Abstract
Background
The efficacy of intravenous acetaminophen compared to its oral formulation for postoperative analgesia is unknown. We hypothesized that the addition of acetaminophen to a multimodal analgesia regimen would provide improved pain management in patients following total knee arthroplasty (TKA) and that the effect of acetaminophen would be variable based upon route of delivery.
Methods
The study was a single center, randomized, double-blinded, placebo-controlled clinical trial on the efficacy of intravenous versus oral acetaminophen in patients undergoing unilateral TKA. One hundred and seventy-four subjects were randomized to one of three groups: intravenous acetaminophen group (IV Group, n=57) received 1-gram intravenous acetaminophen and oral placebo prior to post-anesthesia care unit (PACU) admission; oral acetaminophen group (PO Group, n=58) received 1-gram oral acetaminophen and volume-matched intravenous normal saline; placebo group (Placebo Group, n=59) received oral placebo and volume-matched intravenous normal saline. Pain scores were obtained every 15 minutes during PACU stay. Average pain scores, maximum pain score, and pain scores before physical therapy were compared among the three groups. Secondary outcomes included total opiate consumption, time to PACU discharge, time to rescue analgesia, and time to breakthrough pain.
Results
The average PACU pain score was similar in the IV Group (0.56 ±0.99 [mean ±SD]) compared to the PO Group (0.67 ±1.20) (P=0.84) and Placebo Group (0.58 ±0.99) (P=0.71). Total opiate consumption at 6 hours (0.47mg hydromorphone equivalents ±0.56 vs 0.54 ±0.53 vs 0.54 ±0.61; P=0.69) and 24 hours (1.25 ±1.30 vs 1.49 ±1.34 vs 1.36 ±1.31; P=0.46) were also similar between the IV, PO, and Placebo Groups. No significant differences were found between all groups for any other outcome.
Conclusion
Neither intravenous nor oral acetaminophen provides additional analgesia in the immediate postoperative period when administered as an adjunct to multimodal analgesia in patients undergoing TKA in the setting of a spinal anesthetic.
Introduction
Over 80% of patients undergoing surgery experience some degree of pain postoperatively with a majority of these patients rating the pain as moderate to severe [1, 2]. Inadequate pain control following surgery may contribute to several complications including a higher incidence of myocardial infarction, impaired wound healing, and poor respiratory effort [3]. Treating postoperative pain with analgesics such as opioids is not without adverse effects as opioids may cause drowsiness, postoperative nausea and vomiting, ileus, respiratory depression, and bladder dysfunction [4]. The use of intravenous acetaminophen has been shown to reduce pain scores and consumption of opioids following surgery [5-7]; however, oral acetaminophen has demonstrated similar results to its intravenous counterpart in some patient populations [8].
When comparing intravenous and oral acetaminophen, the bioavailability in cerebrospinal fluid and plasma is superior with the intravenous formulation [3, 9]. How this translates clinically is unclear as studies between intravenous and oral acetaminophen are lacking and have yet to demonstrate a benefit of intravenous acetaminophen when compared to oral acetaminophen [10, 11]. The cost of intravenous acetaminophen is much higher than the oral formulation, and the reimbursement is typically lower [12, 13].
Intravenous acetaminophen has the potential to be a versatile addition to multimodal analgesia regimens in the perioperative setting. Therefore, we conducted a randomized, double-blind, clinical trial to compare intravenous acetaminophen to the oral formulation. We hypothesized that the addition of acetaminophen to a multimodal analgesia regimen would provide improved pain management in patients following total knee arthroplasty (TKA) and that the effect of acetaminophen would be variable based upon route of delivery.
Study Methods
Study Overview
The study was a single center, randomized, double-blind, placebo-controlled clinical trial to assess the efficacy of intravenous acetaminophen compared to oral acetaminophen in patients undergoing unilateral total knee arthroplasty (TKA) under spinal anesthesia. Institutional review board approval was obtained prior to patient recruitment. All TKA surgeries were completed by a single orthopedic surgeon. Standard preoperative pain medication regimen included doses of celecoxib and OxyContin. Exceptions to administration of celecoxib included documented allergy, previous gastrointestinal bleeding, or intolerance to this medication. Exceptions to OxyContin administration included known allergy or intolerance to this medication. Intraoperatively, all patients received a peri-capsular injection of 300 mg ropivicaine, 30 mg ketorolac, 0.08 mg clonidine, 1 mg epinephrine in a total volume of 100cc of 0.9% sodium chloride 0.9% into the knee joint [14]. In addition, a majority of patients received intravenous dexamethasone (4mg to 10mg) intraoperatively at the discretion of the in-room anesthesia provider prior to surgical incision. Following surgery, all subjects were administered study medications in the operating room prior to admission in the post-anesthesia care unit (PACU). Data was then collected throughout the PACU stay. The study was registered at ClinicalTrials.gov (NCT02216682) prior to patient enrollment.
Subject Selection
Male and female patients 18 years of age or older undergoing unilateral TKA under spinal anesthesia were eligible for the study. Subjects were excluded from the study if spinal anesthesia failed intraoperatively; were pregnant; weighed less than 50 kilograms; had a history of chronic opiate use (greater than 60 mg morphine equivalents per day); had a history of liver disease; had a known allergy/hypersensitivity to acetaminophen or opiates; had a history of dementia; had a history of alcohol abuse; had a history of renal impairment; or used acetaminophen within 24 hours of surgery. Written informed consent was obtained for every participating subject. A member of the study staff monitored data collection biweekly to ensure the safety of study subjects and validity of data collection.
Study Design
A total of 174 eligible subjects were randomized to one of three groups with a computerized random number generator prior to their surgery. The intravenous acetaminophen group (IV Group, n=57) received 1-gram intravenous acetaminophen and oral placebo. The oral acetaminophen group (PO Group, n=58) received 1-gram oral acetaminophen and volume-matched intravenous normal saline (100mL). The placebo group (Placebo Group, n=59) received oral placebo and volume-matched intravenous normal saline (100mL). Study medications were prepared and blinded with study labels by the pharmacy for each subject based on group randomization. Study medications were administered at the conclusion of surgery and prior to admission in the post-anesthesia care unit (PACU) by the in-room anesthesia provider.
Postsurgical Assessments
Pain intensity was assessed using the numeric rating scale (NRS). The 11-point NRS score (0=no pain; 10=worst pain possible) was collected every 15 minutes in the PACU for up to four hours by a research assistant. Pain scores were also obtained prior to the first physical therapy session done in the PACU. The total duration of spinal block was reported in minutes from time of placement to the time motor function returned to baseline.
Outcomes Measures
The primary outcome of pain score was measured with the NRS. Secondary outcomes included total opiate consumption (converted to intravenous hydromorphone equivalents in milligrams) within 6 and 24 hours of surgery, time to rescue analgesia (minutes), time to breakthrough pain (minutes), time until ready for PACU discharge (minutes) defined by resolution of spinal anesthetic, hemodynamic stability, and adequate pain control.
Statistical Analysis
As part of the study design, a power analysis was conducted. Under the assumption that the 3 subject groups were normally distributed with a pain score standard deviation of 2.5, and if there was true association between the treatment group and pain reduction, we calculated 174 subjects were necessary to be able to reject the null hypothesis with a power of 0.8. The Type I error probability associated with this test of the null hypothesis was 0.025.
Baseline characteristics were compared between the 3 study groups. Categorical data were compared using the Fisher's exact test, and the Kruskal-Wallis procedure was used for continuous data. The primary outcome of this analysis was compared between all 3 groups using the Kruskal-Wallis procedure. Additionally, differences were compared between the following groups using the Wilcoxon-rank sum test: IV acetaminophen vs. PO acetaminophen; IV acetaminophen vs. placebo; and PO acetaminophen vs. placebo. Statistical significance was defined as p ≤ 0.05. SAS (SAS Institute, Cary, NC) version 9.4 was used for all analyses.
Results
One hundred and seventy-five subjects were recruited for the study, but one was excluded due to failed spinal anesthesia (Figure 1). Baseline patient demographics including age, body mass index, gender, and American Society of Anesthesiologists physical status were similar across the three groups (Table 1). There were no differences among the groups with respect to return of motor function or length of surgery. Also, preoperative and intraoperative analgesics administered to patients were similar between the groups (Table 2).
Figure 1.
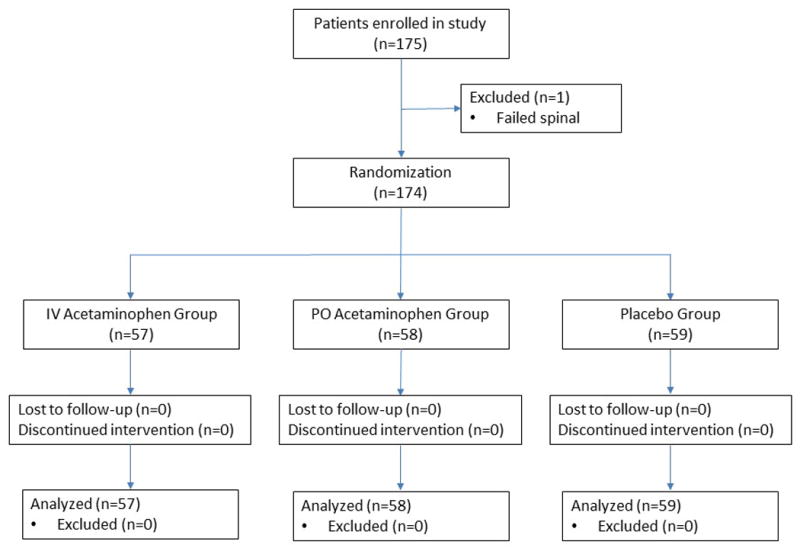
Table 1. Patient demographics.
| IV Group (n=57) |
PO Group (n=58) |
Placebo Group (n=59) |
P* | |
|---|---|---|---|---|
| Age, years | 0.15 | |||
| mean ±SD | 68 ±8.3 | 67 ±9.0 | 70 ±8.8 | |
| median (25th-75th percentiles) | 68 (64-72) | 68 (59-73) | 70 (63-77) | |
| Male, n (%) | 32 (56) | 26 (45) | 25 (42) | 0.30 |
| Body mass index, kg/m2 | 0.056 | |||
| mean ±SD | 28 ±4.7 | 31 ±5.1 | 29 ±4.8 | |
| median (25th-75th percentiles) | 29 (25-32) | 30 (28-34) | 28 (25-31) | |
| ASA Physical Status, n (%) | 0.95 | |||
| I | 3 (5) | 2 (3) | 3 (5) | |
| II | 40 (70) | 43 (74) | 40 (68) | |
| III | 14 (25) | 13 (23) | 16 (27) | |
| Length of surgery, minutes | 0.21 | |||
| mean ±SD | 73 ±10 | 79 ±18 | 78 ±11 | |
| median (25th-75th percentiles) | 72 (66-82) | 73 (68-86) | 77 (71-86) | |
| Return of motor function, minutes | ||||
| mean ±SD | 229 ±81 | 216 ±72 | 233 ±75 | 0.50 |
| median (25th-75th percentiles) | 225 (165-280) | 230 (145-270) | 240 (180-280) |
SD = standard deviation; ASA = American Society of Anesthesiologists.
Categorical data compared using the Fisher's exact test and the Kruskal-Wallis test was used to compare continuous data.
Table 2. Perioperative characteristics.
| IV Group (n=57) |
PO Group (n=58) |
Placebo Group (n=59) |
P | |
|---|---|---|---|---|
| Preoperative analgesics, n (%) | ||||
| Celecoxib | 47 (82) | 48 (83) | 51 (86) | 0.84 |
| OxyContin | 39 (68) | 42 (72) | 46 (77) | 0.51 |
| Intraoperative analgesics, n (%) | ||||
| Fentanyl | 5 (9) | 3 (5) | 3 (5) | 0.68 |
| Hydromorphone | 0 (0) | 2 (3) | 2 (3) | 0.55 |
| Morphine | 0 (0) | 0 (0) | 1 (2) | 1.0 |
| Dexamethasone | 51 (89) | 51 (88) | 52 (88) | 1.0 |
Data compared using the Fisher's exact test.
Pain scores were similar when compared across the groups (Table 3). The average pain scores in the PACU were 0.56 ±0.99 [mean ±SD] in the IV Group, 0.67 ±1.20 in the PO Group and 0.58 ±0.99 in the Placebo group (P=0.71). The pain scores prior to the first physical therapy session were 2.55 ±1.73 in the IV Group, 2.85 ±1.79 in the PO Group, and 2.32 ±1.90 in the Placebo Group (P=0.09). When comparing all collected pain scores, no statistically significant differences were seen across the groups or between each group compared to one another.
Table 3. Pain scores across all groups.
| IV Group (n=57) |
PO Group (n=58) |
Placebo Group (n=59) |
P-value* | P-value† | P-value‡ | P-value‖ | |
|---|---|---|---|---|---|---|---|
| Average PACU pain score | 0.93 | 0.84 | 0.92 | 0.71 | |||
| mean ±SD | 0.56 ±0.99 | 0.67 ±1.20 | 0.58 ±0.99 | ||||
| median (25th-75th percentiles) | 0 (0-0.82) | 0 (0-0.69) | 0 (0-0.82) | ||||
|
| |||||||
| Maximum PACU pain score | 0.95 | 0.99 | 0.79 | 0.78 | |||
| mean ±SD | 1.60 ±2.19 | 1.55 ±2.10 | 1.47 ±2.05 | ||||
| median (25th-75th percentiles) | 0 (0-3) | 0 (0-3) | 0 (0-3) | ||||
|
| |||||||
| Pain score before PT | 0.19 | 0.39 | 0.29 | 0.085 | |||
| mean ±SD | 2.55 ±1.73 | 2.85 ±1.79 | 2.32 ±1.90 | ||||
| median (25th-75th percentiles) | 2.5 (1.3-3.5) | 3.0 (1.5-4.0) | 2.0 (1.0-3.5) | ||||
PT = physical therapy; SD = standard deviation; PACU = post-anesthesia care unit.
Comparison between all groups was performed with the Kruskal-Wallis test.
Comparison between IV Group and PO Group was performed with the Wilcoxon rank sum.
Comparison between IV Group and Placebo Group was performed with the Wilcoxon rank sum.
Comparison between PO Group and Placebo Group was performed with the Wilcoxon rank sum.
The times to breakthrough pain were 234 ±207 in the IV Group, 245 ±269 minutes in the PO Group, and 204 ±146 minutes in the Placebo Group (P=0.92) (Table 4). While times to rescue analgesia were 270 ±230 minutes in the IV Group, 283 ±271 minutes in the PO Group, and 239 ±194 minutes in the Placebo Group (P=0.47). The difference in time to breakthrough pain and time to rescue analgesia were not significant between each group compared to one another either.
Table 4. Secondary outcomes across all groups.
| IV Group (n=57) |
PO Group (n=58) |
Placebo Group (n=59) |
P-value* | P-value† | P-value‡ | P-value‖ | |
|---|---|---|---|---|---|---|---|
| Time to breakthrough pain, minutes | 0.80 | 0.64 | 0.52 | 0.92 | |||
| mean ±SD | 234 ±207 | 245 ±269 | 204 ±146 | ||||
| median (25th-75th percentiles) | 170 (118-299) | 165 (95-295) | 170 (105-260) | ||||
|
| |||||||
| Time to rescue analgesia, minutes | 0.72 | 0.98 | 0.50 | 0.47 | |||
| mean ±SD | 270 ±230 | 283 ±271 | 239 ±194 | ||||
| median (25th-75th percentiles) | 203 (113-370) | 216 (120-325) | 175 (111-300) | ||||
|
| |||||||
| Total opiate consumption, 6 hours | 0.60 | 0.30 | 0.57 | 0.69 | |||
| mean ±SD | 0.47 ±0.56 | 0.54 ±0.53 | 0.54 ±0.61 | ||||
| median (25th-75th percentiles) | 0.38 (0-0.75) | 0.38 (0-0.75) | 0.38 (0-0.75) | ||||
|
| |||||||
| Total opiate consumption, 24 hours | 0.48 | 0.23 | 0.67 | 0.46 | |||
| mean ±SD | 1.25 ±1.30 | 1.49 ±1.34 | 1.36 ±1.31 | ||||
| median (25th-75th percentiles) | 0.75 (0.38-1.88) | 1.14 (0.38-2.25) | 0.94 (0.38-2.38) | ||||
|
| |||||||
| Time to PACU discharge, minutes | 0.39 | 0.17 | 0.46 | 0.57 | |||
| mean ±SD | 175 ±75 | 155 ±55 | 164 ±70 | ||||
| median (25th-75th percentiles) | 165 (129-205) | 145 (110-195) | 150 (120-210) | ||||
Total opiate consumption reported in IV hydromorphone (millgrams) equivalents.
SD = standard deviation; PACU = post-anesthesia care unit.
Comparison between all groups was performed with the Kruskal-Wallis test.
Comparison between IV Group and PO Group was performed with the Wilcoxon rank sum.
Comparison between IV Group and Placebo Group was performed with the Wilcoxon rank sum.
Comparison between PO Group and Placebo Group was performed with the Wilcoxon rank sum.
Opiate consumptions at 6 hours following surgery were 0.47 ±0.56mg in the IV Group, 0.54 ±0.53mg in the PO Group, and 0.54 ±0.61mg in the Placebo Group (P=0.69). At 24 hours following surgery, opiate consumptions were 1.25 ±1.30mg in the IV Group, 1.49 ±1.34mg in the PO Group, and 1.36 ±1.31mg in the Placebo Group (P=0.46). There were no statistically significant differences in total opiate consumption within 6 or 24 hours or time to PACU discharge when comparing the groups to one another or between all three groups.
Discussion
The results of our randomized, double-blind, clinical trial do not suggest a benefit of using acetaminophen, either intravenously or orally, for pain control in the perioperative period following TKA when compared to placebo. Additionally, pain scores and opiate consumption were not statistically different between the interventional groups.
The findings of this analysis are consistent with other studies investigating a difference between the two formulations. A randomized non-inferiority clinical trial on patients undergoing lower third molar extractions found similar pain scores and opiate consumption between patients receiving oral acetaminophen versus intravenous acetaminophen [10]. A study in which patients undergoing laparoscopic cholecystectomy were randomized to intravenous or oral acetaminophen also did not find a significant difference in pain scores or opiate consumption within 24 hours between the groups, although this was a preliminary trial [15]. Another clinical trial with a similar design to our study randomized patients undergoing total knee arthroplasty to receive either intravenous or oral acetaminophen and showed lower pain scores immediately after surgery in the intravenous acetaminophen group (P=0.03), but pain scores over the first 24 hours and opiates consumed did not differ between the two groups [11]. Our data provide further evidence that intravenous acetaminophen does not offer a benefit compared to the oral formulation and neither formulation provided value as an adjunct analgesic following TKA.
Multiple studies have shown reduced pain scores and decreased postoperative opiate consumption in surgical patients receiving either formulation of acetaminophen when used as a component of a multimodal approach in various surgical settings [3, 6, 16]. A majority of these studies administered acetaminophen pre-operatively, whereas in our study, it was administered postoperatively. Pre-emptive analgesia, or analgesia received before a surgical stimulus, may affect nociceptive receptors differently than preventive or rescue analgesia and potentially result in improved outcomes. However, a previous study on pre-emptive versus preventive use of IV acetaminophen in patients undergoing lower extremity orthopedic surgery under spinal was not shown to have a difference in pain control [17]. This study did show that both the preemptive and preventive groups had a decrease in pain scores at 6 hours after surgery compared with placebo (p<0.001). Thus, the timing of our study drug administration likely did not influence the results of the study.
Another consideration for our null findings was the use of a multimodal analgesia regimen on all subjects in the study. Every subject received a peri-articular injection of ropivacaine/ketorolac/epinephrine/clonidine at the end of the operation. Several studies have demonstrated better pain relief and less opiate consumption in total knee replacement patients receiving multimodal drug injections [14]. Also, a majority of patients received both celecoxib and OxyContin pre-operatively, and these medications when given pre-emptively have been shown to improve pain relief in surgical patients [18-20]. A concern raised by the institutional review board (IRB), was uncontrolled pain in patients undergoing knee replacement surgery without a preoperative regional block, however, regional blocks are no longer standard care at our institution. For IRB approval, standardizing a multimodal approach was the compromise for our study. The standardized approach may have decreased postoperative pain to a degree in which the effect of administering acetaminophen may have been diminished. Future studies should consider a design which eliminates or reduces the multimodal analgesia patients receive to obtain a more focused comparison of the different formulations of acetaminophen and their influence on postoperative pain and opiate consumption.
The multimodal analgesic approach consisting of pre-emptive and peri-capsular injection analgesia provided adequate pain control in our surgical population. This is supported by the low average PACU pain scores (NRS 0.58 ±0.99) and opiate consumption (6h, 0.54 ±0.61 mg equivalents of intravenous hydromorphone; 24h, 1.36 ±1.31) in the placebo group. The pain scores and opiate requirements were similar between the interventional groups and the placebo group, and overall, pain was well controlled across all the groups. Thus, our results do not suggest improvement in pain control with the use of acetaminophen in patients experiencing mild post-operative pain, but do support a multimodal analgesic approach in the peri-operative care of patients undergoing unilateral TKA.
The duration of spinal block did not differ between the three groups, but the return of motor function to baseline was around 3.5 hours on average. Knowing that motor function would return to patients before sensation, the peak effects of intravenous and/or oral acetaminophen still may have been concealed by the spinal anesthesia. This was a consideration while formulating the protocol for our study and the main factor in administering the study drug after conclusion of the procedure and just prior to arrival in the PACU and not before surgery. Even so, the duration of acetaminophen should have lasted well beyond the spinal block.
The limitations of this study include a lack of standardization for preoperative and intraoperative care for patients in the study. However, there were no differences between preoperative analgesics and intraoperative analgesics administered across the groups. Another consideration is that patients received peri-articular injections, as it is considered standard of care at our institution. Average pain scores were less than 1 across all groups, and the analgesic effects from this injection and the period of sensory loss with spinal anesthesia may have mitigated the ability to detect a difference in pain control between intravenous and oral acetaminophen even if one was present.
Conclusion
Acetaminophen, irrespective of formulation, does not have adjunctive value as a component of a multimodal analgesic approach when administered immediately following surgery in patients undergoing TKA in the setting of a spinal anesthetic. A multimodal analgesic regimen in TKA patients does provide adequate post-operative pain relief.
Acknowledgments
The authors thank Katherine Koury for her assistance with IRB submission and study design.
Funding: This work was supported by the Department of Anesthesia and Critical Care and Pain Management Pilot Grant at Massachusetts General Hospital. This work was also supported by the National Institutes of Health under award numbers T32GM108554 (JBO) and F32HL134290 (WTO). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interests: none
References
- 1.Gan TJ, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–60. doi: 10.1185/03007995.2013.860019. [DOI] [PubMed] [Google Scholar]
- 2.Apfelbaum JL, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. table of contents. [DOI] [PubMed] [Google Scholar]
- 3.Smith HS. Perioperative intravenous acetaminophen and NSAIDs. Pain Med. 2011;12(6):961–81. doi: 10.1111/j.1526-4637.2011.01141.x. [DOI] [PubMed] [Google Scholar]
- 4.Dahl JB, et al. Prevention of postoperative pain by balanced analgesia. Br J Anaesth. 1990;64(4):518–20. doi: 10.1093/bja/64.4.518. [DOI] [PubMed] [Google Scholar]
- 5.Memis D, et al. Intravenous paracetamol reduced the use of opioids, extubation time, and opioid-related adverse effects after major surgery in intensive care unit. J Crit Care. 2010;25(3):458–62. doi: 10.1016/j.jcrc.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Pergolizzi JV, Jr, et al. Continuous multimechanistic postoperative analgesia: a rationale for transitioning from intravenous acetaminophen and opioids to oral formulations. Pain Pract. 2012;12(2):159–73. doi: 10.1111/j.1533-2500.2011.00476.x. [DOI] [PubMed] [Google Scholar]
- 7.Sinatra RS, et al. Intravenous acetaminophen for pain after major orthopedic surgery: an expanded analysis. Pain Pract. 2012;12(5):357–65. doi: 10.1111/j.1533-2500.2011.00514.x. [DOI] [PubMed] [Google Scholar]
- 8.Toms L, et al. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev. 20084 doi: 10.1002/14651858.CD004602.pub2. Cd004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singla NK, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12(7):523–32. doi: 10.1111/j.1533-2500.2012.00556.x. [DOI] [PubMed] [Google Scholar]
- 10.Fenlon S, et al. Oral vs intravenous paracetamol for lower third molar extractions under general anaesthesia: is oral administration inferior? Br J Anaesth. 2013;110(3):432–7. doi: 10.1093/bja/aes387. [DOI] [PubMed] [Google Scholar]
- 11.Politi JR, Davis RL, 2nd, Matrka AK. Randomized Prospective Trial Comparing the Use of Intravenous Versus Oral Acetaminophen in Total Joint Arthroplasty. J Arthroplasty. 2016 doi: 10.1016/j.arth.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 12.O'Neal JB. The utility of intravenous acetaminophen in the perioperative period. Front Public Health. 2013;1:25. doi: 10.3389/fpubh.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koons C. This Drugmaker Suffered the Consequences of Price Increases. 2015 [cited 2017 3/1/2017]; Available from: https://www.bloomberg.com/news/articles/2015-10-12/how-one-drugmaker-learned-the-consequences-of-price-increases.
- 14.Jiang J, et al. The efficacy of periarticular multimodal drug injection for postoperative pain management in total knee or hip arthroplasty. J Arthroplasty. 2013;28(10):1882–7. doi: 10.1016/j.arth.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Plunkett A, et al. A Preliminary Examination of the Comparative Efficacy of Intravenous vs Oral Acetaminophen in the Treatment of Perioperative Pain. Pain Med. 2016 doi: 10.1093/pm/pnw273. [DOI] [PubMed] [Google Scholar]
- 16.Lee Z, et al. A Non-narcotic Pathway for the Management of Postoperative Pain Following Pediatric Robotic Pyeloplasty. J Endourol. 2017 doi: 10.1089/end.2016.0846. [DOI] [PubMed] [Google Scholar]
- 17.Khalili G, et al. Effect of preemptive and preventive acetaminophen on postoperative pain score: a randomized, double-blind trial of patients undergoing lower extremity surgery. J Clin Anesth. 2013;25(3):188–92. doi: 10.1016/j.jclinane.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Garcia RM, et al. A multimodal approach for postoperative pain management after lumbar decompression surgery: a prospective, randomized study. J Spinal Disord Tech. 2013;26(6):291–7. doi: 10.1097/BSD.0b013e318246b0a6. [DOI] [PubMed] [Google Scholar]
- 19.Trabulsi EJ, et al. Preemptive multimodal pain regimen reduces opioid analgesia for patients undergoing robotic-assisted laparoscopic radical prostatectomy. Urology. 2010;76(5):1122–4. doi: 10.1016/j.urology.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Kim SI, Ha KY, Oh IS. Preemptive multimodal analgesia for postoperative pain management after lumbar fusion surgery: a randomized controlled trial. Eur Spine J. 2016;25(5):1614–9. doi: 10.1007/s00586-015-4216-3. [DOI] [PubMed] [Google Scholar]


