluating Pertuzumab Combined With Chemotherapy for Low Tumor Human Epidermal Growth Factor Receptor 3 mRNA-Expressing Platinum-Resistant Ovarian Cancer (PENELOPE) J Clin Oncol. 2016;34:2516–25. doi: 10.1200/JCO.2015.66.0787. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2078–99. doi: 10.1200/JCO.2013.54.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J Clin Oncol. 2017;35:141–8. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Breast cancer version 2.2016. doi: 10.6004/jnccn.2016.0051. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp - site. [DOI] [PubMed]
- 34.Montero JC, Garcia-Alonso S, Ocana A, Pandiella A. Identification of therapeutic targets in ovarian cancer through active tyrosine kinase profiling. Oncotarget. 2015;6:30057–71. doi: 10.18632/oncotarget.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sims AH, Zweemer AJ, Nagumo Y, Faratian D, Muir M, Dodds M, et al. Defining the molecular response to trastuzumab, pertuzumab and combination therapy in ovarian cancer. Br J Cancer. 2012;106:1779–89. doi: 10.1038/bjc.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.